Abstract
Exercise showed the beneficial effects on mental health in depressed sufferers, whereas, its underlying mechanisms remained unresolved. This study utilized the chronic unpredictable stress (CNS) animal model of depression to evaluate the effects of exercise on depressive behaviors and spatial performance in rats. Furthermore, we tested the hypothesis that the capacity of exercise to reverse the harmful effects of CNS was relative to the hypothalamo–pituitary–adrenal (HPA) system and brain-derived neurotrophic factor (BDNF) in the hippocampus. Animal groups were exposed to CNS for 4 weeks with and without access to voluntary wheel running. Stressed rats consumed significantly less of a 1% sucrose solution during CNS and exhibited a significant decrease in open field behavior. On the other hand, they showed impaired spatial performance in Morris water maze test 2 weeks after the end of CNS. Further, CNS significantly decreased hippocampal BDNF mRNA levels. However, voluntary exercise improved or even reversed these harmful behavioral effects in stressed rats. Furthermore, exercise counteracted a decrease in hippocampal BDNF mRNA caused by CNS. In addition, we also found that CMS alone increased circulating corticosterone (CORT) significantly and decreased hippocampal glucocorticoid receptor (GR) mRNA. At the same time, exercise alone increased CORT moderately and did not affect hippocampal GR mRNA levels. While, when both CNS and exercise were combined, exercise reduced the increase of CORT and the decrease of GR caused by CMS.
The results demonstrated that: (1) exercise reversed the harmful effects of CNS on mood and spatial performance in rats and (2) the behavioral changes induced by exercise and/or CNS might be associated with hippocampal BDNF levels, and in addition, the HPA system might play different roles in the two different processes.
Keywords: Open field test, Morris water maze, BDNF, Corticosterone, Glucocorticoid receptor, Hippocampus
1. Introduction
Exercise was shown to be beneficial to improve depressive symptoms in depressed sufferers [1,2], but the biological mechanisms sustained unclear. Animal models of depression were useful for examining the effects of exercise on brain function and investigating their mechanisms. Chronic unpredictable stress (CNS), consisting of several relatively mild and unpredictable stressors, was considered to be a valid animal model of depression [3,4] and could induce anhedonia, a core symptom of depression in human, as measured by changes in sucrose consumption in rats [5]. The model was proven to be successful in the functional identification of antidepressant, and therefore had a high degree of predictive validity [6,7]. The alterations reported in the model were stable for a long time [8], which made a significant difference with respect to the transitory disturbances observed in other animal models of depression. Aforementioned characteristic of CMS as an animal model of depression made it possible for the more profound studies for the etiology of depression and the mechanisms of chronic antidepressive treatments.
Apart from mood disorder, cognitive impairment was frequently observed in depressed sufferers [9,10]. However, only a few studies investigated memory performance in animal models of depression. Previous studies reported a deficiency in spatial leaning in the Morris water maze and/or the eight-arm radial maze in olfactory bulbectomized rats and Wistar-Kyoto rats [11,12]. On the other hand, voluntary exercise reported could improve cognitive function in human and in animals [13–15], but few studies had investigated the effect of exercise on cognitive function in animal models of depression.
Chronic stress was thought to play an important role in the etiology of depression [16]. Depressed patients often exhibited hyperactivity in the HPA axis such as hypersecretion of basal CORT [17] and increased adrenal weight [18]. Sustained exposure to chronic stress or excessive glucocorticoids had adverse effects on the hippocampus [19–21], a region of the brain that is intrinsically linked to mood and cognitive function, and might cause the hippocampal atrophy. There was a significant correlation between the duration of the depression and the extent of hippocampal atrophy [22]. The effect of CORT on hippocampus might be relative to hippocampal GR. Under a stress situation, hippocampal GR was sensitive to elevated CORT levels and played a crucial role in the normalization of the HPA axis [23].
Recent studies had indicated that brain-derived neurotrophic factor (BDNF), expressed at high levels in the hippocampus [24], was involved in the pathogenesis of depression. BDNF was decreased in serum of depressed sufferers [25] and increased by antidepressant treatments [26]. Local cerebral administration of BDNF was reported exert antidepressant-like effects in animal models of depression [27]. Previous studies had examined the effects of various forms of chronic stress and CORT on hip-pocampal BDNF mRNA. Repeated immobilization stress was shown to result in a significant decrease in hippocampal BDNF mRNA [28,29]. Similarly, the administration of high doses of CORT resulted in a transient decrease in BDNF mRNA in multiple subregions of the hippocampus [30,31]. The interaction of stress and CORT on BDNF might, therefore, be relative to hip-pocampal atrophy and contribute to mood disorder and memory impairments.
It was reported that voluntary exercise increased BDNF mRNA level in the hippocampus in normal rats [32,33]. It was possible that exercise might protect against the effects of chronic stress and exert antidepressant-like effects by counteracting a decrease in hippocampal BDNF mRNA caused by chronic stress. The present study was designed (1) to evaluate the effects of exercise on depressive behaviors and spatial performance in chronic unpredictable stress rats and (2) to identify whether these effects were associated with the circulating CORT and the hippocampal BDNF mRNA.
2. Materials and methods
2.1. Subjects
Male Sprague-Dawley rats, weighing 150–200 g, were used. Rats were acclimated to the surroundings for 1 week before experimentation and were individually housed in a temperature (22 ± 2 °C), humidity (55 ± 10%), and light (12 h light:12 h dark cycle; lights on at 7 a.m.) controlled environment and were fed food and water ad libitum. All animal experiments were performed in accordance with the local, international and institutional guidelines. The experiments in the present study were designed to minimize the number of animals used and their suffering.
2.2. Chronic unpredictable stress (CNS)
The CNS procedure, adapted from Murua et al. [34], was designed to maximize the unpredictable nature of the stressors and consisted of the following stressors in random order: 24-h water deprivation, 5-min tail suspension, 2-h restraint, 5-min forced swim in cool water (4 °C), 24-h food deprivation, 5-min hot environment (45 °C), continuous overnight illumination and 20-min inescapable footshocks (0.8 mA in intensity, 4 s in duration and 5 s in interval). The CNS procedure was carried out in stressed animals once per day for 4 weeks. Non-stressed animals were left undisturbed in the home cages except for the necessary controls such as regular cage cleaning and weighing.
