Abstract
A new acoustically-active delivery vehicle was developed by conjugating liposomes and microbubbles, using the high affinity interaction between avidin and biotin. Binding between microbubbles and liposomes each containing 5% DSPE-PEG2kBiotin was highly dependent on avidin concentration and observed above an avidin concentration of 10 nM. With an optimized avidin and liposome concentration, we measured and calculated as high as 1000 to 10,000 liposomes with average diameters of 200 and 100 nm, respectively, attached to each microbubble. Replacing avidin with neutravidin resulted in 3-fold higher binding, approaching the calculated saturation level. High-speed photography of this new drug delivery vehicle demonstrated that the liposome-bearing microbubbles oscillate in response to an acoustic pulse similar to microbubble contrast agents. Additionally, microbubbles carrying liposomes could be spatially concentrated on a monolayer of PC-3 cells at the focal point of ultrasound beam. As a result of cell-vehicle contact, the liposomes fused with the cells and internalization of NBD-cholesterol occurred shortly after incubation at 37°C, with internalization of NBD-cholesterol substantially enhanced in the acoustic focus.
Keywords: Microbubble, Liposome, Ultrasound Radiation Forces, Delivery Vehicle
1. Introduction
Over the last decade, encapsulated microbubbles have been developed as powerful contrast agents for ultrasound imaging [1–4]. These microbubbles are injected intravenously and travel through the circulation with rheological properties similar to erythrocytes, enhancing blood-to-tissue contrast [5]. Current FDA-approved microbubble contrast agents are based on a lipid monolayer or albumin shell encapsulating a high-molecular weight gas [6–8]. The gas core provides the acoustic impedance mismatch which makes microbubbles highly echogenic and allows the microbubbles to be manipulated with acoustic radiation force [9–12].
In addition to the use of microbubbles with ultrasound for molecular imaging approaches, combining microbubbles and therapeutics has recently been considered [13–17]. The thin microbubble shell and gas core each have limited drug-carrying capacity, and therefore, do not yield an efficient therapeutic delivery vehicle. Recent pre-clinical studies of gene delivery with plasmids attached to the microbubble shell have utilized on the order of 10–100 times the typical human dose of microbubbles [18]. Liposomes are membrane-enclosed vesicles composed of a lipid bilayer shell (which can trap hydrophobic and amphipathic drugs) surrounding an aqueous core (which can encapsulate hydrophilic drugs) [19–21]. In contrast to microbubbles, liposomes have been shown to efficiently carry drugs such as doxorubicin and vincristine [22–24], and intravenous injection of drug-carrying liposomes can increase accumulation at the tumor site by 50 to 100 fold compared to the administration of free drug [25–27]. Moreover, monodisperse liposomes can be manufactured with an optimized diameter, typically chosen to be near 100 nm to maximize extravasation from tumor vasculature and fusion with cell membranes. Unfortunately, the fluid structure of liposomes makes them weakly echogenic and not readily manipulated with ultrasound radiation force.
Since spatial localization with ultrasound radiation force permits higher vehicle delivery rates than can be achieved by flow/adhesion dynamics alone, it is desirable to produce a drug-carrier vehicle which retains the capability to be acoustically concentrated [4, 10, 11, 28]. Taking advantage of the acoustic properties of lipid-coated microbubbles and the fusogenicity of liposomes motivated us to create a new drug delivery vehicle by mounting the liposomes on microbubble shells. This new vehicle possesses the drug payload capacity of liposomes, yet can still be spatially concentrated and delivered with ultrasound radiation force.
The lipid composition and surface properties play an important role in the binding of biotinylated liposomes to avidin, in vivo blood circulation, and the targeting efficacy of liposomes [29–32]. In this study, we have used a simple formulation consisting of DPPC as the core lipid, DSPE-PEG2kBiotin as a linker, and NBD-cholesterol as a fluorophore, to investigate the binding efficiency and construction of the new vehicle. However, further optimization in liposomal formulation and design, e.g. addition of negatively charged lipids, cholesterol, targeting ligand, etc., is required depending on the specificity of the vehicle applications.
In order to characterize and evaluate the performance of this hybrid vehicle, the simple but useful noncovalent avidin-biotin conjugation technique was employed to attach the biotinylated liposomes to biotinylated microbubbles. The avidin-biotin interactions have been used successfully in many biological and analytical systems but have the disadvantage of being highly immunogenic, which limits their applications in vivo [33].
In order to demonstrate the efficiency of microbubble-liposome conjugation, the release of the liposomes by ultrasound, and the internalization of the liposomes, NBD-cholesterol was incorporated into the lipid bilayer of the liposomes. The delivery of NBD-cholesterol to a cell monolayer with and without insonation was observed with fluorescence microscopy and flow cytometry.
2. Material and Methods
2.1 Materials
1,2 Distearoyl-sn-glycero-3-phosphatidylcholine (DSPC), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2 distearoyl-sn-glycero-3-phosphoethanolamine-N-[Methoxy(Polyethylene glycol)-2000] (DSPE-PEG2k), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[Biotinyl(Polyethylene Glycol)2000] (DSPE-PEG2kBiotin), and 25-[N-[(7-nitro-2-1,3-benzoxadiazol-4-yl)methyl]amino]-27-norcholesterol (25-NBD-cholesterol), all in chloroform, were purchased from Avanti Polar Lipids Inc. (Alabaster, AL). Avidin from egg white was obtained from Sigma-Aldrich (St. Louis, MO) and neutravidin was from Pierce Biotechnology (Rockford, IL). All other reagents were of analytical grade and were used as received.
