Abstract
Morelloflavone, a biflavonoid extracted from Garcinia dulcis, has shown anti-oxidative, antiviral, and anti-inflammatory properties. However, the function and the mechanism of this compound in cancer treatment and tumor angiogenesis have not been elucidated to date. In this study, we postulated that morelloflavone might have the ability to inhibit angiogenesis, the pivotal step in tumor growth, invasiveness and metastasis. We demonstrated that morelloflavone could inhibit vascular endothelial growth factor (VEGF)-induced cell proliferation, migration, invasion, and capillary-like tube formation of primary cultured human umbilical endothelial cells (HUVECs) in a dose-dependent manner. Morelloflavone effectively inhibited microvessel sprouting of endothelial cells in the rat aortic ring assay and the formation of new blood microvessels induced by VEGF in the mouse Matrigel plug assay. Furthermore, morelloflavone inhibited tumor growth and tumor angiogenesis of prostate cancer cells (PC-3) in xenograft mouse tumor model in vivo, suggesting that morelloflavone inhibited tumorigenesis by targeting angiogenesis. To understand the underlying mechanism of morelloflavone on the inhibitory effect of tumor growth and angiogenesis, we demonstrated that morelloflavone could inhibit the activation of both RhoA and Rac1 GTPases, but have little effect on the activation of Cdc42 GTPase. Additionally, morelloflavone inhibited the phosphorylation and activation of Raf/MEK/ERK pathway kinases without affecting VEGFR2 activity. Together, our results indicate that morelloflavone exerts anti-angiogenic action by targeting the activation of Rho-GTPases and ERK signaling pathways. These findings are the first to reveal the novel functions of morelloflavone in tumor angiogenesis and its molecular basis for the anticancer action.
Keywords: Morelloflavone, angiogenesis, Rho GTPases, ERK pathway, antitumor agent
Introduction
Tumor angiogenesis, the development of new blood vessels from the existing vasculature, is considered a key step in tumor growth, invasion, and metastasis, which is required for proper nourishment and removal of metabolic wastes from tumor sites (1–3). Advances in this field are leading to novel treatments for many cancers (4). Angiogenesis is initiated by cell proliferation and migration in response to chemotactic agents, such as vascular endothelial growth factor (VEGF), which is expressed and generated by most cancer cell types and is a potent proangiogenic factor that functions in tumor vascular development (5). VEGF exerts its biological effects by binding to its receptor tyrosine kinases, expressed on endothelial cells. The biologically relevant VEGF signaling events are mediated mainly via VEGFR-2 (6). Activation of VEGFR-2 leads to the activation of various downstream signal transduction proteins, including extracellular signal-regulated kinases (ERK), protein kinase C, Src family kinase, focal adhesion kinase (FAK) (7, 8), and phosphoinositide 3-kinase (PI3K)/AKT/eNOS pathway. Raf/mitogen extracellular kinase (MEK)/ERK signaling pathway mediated by VEGF mainly regulate cellular proliferation and survival, and have been the focus of cancer chemotherapy because of its relevance in tumor angiogenesis and carcinogenesis (9).
Cellular functions of the Rho family of small GTPases, including RhoA, Rac1 and Cdc42, have been demonstrated to regulate a vast spectrum of biological functions, including cell actin cytoskeleton, cell polarity and migration (10, 11), which are all involved in tumor angiogenesis and metastasis (12). In addition, Rho GTPases have also been implicated in the control of gene transcription (13), in cell cycle progression, cell growth and proliferation (14, 15). Since Rho proteins are key regulators of angiogenesis, drugs targeting Rho GTPase signaling pathways are potential pharmacological and therapeutic agents (10).
A variety of polyphenolic substances, particularly those present in dietary and medical plants, are hypothesized to exhibit a preventive effect on cancer (16). Morelloflavone is a bioactive biflavonoid from Garcinia dulcis, which is a traditional herb medicine belonging to the Guttiferae family and is widely distributed in Thailand, Philippines, and other Southeast Asian countries. It has been documented that morelloflavone demonstrates significant antiviral activity against HIV-1 in phytohemagglutinin-stimulated primary human peripheral blood mononuclear cells (17), exerts inhibitory function in human secretory phospholipase A2, and ameliorates TPA-induced ear inflammation and carrageenan-induced paw edema in mice (18). However, whether morelloflavone has any functions in tumor angiogenesis and cancer prevention have not been reported yet.
In this study, we examined how morelloflavone inhibited tumor angiogenesis by targeting key signaling pathways and genes. Our results demonstrate that morelloflavone could significantly inhibit VEGF-stimulated endothelial cell proliferation, migration, invasion, tube formation, and tumor angiogenesis by targeting Rho family proteins, especially the activation of Rac1 and RhoA activities, and by interfering with the Raf/MEK/ERK pathway, leading to the suppression of tumor growth and tumor angiogenesis.
Materials and Methods
Cell lines, cell culture and reagents
Morelloflavone (MF) was kindly provided by Dr. Fujise, 98% by TLC/high performance liquid chromatography. A 10 mmol/L solution of morelloflavone was prepared in DMSO, stored at −20°C and protected by light, and then diluted as needed concentrations in cell culture medium. Primary human umbilical vascular endothelial cells (HUVECs) were kindly gifted from Dr. Xinli Wang (19, 20) (Cardiothoracic Surgery Division of Michael E. DeBakey Department of Surgery at Baylor College of Medicine in Houston). HUVECs were cultured in endothelial cell growth medium (ECGM): M199 medium (Gibco, Invitrogen) supplemented with 20% fetal bovine serum (FBS, Hyclone Laboratories), 20μg/mL bovine endothelial cell growth factor (bECGF, Roche), 0.1mg/mL Heparin (Sigma), 15mmol/L HEPES buffer, penicillin (50 IU/L), streptomycin (50 mg/L), NaHCO3 (44mmol/L), and 50μg/mL Amphotericin B at 37°C under a humidified 95%:5% (v/v) mixture of air and CO2. The human prostate cancer cell line (PC-3) was purchased from the American Type Culture Collection (ATCC) and cultured in RPM1640 medium supplemented with 10% FBS. VEGF165 was obtained from NIH experimental branch. Matrigel was purchased from BD Biosciences, and mitomycin C was ordered from Roche.
Cell viability assay
HUVECs and PC-3 cell (2×104 cells/well) were treated with or without VEGF (4 ng/mL) and different concentrations of morelloflavone for 24 hours. Cell viability was determined by MTS method, following the manual of CellTiter 96 Aqueous One Solution Cell Proliferation assay (Promega) with VERSAmax microplate reader (Molecular Devices).
Flow cytometry fluorescence-activated cell sorting analysis
HUVECs (1×106) were treated with different concentrations of morelloflavone for 24 hours and then collected and performed in a FACS flow cytometer (BD Sciences) with propidium iodile (PI) staining. The percentages of cell population at G0/G1 and G2/M phases were observed.
Migration assay
HUVECs were allowed to grow into full confluence in 6-well plates pre-coated with 0.1% gelatin (Sigma) and then incubated with 10 μg/mL mitomycin C for 2 hours to inactivate HUVECs proliferation. After that, cells were wounded by pipette tips and washed with PBS. ECGM supplemented with 0.5% FBS was added into well with or without 4ng/mL VEGF and different concentrations of morelloflavone. Images were taken after about 8–10 hours of incubation at 37°C, 5% CO2. The migrated cells were quantified by manual counting and percentage inhibition was expressed using untreated wells at 100%. Three independent experiments were preformed.
Transwell migration assay
The transwell (Corning Incorporated) were coated with 0.1% gelatin for 30min in cell incubator. The bottom chambers of transwell were filled with ECGM with 0.5% FBS supplemented with 4ng/mL VEGF and the top chambers were seeded inactivated 4 ×104 cells/well HUVECs (Pre-treated with mytomycin C) in 100 μL ECGM (0.5% FBS) plus different concentrations of morelloflavone. After 8–10 hours migration, the cells on the top surface of the membrane (non-migrated cells) were scraped with a cotton swab and the cells spreading on the bottom sides of the membrane (invasive cells) were fixed with cold 4% paraformaldehyde for 30 minutes. After that, those migrated cells were stained with Haematoxylin. Images were taken using OLYPUS inverted microscope and invasive cells were quantified by manual counting. Percentage inhibition of invasive cells was quantified and expressed on the basis of untreated control wells.
Tube formation assay
Matrigel (Growth factor reduced, BD Biosciences) were thawed at 4°C, and each well of prechilled 24-well plates was coated with 50 μL Matrigel and incubated at 37°C for 45 min. HUVECs (4×104 cells) were added in 1 mL ECGM (supplemented with 0.5% FBS and 4ng/mL VEGF) with various concentrations of morelloflavone. After 4–6 hours of incubation at 37°C, 5% CO2, endothelial cell tubular structure formation was quantified by calculating the tube length of high power fields (200×) with OLYMPUS inverted microscope, and inhibition percentage was expressed using untreated wells as 100%.
Aortic ring assay
Aortic ring assay was performed, as previously described with some modifications (21, 22). 48-well plates were covered with 100 μL of Matrigel (Supplemented with growth factor, BD Biosciences) at 4°C and incubated at 37°C, 5% CO2 for 30 min. Aortas isolated from mice were cleaned of periadventitial fat and connective tissues, and cut into 1–1.5 mm long rings. After being rinsed five times with endothelial cell-based medium, the aortas were placed on the Matrigel-covered wells and covered with another 100 μL of Matrigel. After these aortic rings being cultured for 24 hours, replace the medium with or without morelloflavone. After 4 days of incubation, the microvessel growth was quantified by taking photographs with Olympus invert microscope. After images were acquired, the outgrowth area was delineated and measured with the Pro plus software (Media Cybernetics).
Matrigel plug assay
Matrigel plug assay was performed as described by Yi et al. (22). In brief, Matrigel (0.5 mL/plug) with no VEGF or morelloflavone, VEGF (4 ng/mL) but no morelloflavone, VEGF (4 ng/mL) and different concentrations of morelloflavone in liquid form at 4°C respectively, were injected s.c. in the midventral abdominal region of 5- to 6-week-old C57BL/6 mice (n=5 each group). After 7 days, the mice were sacrificed and the plugs were removed. Each group had four to five Matrigel plugs. The Matrigel plugs were fixed and embedded with paraffin. The 5-μm sections were stained with H&E staining. The number of erythrocyte-filled blood vessels in high power field (HPF; 200×) was counted (plug number, 4–5)
Xenograft mouse model
Xenograft mouse model assay was performed as described by Yi et al. (23). The 5-week-old to 6-week-old severe combined immune deficiency (SCID) male mice (ordered from NIH) weighing about 20g were divided with five mice per group. PC-3 cells were s.c. injected (2×106 cells per mouse) into the mice. After the tumors had established (about 50 mm3), the mice were s.c. injected with or without 8 mg/Kg morelloflavone everyday. The mice body weight and tumor sizes were recorded everyday and the tumor sizes were determined by Vernier caliper measurements and calculated as length × width × height. After 15 days, mice with tumors not greater than 1.5 cm in diameter were sacrificed.
Histology and immnohistochemistry
Tumor were removed and fixed with Histochoice MB (Molecular Biology) tissue fixative (Amresco) and embedded with paraffin. Specific blood vessel staining was performed on the 5-μm sections with Chemicon’s blood vessel staining kit (von Willebrand Factor, Chemicon International). Images were taken with ZEISS Axioskop 40 photo microscope. The number of blood vessels was counted (plug number, 4–5).
GST-PBD pull-down assay
GTPase activation assay in the cells were performed by GST-Pak1 or GST-RBP pull-down assays as described by Guo et al. (24). Briefly, HUVEC cells were starved overnight with 0.1% FBS medium. Cells were washed, and pretreated with different concentration of morelloflavone (5, 10, 20 μmol/L) for 30 minutes, and then stimulated by 50 ng/mL VEGF for 1 hour. After that, cells were washed with cold PBS and lysed on the dish in RIPA buffer. About 500 μg protein extracts were used for various pull-down assays. GTP-bound Rac1 or Cdc42 was pulled down using the GST-PBD of PAK1 immobilized on glutathione beads. GTP-bound Rho was pulled down using the GST-RBP immobilized on glutathione beads. The amount of active Rac1, Cdc42, and Rho (GTP-bound form) was detected by Western blot using specific antibodies against Rac1, Cdc42, and Rho (Santa Cruz, CA).
Western immunoblotting
To determine the effects of morelloflavone on VEGF-dependent Raf/MEK/ERK pathway phosphorylation, HUVECs were first starved with 0.1% FBS medium for 12–14 hours. After being washed with new fresh medium, cells were pretreated with or without different concentrations of morelloflavone for 30 minutes and then stimulated with 50ng/mL VEGF 20 minutes for ERK pathway phosphorylation. The whole cell extracts were prepared by RIPA buffer supplemented different kinds of proteinase inhibitors. Specific antibodies were used for different Western blot analyses, including pSer338-c-Raf, pSer217/221-MEK1/2, pSer380p90RSK, pThr202/Tyr204p44/42, and ERK1 (Cell Signaling Technology).
AP-1 (activator protein 1) luciferase reporter assay
Luciferase reporter assay was described by Mitchell et al. (25). 293T cells were seeded in 24-well plates with Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS. After cells were 60% confluent, reporter gene constructs were transfected using Lipofectamine reagent according to the manufacturer’s protocol (Invitrogen). Luciferase activity of protein lysates was measured following the manufacturer’s protocol (Luciferase Assay System, Promega). To normalize the differences of transfection efficiencies, all cells were transfected with pRSV-β-gal control vector (Promega). β-Galactosidase levels were then measured following the manufacturer’s protocol (Galacto-Light Plus, Bedford, MA). All luciferase experiments were performed in triplicate and repeated three times.
VEGF receptor 2 inhibition assay
VEGFR2 inhibition assay was performed as previously reported by Yi et al. (23) and followed the manual of HTScan® VEGF receptor 2 kinase assay kit (Cell Signaling Technology, USA).
Statistical analysis
The data are presented as mean ± SE, and statistical comparisons between groups were performed using one-way ANOVA followed Dunnet test. P value ≤0.05 was considered statistically significance.
Results
Morelloflavone is more effective to inhibit cell viability in HUVECs than in PC-3 cancer cells
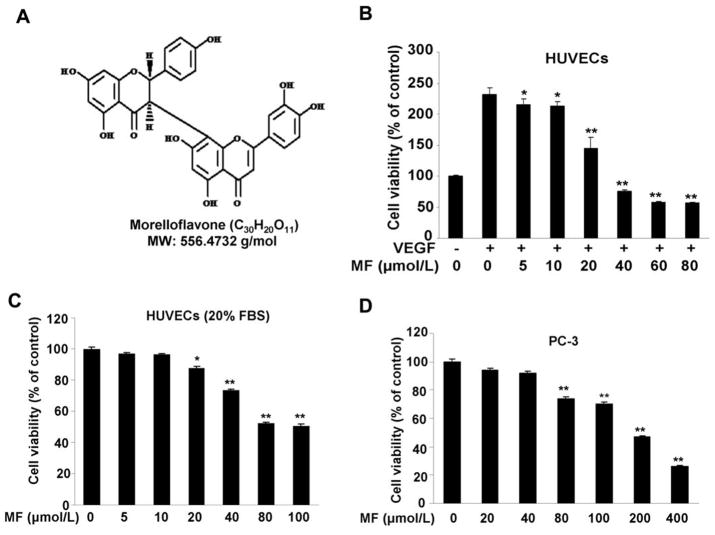
Morelloflavone is a bioactive biflavonoid from Garcinia dulcis with a molecular weight of 556.4732 g/mol (Fig. 1A). To assess the anti-angiogenic property of morelloflavone in vitro, we examined the inhibitory effects of morelloflavone on cell viability in both HUVECs and PC-3 prostate cancer cells using MTS assay. Morelloflavone significantly inhibited VEGF-induced endothelial cell viability from 5 μmol/L and with half-maximal inhibition at 20 μmol/L (Fig. 1B b-1). However, under normal HUVEC culture condition (20% serum), morelloflavone can significantly inhibit cell viability at a much higher concentration with a half-maximal inhibition at 80 μmol/L (Fig. 1C), indicating morelloflavone are more effective in angiogenesis disease condition. As a control, we also performed the cell viability assays with culture medium containing 0.5% DMSO. Our data indicate that 0.5% DMSO has no effect on cell growth, proliferation and viability (data not shown). To examine whether morelloflavone regulated cell cycle progression, we performed fluorescence-activated cell sorting and the result revealed that morelloflavone induced a depletion of cells in the G0/G1 phase, from 82.06% to 50.23%, and a concomitant accumulation of cells in G2/M phase, from 12.57% to 29.99% (Table 1). These data suggested that morelloflavone could arrest endothelial cell proliferation. Furthermore, we examined the inhibitory effect of morelloflavone on human prostate cancer cells and the results demonstrated that the half-maximal inhibition of PC-3 cells was over 100 μmol/L (Fig 1D), suggesting morelloflavone is more effective to regulate the proliferation of HUVECs than that of cancer cells.
Figure 1. Morelloflavone inhibits cell viability both in HUVECs and in PC-3 cells.
A. The chemical structure of morelloflavone with a molecular weight 556.4732 g/mol. B, Morelloflavone inhibits VEGF induced cell viability in dose-dependent manner. HUVECs (2×104 cells/well) were starved with 0.1% FBS medium and then treated with or without VEGF (4 ng/mL) and different concentrations of morelloflavone for 24 hours. Cell viability was quantified by MTS assay (*, P<0.05; **, P<0.01 versus VEGF alone). C, Effects of morelloflavone on HUVEC viability under normal culture condition. HUVECs (2×104 cells/well) were treated with different concentrations of morelloflavone for 24 hours (*, P<0.05; **, P<0.01 versus control). D, Morelloflavone inhibits prostate cancer cell (PC-3) viability (the treatment is the same as C). Column, mean from three different experiments with 6 duplicates; bar, SE.
Table 1.
Morelloflavone arrests cell cycle progression in HUVECs
| Population (%) | Morelloflavone (μmol/L) | ||||
|---|---|---|---|---|---|
| 0 | 10 | 20 | 50 | 100 | |
| G0/G1 phase | 82.06±2.33 | 81.60±3.34 | 79.78±4.92 | 68.15±3.78 | 50.23±6.34 |
| G2/M phase | 12.57±1.54 | 13.08±0.34 | 14.14±3.23 | 18.09±1.23 | 29.99±3.28 |
Morelloflavone inhibits VEGF-induced migration, invasion, and capillary structure formation of endothelial cells
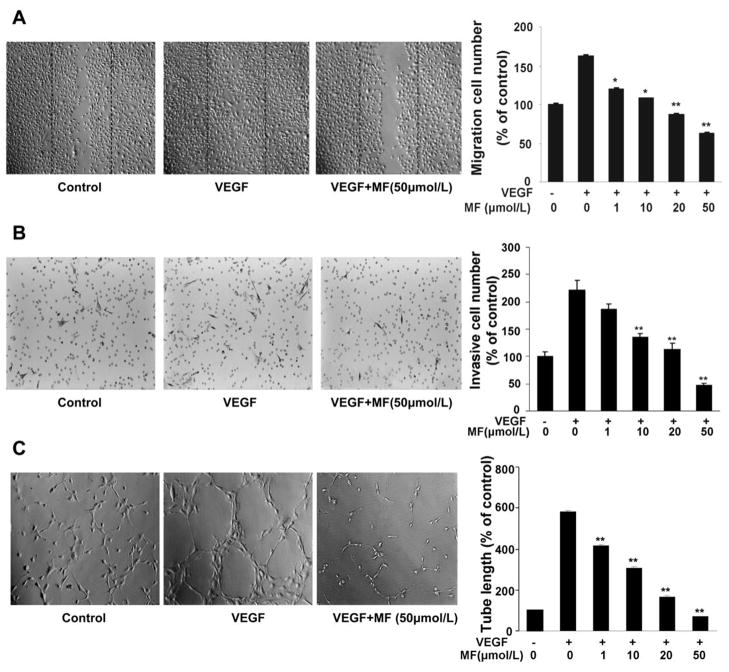
Cell migration is critical for endothelial cell to form blood vessels in angiogenesis and thus is necessary for tumor growth and metastasis. The effects of morelloflavone on the chemotactic motility of HUVECs were measured by wound-healing migration assay and transwell cell invasion assay. As shown in Figure 2, morelloflavone inhibits VEGF-induced HUVEC migration in a concentration-dependent manner. Morelloflavone significantly inhibited HUVEC migration at 1 μmol/L in scratching cell assays (Fig. 2A) while the compound inhibited HUVEC invasion at 10μmol/L in transwell assays (Fig. 2B). Although angiogenesis is a very complex process, tube formation of endothelial cells is one of the key steps (26). To examine the potential effects of morelloflavone on the tubular structure formation of endothelial cells, we investigated how morelloflavone affects HUVEC tube formation using two-dimensioned Matrigel assay. When HUVECs were placed on the growth factor-reduced Matrigel, elonged and robust tube-like structures were formed after incubation in the presence of VEGF (Fig. 2C). The ability of endothelial cells to form tubular structures was assessed in the presence or absence of different concentrations of morelloflavone by calculating the length of tubes with an inverted photomicroscope. As shown in figure 2C, 10 μmol/L of morelloflavone inhibited 50% tube formation of HUVECs on Matrigel and 50 μmol/L of morelloflavone can completely inhibited the formation of tubular structures. These results demonstrated that morelloflavone could block VEGF-induced in vitro angiogenesis by inhibiting cell migration, invasion, and tube formation.
Figure 2. Morelloflavone inhibits VEGF-induced migration, invasion, and tubular structure formation of endothelial cells.
A, Morelloflavone inhibits HUVEC migration. HUVECs were allowed to grow into full confluence in 6-well plates, and inactivated with 10 μg/mL mitomycin C for 2 hours. Cells were wounded with pipette and treated with or without 4ng/mL VEGF and different concentration of morelloflavone in ECGM supplemented with 0.5% FBS. After incubation, the migrated cells were quantified by manual counting. B, Morelloflavone inhibits the invasion of HUVEC. HUVECs were seeded in the upper chamber of transwell and treated with different concentrations of morelloflavone. The bottom chamber was filled with ECGM supplemented with VEGF. After about 8–10 hours, the invasive HUVECs passed through the membrane and were quantified by counting the cells that migrated onto the membrane. C, Morelloflavone inhibits VEGF-induced tube formation of HUVECs. HUVECs were placed in the 24-well plates coated with Matrigel at the density of 4×104 cells/well. After 4–6 hours, cells were fixed and tubular structure was quantified by manual counting of high power fields (200×). Column, mean from three different experiments with duplicates; bar, SE (*, P<0.05; **, P<0.01 versus VEGF alone)
Morelloflavone inhibits vessel sprouting ex vivo and angiogenesis in vivo
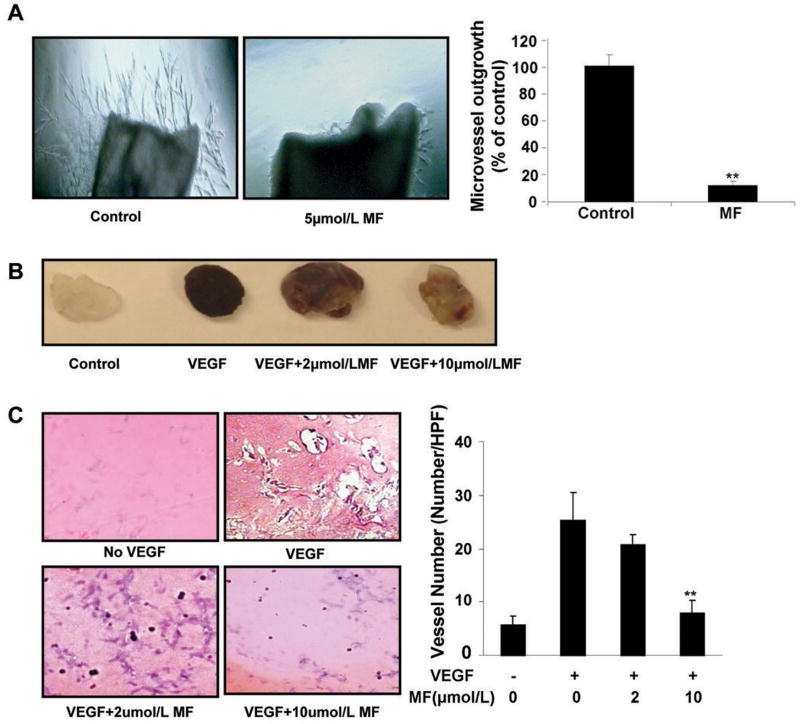
To confirm that morelloflavone inhibits angiogenesis ex vivo, we examined the effect of the compound on the sprouting of microvessel from aortic rings. As shown in Figure 3A, growth factors in Matrigel can dramatically induce microvessel sprouting while addition of morelloflavone significantly antagonizes microvessel sprouting using the aortic ring assay. At 5μmol/L, morelloflavone can inhibit almost all microvessel sprouting in the assays comparing with the control group (Fig. 3A).
Figure 3. Morelloflavone inhibits microvessel sprouting ex vivo and angiogenesis in vivo.
A, Morelloflavone inhibits microvessel sprouting in mouse aortic ring assay. Approximately 1- to 1.5-mm-long cleaned mice aortic rings were placed in the Matrigel-covered wells. After 4 days of incubation with ECGM in the absence or presence of morelloflavone, representative endothelial cell sprout forming branching cords from the margins of aortic ring were photographed and the microvessels were scored. Column, mean, n=4; bar, SE (*, P<0.05; **, P<0.01 versus control). B and C, Morelloflavone inhibits angiogenesis in Matrigel plug assay. 5 -week-old to 6-week-old C57BL/6 mice were injected with Matrigel containing VEGF and morelloflavone (2μmol/L and 10μmol/L) in the midventral abdominal region (5 mice per group). After 7 days, representative Matrigel plugs were removed and photographed in B. The Matrigel plugs were fixed with formalin and 5-μm sections were stained with H&E staining in C. The number of vessels in HPF (magnification, 200×) was counted in the absence or presence of morelloflavone at 2μmol/L and 10μmol/L, respectively. Column, mean, n=4; bar, SE (*, P<0.05; **, P<0.01 versus VEGF alone)
To determine the effects of morelloflavone on VEGF-induced angiogenesis in vivo, we performed the mouse Matrigel plug assay to analyze how morelloflavone regulated VEGF-induced angiogenesis in the presence or absence of morelloflavone (2μmol/L and 10μmol/L, respectively) using 5–6-week-old C57BL/6 mice. After 7 days, the Matrigel plugs were removed. The plugs containing VEGF alone appeared dark red after being fixed (Fig. 2B, VEGF). The vessels were abundantly filled with intact red blood cells (RBCs), indicating the formation of a functional vasculature inside the Matrigel via angiogenesis induced by VEGF. In contrast, the color of Matrigel plugs containing VEGF plus morelloflavone groups, especially the group with 10 μmol/L morelloflavone, were significantly pale, indicating less blood vessel formation (Fig. 2B, VEGF+MF). The number of neovessels was analyzed and quantified after being H&E stained (Fig. 3C). Morelloflavone at 10μmol/L strongly inhibited the vessel number and the formation of microvessels. Together, these results indicated that morelloflavone was capable of suppressing VEGF-induced neovessel formation in vivo.
Morelloflavone inhibits tumor angiogenesis and tumor growth in vivo
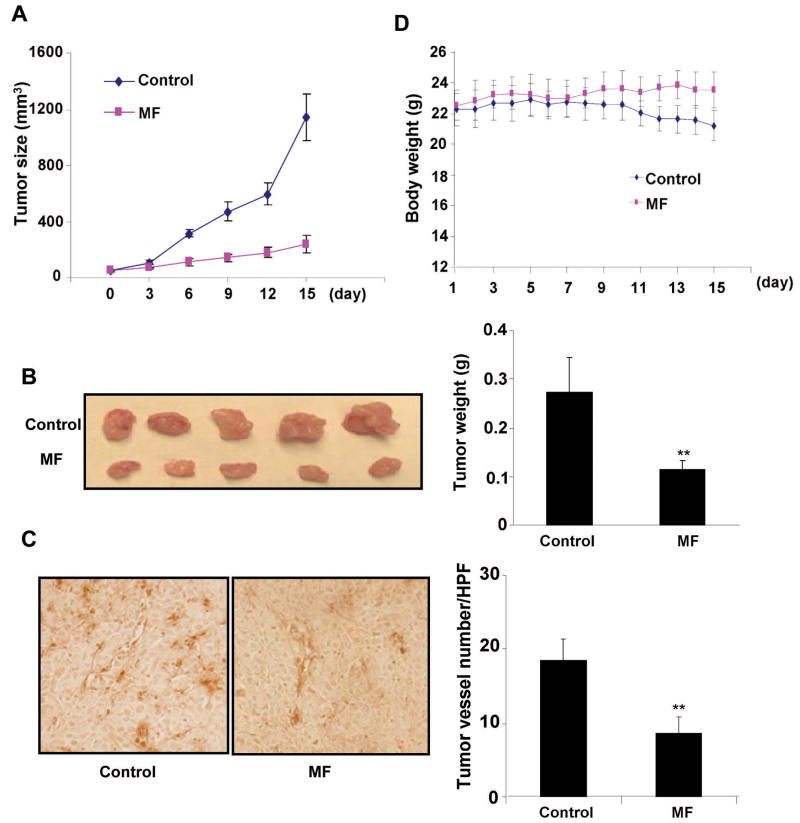
Tumor angiogenesis provides oxygen, nutrients, and main routes for tumor growth, invasiveness and metastasis, and acts as a rate-limiting step in tumor progression (27). To determine the effect of morelloflavone on tumor angiogenesis and tumor growth at nontoxic dosage, we use a xenograft mouse prostate tumor model. PC-3 prostate cancer cells were injected (2×106 per mouse) into the 5- to 6-week-old SCID male mice. After the tumors had established (about 50 mm3), the mice were injected with or without 8 mg/Kg/day morelloflavone everyday. As shown in Figure 4A, tumors in control group increased from 51.18 ± 2.24 to 1143.93 ± 75.60 mm3, whereas tumors in morelloflavone-treated group increased only from 53.80±1.24 to 237.40 ± 26.75 mm3. The average weight of tumors from the control group was 0.272 ± 0.166 gram whereas the average weight in morelloflavone-treated group was only 0.116 ± 0.183 gram (Fig. 4B), suggesting morelloflavone strongly inhibited tumor growth in xenograft mouse prostate tumor model. To further investigate whether morelloflavone inhibited tumor angiogenesis, we stained the solid tumor sections with blood vessel staining kit. Our results indicated that the average number of blood vessel in control group was 18.60±1.47/HPF, whereas the average blood vessel number in morelloflavone-treated group was only 8.6±0.90/HPF (Fig. 4C), indicating that morelloflavone significantly inhibited tumor angiogenesis. However, morelloflavone (8 mg/Kg/day) had little effect on body weight at the concentration tested in the xenograft mouse prostate tumor model (Fig. 4D), suggesting little toxicity of the compound at the tested concentration. The observed mouse body weight decrease in control group was probably due to the tumor burden compared with the morelloflavone-treated group.
Figure 4. Morelloflavone inhibits tumor angiogenesis and tumor growth in vivo.
A. Morelloflavone inhibits solid cancer growth in xenograft prostate cancer mouse model. Prostate cancer cells (PC-3) were injected (2×106 per mouse) into the 5- to 6-week-old SCID male mice. After the tumors had established (about 50 mm3), the mice were injected with or without 8 mg/Kg/d morelloflavone. After 15 days, mice were scarified, tumors were removed and photographed. Tumor sizes in control group and morelloflavone group were calculated and shown in A. Tumors in control group increased from 51.18±2.24 to 1143.93±75.60 mm3, whereas tumors in morelloflavone-treated group increased only from 53.80±1.24 to 237.40±26.75 mm3. B. Solid tumors in morelloflavone-treated group were significantly smaller than the control group. The average weight of tumors from control group was 0.272±0.166 gram, whereas that of morelloflavone-treated group was 0.116±0.183 gram. C. Effects of morelloflavone on tumor angiogenesis in xenograft mouse model. Solid tumor were fixed with histochoice MB tissue fixative and embedded with paraffin. The 5-μm sections were stained with specific blood vessel staining kit and the blood vessel number was calculated. The average vessel number in control group was 18.60±1.47/HPF (magnification, 200×), whereas the average blood vessel number in morelloflavone-treated group was 8.6±0.90/HPF. D. Morelloflavone has little effect on mouse body weight. No significant difference between morelloflavone-treated group (8 mg/Kg/day) and the control group. Column, mean; bar, SE (n=5, *, P<0.05; **, P<0.01 versus control)
Morelloflavone inhibits VEGF-induced activation of Rho GTPases
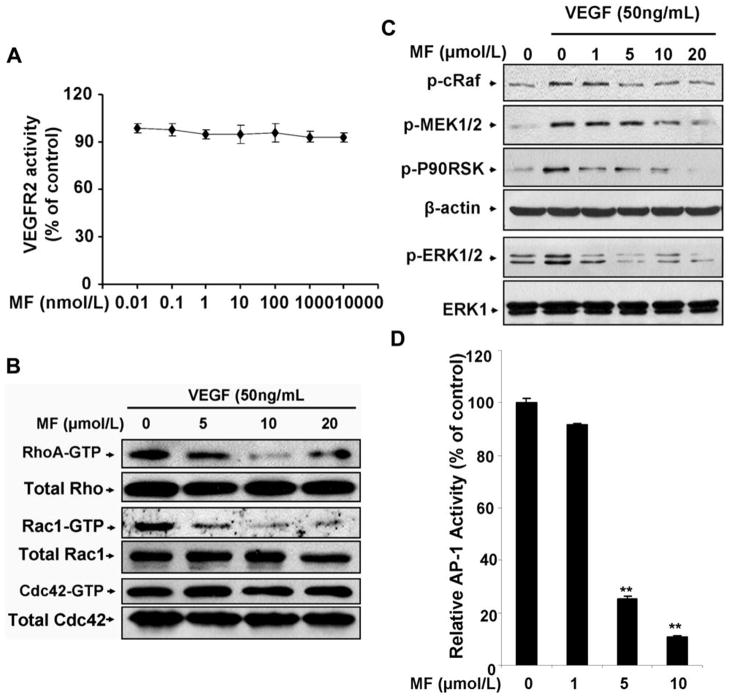
VEGF induces cell proliferation, migration and capillary-structure formation mainly through activation of its cell surface receptor KDR/Flk (VEGFR2) (6, 28). To understand the molecular mechanism of morelloflavone-mediated anti-angiogenic properties, we examined whether different concentrations of morelloflavone could inhibit the activation of VEGFR2 using HTScan VEGFR2 kinase assay kit according to the suggested methods (Cell signaling Technology and PerkinElmer Life Sciences). We found that morelloflavone had no effect on VEGFR2 activation up to 10 μmol/L (Fig. 5A), suggesting that morelloflavone may inhibit angiogenesis by regulating downstream signaling molecules of VEGFR2.
Figure 5. Morelloflavone inhibits VEGF-induced activation of Rho GTPases and the phosphorylation of ERK pathway.
A. Morelloflavone has no effect on VEGFR2 activation. Effect of morelloflavone on VEGFR2 activation was analyzed by a specific VEGFR2 inhibition assay. Blot, mean; bar, SE (n=3). B. Morelloflavone inhibits RhoA and Rac1 GTPases activation while has little effect on Cdc42. HUVECs were pretreated with various concentrations of morelloflavone for 30 minutes before being stimulated with 50ng/mL VEGF for a certain time. After that, cells were washed with cold PBS and lysed on the dish in RIPA buffer. GTP-bound active RhoA was pulled down using the GST-RBP fusion protein immobilized on glutathione beads and detected with anti-RhoA antibody. Active GTP-bound Rac1 or Cdc42 was pulled down using the GST-PBD fusion protein of PAK1 immobilized on glutathione beads and the active Rac1 and Cdc42 was detected with anti-Rac1 and anti-Cdc42 antibodies. C. Effects of morelloflavone on the phosphorylation and activation of Raf/MEK/ERK pathway at different concentrations. Phosphorylation and activation of different protein kinases in Raf/MEK/ERK pathway were examined by specific antibodies, including pSer338-c-Raf antibody, pSer217/221-MEK1/2 antibody, pThr202/Tyr204-p44/42 antibody and pSer380-p90RSK antibody. D. Morelloflavone inhibited AP-1 activity. AP-1 reporter gene construct was transfected into 293T cells, and then the transfected cells were treated with different concentrations of morelloflavone for 24 hours. The relative activity was measured by the luciferase assay as described in Materials and Methods. Column, mean of AP-1 luciferase activities calculated from three independent experiments; bar, SE.
Previous reports have suggested that VEGF-induced endothelial cell migration is mediated by many signaling molecules, including the Rho family of small GTPases (12, 29). To test whether morelloflavone exerted anti-angiogenic actions through inhibition of Rho GTPases, we examined how morelloflavone regulated the activation of RhoA, Rac1, and Cdc42 by GST-fusion protein pull-down assays using specific GST-fusion protein-binding domains for activated small GTPases (GTP-bound forms), respectively. As shown in figure 5B, morelloflavone could significantly reduce the amounts of active RhoA and Rac-1 GTPases in a dose-dependent manner (Fig. 5B). On the other hand, morelloflavone had little effect on the activation of Cdc42 (Fig. 5B). Half-maximal effects were obtained at about 5μmol/L. Together, these data suggest that the inhibitory effects of morelloflavone on the activation of RhoA and Rac1 GTPases might contribute to the anti-angiogenic effects of the compound in HUVECs, especially the migration and invasion of HUVECs.
Morelloflavone inhibits the ERK signaling pathway in endothelial cells
The Raf/MEK/ERK pathway is well-known to be involved in endothelial cell proliferation and cell survival, important in angiogenesis. To investigate whether morelloflavone could suppress the activation of ERK cascade in tumor angiogenesis, we examined the phosphorylation and activation of the protein kinases and transcription factors involved in ERK signaling pathway (Fig. 5C and 5D). Our results indicated that morelloflavone at concentration of 10 μmol/L could significantly inhibit the phosphorylation of protein kinases involved in the activation of ERK signaling pathway induced by VEGF, including pSer338-c-Raf, pSer217/221-MEK1/2, and pThr202/Tyr204-pERK1/2 (Fig. 5C). All these kinases can be down-regulated by morelloflavone with a concentration-dependent manner (Fig. 5C). The downstream protein kinase of ERK, p90RSK, was also significantly inhibited by morelloflavone in a concentration-dependent manner (Fig. 5C), suggesting morelloflavone exerted its anti-angiogenic activity by targeting ERK-regulated down-stream gene expression. To further confirm the transcriptional regulation of morelloflavone on ERK-mediated gene transcription, we examined how morelloflavone affected the activation of AP-1, a key downstream transcription factor of MAPK pathway in cancer development and angiogenesis (30). Our results demonstrated that 5 μmol/L morelloflavone could strongly decrease the transcriptional activity of AP-1 in the AP-1 luciferase reporter assays (Fig. 5D), indicating that morelloflavone can inhibit the ERK-mediated AP-1 transcriptional activity in endothelial cells.
Discussion
Recent years, substantial effort has been dedicated to identifying anticancer agents that can be used to either prevent insurgence of primary tumors or prevent tumor relapse. Current interest is focusing on the beneficial health effects of phytochemicals (31, 32), for example, flavonoids, because this kind of plant polyphenols has been found to influence some steps in cancer angiogenesis (31) beyond their traditional use. Morelloflavone is a bioactive biflavonoid from Garcinia dulcis, however, little information is known about its functions in tumor angiogenesis and tumor growth. In this study, we report the novel biological functions of morelloflavone as an inhibitor of tumor angiogenesis by inhibiting Rho GTPases and ERK signaling pathways. Our research comprehensively focuses on the inhibitory effects of morelloflavone on endothelial cell proliferation, migration, invasion and capillary structure formation in response to VEGF. Furthermore, we demonstrate that morelloflavone can inhibit ex vivo and in vivo angiogenesis and tumor angiogenesis in xenograft mouse prostate tumor model.
Unlike those widely used anticancer agents that have adverse effects (33) or severe cytotoxicity to induce cell apoptosis in modern cancer chemotherapy, morelloflavone presents low cytotoxicity but with outstanding anti-angiogenic actions. FACS analysis indicates that morelloflavone is weak to induce sub G0 distribution in both HUVECs and prostate cancer PC-3 cell lines (results not shown). And, in SCID mouse model, we also found that morelloflavone (8 mg/Kg/day) did not affect the body weight of the mice, but demonstrated significantly inhibitory effects on solid tumor growth and tumor angiogenesis. Thus, we assume morelloflavone may be a novel anticancer agent with limited toxicity. In most cases, morelloflavone effectively induce cell growth inhibition of cultured tumor PC-3 cells at concentrations over 80μmol/L. In contrast, morelloflavone sufficiently inhibited VEGF-induced angiogenic responses only at or less than 10μmol/L in ex vivo and in vitro angiogenesis assays. Such concentration has little effect on endothelial cell viability. Because VEGF is a major inducer for the formation of tumor vasculature (34, 35), our results demonstrate that morelloflavone inhibits tumor growth in vivo via their anti-angiogenic activity at a much lower concentration and much earlier than their cytotoxicity effects on tumor cells.
Rho family of small GTPases, including RhoA, Rac1 and Cdc42, as part of the Ras superfamily, are molecular switches and involved in almost every stage of tumor progression and tumor angiogenesis (10, 12, 36). Coordinated activation of individual Rho GTPase is required for cancer cells to successfully complete the extravasations and distant metastasis (37, 38). Also, in endothelial cells, both Rac1 and RhoA activation lead to stress fiber formation and increased focal adhesion (29). And, Rac1 is required and sufficient for the activation of endothelial cell haptotaxis and VEGF-stimulated chemotaxis (29, 39). Therefore, turning off these molecular switches or interfering with their functions by depressing these small GTPase activities can reverse the cell pro-angiogenic motility and migration (12). We found that morelloflavone could depress the RhoA and Rac1 GTPase activation with concentration-dependent manner. In agreement with the above results, the motility of endothelial cells triggered by VEGF n our ex vivo and in vivo assays can be significantly inhibited by morelloflavone at low concentrations, suggesting that small Rho GTPases are rational molecular targets of morelloflavone for modulating angiogenesis in endothelial cells.
One of the key cell signaling pathways involved in cell proliferation, survival, and tumorigenesis is the Raf/MEK/ERK pathway (12, 40–43). This pathway links extracellular signals directly to nuclear transcription factors and regulation of gene expression (44). Our results illustrated that morelloflavone could significantly inhibit the activation of Raf/MEK/ERK protein kinases in a concentration-dependent manner with the effective concentration of 10 μmol/L. Moreover, morelloflavone can suppress the activation of ERK downstream transcription factors, such as AP-1 and p90RSK that regulate gene expression involved in angiogenesis (30, 45). These results are consistent with the anti-proliferation functions of morelloflavone by inducing a depletion of endothelial cells in the G0/G1 phase and a concomitant accumulation in G2/M phase. Taken together, our studies indicate that morelloflavone is a potential inhibitor of tumor angiogenesis by targeting the Rho GTPase and ERK signaling pathways.
Acknowledgments
This work is partially supported by the Research Platform for Cell Signaling Networks from Science and Technology Commission of Shanghai Municipality, and by a grant from National Cancer Institute, NIH (1R01CA106479). We thank Dr. Xinli Wang (Baylor College of Medicine) for providing us the HUVECs used in our experiments.
Abbreviation
- GTPase
Guanine nucleotide transfer proteinase
- VEGF
Vascular endothelial growth factor
- MAPK
Mitogen-activated protein kinase
- ERK
Extracellullar signal-regulated kinase
- MEK
Mitogen extracellular kinase
- PI3K
Phosphoinositide 3-kinase
- FAK
Focal adhesion kinase
- AP-1
Activator protein 1
References
- 1.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–57. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet P, Base M. Metabolism and therapeutic angiogenesis. N Engl J Med. 2008;358:2511–2. doi: 10.1056/NEJMcibr0802500. [DOI] [PubMed] [Google Scholar]
- 4.Burstein HJ, Schwartz RS. Molecular origins of cancer. N Engl J Med. 2008;358:527. doi: 10.1056/NEJMe0800065. [DOI] [PubMed] [Google Scholar]
- 5.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–49. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrara N, Gerver HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 7.Abu-Ghazaleh R, Kabir J, Jia H, et al. Src mediates stimulation by vascular endothelial growth factor of the phosphorylation of focal adhesion kinase at tyrosine 861, and migration and anti-apoptosis in endothelial cells. Biochem J. 2001;360:255–64. doi: 10.1042/0264-6021:3600255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pyun BJ, Choi S, Lee Y, et al. Capsiate, a nonpungent capsaicin-like compound, inhibits angiogenesis and vascular permeability via a direct inhibition of Src kinase activity. Cancer res. 2008;68:227–35. doi: 10.1158/0008-5472.CAN-07-2799. [DOI] [PubMed] [Google Scholar]
- 9.Lee KW, Kang NJ, Heo YS, et al. Raf and MEK protein kinase are direct molecular targets for the chemopreventive effect of quercetin, a major flavonol in red wine. Cancer Res. 2008;68:946–55. doi: 10.1158/0008-5472.CAN-07-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahai E, Marshall CJ. Rho-GTPases and cancer. Nat Rev Cancer. 2002;2:133–42. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 11.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–79. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 12.Merajver SD, Usimani SZ. Multifaceted role of Rho protein in angiogenesis. J Mammary Gland Biol Neoplasia. 2005;10:291–8. doi: 10.1007/s10911-006-9002-8. [DOI] [PubMed] [Google Scholar]
- 13.Ridley AJ. Rho family proteins: coordinating cell responses. Trends Cell Biol. 2001;11:471–77. doi: 10.1016/s0962-8924(01)02153-5. [DOI] [PubMed] [Google Scholar]
- 14.Olson MF, Ashworth A, Hall A. An essential role for Rho, Rac and Cdc42 GTPases in cell cycle progression through G1. Science. 1995;269:1270–72. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- 15.Welsh CF. Rho GTPases as key transducers of proliferative signals in g1 cell cycle regulation. Breast Cancer Res Treat. 2004;84:33–42. doi: 10.1023/B:BREA.0000018425.31633.07. [DOI] [PubMed] [Google Scholar]
- 16.Mojzis J, Varinska L, Mojzisova G, et al. Antiangiogenic effects of flavonoids and chalcones. Pharmacol Res. 2008;57:259–65. doi: 10.1016/j.phrs.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Lin YM, Anderson H, Flavin MT, et al. In vitro anti-HIV activity of biflavonoids isolated from Rhus succedanea and Garcinia multiflora. J of Nat Prod. 1997;60:884–8. doi: 10.1021/np9700275. [DOI] [PubMed] [Google Scholar]
- 18.Gil B, Sanz MJ, Terencio MC, et al. Morelloflavone, a novel biflavonoid inhibitor of human secretory phospholipase A2 with anti-inflammatory activity. Biochem Pharmacol. 1997;53:733–40. doi: 10.1016/s0006-2952(96)00773-3. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Wilcken DE, Wang XL. Cigarette smoke activates caspase-3 to induce apoptosis of human umbilical venous endothelial cells. Mol Genet Metab. 2001;72:82–8. doi: 10.1006/mgme.2000.3115. [DOI] [PubMed] [Google Scholar]
- 20.Senthil D, Raveendran M, Shen YH, et al. Genotype dependent expression of eNOS in cultured endothelial cells. DNA and Cell Biol. 2005;24:218–24. doi: 10.1089/dna.2005.24.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masson V, Devy L, Grignet-Debrus C, et al. Mouse aortic ring assay: a new approach of the molecular genetics of angiogenesis. Biol Proced Online. 2002;4:24–31. doi: 10.1251/bpo30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yi T, Cho SG, Yi Z, et al. Thymoquinone inhibits tumor angiogenesis and tumor growth through suppressing AKT and extracellular signal-regulated kinase signaling pathways. Mol Cancer Ther. 2008;7:1789–96. doi: 10.1158/1535-7163.MCT-08-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yi T, Yi Z, Cho SG, et al. Gambogic acid inhibits angiogenesis and prostate tumor growth by suppressing vascular endothelial growth factor receptor 2 signaling. Cancer Res. 2008;68:1843–50. doi: 10.1158/0008-5472.CAN-07-5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo X, Stafford LJ, Bryan B, et al. A Rac/Cdc42-specific exchange factor, GEFT, induces cell proliferation, transformation, and migration. J Biol Chem. 2003;278:13207–15. doi: 10.1074/jbc.M208896200. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell DC, Abdelrahim M, Weng J, et al. Regulation of KiSS-1 metastasis suppressor gene expression in breast cancer cells by direct interaction of transcription factors activator protein-2alpha and specificity protein-1. J Bio Chem. 2006;281:51–8. doi: 10.1074/jbc.M506245200. [DOI] [PubMed] [Google Scholar]
- 26.Patan S. Vasculogenesis and angiogenesis. Cancer Treat Res. 2004;117:3–32. doi: 10.1007/978-1-4419-8871-3_1. [DOI] [PubMed] [Google Scholar]
- 27.Tozer GM, Kanthou C, Baguley BC. Disrupting tumor blood vessels. Nat Rev Cancer. 2005;5:423–35. doi: 10.1038/nrc1628. [DOI] [PubMed] [Google Scholar]
- 28.Meyer RD, Rahimi N. Comparative structure of VEGFR-1 and VEGFR-2: What have we learned from chimeric systems? Ann N Y Acad Sci. 2003;995:200–7. doi: 10.1111/j.1749-6632.2003.tb03223.x. [DOI] [PubMed] [Google Scholar]
- 29.Soga N, Namba N, McAllister S, et al. Rho family GTPases regulates VEGF-stimulated endothelial cell motility. Exp Cell Res. 2001;269:73–87. doi: 10.1006/excr.2001.5295. [DOI] [PubMed] [Google Scholar]
- 30.Young MR, Li JJ, Rincon M, et al. Transgenic mice demonstrate AP-1 (activator protein-1) transactivation is required for tumor promotion. Proc Natl Acad Sci. 1999;96:9827–32. doi: 10.1073/pnas.96.17.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren W, Qiao Z, Wang H, et al. Flavonoids: promising anticancer agents. Med Res Rev. 2003;23:519–34. doi: 10.1002/med.10033. [DOI] [PubMed] [Google Scholar]
- 32.Aggarwal BB, Shisodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Heffeter P, Jungwirth U, Jakupec M, et al. Resistance against novel anticancer metal compounds: differences and similarities. Drug Resist Updat. 2008;11:1–16. doi: 10.1016/j.drup.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Ferrrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–74. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 35.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–6. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 36.Jamora C, Fuchs E. Intercellular adhesion, signaling and the cytoskeleton. Nat Cell Biol. 2002;4:E101–8. doi: 10.1038/ncb0402-e101. [DOI] [PubMed] [Google Scholar]
- 37.Sequeira L, Dubyk CW, Riesenberger TA, et al. Rho GTPases in PC-3 prostate cancer cell morphology, invasion and tumor cell diapedesis. Clin Exp Metastasis. 2008 doi: 10.1007/s10585-008-9173-3. (In press) [DOI] [PubMed] [Google Scholar]
- 38.Vega FM, Ridley AJ. Rho GTPases in cancer cell biology. FEBS Lett. 2008;582:2093–101. doi: 10.1016/j.febslet.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 39.Connolly JO, Simpson N, Hewlett L, Hall A. Rac regulates endothelial morphogenesis and capillary assembly. Mol Biol Cell. 2002;13:2474–85. doi: 10.1091/mbc.E02-01-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giehl K. Oncogenic Ras in tumor progression and metastasis. Biol Chem. 2005;386:193–205. doi: 10.1515/BC.2005.025. [DOI] [PubMed] [Google Scholar]
- 41.Murphy DA, Makonnen S, Lassoued W, et al. Inhibition of tumor endothelial ERK activation, angiogenesis, and tumor growth by sorafenib (BAY43-9006) Am J Pathol. 2006;169:1875–85. doi: 10.2353/ajpath.2006.050711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang D, Ding Y, Luo WM, et al. Inhibition of MAPK kinase signaling pathways suppressed renal cell carcinoma growth and angiogenesis in vivo. Cancer Res. 2008;68:81–8. doi: 10.1158/0008-5472.CAN-07-5311. [DOI] [PubMed] [Google Scholar]
- 44.Schreck R, Rapp UR. Raf kinases: oncogenesis and drug discovery. Int J Caner. 2006;119:2261–71. doi: 10.1002/ijc.22144. [DOI] [PubMed] [Google Scholar]
- 45.Frödin M, Gammeltoft S. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol Cell Endocrinol. 1999;151:65–77. doi: 10.1016/s0303-7207(99)00061-1. [DOI] [PubMed] [Google Scholar]