2.3. Sucrose test
Sucrose test was used to measure anhedonia, which was defined as a reduction in sucrose consumption relative to the control group. The protocol for this test was based on that reported previously [35]. Rats were first trained to consume 1% sucrose solution before beginning the experimental procedures. Training consisted of five 1-h baseline tests in which sucrose was presented in the home cage, following 24 h food and water deprivation; intake was measured by weighing preweighted bottles containing the sucrose solution at the end of the test. Subsequently, sucrose consumption was monitored once each week, under similar conditions throughout the whole experiment. All sucrose tests were carried out every Saturday at 7 p.m.
2.4. Experimental protocol
Rats were first trained to consume a 1% sucrose solution for 10 days. On the basis of their sucrose intakes in the final baseline test, rats were divided into four groups, including Control (Con), Exercise (Exe), CNS and CNS/Exercise (CNS/E), and housed individually in standard polyethylene cages. Animals engaged in voluntary exercise had free access to a running wheel (diameter = 33 cm and width = 10 cm). They were allowed 1 week to adapt to the wheels. Wheel revolutions were recorded automatically by computer using homemade Data Acquisition System software. From week 2 on, stressed animals were subjected to a 4-week CNS procedure. Non-stressed animals remained undisturbed during the 4 weeks except for necessary operation such as regular cage cleaning and weighing. The CNS procedure concluded at the end of the 5th week, and then five animals per group were sacrificed on the subsequent day (between 9 and 11 a.m.) for blood and organ collection. Other rats were left undisturbed in the home cages for 2 weeks (recovery period). At the end of the 7th week, another five animals per group were sacrificed as above way. For assessing the long-term effect of CNS on spatial performance, surplus rats (eight for each experimental group) were used for the Morris water maze (MWM) test in the 8th week. Sucrose tests were performed once per week, and weighing and open field test (OFT) were performed once per 2 weeks during the CNS period and the recovery period.
2.5. Open field test (OFT)
The open field test evaluated the general locomotor and exploratory behavior of rats and the experiments were performed as described previously [36]. Each rat was placed at the centre of the open field (76 cm square chamber, 40-cm-high walls, light of 80 lux. with its floor divided into 25 equal squares) for 3 min in a quiet room after weighed. Parameters assessed were the time in the centre square, the number of crossing squares, the times of rearing. Next test was performed after cleaning the chamber.
2.6. Morris water maze (MWM) test
The Morris water maze test was used for assessing cognitive function. The water maze was a 1.5 m diameter, 0.5 m height white circular pool filled with opaque water (30 cm depth) at 22 ± 2 °C. The pool was divided into four quadrants of equal size. An invisible escape platform (11 cm diameter) was placed in the middle of one of the quadrants (2 cm below the water surface). The behavior of the animal was monitored by a video camera mounted in the ceiling above the centre of the pool and a computerized tracking system. The experimental procedure was adapted from that described by [37]. The rats were trained to find the position of the hidden platform taking six consecutive trials for 1 day. The animals were placed into the pool facing the wall sequentially from three different entry points, equally set around the pool. The order of the three entry points was kept the same for all animals and this process was repeated once again to achieve a total number of six trials per rat in the learning session. If the rat failed to escape within 120 s, it was guided to the platform by the experimenter. When the rat escaped onto the platform, it was left there for 30 s, and then it was placed for 30 s in a holding cage. By the end of the training session the animals were returned to their home cages. The day following training, the rats were given a probe trial for 120 s in the absence of the platform to assess spatial memory retention. The escape latency during training and the times of crossing the quadrant position in the probe test were recorded by a computerized video tracking system.
2.7. Blood and organ collection
On the day following end of CNS and after 2 weeks following end of CNS, five rats per group were sacrificed (between 9 and 11 a.m.) and trunk blood was collected and then processed. Serum was collected and stored at −20 °C for corticosterone measurement. Brains were rapidly removed. The hippocampus area was then microdissected and frozen on dry ice and subsequently stored at −80 °C for future analysis.
2.8. Serum corticosterone radioimmunoassay
Serum corticosterone levels were measured by a radioimmunoassay kit (ICN Biomedicals, Costa Mesa, CA). The intra-assay variability of the RIA ranged between 3.1 and 4.5%. Its sensitivity was 5.7 ng/ml.
2.9. Hippocampus GR and BDNF RNA isolation and RT-PCR analysis
Total RNA from the hippocampus was extracted using the Trizol reagent in accordance with the manufacturer’ instructions. The RNA product was resus-pended in 20 μl diethyl pyrocarbouate (DEPC) treated water. The quality of RNA was judged from the pattern of ribosomal RNA after electrophoresis of RNA through 1.5% agarose gel containing ethidium bromide (EB) and visualization by UV illumination. RNA was stored at −80 °C until use. Total RNA was reverse transcribed at 50 °C for 30 min, 99 °C for 5 min, 5 °C for 5 min with AMV Reverse-Transcriptase XL according to the instruction of the manufacturer of the reagent (TAKARA, Biotechnology Co., Ltd.). Following the RT reaction, the cDNA products were stored at −20 °C until use.
cDNA was amplified using adequate primers. GR primers were: forward, 5′-GAAATGGGCAAAGGCGATAC-3′; reverse, 5′-AGGAGCAAAGCAAG-AGCAGGT-3′. BDNF primers were: forward, 5′-GTGACAGTATAGCGA-GTGGG-3′; reverse, 5′-GATTGGGTAGTTCGGCATT-3′. As a control to eliminate variations for sample-to-sample differences in RNA extraction and conversion to cDNA, the housekeeping gene β-action was amplified. β-action primers were: forward, 5′-CAACTGGGACGATATGGAGAAG-3′; reverse, 5′-AGGAAGGAAGCTGGAAGAG-3′. The PCR reaction was carried out with the following cycle parameters: BDNF: 94 °C, 30 s; 53 °C, 30 s; 72 °C, 0.5 min, 30 cycles; GR: 94 °C, 30 s; 57 °C, 30 s; 72 °C, 0.5 min, 28 cycles. Amplified products were separated on 1.5% agarose gels stained with ethidium bromide and photographed under UV illumination with gel-documentation system. Results were evaluated as a relative unit determined by normalization of the optical density (OD) of BDNF or GR band to that of the β-action band.