2.2 Methods
2.2.1 Biotinylated microbubbles
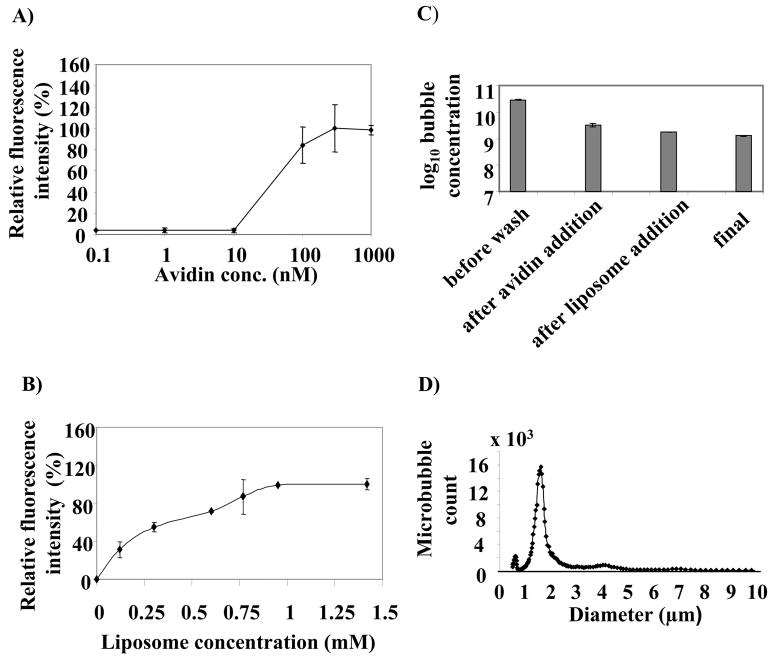
An appropriate amount of DSPC, DSPE-PEG2k, and DSPE-PEG2kBiotin (90:5:5, molar ratio) in chloroform was added to a test tube. Chloroform was removed under N2 followed by evaporation under a vacuum for at least 2 h. A buffer diluent consisting of 100 mM Tris (pH 7.4): glycerol: propyleneglycol (80:10:10, volume ratio) was added to the dried lipids to create a lipid concentration of 1 mM (1 mg/mL). The lipid suspension was mixed well above the phase transition temperature of the lipids (60°C) to form a milky solution of multilamellar vesicles. The suspension was sonicated to clarity using a bath sonicator (40 kHz, 130 W, 10 min). The liposome solution at a final concentration of 1 mg/mL was aliquoted in 1 mL to a 2-mL vial. Then, 10 cm3 of decafluorobutane gas was slowly injected into the vial through the rubber cap and air was exchanged using a needle (20G1, short Bevel, Becton-Dickinson) as a vent. The vial was immediately capped using an aluminum seal on the rubber cap. The sealed vial containing the liposome solution with the decafluorobutane headspace was stored at 4°C until use. Microbubbles were formed via mechanical agitation of the vials of liposome solution using a VialMix shaker as previously described [34]. Upon shaking the vial for 45 seconds, the solution became milky, and was then drawn into a 3-mL syringe and diluted to a final volume of 3 mL using 10 mM phosphate buffered saline (PBS, pH 7.4). Quantification of particle concentration using an Accusizer (770A, Particle Sizing System, Santa Barbara, USA) indicated ~1–5 × 1010 microbubbles formed per milliliter of solution. The remaining liposomes (unincorporated into microbubbles) and submicron-sized bubbles were removed from the solution by flotation at 300 × g for 3 min. After 4 washes, a major peak was observed in the size distribution of the remaining microbubbles at 1.76 microns with mode and median of 1.6 and 1.56 microns, respectively (Figure 2D).
Figure 2.
Relative fluorescence intensity of NBD-cholesterol liposomes attached to microbubbles as a function of A) avidin concentration at 1.2 mM of DPPC:DSPE-PEG2kBiotin:NBD-chol (90:5:5) liposomes with an average diameter of 200 nm; B) Liposome concentration at avidin concentration of 333 nM. C) Microbubble stability throughout the conjugation process of liposomes to microbubbles. D) Size distribution of liposome-bearing microbubbles at a final concentration of 1.5 × 109 microbubbles in 1 mL. Each data point represents the average of triplicate measurements and the error bars are the standard deviations of the triplicate measurements.
2.2.2 Biotinylated liposomes
A multilamellar lipid solution consisting of DPPC: DSPE-PEG2kBiotin: 25-NBD-cholesterol (90:5:5) was prepared, as described in 2.2.1., in 10 mM PBS (pH 7.4) at a final lipid concentration of 12 mM (10 mg/mL). The solution was then extruded through a polycarbonate membrane with pore diameter of 100 or 200 nm above the phase transition temperature of the lipid mixture. A 200 nm polycarbonate membrane was used unless otherwise stated. The pressurized microbubble or liposome solutions were each stable at 4°C for more than 2 months
2.2.3 Conjugation of biotinylated liposomes with biotinylated microbubbles via avidin
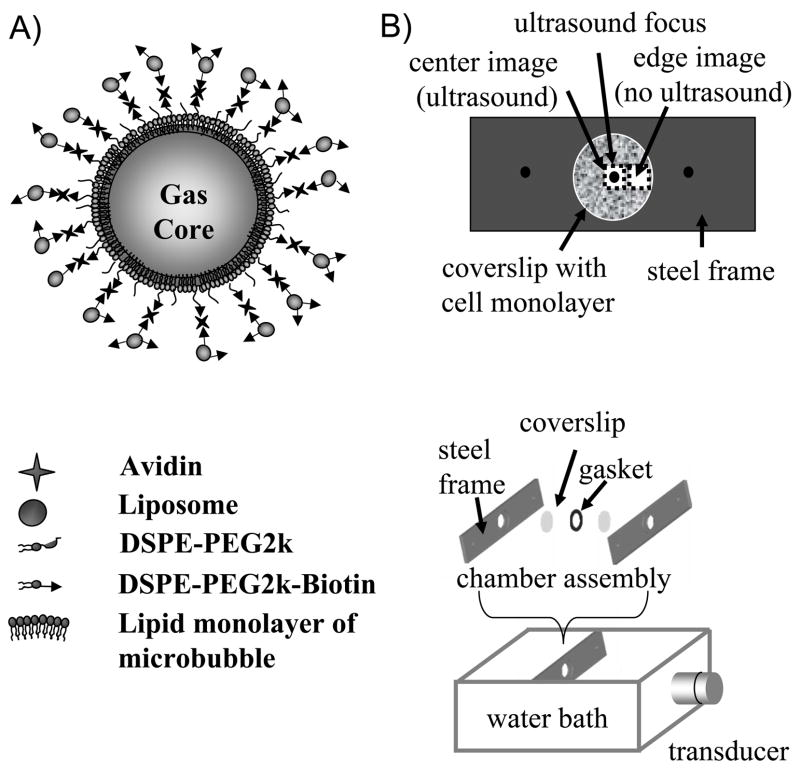
Approximately 3 × 1010 microbubbles in 3 mL were washed 4 times in 10 mM PBS in a 3 mL-luer-lock syringe using a bucket centrifuge at 300 × g for 3 minutes. Centrifugation forced the bubbles to float at the top of the syringe and unincorporated lipids were removed from the bottom of the syringe. Avidin was added at 0.022 mg/mL (333 nM) to the washed microbubbles, mixed well and incubated for 15 minutes at room temperature with gentle shaking. The avidin-bound biotinylated microbubbles were separated from free avidin via 4 times washing. 1.2 mM (1.0 mg) of the extruded liposomes (100 nm or 200 nm size) was added to this suspension and incubated for 10 minutes at room temperature with gentle shaking. Microbubbles carrying liposomes were washed 3–4 additional times to remove free liposomes. Figure 1A provides a schematic presentation of the constructed hybrid vehicle. Microbubble size, concentration and size distribution were determined using the Accusizer as above. Characterization of the agents was then performed in triplicate and the average results were presented. Each data point represents the average of triplicate measurements and the error bars are the standard deviations of the triplicate measurements.
Figure 1.
A) Concept image of a microbubble carrying liposomes on its surface. Microbubbles coated with 5% DSPE-PEG2k and 5% DSPE-PEG2k-Biotin were conjugated with liposomes carrying 5% DSPE-PEG2k-Biotin and 5% NBD-cholesterol via avidin molecules. NBD-cholesterol provides a fluorescent measure for optimization of hybrid vehicle construction, for binding quantification, and also for cellular internalization as a result of cell-vehicle interaction. B) Diagram of the experimental setup. A cell monolayer on a Thermanox coverslip was mounted on the stainless steel frame. The well created by an o-ring gasket was filled with 1 mL of DPBS containing ~ 107 delivery vehicles. The well was then sealed with the second Thermanox coverslip. The second frame was placed on top and the chamber was closed. The chamber was placed in a 37°C water bath perpendicular to ultrasound radiation forces generated from transducer.
2.2.4 Binding quantification
The relative level of liposomes bound to microbubbles was determined using the mean fluorescence intensity of the NBD-cholesterol, measured with a FACScan flow cytometer (Becton Dickson; San Jose, CA) system.
To estimate the number of liposomes bound to one microbubble, an aliquot of 400 μL of liposome-bearing microbubbles at a concentration of 2–3 × 108, carrying a known concentration of NBD-cholesterol in the liposomes, was lysed with 80 μL of 20% sodium deoxycholate at 80°C for 5 minutes. The relative fluorescence intensity of NBD-cholesterol was detected by a microplate reader (FLx800, BioTek Instruments, Winooski, VT) at Ex. 460 nm, Em. 534 nm and converted to the liposome concentration based on the correlation between relative fluorescence intensity of the lysed NBD-cholesterol liposomes with varying concentrations versus liposome concentration. Assuming a single lipid bilayer structure for the extruded liposomes with 100 or 200 nm diameter, and the area of one DPPC molecule of approximately 65 × 10−2 nm2 [35, 36], the number of lipid vesicles created from 1 mg total lipid with the composition used in this study was estimated to be 8 × 1012 and 2 × 1012 for 100 nm and 200 nm liposomes, respectively. The number of bound liposomes was then divided by the count of microbubbles to which liposomes were attached in each experiment.
2.2.5 Cell culture
PC-3, a human prostate cancer cell line, was purchased from ATCC (CRL-1435). The cells were cultured as monolayers in 90% Ham’s F12K medium with 2 mM L-glutamine adjusted to contain 1.5 g/L sodium bicarbonate and 10% fetal bovine serum. The mixture was supplemented with 1% penicillin/streptomycin and maintained in a humidified atmosphere containing 5% CO2 at 37°C.
2.2.6 High-speed photography of delivery vehicles
The acoustic activity of the combination microbubble-liposome delivery vehicle was quantified by observation of vehicle oscillation in response to an acoustic pulse. An ultra high-speed video microscopy system was utilized to record images of bubbles oscillating at MHz frequencies. This system and the procedure for optical analysis of oscillating microbubbles during insonation have been previously described [37, 41]. Briefly, an Imacon 468 (DRS Hadland, Cupertino, CA) camera system with a temporal resolution of 10 nanosecond was coupled to an inverted microscope (IX70, Olympus, Melville, NY). Delivery vehicles were contained within a 200 micron cellulose tube suspended in a water bath on the microscope. A water immersion Achroplan 100× objective with a numerical aperture of 1.0 (Carl Zeiss, Thornwood, NY) provided magnification. Delivery vehicles were back illuminated by a xenon strobe light (DRS Hadland, Cupertino, CA) synchronized to the camera shutter. The ultrasound source, a 2.25 MHz, 2” (~5 cm) spherically focused transducer (V305, Panametrics, Waltham, MA), was positioned in the water bath such that the acoustic focus overlapped the optical focus.
The transducer was energized with 3-cycle sinusoidal pulses with a center frequency of 2.25 MHz from an arbitrary waveform generator (AWG2021 Tektronix, Beaverton, OR) and a 55 db RF amplifier (3200L, ENT). Images were recorded during bubble insonation, and imaging data were analyzed offline via MATLAB (Mathworks, Natick, MA) to quantify initial vehicle radius and maximum expansion the acoustic pulse. Calibration of the system with a 400 micron needle hydrophone (PZT-0400, Onda Corp., Sunnyvale, CA) quantified acoustic pressures and allowed simultaneous alignment of optical and acoustical sample volume.
2.2.7 Vehicle delivery enhancement using ultrasound radiation force
Thermanox coverslips with cell monolayers were mounted into a stainless steel frame, which provided a 2 mm well depth above the cell layer as illustrated in Figure 1B. The well was filled with approximately 1 mL of Dulbecco’s phosphate buffered saline with Ca2+ and Mg2+ (DPBS, Invitrogen) solution containing approximately 107 delivery vehicles (either liposome-bearing microbubbles or microbubbles only). In order to compare the delivery efficiency of liposome-bearing microbubbles with liposomes using ultrasound, liposomes with a similar formulation to those carried by microbubbles were prepared with an average diameter of 200 nm. In order to match the concentration of liposomes with the concentration of liposomes carried by microbubbles, the liposome concentration was calculated by multiplying the number of liposomes attached to one microbubble by the concentration of microbubbles applied to the well, resulting in a final liposome concentration of 50 μM. The well was then sealed with a second 25 mm Thermanox coverslip, and the sample was placed in a 37ºC water bath.
A ¾” (~1.9 cm) single-element 5.0 MHz transducer (IL0506HP, Valpey Fisher, Hopkinton, MA), spherically focused at 2” (5 cm), was mounted in the water bath and aligned confocally with the center of the cell layer sample. The transducer was energized with sinusoidal pulses from a Tektronix AWG2021 arbitrary waveform generator and an ENI 3200L RF amplifier, as in section 2.2.6. Pulse sequences consisted of either a long, low intensity pulse (peak negative pressure of 150 kPa, pulse length of 5×106 cycles, center frequency of 5.0 MHz), optimized to localize the carrier vehicles upon the cell monolayer, termed a “radiation force pulse”, or this push pulse followed immediately by three short, high acoustic pressure pulses designed to disrupt the carrier vehicles (peak negative pressure of 1.9 MPa, with 50 microseconds between five-cycle pulses), termed a “radiation force/fragmentation pulse”. Acoustic pressures in the water bath at the cell sample position were calibrated before the experiment using the needle hydrophone.
The sample was exposed to ultrasound for 120 seconds at a sequence repetition rate of 0.25 Hz (either the radiation force pulse or radiation force/fragmentation pulse was repeated at 0.25 Hz), after which the chamber was removed from the water bath and disassembled. The cell monolayer was then gently rinsed with DPBS to remove remaining carrier vehicles, and transferred to a microscope (Mikron IV 600L, San Diego, CA) for optical observation. Images were recorded with a Cascade 512b (Photometrics, Tucson, AZ) digital camera in both bright field and fluorescence (mercury arc light source). Samples were analyzed optically with 5× and 20× objective magnification. After initial observation, samples were returned to the incubator for an additional 2 hours, after which they were observed again to determine fluorescence internalization.
2.2.8 Observation of liposome internalization
Confocal microscopy using a LSM-5 (Zeiss, Thornwood, NY) confocal microscope was performed in order to determine the localization of the liposomes in relation to the cells after incubation. Z-stacks of 6 images with a 4.0 micron step size were recorded using a 60× oil-immersion objective (NA=1.3).
2.2.9 Cell viability after ultrasound exposure
Ultrasound radiation force and radiation force/fragmentation pulses were applied to PC-3 cell plates in chambers containing liposome-microbubble hybrid vehicles for 2 minutes. The cells were rinsed and incubated with DPBS buffer for 2 h at 37°C. For the cell viability assay, the cells were treated with a 4% (w/v) Trypan Blue solution, washed with DPBS buffer and examined by microscopy. Cells were deemed non-viable if they had internalized the Trypan blue stain.
2.2.10 Statistical analysis
Statistical analysis was performed using two-tailed t-Test assuming unequal variances. A p value of <0.05 was considered to be statistically significant.
3. Results and Discussion
3.1 Optimization of liposome attachment to microbubbles
3.1.1 Avidin concentration
In this model system, we employed avidin-biotin adhesion between liposomes and microbubbles, as this is the strongest known non-covalent interaction between a protein and a ligand with an affinity of 1015 M−1 at pH 5. Binding of microbubbles and liposomes was observed above an avidin concentration of 10 nM and reached saturation around 300 nM (Figure 2A). The high concentration of avidin required for optimum binding between biotinylated liposomes and biotinylated microbubbles was several orders of magnitude higher than the reported binding constant for a free avidin-free biotin system. This phenomenon is consistent with the observations made by Kitano et al. and can be explained by the insufficient accessibility of either free avidin or avidin bound to liposomes to the biotin bound to the surface of liposome [38]. Avidin is a glycosylated protein with an isoelectric point of 10, and thus is positively charged at physiological pH. Although the electrostatic attraction between positively-charged avidin and negatively-charged biotinylated liposome favors binding, the repulsion among the charged avidin as well as the carbohydrate branches may hinder the compact distribution of avidin on the surface of biotinylated microbubbles. In order to determine whether greater binding can be achieved by avoiding the repulsive effect of the charged proteins and eliminating the carbohydrate brushes, neutravidin, the nonglycosylated avidin with a lower isoelectric point of 6.3, was compared with avidin at a similar concentration of 333 nM. Binding increased by more than 3-fold with neutravidin with p<0.01 (data not shown).
In experiments described within the remaining sections, avidin was used at a concentration of 333 nM.
3.1.2 Liposome concentration
Based on the surface area of one PC molecule, the liposome size, and the microbubble size and count, we estimated the ratio of liposomes to microbubbles to be approximately 105. Therefore, the liposome stock solution was prepared at 12 mM (10 mg/mL) total lipids, and this solution was added at different concentrations to approximately 109 avidin-conjugated microbubbles in a syringe with a total volume of 3 mL. Measurement of the fluorescence intensity of the resulting vehicle demonstrated that the number of bound liposomes increased with their initial concentration (Figure 2B), reaching a maximum value at a liposome concentration of ~ 1.0 mM. The concentration of 1.2 mM (1 mg/mL) was used throughout the optimization procedure.
No significant loss in microbubble count was observed throughout the conjugation procedure (Figure 2C). The microbubble count dropped considerably during washes to remove the unincorporated lipids and submicron size microbubbles. However, the population remained stable throughout the addition of avidin and biotinylated liposomes. The size distribution of the microbubbles was not significantly changed by the addition of avidin and liposomes in the concentration range studied, which suggests little or no cross linking between microbubbles (Figure 2D).
3.1.3 Lipid composition in liposomal formulation
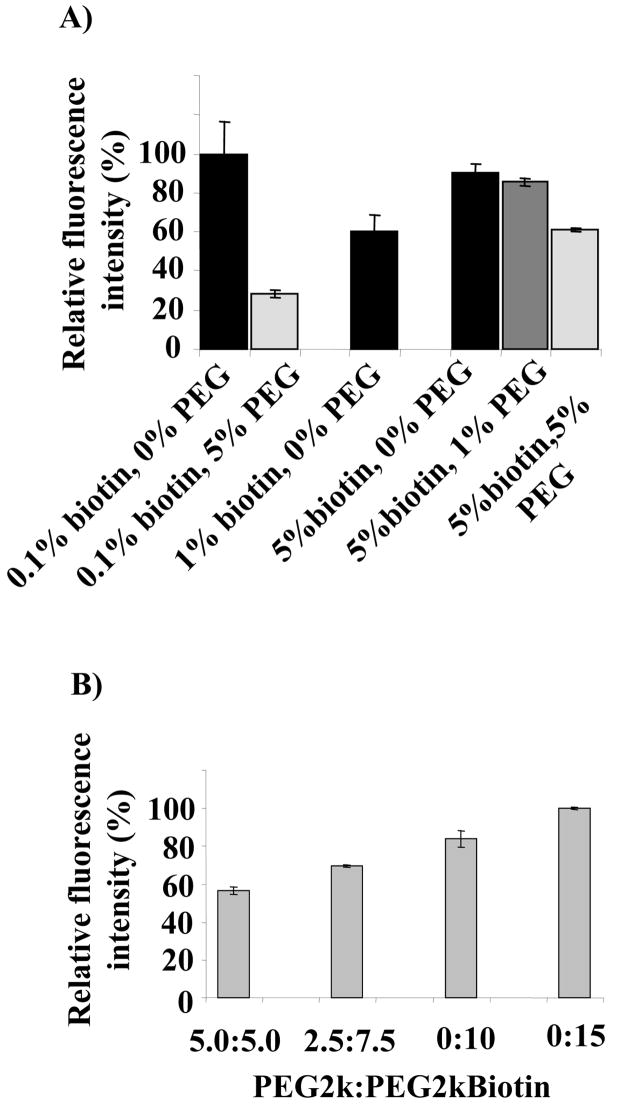
PEG lipids provide a steric barrier on a liposome surface to reduce immune recognition in vivo and thus to prolong blood circulation. Therefore, it is of interest to study the effect of PEG lipids, either in biotinylated or non-biotinylated form, on binding of liposomes to microbubbles. In addition to the PEG2kBiotin used to conjugate the microbubbles and liposomes, the effect of extra PEG2k within the liposomes and microbubbles was evaluated in Figures 3A and 3B, respectively.
Figure 3.
Effect of mole percent of DSPE-PEG2k and DSPE-PEG2kBiotin a) in liposomal formulation with DPPC and 5% NBD-cholesterol; and b) in microbubble formulation with DSPC as the core lipid, at avidin and liposome concentrations of 333 nM and 1.2 mM, respectively.
Without the presence of additional PEG2k (0%), increasing the concentration of PEG2kBiotin from 0.1% to 1% and 5% decreased and then increased liposome-microbubble binding (Figure 3A). We hypothesize that this increase in biotin does not monotonically increase the fluorescence intensity due to two competing factors, steric hindrance and availability of binding sites. With 5% PEG2kBiotin, additional PEG2k did decrease microbubble-liposome binding. Since the presence of 5% PEG2kBiotin in the liposomal formulation provides efficient binding and steric stability, 5% PEG2kBiotin was maintained in our liposomal formulation throughout the optimization and vehicle evaluation procedures.
3.1.4 PEG2k/PEG2kBiotin ratio in biotinylated microbubble
The molar ratios of PEG2k and PEG2kBiotin were then optimized on the microbubble, holding the total PEG concentration to 10%, generally considered to be nearly an optimum percentage for microbubble stability and circulation. An increase in the fraction of PEG2kBiotin present resulted in increased binding (Figure 3B), however PEG2kBiotin at a concentration of 15% reduced the microbubble concentration by 30% as compared to a total PEG lipid concentration of 10%. PEG lipids at a high concentration have the potential to destabilize the microbubble lipid shell [39, 40]. As a result, the concentration of PEG2k and PEG2kBiotin was 5% in all experiments.
3.1.5 Liposome size and its effect on the fluorescence intensity of the bubble and on liposome binding
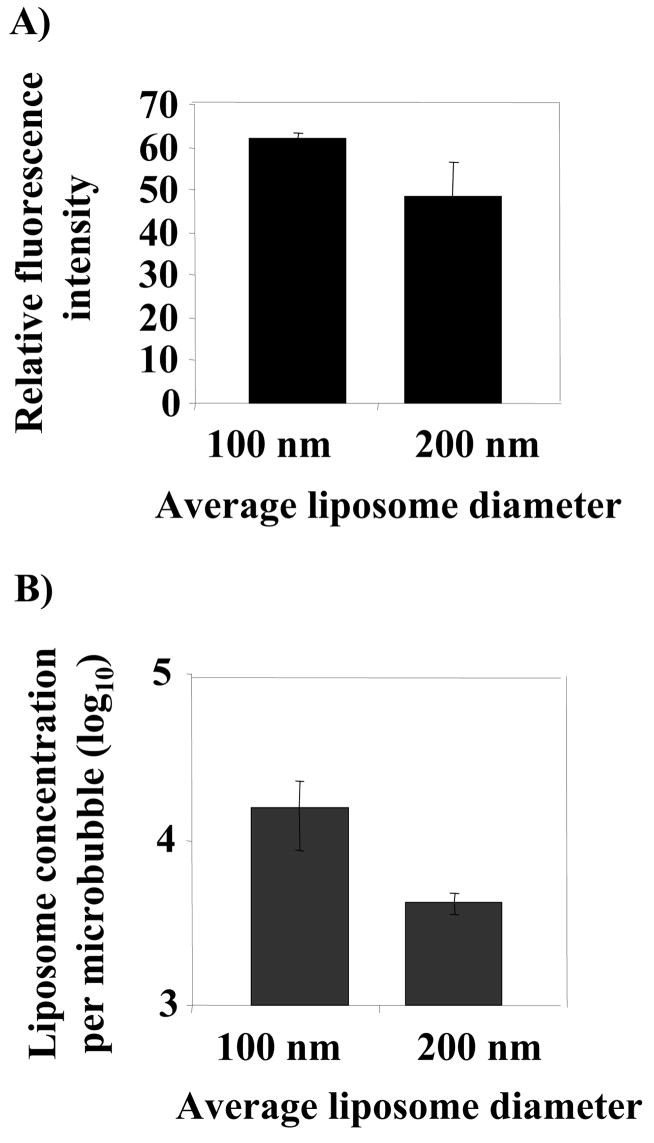
The effect of the average diameter of extruded liposomes on liposomal fluorescence intensity and microbubble-liposome binding was then evaluated by flow cytometry and fluorescence amplitude. For a constant concentration of NBD-cholesterol, the fluorescence intensity of the hybrid vehicles carrying liposomes with average diameters of 100 nm was not significantly different from those with 200 nm (Figure 4A).
Figure 4.
Comparison between liposomes with average sizes of 100 and 200 nm attached to microbubbles, a) relative fluorescence of microbubbles carrying 5% NBD-cholesterol liposomes; b) number of liposomes attached to one microbubble. The experiments were carried out at avidin and liposome concentrations of 333 nM and 1.2 mM, respectively.
The number of liposomes bound to one microbubble was then estimated from our experimental data (Figure 4B). As noted in the methods, 8 × 1012 and 2 × 1012 liposomes were created from 1 mg total lipid for 100 nm and 200 nm liposomes, respectively, and attached to 1–5 × 108 microbubbles. According to these data, under the optimized conditions, a greater number of 100 nm liposomes bound per microbubble (p<0.05). While the binding efficiency of 100 nm liposomes appeared to be higher than that of 200 nm liposomes, the larger liposomes have a 4-fold higher surface area, increasing the unit fluorescence. Using neutravidin, which provides 3-fold higher binding than avidin, the binding of liposomes with an average diameter of 100 nm approaches the saturation level of ~105 sites per microbubble.
Quantification of the number of liposomes attached to each microbubble is based on the original lipid concentration. One possible source of error (10–15% lipid loss during extrusion) is not accounted for in this measurement.
3.1.6 Interaction of liposome-bearing microbubbles with PC-3 cells
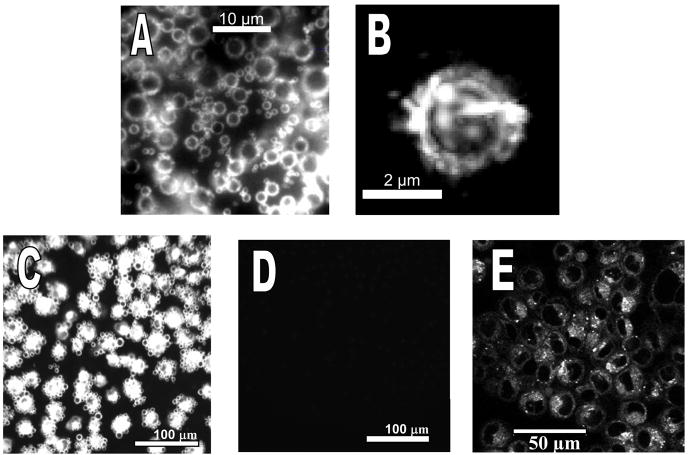
Inverting the chamber (Figure 1B) provided the opportunity to evaluate transfer for cells in contact or not in contact with hybrid vehicles. Initially, the cells are in the bottom of the chamber, and the bubbles tend to float to the opposite side from the cells. When the chamber is inverted, microbubbles rise up due to flotation and contact the cells. Microbubbles carrying NBD-cholesterol liposomes in suspension are shown in Figures 5A and 5B. The optimized hybrid vehicles efficiently transferred the fluorescent cholesterol into a monolayer of PC-3 cells only when forced into contact with the cells by inverting the chamber during incubation (Figure 5C). In the case where the chamber was not inverted, the microbubbles were not forced into contact with the cells and fluorescent cholesterol transfer did not occur (Figure 5D). These results clearly show that transfer did not occur when the cells were not in contact with microbubbles (Figure 5D). Fluorescence transfer during contact was followed by transfer and internalization of NBD-cholesterol (Figure 5E).
Figure 5.
Fluorescence images of A) microbubbles carrying 5% NBD-cholesterol liposomes with an average diameter of 200 nm in suspension (100× oil); B) a single microbubble (100× oil); C, D) interaction between liposomes attached to microbubbles with PC-3 cells when cells incubated with bubbles in contact (C) and cells incubated without bubbles in contact (D); E) confocal image of the cells after contact with liposomes.
3.2 Acoustic response and delivery of liposome-attached microbubbles
3.2.1 Acoustic activity of liposome-bearing microbubbles
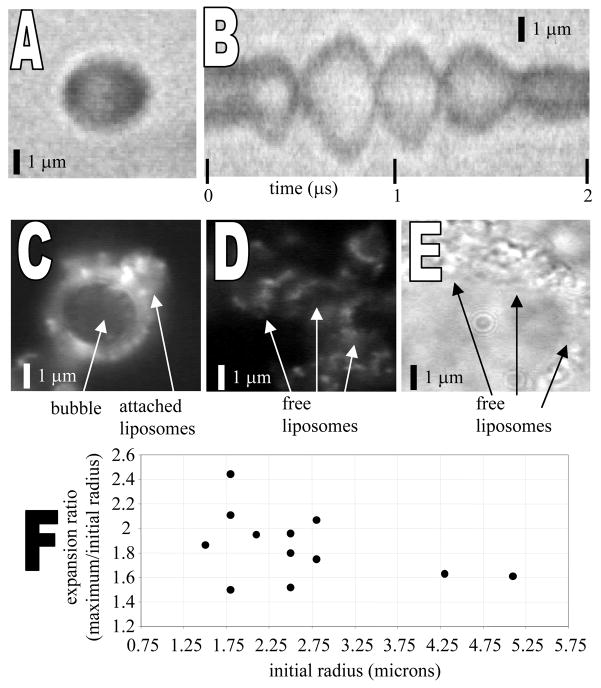
Liposome-bearing microbubbles, shown in Figure 6A in brightfield and 6C in fluorescence, were observed to oscillate in response to an acoustic pulse (Figure 6B). Figure 6B is a radius-time image in which a single line through the center of the microbubble is sampled with 10 nanosecond resolution for a 2 microsecond window. The expansion ratio (maximum diameter/initial diameter) for insonified liposome-bearing microbubbles was on the order of 1.5–2.5 for a peak negative transmission pressure of 200 kPa and center frequency of 2.25 MHz (Figure 6F), similar to that observed previously for lipid-encapsulated perfluorocarbon contrast agents [41]. After bubble oscillation and/or disruption, liposomes were released into solution (Figures 6D in fluorescence and 6E in brightfield). Future studies will quantify the oscillation and release characteristics of liposome-bearing microbubbles over a broad range of imaging parameters.
Figure 6.
A) Brightfield image of liposome-bearing microbubble before insonation. B) Diameter vs. time image of an oscillating liposome-bearing microbubble during insonation with a single 3-cycle, 200 kPa, 2.25-MHz acoustic pulse. Image acquired with a 10 nanosecond sampling rate over the acoustic pulse interval. C) Fluorescence image of a liposome-bearing microbubble before insonation showing adherent fluorescent liposomes. D) Fluorescence image following (C) after insonation and bubble disruption, showing remaining fluorescent liposomes. E) Brightfield image of liposomes in (D) remaining after bubble disruption (liposomes have moved further apart than in (D). (F) Expansion ratio of liposome-bearing microbubbles during insonation with a single 200 kPa, 2.25 MHz acoustic pulse.
3.2.2 Acoustically-mediated delivery of liposome-bearing microbubbles to a cell monolayer
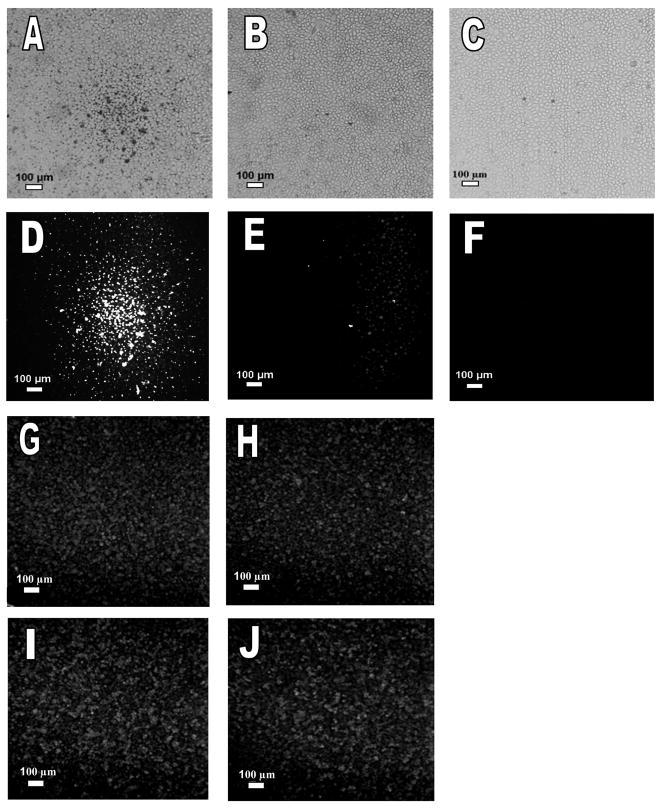
Liposome-bearing microbubbles were readily displaced from solution by ultrasound radiation force and were localized on the cells at the acoustic focus (Figure 7A, B, D, E). Brightfield and fluorescence images of cell monolayers after radiation force enhanced-localization illustrate the localized adhesion of the delivery vehicles within the ultrasound focus (Figures 7A and 7D). Bubbles carrying liposomes were concentrated in a circular region at the focal point of the beam (corresponding to the beam width), and were not desorbed from the cells after the monolayer was rinsed. In contrast, samples not exposed to ultrasound showed minimal vehicle retention (Figures 7B and 7E).
Figure 7.
Fluorescence images of liposome-bearing microbubbles, microbubbles only, and liposomes only on a monolayer of PC-3 cells following insonation with ultrasound radiation force or control. Ultrasound was used to concentrate the vehicles in the center of the monolayer (5 MHz and 150 kPa). (A, B, D, E) NBD-cholesterol was incorporated into the lipid bilayer of liposomes attached to microbubbles. (A) Brightfield image of cells exposed to ultrasound. (B) Brightfield image of cells without ultrasound exposure. (D) Fluorescence image of cells exposed to ultrasound and (E) fluorescence image of cells not exposed to ultrasound. (C, F) Microbubbles carry 5% NBD-cholesterol in their lipid monolayer shell without attached liposomes. (C) Brightfield image of the cells exposed to ultrasound, F) Fluorescence image of cells exposed to ultrasound. (G-J) Fluorescence images of cells incubated with liposomes carrying NBD-cholesterol in the lipid bilayer, (G-H) exposed to ultrasound radiation force/fragmentation pulses, (G) within the ultrasound focus, (H) outside the ultrasound focus, (I-J) without ultrasound exposure, (I) at same location as (G), (J) at same location as (H).
When microbubbles lacking liposomes but containing NBD-cholesterol in the monolayer were deflected to the cell monolayer using similar ultrasound parameters, no significant bubble-cell adhesion or transfer of cholesterol between microbubbles and cells was observed (Figure 7C and 7F). We hypothesize that the radius of curvature of the liposomes makes the surface of the hybrid vehicle more fusogenic [42, 43].
A concentration of liposomes, similar to that carried by microbubbles, was insonified during incubation with the cell monolayer. Fluorescence transfer from the liposomes to the cell monolayer was similar within and outside the ultrasound focus (Figures 7G and 7H). Fluorescence transfer from the liposomes to the cell monolayer was also similar in the presence (Figure 7G and 7H) and absence of ultrasound (Figure 7I and Figure 7J) in both the focal and non-focal areas. These results clearly show the increased transfer from the hybrid vehicle to the monolayer compared with either liposomes only or microbubbles only. The results also exclude the disruption of liposomes or the cell membrane by ultrasound in the absence of microbubbles.
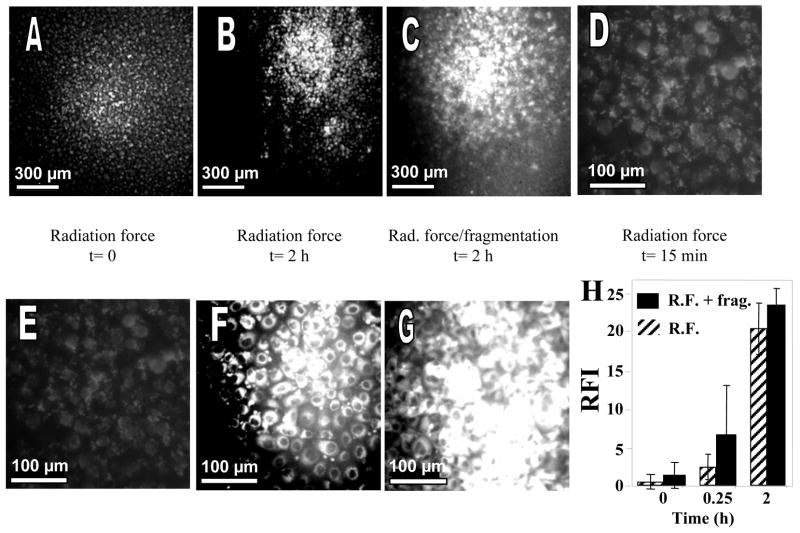
Transfer of fluorescence was investigated over time and then compared for a radiation force pulse and the combination of a radiation force with a fragmentation pulse. Fluorescence transfer near the transducer focus was visualized following 15 minutes of incubation at 37°C after insonation (Figures 8A, 8E at t=0 and Figure 8D at t=15 min) and increased with time for 2 hours with either the radiation force only (Figures 8B, 8F) or the combined radiation force/fragmentation pulses (Figures 8C, 8G).
Figure 8.
Fluorescence images of PC3 cells exposed to ultrasound pulse sequences ending at t=0, where insonation was performed in a solution containing liposome-bearing microbubbles and liposomes contain NBD-cholesterol. After insonation, cells were rinsed several times and further incubated with DPBS buffer at 37°C. (A, E) Images acquired at t=0 following insonation with radiation force pulse sequence. Upper panel 5×, lower panel 20×. (B, F) images acquired at t=2 h following insonation with radiation force pulse sequence. Upper panel 5×, lower panel 20×. (C, G) Images acquired at t=2 h following insonation with radiation force/fragmentation pulse sequence. Upper panel 5×, lower panel 20×. (D) Image acquired at t=15 min following insonation with radiation force pulse sequence, 20×. (H) Summary of relative fluorescence intensity (RFI) versus time of incubation using either the radiation force-only (R.F.) or the combined radiation force/fragmentation pulses (R.F. + frag.). Fluorescence intensity was quantified by counting pixels with an intensity threshold over 0.5, which correlated with bright cellular staining.
Fluorescence transfer was further quantified in terms of image pixel count above a threshold of 0.5 (corresponding to bright cellular staining) using MATLAB (Figure 8H). The extent of internalization of NBD-cholesterol into the cells at the ultrasound focus increased significantly after 15 minutes and 2hours of incubation with each pulse sequence (p<0.05).
Using Trypan blue, we examined the viability of the cells exposed to ultrasound radiation force and radiation force/fragmentation pulses. Blue cell staining was not detected with the insonified or control cells (data not shown), indicating viable cells in both cases.
4. Conclusions
The work presented here supports the idea that the newly designed hybrid vehicle holds promise as a therapeutic delivery vehicle. These vehicles, which combine the advantages of liposomes and microbubbles, are both fusogenic and responsive to ultrasound, overcoming limitations observed with either microbubbles or liposomes individually. The large number of liposomes attached to one bubble (>1000) provides the opportunity to incorporate hydrophilic or hydrophobic therapeutics, without affecting their acoustic properties. Repeated use of avidin or streptavidin could lead to an immunogenic response in some individuals. Alternate methods of microbubble-liposome conjugation, the therapeutic response and the in vivo performance of the liposome-bearing microbubbles are now under evaluation.
Acknowledgments
This work was funded by NIH CA 103828. We wish to extend our appreciation to Prof. Scott Simon for providing the flow cytometry equipment. We would also like to thank Dr. Roger Adamson for his assistance with confocal microscopy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lindner JR. Microbubbles in medical imaging: Current applications and future directions. Nat Rev Drug Discov. 2004;3:527–532. doi: 10.1038/nrd1417. [DOI] [PubMed] [Google Scholar]
- 2.Bloch SH, Dayton PA, Ferrara KW. Targeted imaging using ultrasound contrast agents. Progress and opportunities for clinical and research applications. IEEE Eng Med Biol Mag. 2004;23:18–29. doi: 10.1109/memb.2004.1360405. [DOI] [PubMed] [Google Scholar]
- 3.Klibanov AL. Ligand-carrying gas-filled microbubbles: ultrasound contrast agents for targeted molecular imaging. Bioconjug Chem. 2005;16(1):9–17. doi: 10.1021/bc049898y. [DOI] [PubMed] [Google Scholar]
- 4.Rychak JJ, Klibanov AL, Hossack JA. Acoustic radiation force enhances targeted delivery of ultrasound contrast microbubbles in vitro verification. IEEE Trans Ultrason Ferroelectr Freq Control. 2005;52(3):421–433. doi: 10.1109/tuffc.2005.1417264. [DOI] [PubMed] [Google Scholar]
- 5.Ismail S, Jayaweera AR, Gimple LW, Powers E, Kaul S. Relation between air-filled albumin microbubble and red cell rheology in the human myocardium: influence of echocardiographic systems and chest wall attenuation. Circul. 1996;94:445–451. doi: 10.1161/01.cir.94.3.445. [DOI] [PubMed] [Google Scholar]
- 6.Podell S, Burrascano C, Gaal M, Golec B, Maniquis J, Mehlhaff P. Physical and biochemical stability of Optison, an injectable ultrasound contrast agents. Biotechnol Appl Biochem. 1999;30:213–223. [PubMed] [Google Scholar]
- 7.Unger EC, Porter T, Culp W, Labell R, Matsunaga T, Zutshi R. Therapeutic applications of lipid-coated microbubbles. Adv Drug Deliv Rev. 2004;56(9):1291–1314. doi: 10.1016/j.addr.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Fritz TA, Unger EC, Sutterland G, Sahn D. Phase 1 clinical trials of MRX-115. A new ultrasound contrast agent. Invest Radiol. 1997;32(12):735–740. doi: 10.1097/00004424-199712000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Christiansen JP, Lindner JR. Molecular and cellular imaging with targeted contrast ultrasound. Proc IEEE. 2005;93(4):809–818. [Google Scholar]
- 10.Shortencarier MJ, Dayton PA, Bloch SH, Schumann PA, Matsunaga TO, Ferrara KW. A method for radiation-force localized drug delivery using gas-filled lipospheres. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51(7):822–831. doi: 10.1109/tuffc.2004.1320741. [DOI] [PubMed] [Google Scholar]
- 11.Zhao S, Borden M, Bloch SH, Kruse D, Ferrara KW, Dayton PA. Radiation force assisted targeting facilitates ultrasonic molecular imaging. Mol Imaging. 2004;3(3):135–148. doi: 10.1162/1535350042380317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dayton PA, Ferrara KW. Targeted imaging using ultrasound. Magn Res Imaging. 2002;16:362–377. doi: 10.1002/jmri.10173. [DOI] [PubMed] [Google Scholar]
- 13.Bekeredjian R, Chen S, Grayburn PA, Shohet RV. Augmentation of cardiac protein delivery using ultrasound targeted microbubble destruction. Ultras Med Biol. 2005;31(5):687–691. doi: 10.1016/j.ultrasmedbio.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Leong-Poi H, Christiansen J, Heppner P, Lewis CW, Klibanov AL, Kaul S, Lindner JR. Assessment of endogenous and therapeutic arteriogenesis by contrast agent ultrasound molecular imaging of integrin expression. Circul. 2005;111:3248–3254. doi: 10.1161/CIRCULATIONAHA.104.481515. [DOI] [PubMed] [Google Scholar]
- 15.Unger EC, Porter T, Culp W, Labell R, Matsunaga T, Zutshi R. Therapeutic applications of lipid-coated microbubbles. Adv Drug Deliv Rev. 2004;56(9):1291–1314. doi: 10.1016/j.addr.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Klibanov AL. Microbubble contrast agents; Targeted ultrasound imaging and ultrasound-assisted drug-delivery applications. Invest Radiol. 2006;41(3):354–362. doi: 10.1097/01.rli.0000199292.88189.0f. [DOI] [PubMed] [Google Scholar]
- 17.Cavalieri F, El Hamassi A, Chiessi E, Paradossi G, Villa R, Zaffaroni N. Tethering functional ligands onto shell of ultrasound active polymeric microbubbles. Biomacromolecules. 2006;7(2):604–611. doi: 10.1021/bm050723g. [DOI] [PubMed] [Google Scholar]
- 18.Chen S, Ding J, Bekeredjian R, Yang B, Shohet RV, Johnston SA, Hohmeier HE, Newgard CB, Grayburn PA. Efficient gene delivery to pancreatic islets with ultrasonic microbubble destruction technology. Proc Natl Acad Sci U S A. 2006;103(22):8469–8474. doi: 10.1073/pnas.0602921103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshinaishii C, Miller GP, Kraft ML, Kool ET, Boxer SG. General method for modification of liposomes for encoded assembly on supported bilayers. J Am Chem Sc. 2005;127(5):1356–1357. doi: 10.1021/ja043299k. [DOI] [PubMed] [Google Scholar]
- 20.Nickels LS, Palmer AF. Liposome-encapsulated actin-hemoglobin (LEAcHb) artificial blood substitutes. Biomaterials. 2005;26(17):3759–3769. doi: 10.1016/j.biomaterials.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 21.Heyes J, Palmer L, Bremner K, Maclachlan I. Cationic lipid saturation influences intracellular delivery of encapsulated nucleic acids. J Control Release. 2005;107:276–287. doi: 10.1016/j.jconrel.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Harrison M, Tomlinson D, Stewart S. Liposomal-entrapped doxorubicin: an active agent in AIDS-related Kaposi’s sarcoma. J Clin Oncol. 1995;13(4):914–920. doi: 10.1200/JCO.1995.13.4.914. [DOI] [PubMed] [Google Scholar]
- 23.Halm U, Etzrodt G, Schiefke I, Schmidt F, Witzigmann H, Mossner J, Berr F. A phase II study of pegylated liposomal doxorubicin for treatment of advanced hepatocellular carcinoma. Am Oncol. 2000;11(1):113–114. doi: 10.1023/a:1008386822906. [DOI] [PubMed] [Google Scholar]
- 24.Goebel FD, Goldstein D, Goos M, Jablonowski H, Stewart JS. Efficacy and safety of stealth liposomal doxorubicin in AIDS-related Kaposi’s sarcoma, The International SL-DOX Study Group. Br J Cancer. 1996;73(8):989–994. doi: 10.1038/bjc.1996.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayer LD, Thies RL, Boman NL, Cullis PR, