Abstract
The mechanisms underlying spontaneous neurotransmitter release are not well understood. Under physiological as well as pathophysiological circumstances, spontaneous fusion events can set the concentration of ambient levels of neurotransmitter within the synaptic cleft and in the extracellular milieu. In the brain, unregulated release of excitatory neurotransmitters, exacerbated during pathological conditions such as stroke, can lead to neuronal damage and death. In addition, recent findings suggest that under physiological circumstances spontaneous release events can trigger postsynaptic signaling events independent of evoked neurotransmitter release. Therefore, elucidation of mechanisms underlying spontaneous neurotransmission may help us better understand the functional significance of this form of release and provide tools for its selective manipulation. For instance, our recent investigations indicate that the level of cholesterol in the synapse plays a critical role in limiting spontaneous synaptic vesicle fusion. Therefore, alterations in synaptic cholesterol metabolism can be a critical determinant of glutamatergic neurotransmission at rest. This article aims to provide a closer look into our current understanding of the mechanisms underlying spontaneous neurotransmission and the signaling triggered by these unitary release events.
Keywords: Spontaneous neurotransmission, synaptic vesicle recycling, SNARE, miniature synaptic transmission, synaptotagmin, cholesterol
Introduction
Spontaneous neurotransmitter release in the absence of presynaptic action potentials is a common property of all synapses (Katz, 1969). Several studies, for more than five decades, have examined this form of vesicle fusion to elucidate the mechanisms of neurotransmitter release. In most cases, these low probability release events correspond to a single quantum of neurotransmitter that presumably originates from fusion of a single synaptic vesicle (Frerking et al., 1997). In this respect, spontaneous release events provide hard to obtain information on the unitary properties of neurotransmitter release such as the neurotransmitter content of individual vesicles and the number of postsynaptic receptors that respond to single vesicle release. Therefore, spontaneous neurotransmission is widely studied, though most often as a simpler proxy for the more complicated action potential driven synchronized release of neurotransmitters. However, only few studies have addressed the functional importance of these random unitary release events. These small number of studies have revealed that spontaneous release events may be required for signaling leading to maturation and stability of synaptic networks (McKinney et al., 1999; Verhage et al., 2000), inhibition of local dendritic protein synthesis (Sutton et al., 2004) or may even drive action potential firing in cells with high membrane resistance (Carter and Regehr, 2002; Otsu and Murphy, 2003). In contrast to the highly regulated and precisely timed nature of evoked neurotransmitter release, spontaneous synaptic vesicle fusion can only be loosely regulated by extracellular Ca2+, fluctuations in intracellular calcium and neuromodulators (Dittman and Regehr, 1996; Llano et al., 2000; Angleson and Betz, 2001). This dichotomy led to a debate on the mechanism and location of spontaneous fusion (Colmeus et al., 1982; Van der Kloot, 1996; Deitcher et al., 1998). In rat anterior pituitary lactotrophs, spontaneous neuropeptide discharge from a single vesicle is slower than that of stimulated release, because of the kinetic constraints of fusion pore opening as well as the subnanometer size of the fusion pores that form during spontaneous exocytosis (Vardjan et al., 2007). Accordingly, recent studies in central synapses have shown that spontaneously fused vesicles are swiftly retrieved by endocytosis (Ryan et al., 1997; Murthy and Stevens, 1999; Prange and Murphy, 1999; Sun et al., 2002) suggesting the presence of an effective recycling mechanism that operates at rest. These spontaneous release events are generally assumed to be due to low probability fusion of docked synaptic vesicles that are already primed for release (Murthy and Stevens, 1999), with a rate of one vesicle per synapse every 60–90 seconds (Geppert et al., 1994; Murthy and Stevens, 1999). This rate is extremely slow in comparison to the rate of evoked release at CNS synapses that can exceed 100 vesicles per second (Saviane and Silver, 2006).
Despite the extensive number of studies taking advantage of these spontaneous release events to assess alterations in the pre- or postsynaptic properties of neurotransmission, the mechanism(s) underlying spontaneous fusion are not well understood. Under physiological as well as pathophysiological circumstances, spontaneous fusion events can set the concentration of ambient levels of neurotransmitter within the synaptic cleft and in the extracellular milieu. In the brain, unregulated release of excitatory neurotransmitters, exacerbated during pathological conditions such as stroke, can lead to neuronal damage and death (Lo et al., 2003). In addition, recent work suggests that spontaneous neurotransmitter release may activate a distinct set of postsynaptic signaling cascades compared to evoked neurotransmission (Sutton et al., 2004; Sutton et al., 2007). This article aims to provide a closer look into our current understanding of the mechanisms underlying spontaneous neurotransmission and signaling mediated by spontaneous neurotransmitter release events.
The origin of synaptic vesicles that give rise to spontaneous fusion
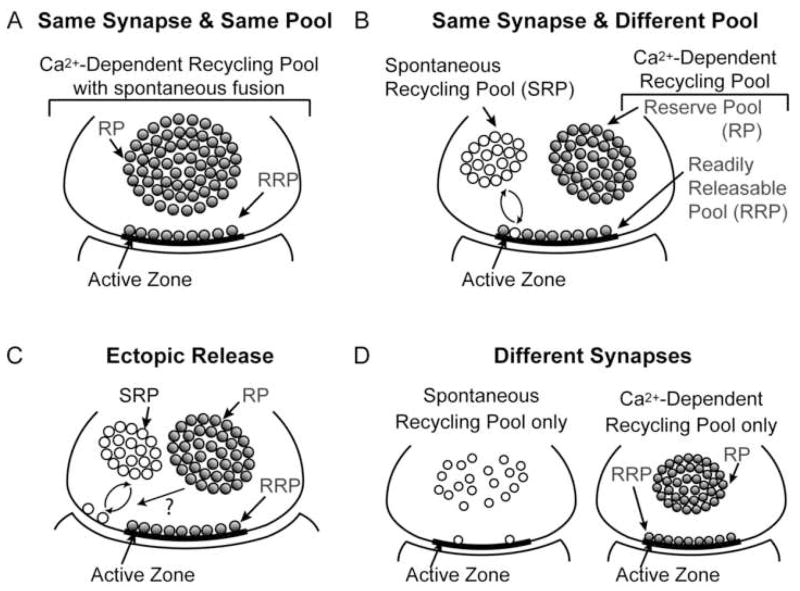
In order to visualize the pathways underlying spontaneous fusion, it is important to pay particular attention to the organization of synaptic vesicles within a synapse. Vesicles in a CNS nerve terminal can be divided into two pools (Fig. 1). The first pool contains a relatively small fraction (5–10%) of vesicles close to release sites. These vesicles are generally thought to be ready for release since they can be fused by rapid uncaging of intrasynaptic Ca2+ (Schneggenburger et al., 1999), a 10-millisecond Ca2+-current pulse (Wu and Borst 1999), a brief high-frequency train of action potentials (Murthy and Stevens 1999) or by hypertonic stimulation (Rosenmund and Stevens 1996). This release-ready pool of vesicles is referred to as the immediately releasable pool or the readily releasable pool (RRP). RRP vesicles are usually considered to be in a morphologically docked state, although not all morphologically docked vesicles are necessarily release competent at any given time (Schikorski and Stevens 2001). In addition to the morphological docking, a “priming” step is required to make vesicles fully release competent (Jahn et al., 2003). A secondary pool of vesicles, the reserve pool (RP), is thought to be spatially distant from the release sites and replenishes the vesicles in the RRP that have exocytosed. The number of vesicles contained in the RRP is a critical parameter that regulates the probability of release, which is defined as the probability that a presynaptic action potential can result in an exocytotic event. In addition, several lines of evidence support the presence of a non-recycling pool of vesicles in the synapse. Mechanisms that can render this “resting” (or “dormant”) pool functional remain to be determined (Sudhof 2000; Harata et al., 2001).
Figure 1. Four possible scenarios for the structural origin of spontaneous neurotransmission.
(A) Same synapse & same pool: spontaneous fusion events may originate from the same pool as the evoked fusion and therefore the extent of spontaneous fusion may reflect the number and fusion propensity of readily releasable vesicles in the synapse
(B) Same synapse & different pool: spontaneous and evoked fusion may occur in the same synapses but may be carried out via a separate pool of vesicles, which may recycle independently. This scenario suggests that spontaneous fusion may require different molecular machinery and recycle uniquely from vesicles fusing in response to an action potential
(C) Ectopic release: spontaneous synaptic vesicle fusion may occur away from the active zone and thus releasing neurotransmitter ectopically.
(D) Different synapses: some synapses may have a strong propensity for spontaneous fusion whereas others may preferentially release neurotransmitter in response to action potentials.
Within the synapse, neurotransmitter release is restricted to active zones. The presynaptic active zone is precisely aligned with the postsynaptic neurotransmitter receptors and the post-synaptic density (PSD). The fusion of synaptic vesicles with these electron-dense regions of the presynaptic plasma membrane is spatially and temporally regulated. After docking at the active zone, synaptic vesicles undergo a series of priming reactions to mature to a fusion-competent state. Recent evidence suggests that docking and priming reactions can occur within 300 ms (Zenisek et al., 2000). At this point, the influx of Ca2+ ions through voltage-gated Ca2+-channels in response to action potentials triggers rapid exocytosis of fusion-competent vesicles. The initial release of vesicles from the RRP or docked-primed pool, and subsequent replenishment and release from the reserve pool, results in biphasic release kinetics, with a rapidly depressing release phase corresponding to release from the RRP and a slow depressing release phase due to the mobilization and release of vesicles from the reserve pool.
This functional allocation of synaptic vesicles into pools aims to account for the properties of evoked neurotransmitter release during activity. On the other hand, studies examining spontaneous neurotransmission typically rely on three assumptions with respect to the pool organization of the vesicles giving rise to spontaneous release. First, it is generally assumed that in a nerve terminal, vesicles that fuse spontaneously originate from the same readily releasable pool as the vesicles that fuse in response to stimulation. This is a key assumption used to justify the analysis of spontaneous release kinetics as a reporter of the number and fusion propensity of vesicles in the readily releasable pool. Second, the priming mechanisms that prepare synaptic vesicles for fusion are believed to be the same for both spontaneous and evoked fusion. In addition, the fusion machinery leading to spontaneous and evoked release are thought to be identical and subject to similar types of regulation aside from evoked release’s steep Ca2+-dependence and reliance on Ca2+ influx (Lou et al., 2005). Finally, the analysis of spontaneous neurotransmission relies on the assumption that evoked fusion events and spontaneous fusion events in a given synapse activate the same set of postsynaptic receptors.
Although only a small number of studies have questioned the validity of the last assumption (Colmeus et al., 1982; but see Van der Kloot, 1996), recent studies have addressed the first two assumptions and provided further insight to the complex relationship between evoked and spontaneous release. For instance, a recent study from our group proposed that a large fraction of vesicles that fuse spontaneously do not originate from the RRP, which gives rise to evoked release in response to action potential firing and Ca2+ influx into nerve terminals (Sara et al., 2005). In this study, synaptic vesicle recycling at rest was detected by the uptake and re-availability of an antibody against the lumenal domain of synaptic vesicle protein synatotagmin-1 as well as the internalization and release of styryl dye FM2-10. Results of these experiments indicated that vesicles recycling spontaneously were more likely to re-fuse spontaneously. Vesicles that took up dye during spontaneous exo-endocytosis were swiftly mobilized in the absence of activity compared to vesicles that recycle during activity. Moreover, spontaneous dye release after spontaneous dye uptake closely followed the kinetics of spontaneous neurotransmission as estimated by previous work (Murthy and Stevens, 1999). In contrast, vesicles that fuse in response to action potentials were not as readily available for release in the absence of stimulation. Taken together, this study proposed that spontaneously endocytosed vesicles preferentially populate a reluctant/reserve pool, which has limited cross talk with vesicles in the activity-dependent recycling pool. However, a later study using simultaneous multicolor imaging of spontaneous and evoked uptake of FM1-43 (a green dye) and FM5-95 (a red dye) argued that vesicles that fuse and endocytose spontaneously populate the same pool as vesicles that fuse in response to action potentials (Groemer and Klingauf, 2007), corroborating an earlier study (Prange and Murphy, 1999). Although, this elegant study disagreed with the proposal that spontaneously endocytosed vesicles recycled independently of the RRP vesicles, it did not examine the resistance of RRP vesicles to spontaneous fusion. Our recent studies showed that after activity-dependent dye uptake stained synapses were resistant to spontaneous dye loss up to 6 hours, consistent with the resilience of RRP vesicles to spontaneous fusion (Wasser, Chung and Kavalali unpublished observations). Sustained resistance of RRP vesicles to spontaneous fusion implies that most synaptic vesicles are actively prevented from fusing spontaneously by an unknown mechanism. To better understand this potential mechanism, we recently investigated the role of synaptic cholesterol levels in the regulation of spontaneous fusion propensity and found that synaptic vesicle cholesterol is a major factor impeding spontaneous fusion (Wasser et al., 2007).
Taken together, currently there are multiple scenarios that can account for the origin of spontaneous release, which do not necessarily constitute mutually exclusive possibilities. First, spontaneous fusion events may originate from the same pool as vesicles giving rise to evoked fusion, (Fig. 1A) and therefore the extent of spontaneous fusion may reflect the number and fusion propensity of readily releasable vesicles in the synapse (Prange and Murphy, 1999; Groemer and Klingauf, 2007). Second, spontaneous and evoked fusion may occur in the same synapse but may be carried out via a separate pool of vesicles, which may recycle independently. This scenario suggests that spontaneous fusion may require different molecular machinery and recycle uniquely from vesicles that fuse in response to action potentials (Fig. 1B) (Hua et al., 1998; Angleson and Betz, 2001; Sara et al., 2005). Third, spontaneous synaptic vesicle fusion may occur away from the active zone, thus releasing neurotransmitter ectopically (Fig. 1C). Earlier results in the neuromuscular junction (NMJ) support the hypothesis that spontaneous fusion occurs at an ectopic site (Colmeus et al., 1982; but see Van der Kloot, 1996). In addition, recent evidence for ectopic neurotransmitter release brings further credence to this scenario (Matsui and Jahr, 2003; Coggan et al., 2005). Finally, some synapses may have a strong propensity for spontaneous fusion, whereas others may preferentially release neurotransmitter in response to action potentials (Fig. 1D). In the frog NMJ, the lack of a strong correlation between the amounts of spontaneous versus evoked fusion at individual release sites is consistent with the notion that some release sites may preferentially support spontaneous or evoked release (Zefirov et al., 2005). Future experiments will clearly need to discern these possibilities. This effort will also help us gain important insight to the functional organization of synaptic transmission at the level of individual synapses.
The role of SNAREs in spontaneous neurotransmission
Priming is a process that occurs after vesicle docking at the active zone and before the execution of fusion. Molecularly, this process is thought to correspond to the formation of SNARE (acronym for soluble N-ethylmaleimide-sensitive factor attachment protein receptor) complexes and dissolution of SNARE interactions that typically hinder individual SNAREs from participating in SNARE complexes. Synaptic vesicle fusion is mediated by the formation of SNARE complexes (Sollner et al., 1993) from the SNARE proteins synaptobrevin/VAMP, syntaxin-1 and SNAP-25 (Rizo and Sudhof, 2002; Jahn et al., 2003; Jahn and Scheller, 2006). Syntaxin and synaptobrevin are anchored on the plasma and the synaptic vesicle membrane, respectively, by a transmembrane region, whereas SNAP-25 is attached to the plasma membrane by palmitoylated cysteines (Hess et al., 1992). All SNARE proteins contain a sequence called the SNARE motif that associates into parallel four-helical bundles to form SNARE complexes, with SNAP-25 contributing two SNARE motifs, and syntaxin and synaptobrevin each contributing one SNARE motif to the synaptic SNARE complex (Sutton et al., 1998). SNARE complexes are thought to assemble by ‘zippering’ in an N- to C-terminal direction, thereby forcing their resident membranes closely together (Sorensen et al., 2006). SNAREs alone appear sufficient to fuse lipid bilayer vesicles (Weber et al., 1998) as well as fibroblast plasma membranes (Hu et al., 2003), and so may represent the minimal fusion machinery. It is less clear whether SNAREs alone are sufficient to execute fusion physiologically, but likely cooperate with SM-proteins (Sec1/Munc18-like proteins) in a poorly understood reaction (Rizo and Sudhof, 2002).
Analyses of genetic deletions of individual SNARE proteins present a complicated view of SNARE function in spontaneous and evoked neurotransmitter release. In Drosophila, loss of either syntaxin or synaptobrevin results in a complete loss of Ca2+-evoked release, but some spontaneous release persists (Schulze et al., 1995; Deitcher et al., 1998) along with a residual hyperosmotic saline response (Broadie et al., 1995). In C. elegans, syntaxin null mutants were almost completely paralyzed (Saifee et al., 1998), whereas synaptobrevin nulls exhibit reduced but not absent movements, such as pharyngeal pumping (Nonet et al., 1998). In mice, loss of syntaxin 1A results in normal basic neurotransmitter release, but there is a deficiency in hippocampal long term potentiation as well as conditioned fear memory (Fujiwara et al., 2006). This relatively weak phenotype might be due to compensation by syntaxin 1B. Deletion of mouse synaptobrevin-2, the major vesicular SNARE protein in the brain, also causes only a partial impairment of neurotransmitter release. Here, spontaneous and hypertonic sucrose-induced neurotransmitter release is relatively less affected than evoked release (Schoch et al., 2001). Moreover, both in flies and mice, synaptobrevin null mutants exhibit a facilitation of release during 10 Hz stimulation (Yoshihara et al., 1999; Deak et al., 2004). In contrast to synaptobrevin, loss of SNAP-25 in flies did not diminish neurotransmission substantially, partly due to potential compensation from SNAP-24, a protein closely related to SNAP-25 (Niemeyer and Schwarz, 2000; Vilinsky et al., 2002). In mice, SNAP-25 deletion leads to lethality at birth, and secretion, in particular stimulus evoked secretion, is severely impaired (Washbourne et al., 2002; Sorensen et al., 2003; Tafoya et al., 2006). Our group has recently analyzed the remaining neurotransmission in mature hippocampal cultures from SNAP-25 deficient mice and tested whether loss of SNAP-25 causes a differential impairment of Ca2+-dependent and -independent synaptic vesicle trafficking (Bronk et al., 2007). In SNAP-25 knockout neuronal cultures, we detected almost no Ca2+-evoked release, which agrees with earlier findings (Washbourne et al. 2002). Even strong stimulation with elevated potassium could barely elicit responses. In contrast, spontaneous neurotransmission occurs reliably in SNAP-25 knockout neurons, albeit at a lower frequency than controls. In addition, SNAP-25 mutants always responded to hypertonic sucrose application, a calcium-independent form of stimulation. Furthermore, SNAP-25 deficient synapses are capable of synaptic vesicle recycling monitored by uptake and release of FM dyes in response to hypertonic sucrose stimulation. Together, these results suggest that SNAP-25 has a more significant role in calcium-secretion coupling than synaptobrevin-2.
One puzzling finding in SNARE loss of function studies was the persistence of spontaneous neurotransmitter release. This result was interpreted as the presence of alternative pathways presumably requiring a distinct set of SNARE proteins that mediate this unregulated form of release. Earlier results have shown that after the loss of synaptobrevin-2 and of SNAP-25, spontaneous transmission is largely preserved at a diminished level and the properties of individual events are essentially unaffected. However, we have only limited information on the role of SNAREs in the regulation of unitary release events in central synapses. A new insight to this issue came from experiments designed to test whether the distance between the SNARE motif and transmembrane region is critical for fusion. This distance is expected to be an important determinant of a vesicle’s fusion propensity if SNARE complexes force membranes into close proximity. To test this hypothesis, insertions of 12 or 24 residues were introduced between the SNARE motif and the transmembrane region of synaptobrevin-2 (Deak et al., 2006). These mutants revealed that the physical distance between the two regions of synaptobrevin-2 is indeed critical for the rescue of evoked fusion, which agrees with the proposal that the assembly of SNARE complexes provides the energy for membrane fusion. Surprisingly, in contrast to the insertion of 24 amino acids, the 12 amino acid insertion mutant completely rescued spontaneous release, suggesting that constraints on SNARE function during spontaneous fusion are more flexible than for evoked fusion. This finding argues against the traditional notion that spontaneous release events arise from the random low probability exocytosis of docked and fully primed vesicles in the RRP. According to this view, spontaneous fusion should possess the same structural requirements as evoked fusion. This finding could not be ascribed to a selective effect of synaptobrevin-2 in Ca2+-dependence of evoked fusion (Sakaba et al., 2005; Young, 2005), because the synaptobrevin-2 carrying the 12-residue insertion was also largely unable to rescue hypertonic sucrose evoked fusion, which is Ca2+-independent (Rosenmund and Stevens, 1996).
The differential requirement of membrane proximity in Ca2+-evoked and spontaneous synaptic release raises the question of whether the release machinery for spontaneous fusion is identical to the one required for evoked fusion. Clearly, both forms of fusion are impaired in the absence of the same SNARE molecules, albeit to different degrees (Schoch et al., 2001; Washbourne et al., 2002). Taken together with the earlier observations from the SNARE mutant studies, this finding suggests that spontaneous fusion may require an alternative fusion complex (with a different vesicular SNARE) or the same complex formed with less stringency (Xu et al., 1999; Melia et al., 2002). The stronger dependency of evoked release on the close proximity of membranes is also more compatible with its strict Ca2+-dependence. Paradoxically, the mechanism of evoked release appears to be closely related to the evolutionally conserved exocytosis, because yeast vacuolar fusion was similarly strictly dependent on membrane proximity and the length of vesicular SNAREs (McNew et al., 1999).
Ca2+-dependence of spontaneous release
Spontaneous release events persist at reduced levels after the removal of extracellular Ca2+ or after strong chelation of intracellular Ca2+. In contrast, increases in intracellular Ca2+ can robustly augment spontaneous fusion (e.g. Angleson and Betz, 2001). Despite these widespread observations, the mechanism(s) underlying Ca2+-dependent regulation of spontaneous release remains poorly understood (Lou et al., 2005; Glitsch, 2007). At mammalian central synapses, as well as the Drosophila NMJ, fast synchronous Ca2+-dependent vesicle fusion requires synaptotagmin-1 as the Ca2+ sensor (Geppert et al., 1994; Fernandez-Chacon et al., 2001; Nishiki et al., 2001; Yoshihara et al., 2002). In the spinal cord and the brain stem, synaptotagmin-2 is similarly required as a fast Ca2+ sensor (Pang et al., 2006). Genetic studies showed that the spontaneous fusion rate was increased or unchanged in the knockout of synaptotagmin-1 or synaptotagmin-2 (Geppert et al., 1994; Pang et al., 2006), which is consistent with the notion that synaptotagmins contribute to a clamp on spontaneous fusion in addition to their obligatory role in fast evoked fusion. Nevertheless, the exact mechanism underlying this increase in spontaneous neurotransmission is currently unknown. Furthermore, the loss of synaptotagmin-1 or synaptotagmin-2 leads to a prominent increase in asynchronous release (Sun et al., 2007) consistent with the proposal that synchronous and asynchronous release compete for the same pool of vesicles (Otsu et al. 2004). Despite the apparent parallels between the negative regulation of spontaneous and asynchronous release by synaptotagmins, the precise molecular relationship between asynchronous release and spontaneous release remains to be determined. In contrast to the persistence of spontaneous and asynchronous release after deletion of synaptotagmins, these two forms of release are significantly reduced after deletion of synaptobrevin-2/VAMP-2 or completely abolished after genetic deletion of munc-18 or munc-13 isoforms (Verhage et al., 2000; Schoch et al., 2001; Varoqueaux et al., 2002). The selective role of proteins such as synaptotagmin-1 and synaptotagmin-2 in evoked neurotransmitter release is hard to reconcile with the substantial role of synaptobrevin, munc-18 or munc-13 in both forms of vesicle trafficking if indeed the two forms of release were part of the same pathway. In addition to synaptotagmins, complexins, small soluble proteins that compete with synaptotagmins for binding to SNAREs, are key players in regulation of Ca2+-dependent fusion. The crystal structure of complexins suggests that it forms an α-helix that interacts with SNAREs with 1:1 ratio in an anti-parallel manner (Chen et al., 2002). This interaction is thought to stabilize the C-terminal part of the coiled SNARE complex. In complexin knock-out mice, Ca2+-triggered release is compromised but Ca2+-independent hypertonic sucrose triggered release is normal (Reim et al., 2001). Enrichment of complexin at the SNARE complex sites by expression of a synaptobrevin-complexin fusion protein imitated synaptotagmin knockout phenotype (Tang et al, 2006). In agreement with this finding, at the Drosophila NMJ, lack of complexin mimicked the loss of function in phenotype of synaptotagmin-1, by facilitating spontaneous neurotransmitter release (Huntwork and Littleton, 2007). Complexin binds the SNARE complex in the groove between synaptobrevin and syntaxin. Synaptotagmin and complexin compete relatively equally for SNARE complex binding, unless synaptotagmin binds Ca2+ and then it has a much higher affinity for the SNARE complex compared to complexin (Tang et al, 2006). Complexin seems to play a role not only in stabilizing the SNARE complex but also in the inhibition of spontaneous fusion after SNARE assembly.
A non-Ca2+ binding isoform of synaptotagmin, synaptotagmin-12, has recently been implicated as selective regulator of spontaneous neurotransmission (Maximov et al., 2007). Synaptotagmin-12 binds synaptotagmin-1 and specifically increases spontaneous fusion. Overexpression of synaptotagmin-12, in both wild type and synaptotagmin-1-deficient neurons, resulted in a large increase in spontaneous fusion events without affecting the properties of evoked fusion (Maximov et al., 2007) suggesting that this molecule promotes spontaneous vesicle fusion in a synaptotagmin-1-independent manner. Taken together, studies so far indicate that synaptotagmin-1 or –2 in conjunction with complexins constitute a fusion clamp on spontaneous release. However, in the absence of any available structure-function data from synaptotagmins and complexins on their impact on spontaneous neurotransmission, it is currently difficult to propose a precise mechanism for this clamp.
Properties of spontaneous synaptic vesicle recycling
In addition to loss of function studies of SNAREs and presynaptic proteins such as synaptotagmins and complexins, manipulations of key molecules involved in synaptic vesicle endocytosis and trafficking also suggest a divergence in the mechanisms that mediate spontaneous and evoked synaptic vesicle recycling. For instance, Drosophila NMJs mutant in rab5, a small GTPase critical for vesicle trafficking through early endosomes, showed no differences in the frequency and amplitude of miniature excitatory junction potentials compared to wild type junctions (Wucherpfennig et al., 2003). In contrast, evoked neurotransmitter release probability was significantly altered in these mutants supporting the argument that both forms of release operate through distinct vesicle trafficking pathways. This premise is further supported by experiments performed by Koenig and Ikeda in the Drosophila NMJ where they monitored the recovery of evoked and spontaneous synaptic responses after vesicle depletion induced by the temperature sensitive dynamin mutant shibire (Koenig and Ikeda, 1999). In this study, the authors observed that the active zone population of vesicles and evoked neurotransmitter release recovered in parallel within 30 seconds, in contrast, full recovery of spontaneous release took 10 to 15 minutes and required the recovery of the non-active zone population of vesicles. In mice, mutations in dynamin-1 show a disparate effect on spontaneous and evoked neurotransmission. Dynamin-1 is an integral player in clathrin-dependent endocytosis, where it acts to pinch off endocytosing membrane from the plasma membrane. Dynamin-1-deficient cortical neurons display a large attenuation in evoked responses with no change in the frequency of spontaneous events (Ferguson et al., 2007).
In addition to dynamin and rab5 mutants, modifications in clathrin adaptor molecules, such as AP180 and AP3, reveal differences in the vesicle recycling pathways that give rise to evoked versus spontaneous neurotransmission. AP180 binds to clathrin and AP2, and this binding is essential for vesicle recovery from the plasma membrane after full exocytosis. In Drosophila, mutation in the AP180 (lap) gene results in an increase in the rate of spontaneous fusion, while Ca2+-dependent fusion is decreased (Bao et al., 2005). Neurons lacking AP3, which is a clathrin adaptor protein involved in endosomal trafficking, show an increase in spontaneous fusion frequency with a significant decrease in the evoked responses (Scheuber et al., 2006).
In summary, findings from genetic manipulations of nerve terminals agree well with the proposal that the spontaneous recycling and activity-dependent recycling operate independently. The functional segregation of the two sets of vesicles may be mediated by differences in the molecular composition of synaptic vesicles that make up the two pools. However, the observation that the antibodies to synaptotagmin-1 can readily label spontaneously recycling vesicles (Sara et al., 2005) argues against a simple molecular dichotomy (such as absence or presence of synaptotagmin-1) as the underlying reason for this phenomenon. Moreover, a simple replacement of a single molecule would be hard to reconcile with the finding that the readily releasable vesicles have a lower tendency than spontaneously recycling ones to fuse spontaneously. This result implies that the docked vesicle pool is to a large extent stable in the absence of stimulation. A divergence in the molecular composition of vesicles may also make differential regulation of these two recycling pathways a possibility. Such selective regulation may provide neural networks a means to distinguish between evoked and spontaneous synaptic activity.
Regulation of spontaneous neurotransmission
While the absence of spontaneous signaling may compromise neuronal survival and structural stability of synaptic connections (Verhage et al., 2000) in the brain, unregulated enhanced release of excitatory neurotransmitters can lead to neuronal damage and death. This excitotoxic neuronal death induced by increased synaptic glutamate is implicated in the pathology of multiple neurodegenerative diseases including Alzheimer’s and Huntington’s diseases along with ischemia and epilepsy (Nishizawa, 2001; Lo et al., 2003; Hynd et al., 2004). In addition, excess spontaneous fusion may cause presynaptic vesicle depletion and impair synaptic function in the long term. As excess or diminished spontaneous neurotransmitter release may lead to adverse consequences, regulatory mechanisms are necessary to fine-tune this type of fusion. The modulation of spontaneous neurotransmitter release occurs through several signaling pathways (reviewed in Bouron, 2001). Many neuromodulators decrease evoked release through inhibition of voltage gated Ca2+ channels (Gβγ-mediated), but some can also exert inhibition on spontaneous release. For example, adenosine acts through A1 receptors and unlike other neuromodulators attenuates both evoked and spontaneous neurotransmission (Fredholm et al., 2005). To suppress evoked neurotransmission, A1 receptors act through P-type Ca2+ channels to inhibit neurotransmission (Dittman, and Regehr, 1996). The mechanism underlying inhibition of spontaneous neurotransmission is unclear but it may be shared with the action of glutamate through presynaptic group II metabotropic glutamate receptors to inhibit spontaneous vesicle fusion (Glitsch, 2006).
In addition to neuromodulators, membrane lipids such as cholesterol also have a strong impact on the propensity of synaptic vesicle fusion. Cholesterol contributes to membrane dynamics, particularly the regulation of membrane fluidity and microdomains involved in protein interactions. In the central nervous system, cholesterol synthesis is primarily de novo and tightly regulated (Spady and Dietschy, 1983; Dietschy et al., 1993; Jurevics and Morell, 1995; Turley et al., 1998). At the synapse, cholesterol depletion experiments implicate cholesterol in the regulation of the efficiency of Ca2+-dependent exocytosis by mediating SNARE protein localization at the synapse (Chamberlain et al., 2001; Lang et al., 2001; Salaun et al., 2004; Churchward et al., 2005) and the retrieval of exocytosed membrane through clathrin-dependent pathways (Rodal et al, 1999; Subtil et al., 1999).
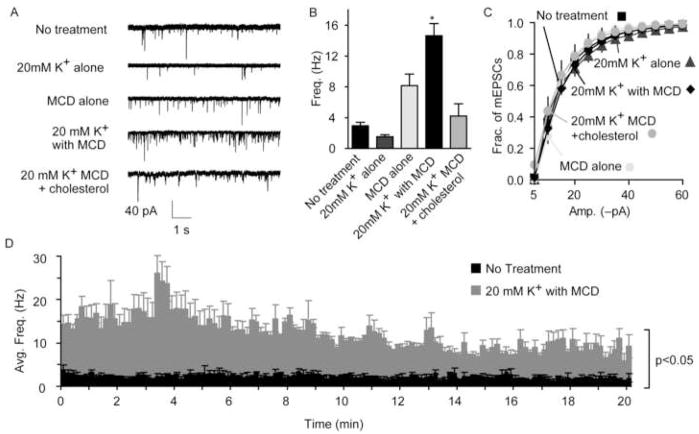
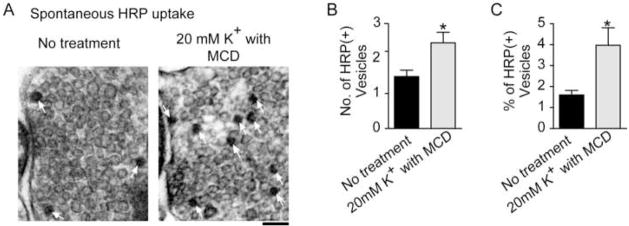
In addition to the role of cholesterol in the efficacy of endocytosis and evoked exocytosis, cholesterol is also thought to act to inhibit spontaneous vesicle fusion (Zamir and Charlton, 2006; Wasser et al., 2007). In hippocampal neurons, decreasing cholesterol by acute depletion with methyl-β-cyclodextrin (MCD, Fig. 2A–C), via inhibition of its synthesis, or by using cultured neurons from mice deficient in cholesterol trafficking all result in a significant increase in the rate of spontaneous vesicle fusion and a substantial decrease in evoked vesicle fusion. Cholesterol addition reversed each of these effects (Wasser et al., 2007). After acute depletion of cholesterol with MCD, the spontaneous release rate potentiates for up to 20 minutes (Fig. 2D), and the number of vesicles that take up horse radish peroxidase (HRP) spontaneously increases compared to untreated synapses (Fig. 3). The increase in spontaneous uptake of HRP and the continued high level of spontaneous release observed after cholesterol removal suggest that the enhanced spontaneous fusion is coupled to an increase in endocytosis indicating an overall alteration in the recycling of spontaneous vesicles rather than an increase spontaneous fusion alone after acute MCD-mediated cholesterol removal.
Figure 2. Cholesterol depletion augments spontaneous fusion rate.
(A) Sample traces of spontaneous miniature excitatory postsynaptic currents (mEPSC) after MCD treatment of dissociated hippocampal cultures.
(B) Summary graph showing a 5-fold increase in the frequency of mEPSCs for MCD-treated cultures compared to non-treated cultures. Treatment with 20mM K+ alone did not alter the frequency of mEPSC events. Cultures treated with MCD alone had an average 3-fold higher frequency of mEPSCs; however the rate was not significantly different from the non-treated cultures. (C) Cumulative histograms of the distribution of mEPSC amplitudes showed no differences using Kolmogorov-Smirnov test (K-S test, p>0.0001).
(D) Summary graph depicting the persistent 5-fold increase of the average mEPSC frequency (integrated per 10-second intervals) for 20 minutes for non-treated cultures and cultures treated in 20mM K+ with MCD. (at least 3 cultures, No treatment n=28, 20 mM K+ Only n=6, MCD alone n=24, 20 mM K+ with MCD n=31, and 20 mM K+ with MCD + cholesterol n=4). Error bars represent the SEM (*p< 0.001). (modified with permission from Wasser et al., 2007 The Journal of Physiology, Blackwell Publishing)
Figure 3. Cholesterol depletion decreases the number of vesicles per synapse and effects depolarization-evoked and spontaneous Horse Radish Peroxidase uptake differentially.
(A) Representative electron micrographs of MCD-treated cultures loaded with Horse Radish Peroxidase (HRP) spontaneously (white arrows indicate HRP-positive (HRP+) vesicles).
(B) Summary graph showing a 1.6-fold increase in the average number of HRP+ vesicles per synapse for cultures treated in 20 mM K+ with MCD.
(C) Summary graph showing a 2.5-fold increase in the average percent of HRP+ vesicles per synapse for cultures treated in 20 mM K+ with MCD. (No treatment n=47 and 20 mM K+ with MCD n=72). Error bars represent the SEM (*p<0.05; scale bar = 100 nm). (modified with permission from Wasser et al., 2007 The Journal of Physiology, Blackwell Publishing)
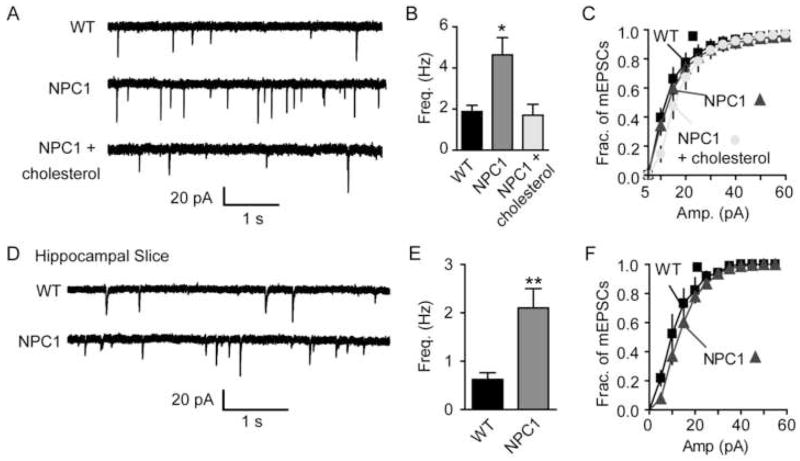
Synaptic neurotransmission in Niemann-Pick type C1 (NPC1)-deficient neuronal cultures mimicked the observations seen after acute cholesterol depletion. These NPC1-deficient neurons have a defect in cholesterol trafficking, which traps cholesterol in the late endosome/lysosome and results in a decreased concentration of cholesterol at the synapse. Along with the attenuation of evoked responses in the cultures, both NPC1-deficient neurons in slice or culture display a higher frequency of spontaneous vesicle fusion compared to the wild-type littermates (Fig. 4).
Figure 4. Altered cholesterol trafficking in Niemann Pick C1-deficient mice causes abnormalities in neurotransmission mimicking the effect of acute cholesterol depletion.
(A–C) mEPSCs from NPC1 and WT cells. (A) Sample traces. (B) Summary plot shows a 2.5-fold increase in the frequency of mEPSCs for NPC1 neurons compared to WT neurons. The increased frequency in NPC1 neurons was reduced to the WT frequency levels after incubation with MCD: cholesterol complexes. (C) The distributions of mEPSC amplitudes were not different under all conditions as determined by the K-S test (p>0.0001) (4 cultures, WT n=16, NPC1 n=17 NPC1 + cholesterol n=9).
(D–F) Electrophysiological recordings from NPC1 and WT hippocampal slices. (D) Sample mEPSC traces. (E) Summary graph of the frequency of mEPSCs showing a 3.4-fold increase in the frequency of mEPSCs in the NPC1 neurons. (F) The distributions of mEPSC amplitudes were not different as determined by the K-S test (p>0.0001) (WT n=6, NPC1 n=5). Error bars represent the SEM (*p<0.01, ** p<0.005). (modified with permission from Wasser et al., 2007 The Journal of Physiology, Blackwell Publishing)
These findings suggest that the presence of cholesterol increases the efficiency of Ca2+-dependent fusion while inhibiting spontaneous fusion. Interestingly, vesicular cholesterol oxidation (instead of complete extraction) also results in an increase in spontaneous fusion frequency (4-fold increase, Wasser and Kavalali unpublished observations). The oxidation of cholesterol does not affect the fluidity of the membrane (Lau and Das, 1995), however alterations in cholesterol-dependent protein interactions could occur suggesting a protein-mediated inhibition of spontaneous fusion regulated by the presence of cholesterol. These impairments in synaptic transmission, especially the large increase in spontaneous neurotransmission, might form the basis for the neurological symptoms and neurodegeneration seen in patients with Niemann-Pick disease.
Spontaneous neurotransmission as an independent pathway for interneuronal signalling
Does spontaneous neurotransmitter release, a common feature of synapses through out the nervous system, serve a purpose? Since Paul Fatt and Bernard Katz in early 1950s discovered neurotransmission in the absence of nerve impulses (Fatt and Katz, 1952), this question has been in the minds of neurophysiologists. A few studies have examined spontaneous neurotransmission for its own sake and have shown that spontaneous release events may trigger action potential firing in cells with high membrane resistance and may also be required for maturation of synapses (Zucker, 2005). For instance, dendritic spine density is susceptible to decreases in spontaneous release or block of postsynaptic glutamate receptors but is largely unaltered after blockade of action potentials (McKinney et al., 1999). Synapses in mice deficient in munc-18 experience no evoked or spontaneous vesicle fusion. Interestingly, although synapse numbers and their morphology are normal before birth, synapses quickly disassemble during development suggesting an integral role for spontaneous neurotransmitter release in synapse stability (Verhage et al., 2000). Another mysterious phenomenon that has puzzled neurophysiologists over a century is the hyperexcitability of target membranes that follows denervation or other disruption of their nervous input (Axelsson and Thesleff, 1959). Early experiments in the NMJ have shown that the increase in sensitivity of muscle tissue to acetylcholine seen after denervation was due to upregulation of acetycholine receptors. Experiments in late 1990s elucidated a similar receptor upregulation at central synapses, revealing a powerful mechanism for maintenance of homeostatic stability of CNS synaptic networks (Turrigiano et al., 1998). Furthermore, chronic blockade of action potential firing in neuronal cultures increases trafficking of the AMPA receptor subunits GluR1 and GluR2 to postsynaptic sites, thus increasing sensitivity to released glutamate (Wierenga et al., 2005).
Recent studies by Sutton and colleagues (Sutton et al., 2006; Sutton et al., 2007) bridged these two persistent questions of neurophysiology through a comprehensive set of experiments and provide a causal link between the two phenomena. This work showed that spontaneous neurotransmitter release, rather than evoked neurotransmission, is a specific regulator of postsynaptic sensitivity to neurotransmitters by suppressing the dendritic protein translation machinery locally and thereby maintaining receptor composition of synapses. The initial set of experiments performed by Sutton et al. documents that, unlike the blockade of action potentials, inhibition of either NMDA receptors or AMPA receptors can increase the amplitude of miniature excitatory postsynaptic currents (“minis”) within mere hours. The frequency of “minis” remains unchanged, and the increased mini amplitude is seen even when action potentials are allowed during receptor blockade. This finding has two surprising aspects. First, it strongly suggests that NMDA receptors are active at rest during spontaneous neurotransmission, despite their reduced ion conductance due to Mg2+ block. Second, the observation that amplitudes of unitary synaptic responses increase rapidly within an hour after NMDA receptor blockade stands in striking contrast to earlier reports that chronic blockade of neuronal firing by tetrodotoxin (TTX) leads to slow rescaling of unitary synaptic efficacy. Moreover, the authors show that this rapid effect of NMDA receptor blockade on unitary transmission is strictly dependent on protein synthesis, which is consistent with earlier findings from the same group (Sutton et al., 2004). A recent study by the same investigators showed that regulation of protein translation by spontaneous release events occurs through the eukaryotic elongation factor-2 (eEF2), which distinguishes unitary Ca2+ currents generated by evoked release from currents mediated by spontaneous release and controls protein synthesis accordingly (Sutton et al., 2007).
An intriguing implication of this work is the divergence of mechanisms underlying synaptic scaling after chronic inhibition of NMDA receptor mediated miniature currents versus chronic inhibition of action potentials. The difference in the time course of action of the two manoeuvres argues for separate mechanisms mediating the increase in AMPA receptor activity. Wierenga et al. (2005) suggested that synaptic scaling in response to chronic action potential blockade does not involve the transient expression of Ca2+ -permeable AMPA receptors. The exact mechanism mediating synaptic scaling induced by chronic TTX treatment remains unknown. It is thought to act more globally (Turrigiano et al., 2004), which is consistent with the idea of large-scale homeostatic maintenance of synaptic circuits. If chronic blockade of action potential firing also mediates its effect synaptically but by activating a different signalling cascade, then how do neurons distinguish evoked and spontaneous neurotransmission? Are the differences in temporal characteristics of the two forms of neurotransmission sufficient? Or, are there distinct postsynaptic detection machineries for spontaneously released neurotransmitters? Better understanding of the presynaptic machinery and its postsynaptic counterparts that underlie spontaneous and evoked neurotransmission will provide us with molecular and pharmacological tools that can selectively manipulate the two forms of neurotransmission. Independent analysis of spontaneous and evoked neurotransmission may uncover more surprises in the intricacies of communication within individual synapses.
Acknowledgments
The studies in our laboratory are supported by an Established Investigator Award from the American Heart Association and grants from the National Institute of Mental Health.
Abbreviations
- RP
reserve pool
- RRP
readily releasable pool
- NPC1
Niemann-Pick type C1
- MCD
methyl-β-cyclodextrin
- NMDAR
N-methyl-D-aspartic acid receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angleson JK, Betz WJ. Intraterminal Ca(2+) and spontaneous transmitter release at the frog neuromuscular junction. J Neurophysiol. 2001;85:287–294. doi: 10.1152/jn.2001.85.1.287. [DOI] [PubMed] [Google Scholar]
- Axelsson J, Thesleff S. A study of supersensitivity in denervated mammalian skeletal muscle. J Physiol. 1959;147:178–193. doi: 10.1113/jphysiol.1959.sp006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao H, Daniels RW, MacLeod GT, Charlton MP, Atwood HL, Zhang B. AP180 maintains the distribution of synaptic and vesicle proteins in the nerve terminal and indirectly regulates the efficacy of Ca2+-triggered exocytosis. J Neurophysiol. 2005;94:1888–1903. doi: 10.1152/jn.00080.2005. [DOI] [PubMed] [Google Scholar]
- Bouron A. Modulation of spontaneous quantal release of neurotransmitters in the hippocampus. Prog Neurobiol. 2001;63:613–635. doi: 10.1016/s0301-0082(00)00053-8. [DOI] [PubMed] [Google Scholar]
- Broadie K, Prokop A, Bellen HJ, O’Kane CJ, Schulze KL, Sweeney ST. Syntaxin and synaptobrevin function downstream of vesicle docking in Drosophila. Neuron. 1995;15:663–673. doi: 10.1016/0896-6273(95)90154-x. [DOI] [PubMed] [Google Scholar]
- Bronk P, Deak F, Wilson MC, Liu X, Sudhof TC, Kavalali ET. Differential effects of SNAP-25 deletion on Ca2+-dependent and Ca2+-independent neurotransmission. J Neurophysiol. 2007;98:794–806. doi: 10.1152/jn.00226.2007. [DOI] [PubMed] [Google Scholar]
- Carter AG, Regehr WG. Quantal events shape cerebellar interneuron firing. Nat Neurosci. 2002;5:1309–1318. doi: 10.1038/nn970. [DOI] [PubMed] [Google Scholar]
- Chamberlain LH, Burgoyne RD, Gould GW. SNARE proteins are highly enriched in lipid rafts in PC12 cells: implications for the spatial control of exocytosis. Proc Natl Acad Sci U S A. 2001;98:5619–5624. doi: 10.1073/pnas.091502398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Tomchick DR, Kovrigin E, Arac D, Machius M, Sudhof TC, Rizo J. Three-dimensional structure of the complexin/SNARE complex. Neuron. 2002;33:397–409. doi: 10.1016/s0896-6273(02)00583-4. [DOI] [PubMed] [Google Scholar]
- Churchward MA, Rogasevskaia T, Hofgen J, Bau J, Coorssen JR. Cholesterol facilitates the native mechanism of Ca2+-triggered membrane fusion. J Cell Sci. 2005;118:4833–4848. doi: 10.1242/jcs.02601. [DOI] [PubMed] [Google Scholar]
- Colmeus C, Gomez S, Molgo J, Thesleff S. Discrepancies between spontaneous and evoked synaptic potentials at normal, regenerating and botulinum toxin poisoned mammalian neuromuscular junctions. Proc R Soc Lond B Biol Sci. 1982;215:63–74. doi: 10.1098/rspb.1982.0028. [DOI] [PubMed] [Google Scholar]
- Coggan JS, Bartol TM, Esquenazi E, Stiles JR, Lamont S, Martone ME, Berg DK, Ellisman MH, Sejnowski TJ. Evidence for ectopic neurotransmission at a neuronal synapse. Science. 2005;309:446–451. doi: 10.1126/science.1108239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak F, Schoch S, Liu X, Sudhof TC, Kavalali ET. Synaptobrevin is essential for fast synaptic-vesicle endocytosis. Nat Cell Biol. 2004;6:1102–1108. doi: 10.1038/ncb1185. [DOI] [PubMed] [Google Scholar]
- Deak F, Shin OH, Kavalali ET, Sudhof TC. Structural determinants of synaptobrevin 2 function in synaptic vesicle fusion. J Neurosci. 2006;26:6668–6676. doi: 10.1523/JNEUROSCI.5272-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitcher DL, Ueda A, Stewart BA, Burgess RW, Kidokoro Y, Schwarz TL. Distinct requirements for evoked and spontaneous release of neurotransmitter are revealed by mutations in the Drosophila gene neuronal-synaptobrevin. J Neurosci. 1998;18:2028–2039. doi: 10.1523/JNEUROSCI.18-06-02028.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD, Spady DK. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J Lipid Res. 1993;34:1637–1659. [PubMed] [Google Scholar]
- Dittman JS, Regehr WG. Contributions of calcium-dependent and calcium-independent mechanisms to presynaptic inhibition at a cerebellar synapse. J Neurosci. 1996;16:1623–1633. doi: 10.1523/JNEUROSCI.16-05-01623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatt P, Katz B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952;117:109–128. [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Brasnjo G, Hayashi M, Wolfel M, Collesi C, Giovedi S, Raimondi A, Gong LW, Ariel P, Paradise S, O’Toole E, Flavell R, Cremona O, Miesenbock G, Ryan TA, De Camilli P. A selective activity-dependent requirement for dynamin 1 in synaptic vesicle endocytosis. Science. 2007;316:570–574. doi: 10.1126/science.1140621. [DOI] [PubMed] [Google Scholar]
- Fernandez-Chacon R, Konigstorfer A, Gerber SH, Garcia J, Matos MF, Stevens CF, Brose N, Rizo J, Rosenmund C, Sudhof TC. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM. Adenosine and brain function. Int Rev Neurobiol. 2005;63:191–270. doi: 10.1016/S0074-7742(05)63007-3. [DOI] [PubMed] [Google Scholar]
- Frerking M, Borges S, Wilson M. Are some minis multiquantal? J Neurophysiol. 1997;78:1293–1304. doi: 10.1152/jn.1997.78.3.1293. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Mishima T, Kofuji T, Chiba T, Tanaka K, Yamamoto A, Akagawa K. Analysis of knock-out mice to determine the role of HPC-1/syntaxin 1A in expressing synaptic plasticity. J Neurosci. 2006;26:5767–5776. doi: 10.1523/JNEUROSCI.0289-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Sudhof TC. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994b;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- Glitsch M. Selective inhibition of spontaneous but not Ca2+-dependent release machinery by presynaptic group II mGluRs in rat cerebellar slices. J Neurophysiol. 2006;96:86–96. doi: 10.1152/jn.01282.2005. [DOI] [PubMed] [Google Scholar]
- Glitsch MD. Spontaneous neurotransmitter release and Ca(2+)-How spontaneous is spontaneous neurotransmitter release? Cell Calcium. 2007 doi: 10.1016/j.ceca.2007.02.008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Groemer TW, Klingauf J. Synaptic vesicles recycling spontaneously and during activity belong to the same vesicle pool. Nat Neurosci. 2007;10:145–147. doi: 10.1038/nn1831. [DOI] [PubMed] [Google Scholar]
- Harata N, Pyle JL, Aravanis AM, Mozhayeva M, Kavalali ET, Tsien RW. Limited numbers of recycling vesicles in small CNS nerve terminals: implications for neural signaling and vesicular cycling. Trends Neurosci. 2001;24:637–643. doi: 10.1016/s0166-2236(00)02030-0. [DOI] [PubMed] [Google Scholar]
- Hess DT, Slater TM, Wilson MC, Skene JH. The 25 kDa synaptosomal-associated protein SNAP-25 is the major methionine-rich polypeptide in rapid axonal transport and a major substrate for palmitoylation in adult CNS. J Neurosci. 1992;12:4634–4641. doi: 10.1523/JNEUROSCI.12-12-04634.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Ahmed M, Melia TJ, Sollner TH, Mayer T, Rothman JE. Fusion of cells by flipped SNAREs. Science. 2003;300:1745–1749. doi: 10.1126/science.1084909. [DOI] [PubMed] [Google Scholar]
- Hua SY, Raciborska DA, Trimble WS, Charlton MP. Different VAMP/synaptobrevin complexes for spontaneous and evoked transmitter release at the crayfish neuromuscular junction. J Neurophysiol. 1998;80:3233–3246. doi: 10.1152/jn.1998.80.6.3233. [DOI] [PubMed] [Google Scholar]
- Huntwork S, Littleton JT. A complexin fusion clamp regulates spontaneous neurotransmitter release and synaptic growth. Nat Neurosci. 2007;10:1235–1237. doi: 10.1038/nn1980. [DOI] [PubMed] [Google Scholar]
- Hynd MR, Scott HL, Dodd PR. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochem Int. 2004;45:583–595. doi: 10.1016/j.neuint.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Jahn R, Lang T, Sudhof TC. Membrane fusion. Cell. 2003;112:519–533. doi: 10.1016/s0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- Jahn R, Scheller RH. SNAREs--engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- Jurevics H, Morell P. Cholesterol for synthesis of myelin is made locally, not imported into brain. J Neurochem. 1995;64:895–901. doi: 10.1046/j.1471-4159.1995.64020895.x. [DOI] [PubMed] [Google Scholar]
- Katz B. The Release of Neural Transmitter Substances. Vol. 10. Liverpool: Liverpool University Press; 1969. [Google Scholar]
- Koenig JH, Ikeda K. Contribution of active zone subpopulation of vesicles to evoked and spontaneous release. J Neurophysiol. 1999;81:1495–1505. doi: 10.1152/jn.1999.81.4.1495. [DOI] [PubMed] [Google Scholar]
- Lang T, Bruns D, Wenzel D, Riedel D, Holroyd P, Thiele C, Jahn R. SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis. Embo J. 2001;20:2202–2213. doi: 10.1093/emboj/20.9.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau WF, Das NP. In vitro modulation of rat adipocyte ghost membrane fluidity by cholesterol oxysterols. Experientia. 1995;51:731–737. doi: 10.1007/BF01941271. [DOI] [PubMed] [Google Scholar]
- Llano I, Gonzalez J, Caputo C, Lai FA, Blayney LM, Tan YP, Marty A. Presynaptic calcium stores underlie large-amplitude miniature IPSCs and spontaneous calcium transients. Nat Neurosci. 2000;3:1256–1265. doi: 10.1038/81781. [DOI] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Lou X, Scheuss V, Schneggenburger R. Allosteric modulation of the presynaptic Ca2+ sensor for vesicle fusion. Nature. 2005;435:497–501. doi: 10.1038/nature03568. [DOI] [PubMed] [Google Scholar]
- Matsui K, Jahr CE. Ectopic release of synaptic vesicles. Neuron. 2003;40:1173–1183. doi: 10.1016/s0896-6273(03)00788-8. [DOI] [PubMed] [Google Scholar]
- Maximov A, Shin OH, Liu X, Sudhof TC. Synaptotagmin-12, a synaptic vesicle phosphoprotein that modulates spontaneous neurotransmitter release. J Cell Biol. 2007;176:113–124. doi: 10.1083/jcb.200607021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney RA, Capogna M, Durr R, Gahwiler BH, Thompson SM. Miniature synaptic events maintain dendritic spines via AMPA receptor activation. Nat Neurosci. 1999;2:44–49. doi: 10.1038/4548. [DOI] [PubMed] [Google Scholar]
- McNew JA, Weber T, Parlati F, Johnston RJ, Melia TJ, Sollner TH, Rothman JE. Close is not enough: SNARE-dependent membrane fusion requires an active mechanism that transduces force to membrane anchors. J Cell Biol. 2000;150:105–117. doi: 10.1083/jcb.150.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melia TJ, Weber T, McNew JA, Fisher LE, Johnston RJ, Parlati F, Mahal LK, Sollner TH, Rothman JE. Regulation of membrane fusion by the membrane-proximal coil of the t-SNARE during zippering of SNAREpins. J Cell Biol. 2002;158:929–940. doi: 10.1083/jcb.200112081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VN, Stevens CF. Reversal of synaptic vesicle docking at central synapses. Nat Neurosci. 1999;2:503–507. doi: 10.1038/9149. [DOI] [PubMed] [Google Scholar]
- Niemeyer BA, Schwarz TL. SNAP-24, a Drosophila SNAP-25 homologue on granule membranes, is a putative mediator of secretion and granule-granule fusion in salivary glands. J Cell Sci. 2000;113( Pt 22):4055–4064. doi: 10.1242/jcs.113.22.4055. [DOI] [PubMed] [Google Scholar]
- Nishiki TI, Augustine GJ. Calcium-dependent neurotransmitter release: synaptotagmin to the rescue. J Comp Neurol. 2001;436:1–3. [PubMed] [Google Scholar]
- Nishizawa Y. Glutamate release and neuronal damage in ischemia. Life Sci. 2001;69:369–381. doi: 10.1016/s0024-3205(01)01142-0. [DOI] [PubMed] [Google Scholar]
- Nonet ML, Saifee O, Zhao H, Rand JB, Wei L. Synaptic transmission deficits in Caenorhabditis elegans synaptobrevin mutants. J Neurosci. 1998;18:70–80. doi: 10.1523/JNEUROSCI.18-01-00070.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsu Y, Murphy TH. Miniature transmitter release: accident of nature or careful design? Sci STKE. 2003;211:pe54. doi: 10.1126/stke.2112003pe54. [DOI] [PubMed] [Google Scholar]
- Otsu Y, Shahrezaei V, Li B, Raymond LA, Delaney KR, Murphy TH. Competition between phasic and asynchronous release for recovered synaptic vesicles at developing hippocampal autaptic synapses. J Neurosci. 2004;24:420–433. doi: 10.1523/JNEUROSCI.4452-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang ZP, Melicoff E, Padgett D, Liu Y, Teich AF, Dickey BF, Lin W, Adachi R, Sudhof TC. Synaptotagmin-2 is essential for survival and contributes to Ca2+ triggering of neurotransmitter release in central and neuromuscular synapses. J Neurosci. 2006;26:13493–13504. doi: 10.1523/JNEUROSCI.3519-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prange O, Murphy TH. Correlation of miniature synaptic activity and evoked release probability in cultures of cortical neurons. J Neurosci. 1999;19:6427–6438. doi: 10.1523/JNEUROSCI.19-15-06427.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reim K, Mansour M, Varoqueaux F, McMahon HT, Sudhof TC, Brose N, Rosenmund C. Complexins regulate a late step in Ca2+-dependent neurotransmitter release. Cell. 2001;104:71–81. doi: 10.1016/s0092-8674(01)00192-1. [DOI] [PubMed] [Google Scholar]
- Rizo J, Sudhof TC. Snares and Munc18 in synaptic vesicle fusion. Nat Rev Neurosci. 2002;3:641–653. doi: 10.1038/nrn898. [DOI] [PubMed] [Google Scholar]
- Rodal SK, Skretting G, Garred O, Vilhardt F, van Deurs B, Sandvig K. Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol Biol Cell. 1999;10:961–974. doi: 10.1091/mbc.10.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Stevens CF. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron. 1996;16:1197–1207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- Ryan TA, Reuter H, Smith SJ. Optical detection of a quantal presynaptic membrane turnover. Nature. 1997;388:478–482. doi: 10.1038/41335. [DOI] [PubMed] [Google Scholar]
- Saifee O, Wei L, Nonet ML. The Caenorhabditis elegans unc-64 locus encodes a syntaxin that interacts genetically with synaptobrevin. Mol Biol Cell. 1998;9:1235–1252. doi: 10.1091/mbc.9.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaba T, Stein A, Jahn R, Neher E. Distinct kinetic changes in neurotransmitter release after SNARE protein cleavage. Science. 2005;309:491–494. doi: 10.1126/science.1112645. [DOI] [PubMed] [Google Scholar]
- Salaun C, James DJ, Chamberlain LH. Lipid rafts and the regulation of exocytosis. Traffic. 2004;5:255–264. doi: 10.1111/j.1600-0854.2004.0162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara Y, Virmani T, Deak F, Liu X, Kavalali ET. An isolated pool of vesicles recycles at rest and drives spontaneous neurotransmission. Neuron. 2005;45:563–573. doi: 10.1016/j.neuron.2004.12.056. [DOI] [PubMed] [Google Scholar]
- Saviane C, Silver RA. Fast vesicle reloading and a large pool sustain high bandwidth transmission at a central synapse. Nature. 2006;439:983–987. doi: 10.1038/nature04509. [DOI] [PubMed] [Google Scholar]
- Scheuber A, Rudge R, Danglot L, Raposo G, Binz T, Poncer JC, Galli T. Loss of AP-3 function affects spontaneous and evoked release at hippocampal mossy fiber synapses. Proc Natl Acad Sci U S A. 2006;103:16562–16567. doi: 10.1073/pnas.0603511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikorski T, Stevens CF. Morphological correlates of functionally defined synaptic vesicle populations. Nat Neurosci. 2001;4:391–395. doi: 10.1038/86042. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Meyer AC, Neher E. Released fraction and total size of a pool of immediately available transmitter quanta at a calyx synapse. Neuron. 1999;23:399–409. doi: 10.1016/s0896-6273(00)80789-8. [DOI] [PubMed] [Google Scholar]
- Schoch S, Deak F, Konigstorfer A, Mozhayeva M, Sara Y, Sudhof TC, Kavalali ET. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science. 2001;294:1117–1122. doi: 10.1126/science.1064335. [DOI] [PubMed] [Google Scholar]
- Schulze KL, Broadie K, Perin MS, Bellen HJ. Genetic and electrophysiological studies of Drosophila syntaxin-1A demonstrate its role in nonneuronal secretion and neurotransmission. Cell. 1995;80:311–320. doi: 10.1016/0092-8674(95)90414-x. [DOI] [PubMed] [Google Scholar]
- Sollner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Sorensen JB, Nagy G, Varoqueaux F, Nehring RB, Brose N, Wilson MC, Neher E. Differential control of the releasable vesicle pools by SNAP-25 splice variants and SNAP-23. Cell. 2003;114:75–86. doi: 10.1016/s0092-8674(03)00477-x. [DOI] [PubMed] [Google Scholar]
- Sorensen JB, Wiederhold K, Muller EM, Milosevic I, Nagy G, de Groot BL, Grubmuller H, Fasshauer D. Sequential N- to C-terminal SNARE complex assembly drives priming and fusion of secretory vesicles. Embo J. 2006;25:955–966. doi: 10.1038/sj.emboj.7601003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spady DK, Dietschy JM. Sterol synthesis in vivo in 18 tissues of the squirrel monkey, guinea pig, rabbit, hamster, and rat. J Lipid Res. 1983;24:303–315. [PubMed] [Google Scholar]
- Subtil A, Gaidarov I, Kobylarz K, Lampson MA, Keen JH, McGraw TE. Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc Natl Acad Sci U S A. 1999;96:6775–6780. doi: 10.1073/pnas.96.12.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle revisited. Neuron. 2000;28:317–320. doi: 10.1016/s0896-6273(00)00109-4. [DOI] [PubMed] [Google Scholar]
- Sun JY, Wu XS, Wu LG. Single and multiple vesicle fusion induce different rates of endocytosis at a central synapse. Nature. 2002;417:555–559. doi: 10.1038/417555a. [DOI] [PubMed] [Google Scholar]
- Sun J, Pang ZP, Qin D, Fahim AT, Adachi R, Südhof TC. A dual-Ca2+-sensor model for neurotransmitter release in a central synapse. Nature. 2007;450:676–682. doi: 10.1038/nature06308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Taylor AM, Ito HT, Pham A, Schuman EM. Postsynaptic decoding of neural activity: eEF2 as a biochemical sensor coupling miniature synaptic transmission to local protein synthesis. Neuron. 2007;55:648–661. doi: 10.1016/j.neuron.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Wall NR, Aakalu GN, Schuman EM. Regulation of dendritic protein synthesis by miniature synaptic events. Science. 2004;304:1979–1983. doi: 10.1126/science.1096202. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125:785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- Tafoya LC, Mameli M, Miyashita T, Guzowski JF, Valenzuela CF, Wilson MC. Expression and function of SNAP-25 as a universal SNARE component in GABAergic neurons. J Neurosci. 2006;26:7826–7838. doi: 10.1523/JNEUROSCI.1866-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Maximov A, Shin OH, Dai H, Rizo J, Sudhof TC. A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell. 2006;126:1175–1187. doi: 10.1016/j.cell.2006.08.030. [DOI] [PubMed] [Google Scholar]
- Turley SD, Burns DK, Dietschy JM. Preferential utilization of newly synthesized cholesterol for brain growth in neonatal lambs. Am J Physiol. 1998;274:E1099–1105. doi: 10.1152/ajpendo.1998.274.6.E1099. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W, Naves LA. Localizing quantal currents along frog neuromuscular junctions. J Physiol. 1996;497:189–198. doi: 10.1113/jphysiol.1996.sp021759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardjan N, Stenovec M, Jorgacevski J, Kreft M, Zorec R. Subnanometer fusion pores in spontaneous exocytosis of peptidergic vesicles. J Neurosci. 2007;27:4737–4746. doi: 10.1523/JNEUROSCI.0351-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F, Sigler A, Rhee JS, Brose N, Enk C, Reim K, Rosenmund C. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc Natl Acad Sci U S A. 2002;99:9037–9042. doi: 10.1073/pnas.122623799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, Vermeer H, Toonen RF, Hammer RE, van den Berg TK, Missler M, Geuze HJ, Sudhof TC. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- Vilinsky I, Stewart BA, Drummond J, Robinson I, Deitcher DL. A Drosophila SNAP-25 null mutant reveals context-dependent redundancy with SNAP-24 in neurotransmission. Genetics. 2002;162:259–271. doi: 10.1093/genetics/162.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washbourne P, Thompson PM, Carta M, Costa ET, Mathews JR, Lopez-Benditó G, Molnár Z, Becher MW, Valenzuela CF, Partridge LD, Wilson MC. Genetic ablation of the t-SNARE SNAP-25 distinguishes mechanisms of neuroexocytosis. Nat Neurosci. 2002;5:19–26. doi: 10.1038/nn783. [DOI] [PubMed] [Google Scholar]
- Wasser CR, Ertunc M, Liu X, Kavalali ET. Cholesterol-dependent balance between evoked and spontaneous synaptic vesicle recycling. J Physiol. 2007;579:413–429. doi: 10.1113/jphysiol.2006.123133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- Wierenga CJ, Ibata K, Turrigiano GG. Postsynaptic expression of homeostatic plasticity at neocortical synapses. J Neurosci. 2005;25:2895–2905. doi: 10.1523/JNEUROSCI.5217-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG, Borst JG. The reduced release probability of releasable vesicles during recovery from short-term synaptic depression. Neuron. 1999;23:821–832. doi: 10.1016/s0896-6273(01)80039-8. [DOI] [PubMed] [Google Scholar]
- Wucherpfennig T, Wilsch-Brauninger M, Gonzalez-Gaitan M. Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J Cell Biol. 2003;161:609–624. doi: 10.1083/jcb.200211087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Rammner B, Margittai M, Artalejo AR, Neher E, Jahn R. Inhibition of SNARE complex assembly differentially affects kinetic components of exocytosis. Cell. 1999;99:713–722. doi: 10.1016/s0092-8674(00)81669-4. [DOI] [PubMed] [Google Scholar]
- Yoshihara M, Littleton JT. Synaptotagmin I functions as a calcium sensor to synchronize neurotransmitter release. Neuron. 2002;36:897–908. doi: 10.1016/s0896-6273(02)01065-6. [DOI] [PubMed] [Google Scholar]
- Yoshihara M, Ueda A, Zhang D, Deitcher DL, Schwarz TL, Kidokoro Y. Selective effects of neuronal-synaptobrevin mutations on transmitter release evoked by sustained versus transient Ca2+ increases and by cAMP. J Neurosci. 1999;19:2432–2441. doi: 10.1523/JNEUROSCI.19-07-02432.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SM., Jr Proteolysis of SNARE proteins alters facilitation and depression in a specific way. Proc Natl Acad Sci U S A. 2005;102:2614–2619. doi: 10.1073/pnas.0409656102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir O, Charlton MP. Cholesterol and synaptic transmitter release at crayfish neuromuscular junctions. J Physiol. 2006;571:83–99. doi: 10.1113/jphysiol.2005.098319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zefirov A, Benish T, Fatkullin N, Cheranov S, Khazipov R. Localization of active zones. Nature. 1995;376:393–394. doi: 10.1038/376393b0. [DOI] [PubMed] [Google Scholar]
- Zenisek D, Steyer JA, Almers W. Transport, capture and exocytosis of single synaptic vesicles at active zones. Nature. 2000;406:849–854. doi: 10.1038/35022500. [DOI] [PubMed] [Google Scholar]
- Zucker RS. Minis: whence and wherefore? Neuron. 2005;45:482–484. doi: 10.1016/j.neuron.2005.02.003. [DOI] [PubMed] [Google Scholar]