2.10. Statistical analysis
Two-way ANOVA (stress versus no stress and exercise versus no exercise) with repeated measures was performed on body weight, open field behaviors and sucrose consumption during the experiment and escape latency in the water maze training session. All other measures were analyzed using two-way ANOVA for a single time point. LSD post hoc tests were used to make significant interactions analysis when necessary. The significance level was set at P < 0.05.
3. Results
3.1. Body weight
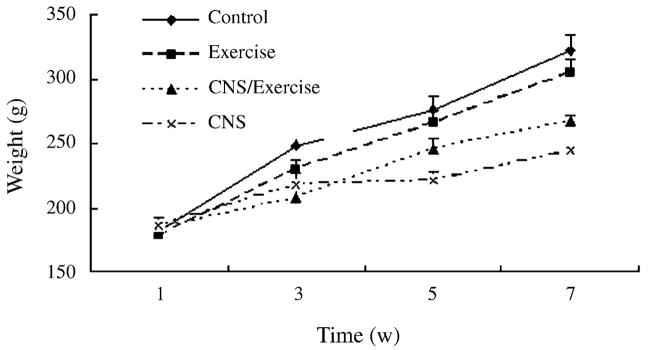
As shown in Fig. 1, the CNS used significantly affected body weight. Stressed animals gained significantly less weight than non-stressed objects, and a more pronounced effect was seen in animals without exercise, whereas, the non-stressed rats with exercise gained less weight than the non-stressed rats without exercise. ANOVA indicated a significant effect of time (F3,26 = 200.78, P < 0.001) and a significant interaction of time × CNS (F3,26 = 18.52, P < 0.001). ANOVA also showed a significant effect of CNS (F1,28 = 24.208, P < 0.001), a lack effect of exercise (F1,28 = 0.083, P = 0.775) and a lack of CNS × exercise interaction (F1,28 = 3.060, P = 0.09).
Fig. 1.
Effects of CNS, exercise, or both combined on animal body weights during the 7-week study period (Exercised rats were made to adapt to the wheels at week 1, CNS procedure were made from week 2 to 5 and animals remained undisturbed at week 6 and 7). Values represent mean ± S.E.M. n = 8 for each experimental group.
3.2. Sucrose test
Fig. 2 displayed the effects of CNS and/or exercise on sucrose consumption in 1 h. During the CNS, stressed animals drank significantly less sucrose than the control, with a more pronounced effect seen in animals without exercise. ANOVA showed an effect of time (F6,23 = 2.180, P = 0.082) and a significant CNS × time interaction (F6,23 = 4.201, P = 0.005). ANOVA also showed significant effects of CNS and exercise (F1,28 = 42.375, P < 0.001 and F1,28 = 9.444, P = 0.005, respectively). Post hoc analysis, through LSD test for each measure separately, revealed that in week 4, 6 and 7, CNS group consumed less sucrose, significantly differing from all other groups (all P < 0.05). However, CNS/Exercise group only showed a sig-nificant difference from the control in week 4 (P = 0.022). There was no significant interaction of CNS × exercise.
Fig. 2.
Effects of CNS, exercise, or both combined on sucrose consumption in 1 h during the 7-week study period (Exercised rats were made to adapt to the wheels at week 1, CNS procedure were made from week 2 to 5 and animals remained undisturbed at week 6 and 7). Values represent mean ± S.E.M. n = 8 for each experimental group. *Statistically different from all other groups in the same time points (LSD post hoc test). Significance was accepted for P < 0.05. The exact P values are given in the text.
3.3. Open field test
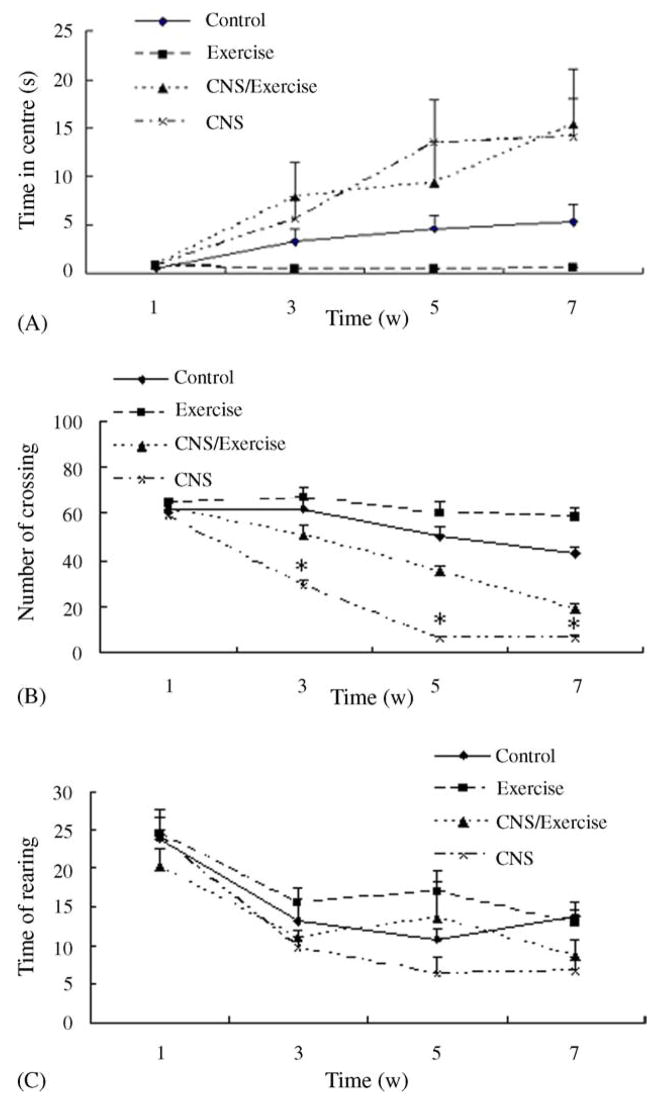
The CNS paradigm increased the time in the centre in the open field significantly and the effect was more pronounced in animals without wheel running, which were shown in Fig. 3(A). ANOVA revealed a significant effect of time (F3,26 = 15.29, P < 0.001) and a significant time × CNS interaction (F3,26 = 7.731, P = 0.001). Otherwise, the effect of CNS (F1,28 = 24.565, P < 0.001) was significant but there were not a significant effect of exercise and a significant exercise × CNS interaction. Post hoc analysis revealed that CNS groups were significantly different from the control week 5 (P = 0.044). No significant differences were observed between CNS/E groups and the control.
Fig. 3.
Effects of CNS, exercise, or both combined on open field behavior during the 7-week study period (Exercised rats were made to adapt to the wheels at week 1, CNS procedure were made from week 2 to 5 and animals remained undisturbed at week 6 and 7). (A) The time in the centre during the 3 min session, (B) the number of crossing during the test session and (C) the times of rearing during the test session. Values represent mean ± S.E.M. n = 8 for each experimental group. *Statistically different from all other groups in the same time points (LSD post hoc test). Significance was accepted for P < 0.05. The exact P values are given in the text.
CNS and exercise significantly affected the number of crossing squares in the open field tests, as shown in Fig. 3(B). ANOVA showed a significant time effect and a significant interaction of time × stress (F3,26 = 70.376, P < 0.001; F3,26 = 19.901, P < 0.001, respectively). ANOVA also showed significant effects of CNS and exercise and a significant CNS × exercise interaction (F1,28 = 192.702, P < 0.001; F1,28 = 47.576, P < 0.001; F1,28 = 5.555, P = 0.026, respectively). Post hoc analysis revealed that from week 3 on, CNS group and CNS/E group showed significantly differences from all other groups, respectively (all P < 0.05).
Fig. 3(C) displayed the effects of CNS and/or exercise on the rearing times in the open field tests. ANOVA showed a significant time effect (F3,26 = 31.597, P < 0.001) and also a CNS effect (F1,28 = 6.541, P = 0.016). Post hoc analysis revealed that CNS group were significantly different from the control week 7 (P = 0.012). No significant differences were observed between CNS/E groups and the control. There was no signifi-cant stress × exercise interaction.
3.4. Morris water maze test
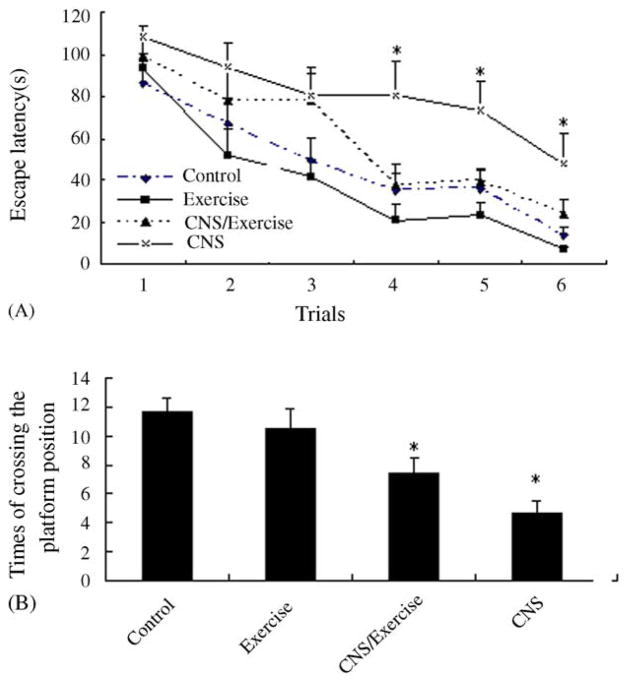
As shown in Fig. 4(A), CNS increased escape latency in Morris water maze and a more pronounced effect was found in animals without exercise. ANOVA showed a significant effect of time (F5,24 = 40.403, P < 0.001). The main effects of stress (F1,28 = 370.754, P < 0.001), exercise (F1,28 = 6.293, P = 0.018) on escape latency also were significant. Post hoc analysis, through LSD test for each trial separately, revealed that in trials 4, 5 and 6, the escape latency of CNS group were significantly higher than all other groups (all P < 0.05). No significant differences between CNS/E group and the control in the learning task except for in trial 4 (P = 0.038).
Fig. 4.
Effects of CNS, exercise, or both combined on the acquisition of spatial learning (A) and the recall of the platform location during the probe test (B) in the Morris water maze. Values represent mean ± S.E.M. n = 8 for each experimental group. (A) Escape latencies required by rats to find hidden platform in the six trials. *Statistically different from all other groups in the same time points (LSD post hoc test). Significance was accepted for P < 0.05. The exact P values are given in the text. (B) The times of crossing the platform position (probe test). *Statistically different from control groups (LSD post hoc test). Significance was accepted for P < 0.05. The exact P values are given in the text.
To check memory retrieval, the times that the animal crossed the platform position during the probe trial (Fig. 4 (B)) was analyzed, indicating a significant effect of CNS (F = 22.002, P < 0.001) and a significant stress × exercise interaction (F = 30.031, P = 0.003), but there was no significant effect of exercise (F = 0.570, P = 0.457). Post hoc analysis revealed that CNS group showed a significant difference from the control (P < 0.001) and Con/E group (P = 0.001).
3.5. Plasma CORT levels
A two-way ANOVA on CORT levels determined on the day following the cessation of CNS procedure (Fig. 5) showed that there was a significant effect of CNS (F1,16 = 58.617, P < 0.001) and a significant interaction of CNS × exercise (F1,16 = 5.675, P = 0.030). Post hoc analysis revealed that CNS/E group and CNS group respectively showed a significant difference from all other groups (P < 0.05). While exercise group did not differ from the control (P = 0.305).
Fig. 5.
Effects of CNS, exercise, or both combined on CORT levels (‘Day 1’ presents the CORT levels on the day following the end of CNS procedure; ‘Day 14’ presents after 2 week following the end of CNS). Values represent mean ± S.E.M. n = 5 for each experimental group in each measure. *P < 0.05, **P < 0.01, compared to control; #P < 0.05, ##P < 0.01, compared to CNS group.
The exact P values are given in the text.
ANOVA on CORT levels determined 2 weeks following the cessation of CNS procedure (Fig. 5) also showed significant effects of exercise (F = 10.585, P = 0.005), CNS (F = 13.334, P = 0.002) and a significant CNS × exercise interaction (F = 5.323, P = 0.035). Post hoc analysis revealed that CNS groups showed a significant difference from all other groups (P < 0.05). Exercise group showed a significant difference from all other groups (P < 0.05). No significant differences were observed between other groups.
3.6. Glucocorticoid receptor (GR) mRNA in the hippocampus
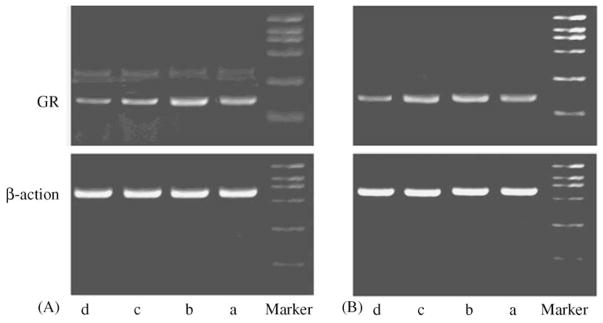
RTPCR of GR with control template β-action in the hippocampus yielded the products of expected length: 151 bp for GR, 573 bp for β-action, which were showed in Fig. 6.
Fig. 6.
Example of RT-PCR products corresponding to GR and β-action on mRNA (‘A’ presents the example of RT-PCR products on the day following the end of CNS procedure; ‘B’ presents after 2 week following the end of CNS) were visualized on an EB stained 1.5% agarose gel (a, Control; b, Exercise; c, CNS/Exercise; d, CNS).
As shown in Fig. 7, a two-way ANOVA on glucocorti-coid receptor mRNA levels determined on the day following the cessation of CNS procedure showed significant effects of CNS (F1,16 = 42.042, P < 0.001) and exercise (F1,16 = 4.798, P = 0.044). Whereas there is not a significant CNS × exercise interaction (F1,16 = 3.716, P = 0.072). Post hoc analysis revealed that CNS/Exercise group and CNS group, respectively showed a significant difference from all other groups (P < 0.05). While GR mRNA levels of exercise rats did not differ from control (P = 0.855).
Fig. 7.
Effects of CNS, exercise, or both combined on GR mRNA after normalization with β-action mRNA levels. (‘Day 1’ presents GR levels on the day following the end of CNS procedure; ‘Day 14’ presents after 2 week following the end of CNS). Values represent mean ± S.E.M. n = 5 for each experimental group in each measure. *P < 0.05, **P < 0.01, compared to control; #P < 0.05, ##P < 0.01, compared to CNS group. The exact P values are given in the text.
In addition, ANOVA on glucocorticoid receptors mRNA levels determined 2 weeks following the cessation of CNS procedure (Fig. 7) showed there was not significant effects of exercise (F1,16 = 1.733, P = 0.207), CNS (F1,16 = 0.770, P = 0.393) but a significant CNS × exercise interaction (F1,16 = 7.321, P = 0.016). Post hoc analysis revealed that CNS group showed a significant difference from control group (P = 0.012) and CNS/Exercise group (P = 0.022). No significant differences were observed between other groups.
3.7. BDNF mRNA in the hippocampus
RT-PCR of BDNF with control template β-action in the hippocampus yielded the products of expected length: 217 bp for BDNF, 573 bp for β-action, which were showed in Fig. 8.
Fig. 8.
Example of RT-PCR products corresponding to BDNF and β-action on mRNA (‘A’ presents the example of RT-PCR products on the day following the end of CNS procedure; ‘B’ presents after 2 week following the end of CNS) were visualized on an EB stained 1.5% agarose gel (a, Control; b, Exercise; c, CNS/Exercise; d, CNS).
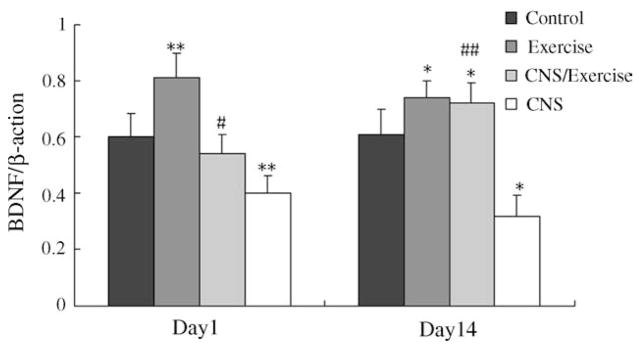
The effects of CNS, exercise, or both combined on BDNF mRNA levels were shown in Fig. 9. Two-way ANOVA on BDNF mRNA levels on the day following the cessation of CNS procedure showed significant effects of CNS (F1,16 = 38.124, P < 0.001) and exercise (F1,16 = 22.001, P < 0.001). While there was not a significant CNS × exercise interaction (F1,16 = 0.961, P = 0.341). Post hoc analysis revealed that CNS group and exercise group, respectively showed a significant difference from all other groups (P < 0.05). While CNS/Exercise group did not differ from control (P = 0.305).
Fig. 9.
Effects of CNS, exercise, or both combined on BDNF mRNA after normalization with β-action mRNA levels. (‘Day 1’ presents the BDNF levels on the day following the end of CNS procedure; ‘Day 14’ presents after 2 week following the end of CNS). Values represent mean ± S.E.M. n = 5 for each experimental group in each measure. *P < 0.05, **P < 0.01, compared to control; #P < 0.05, ##P < 0.01, compared to CNS group. The exact P values are given in the text.
A two-way ANOVA on BDNF mRNA levels determined 2 weeks following the cessation of CNS procedure indicated significant exercise (F1,16 = 59.748, P < 0.001) and CNS (F1,16 = 21.401, P < 0.001) effects and a signifi-cant CNS × exercise interaction (F1,16 = 15.856, P = 0.001), as shown in Fig. 9. Post hoc analysis revealed that CNS group showed a significant difference from all other groups (P < 0.05). While BDNF mRNA levels of the CNS/E groups did not differ from exercise groups (P = 0.305).
4. Discussion
Our results showed that CNS resulted in depressive behaviors and impaired spatial performance in rats. Stressed subjects showed a significant increased circulating CORT levels, reduced GR and BDNF mRNA levels in the hippocampus. Exercise diminished or even reversed the effects of CNS on depressive behaviors, spatial performance, CORT and hippocampal BDNF mRNA.
During the CNS session and the recovery session, stressed rats consumed significantly less 1% sucrose solution, exhibited lower open field activity both crossing and rearing than the control. These behavioral changes were consistent or similar with previous reports [38–43]. In addition, 2 weeks after the end of CNS, in the Morris water maze test, there was a signifi-cant increase in escape latency and a significant decrease in the times of crossing the platform in the stressed rats compared with the control, indicating an impaired spatial performance in these rats.
Among these stressed animals, the rats with exercise showed much less pronounced depressive behaviors than the rats without exercise in present study. They reduced the sucrose consumption slower and less. Two weeks after the end of CNS, their sucrose consumption increased to the controls levels, and the open field activity was improved significantly. These results indicated protective properties of exercise against depressive behaviors. Two weeks after the end of CNS, exercise showed a significant effect on spatial learning in the Morris water maze test. CNS had a much greater effect on spatial learning in rats without exercise. On the other hand, shorter escape latency and more times of crossing the platform were seen in control rats with exercise relative to control rats without exercise, suggesting that the positive effects of exercise on spatial performance, irrespective of exposure to CNS.
In present study, the CNS procedure we used increased CORT levels significantly, suggesting an activation of HPA axis under CNS condition. This was consistent with previous reports [44,45]. While, 2 weeks after termination of stress, the CORT levels in stressed rats came back to normal baseline and were even lower in stressed rats relative to control rats. This result might be tied to an adaptation of HPA axis to the CNS.
The hyperactivity of HPA axis might play an important role in the aforementioned harmful effects of CNS on emotion and spatial performance in rats. Sustained exposure to excessive glu-cocorticoids had adverse effects in the rat hippocampus and there was a significant correlation between the duration of depression and the extent of bilateral hippocampal atrophy [19–22]. The increase in CORT had particular relevance to the hippocampus, which contained the highest density of GC target receptors, which mediate the action of GCs [46]. In the study, we observed that CNS decreased GR mRNA expression in the hippocampus significantly. This was in accordance with previous reports of a decrease in mRNA levels for GR in the rat hippocampus impacted by different chronic stressors [47,48]. The down-regulation of GR mRNA in the hippocampus might be the result of increased CORT levels and represent a protective mechanism toward the damaging effects of chronic stress. On the other hand, this adaptive response to chronic stress might lead to alteration of the negative feedback mechanisms of HPA axis and then resulted in degenerative changes in the hippocampus [49].
CORT levels were higher in stressed rats without exercise compared with stressed rats with exercise. This might be explained that exercise improved the adaptive ability of rats to chronic stress and was linked to a more adaptive HPA axis response to stress and a faster return of CORT to basal levels following CNS. Because of the relatively mild increase in CORT levels, hippocampal GR levels in stressed rats with exercise was higher relative to stressed rats without exercise and came to normal level 2 weeks after end of CNS. Interestingly, we observed that exercise alone also elevated CORT levels compared with control rats. The increase was moderate and did not come to normal level 2 weeks following the end of CNS. At the same time, exercise alone did not affect hippocampal GR levels sig-nificantly. This might be explained that the degree of increased CORT level was not enough to decrease the level of GR significantly.
In the study, CNS significantly reduced hippocampal BDNF mRNA expression. Two weeks after the termination of CNS, the BDNF mRNA level in stressed rats was still lower than normal levels significantly. This was consistent with previous reports that many acute or chronic stress decreases BDNF levels in the hippocampus [28–31]. The increase of CORT levels might be relative to the decrease of hippocampal BDNF mRNA. The BDNF gene contained a specific response element, cAMP response element (CRE) and phosphorylated cAMP response element binding protein (CREB) binds to CRE and enhances transcription. While, the cortisol–GR complex binds to CREB, preventing its phosphorylation and therefore blocking the expression of target gene of BDNF [50,51]. It was known that BDNF had well-established effects on neurotrophic procedure and neurogenesis [52,53]. BDNF might provide the effects by an inhibition of cell death cascades. Decrease of BDNF gene expression might result in hippocampus atrophy by an excess of neural loss (apoptosis) and an altered regulation of the neu-rotrophic processes [54] and be responsible for the harmful effects of CNS on spatial performance and emotion.
Two week after the end of CNS, the CORT levels in stressed rats came to baseline but the BDNF mRNA levels were still lower than normal levels significantly. It seen to be inconsistent with previous hypothesis that increased CORT was relative to decreased BDNF. One reason for this was that 2 weeks were not enough for the normalization of BDNF levels. It need be clari-fied by time course analyse for the CORT level and the BDNF level in the future experiment. In addition, it might also be due to the structural impairment of hippocampus such as hippocampus atrophy after the CNS procedure used, which leaded to the durative decrease of hippocampal BDNF levels. Virtually, just because of the durative decrease in BDNF levels, the effects of CORT on mood and spatial learning were durative even if CORT levels came to baseline.
Exercise showed a significant effect on the hippocampal BDNF mRNA level in rats in the study. The decrease of BDNF in stressed rats with exercise was much less pronounced compared with stressed rats without exercise on the day following the end of CNS. Two weeks after termination of stress, the BDNF level in stressed rats with exercise became higher significantly relative to control rats. Exercise also showed a significant effect on BDNF levels in non-stressed rats. An increase in BDNF mRNA was seen in non-stressed rats with exercise relative to non-stressed rats without exercise. This was consistent with previous study [32,33].
The effect of exercise on counteracting the stress-induced decrease of BDNF level might be associated with the lower CORT level in stressed rats with exercise and contributed to the beneficial behavioral effects of exercise. While, in present study, we observed exercise alone elevated BDNF levels and CORT levels simultaneously. This reflected that CORT might affect hippocampal BDNF expression in different ways and have a two-faced role in the brain. There might be a hypothesis that a significantly increased CORT level after CNS wound decrease BDNF levels in the hippocampus and thereby lead to depressive behaviors and impaired spatial learning, however, a mild increased CORT after exercise wound increase hippocampal BDNF levels and thereby showed a positive role on the emotion and spatial performance. It need further study in the future.
5. Conclusions
This study demonstrated that exercise by free running wheel could reduce adverse effects of CNS on depressive behaviors and spatial performance in rats. Furthermore, the results indicated that both the beneficial effects of exercise and the harmful effects of CNS were relative to a common molecular, BDNF, in the hippocampus. In addition, circulating CORT might play different roles in the two processes. In summary, first, CNS was a good model for the study for biological mechanisms of the etiology of depression and the beneficial effects of exercise on chronic stress. Secondly, exercise, as a non-pharmacological tool for depression, was effective on depressive behaviors and spatial performance in rats. Thirdly, the beneficial effects of exercise might be associated with circulating CORT levels and hippocam-pal BDNF levels. Fourthly, the important role of BDNF in the depression makes it possible to be a new antidepressive in the future.
Acknowledgments
This study was supported by the National Nature Science Foundation of China (no. 39970275; no. 30070288 to Z. Wang; no. 30070198 to C. Wan).
References
- 1.Dunn AL, Trivedi MH, O’Neal H. Physical activity dose-response effects on outcomes of depression and anxiety. Med Sci Sports Exerc. 2001;33(6):587–97. doi: 10.1097/00005768-200106001-00027. [DOI] [PubMed] [Google Scholar]
- 2.Brosse AL, Sheets ES, Lett HS, Blumenthal JA. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med. 2002;32:741–60. doi: 10.2165/00007256-200232120-00001. [DOI] [PubMed] [Google Scholar]
- 3.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology. 1997;134:319–29. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 4.Willner P. The validity of animal models of depression. Psychopharma-cology. 1984;83:1–16. doi: 10.1007/BF00427414. [DOI] [PubMed] [Google Scholar]
- 5.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology. 1987;93:358–64. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 6.Muscat R, Papp M, Willner P. Antidepressant-like effects of dopamine agonist in an animal model of depression. Biol Psychiatry. 1992;31:937–46. doi: 10.1016/0006-3223(92)90119-k. [DOI] [PubMed] [Google Scholar]
- 7.Muscat R, Papp M, Willner P. Reversal of stress-induced anhedonia by the atypical antidepressants, fluoxetine and maprotiline. Psychopharma-cology. 1992;109:433–8. doi: 10.1007/BF02247719. [DOI] [PubMed] [Google Scholar]
- 8.Mariusz Papp, Irena Nalepa, Lucyna Antkiewicz-Michaluk, Connie Sa’nchez. Behavioural and biochemical studies of citalopram and WAY 100635 in rat chronic mild stress model. Pharmacol Biochem Behav. 2002;72:465–74. doi: 10.1016/s0091-3057(01)00778-x. [DOI] [PubMed] [Google Scholar]
- 9.Burt DB, Zembar MJ, Niederehe G. Depression and memory impairment: a meta-analysis of the association, its pattern, and specificity. Psychol Bull. 1995;117:285–305. doi: 10.1037/0033-2909.117.2.285. [DOI] [PubMed] [Google Scholar]
- 10.Kalska H, Punamaki RL, Makinen-Pelli T, Saarinen M. Memory and metamemory functioning among depressed patients. Appl Neuropsychol. 1999;6:96–107. doi: 10.1207/s15324826an0602_5. [DOI] [PubMed] [Google Scholar]
- 11.Grauer E, Kapon Y. Wistar-Kyoto rats in the Morris water maze: impaired working memory and hyper-reactivity to stress. Behav Brain Res. 1993;59:147–51. doi: 10.1016/0166-4328(93)90161-i. [DOI] [PubMed] [Google Scholar]
- 12.Hail RD, Macrides F. Olfactory bulbectomy impairs the rat’s radial maze behaviour. Physiol Behav. 1983;30:793–803. doi: 10.1016/0031-9384(83)90180-4. [DOI] [PubMed] [Google Scholar]
- 13.Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- 14.Radak Z, Kaneko T, Tahara S, Nakamoto H, Pucsok J, Sasvari M, et al. Regular exercise improves cognitive function and decreases oxidative damage in rat brain. Neurochem Int. 2001;38:17–23. doi: 10.1016/s0197-0186(00)00063-2. [DOI] [PubMed] [Google Scholar]
- 15.Churchill JD, Galvez R, Colcombe S, Swain RA, Kramer AF, Gree-nough WT. Exercise, experience and the aging brain. Neurobiol Aging. 2002;23:941–55. doi: 10.1016/s0197-4580(02)00028-3. [DOI] [PubMed] [Google Scholar]
- 16.Mazure CM. Does stress cause psychiatric illness? In: Spiegel D, editor. Progress in Psychiatry. Washington, DC: American Psychiatric; 1995. pp. 270–80. [Google Scholar]
- 17.Carroll BJ, Curtis GC, Mendels J. Cerebrospinal fluid and plasma free cortisol concentrations in depression. Psychol Med. 1976;6(2):235–44. doi: 10.1017/s0033291700013775. [DOI] [PubMed] [Google Scholar]
- 18.Rubin RT, Phillips JJ, Sadow TF, McCracken JT. Adrenal gland volume in major depression. Increase during the depressive episode and decrease with successful treatment. Arch Gen Psychiatry. 1995;52(3):213–8. doi: 10.1001/archpsyc.1995.03950150045009. [DOI] [PubMed] [Google Scholar]
- 19.Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hip-pocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA. 1996;93(9):3908–13. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizoguchi K, Kunishita T, Chui DH, Tabira T. Stress induces neuronal death in the hippocampus of castrated rats. Neurosci Lett. 1992;138:157–60. doi: 10.1016/0304-3940(92)90495-s. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992;588:341–5. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- 22.McEwen BS. Re-examination of the glucocorticoid hypothesis of stress and aging. Prog Brain Res. 1992;93:365–81. doi: 10.1016/s0079-6123(08)64585-9. [DOI] [PubMed] [Google Scholar]
- 23.Paskitti ME, McCreary BJ, Herman JP. S tress regulation of adreno-corticosteroid receptor gene transcription and mRNA expression in rat hippocampus: time-course analysis. Brain Res Mol Brain Res. 2000;80:142–52. doi: 10.1016/s0169-328x(00)00121-2. [DOI] [PubMed] [Google Scholar]
- 24.Conner J, Lauterborn J, Yan Q, Gall C, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997;17:2295–313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109:143–8. doi: 10.1016/s0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- 26.Nibuya M, Morinobu S, Duman R. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive and antidepressant drug treatment. J Neurosci. 1995;11:7539–47. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–61. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith M, Makino S, Kvetnansky R, Post R. Stress and gluco-corticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1768–77. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ueyama T, Kawai Y, Nemoto K, Sekimoto M, Tone S, Senba E. Immobilization stress reduced the expression of neurotrophins and their receptors in the rat brain. Neurosci Res. 1997;28:103–10. doi: 10.1016/s0168-0102(97)00030-8. [DOI] [PubMed] [Google Scholar]
- 30.Schaaf M, Siburg R, Duurland R, Fluttert M, Oitzl M, de Kloet E, et al. Corticosterone effects on BDNF mRNA expression in the rat hippocampus during Morris water maze training. Stress. 1999;3:173–83. doi: 10.3109/10253899909001121. [DOI] [PubMed] [Google Scholar]
- 31.Vellucci S, Parrott R, Mimmack M. Down-regulation of BDNF mRNA, with no effect on TrkB or glucocorticoid receptor mRNAs, in the porcine hippocampus after acute dexamethosone treatment. Res Vet Sci. 2001;70:157–62. doi: 10.1053/rvsc.2001.0456. [DOI] [PubMed] [Google Scholar]
- 32.Oliff S, Berchtold N, Isakson P, Cotman C. Exercise-induced regulation of brain-derived neurotrophic factor (BDNF) transcripts in the rat hippocampus. Mol Brain Res. 1998;61:147–53. doi: 10.1016/s0169-328x(98)00222-8. [DOI] [PubMed] [Google Scholar]
- 33.Molteni R, Ying Z, Gomez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. Eur J Neurosci. 2000;16:1107–16. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- 34.Murua VS, Gomez RA, Andrea ME, et al. Shuttle-box deficits induced by chronic variable stress: reversal by imipramine administration. Phar-macol Biochem Behav. 1991;38:125–30. doi: 10.1016/0091-3057(91)90599-w. [DOI] [PubMed] [Google Scholar]
- 35.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology. 1987;93:358–64. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 36.Kennett GA, Dickinson SL, Curzon G. Enhancement of some 5-HT-dependent behavioural responses following repeated immobilization in rats. Brain Res. 1985;330:253–63. doi: 10.1016/0006-8993(85)90684-5. [DOI] [PubMed] [Google Scholar]
- 37.Morris R. Developments of wate-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 38.Willner P, Nuscat R, Papp N. Chronic mild stress-induced anhedo-nia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992;16:525–34. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- 39.D’Aquila PS, Peana AT, Carboni V, Serra G. Exploratory behaviour and grooming after repeated restraint and chronic mild stress: effect of desipramine. Eur J Pharmacol. 2000;399:43–7. doi: 10.1016/s0014-2999(00)00332-0. [DOI] [PubMed] [Google Scholar]
- 40.Nielsen CK, Arnt J, Sanchez C. Intracranial self-stimulation and sucrose intake differ as hedonic measures following chronic mild stress: inter-strain and interindividual differences. Behav Brain Res. 2000;107:21–33. doi: 10.1016/s0166-4328(99)00110-2. [DOI] [PubMed] [Google Scholar]
- 41.Bielajew C, Konkle ATM, Merali Z. The effects of chronic mild stress on male Sprague-Dawley and Long Evans rats. Behav Brain Res. 2002;136:583–92. doi: 10.1016/s0166-4328(02)00222-x. [DOI] [PubMed] [Google Scholar]
- 42.Jaanus Harro, Riina Haidkind, Maarike Harro, Ali-Reza Modiri, Per-Goran Gillberg, Rein Pahkla, et al. Chronic mild unpredictable stress after noradrenergic denervation: attenuation of behavioural and biochemical effects of DSP-4 treatment. Eur Neuropsychopharmacol. 1999;10:5–16. doi: 10.1016/s0924-977x(99)00043-7. [DOI] [PubMed] [Google Scholar]
- 43.Christel Westenbroek, Horst Gert J Ter, Roos Marjon H, Kuipers Sjoukje D, Trentani Andrea, den Boer Johan A. Gender-specific effects of social housing in rats after chronic mild stress exposure. Prog Neuropsy-chopharmacol Biol Psychiatry. 2003;27:21–30. doi: 10.1016/s0278-5846(02)00310-x. [DOI] [PubMed] [Google Scholar]
- 44.Ayensu WK, Pucilowski O, Mason GA, Overstreet D, Rezvani AH, Janowsky DS. Effects of chronic mild stress on serum complement activity, saccharin preference and corticosterone levels in Flinders lines of rats. Physiol Behav. 1995;57:165–9. doi: 10.1016/0031-9384(94)00204-i. [DOI] [PubMed] [Google Scholar]
- 45.Harris RBS, Zhou J, Youngblood BD, Smagin GN, Ryan DH. Failure to change exploration or saccharin preference in rats exposed to chronic mild stress. Physiol Behav. 1998;63:91–100. doi: 10.1016/s0031-9384(97)00425-3. [DOI] [PubMed] [Google Scholar]
- 46.Bowman R, Zrull M, Luine V. Chronic restraint stress enhances radial arm maze performance in female rats. Brain Res. 2001;904:279–89. doi: 10.1016/s0006-8993(01)02474-x. [DOI] [PubMed] [Google Scholar]
- 47.Sapolsky RM, Krey LC, McEwen BS. Stress down-regulates corticos-terone receptors in a site-specific manner in the brain. Endocrinology. 1984;114:287–92. doi: 10.1210/endo-114-1-287. [DOI] [PubMed] [Google Scholar]
- 48.Kitraki E, Karandrea D, Kittas C. Long-lasting effects of stress on gluco-corticoid receptor gene expression in the rat brain. Neuroendocrinology. 1999;69:331–8. doi: 10.1159/000054435. [DOI] [PubMed] [Google Scholar]
- 49.McEwen BS, De Kloet ER, Rostene W. Adrenal steroid receptors and actions in the nervous system. Physiol Rev. 1986;66(4):1121–88. doi: 10.1152/physrev.1986.66.4.1121. [DOI] [PubMed] [Google Scholar]
- 50.Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 51.Schaaf MJ, De Kloet ER, Vreugdenhil E. Corticosterone effects on BDNF expression in the hippocampus. Implications for memory formation. Stress. 2000;3:201–8. doi: 10.3109/10253890009001124. [DOI] [PubMed] [Google Scholar]
- 52.Barde YA. Trophic factors and neuronal survival. Neuron. 1989;2:1525–34. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- 53.Philippe Fossati, Andrei Radtchenko, Patrice Boyer. Neuroplastic-ity: from MRK to depressive symptoms. Eur Neuropsychopharmacol. 2004;14:503–10. doi: 10.1016/j.euroneuro.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 54.Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–9. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]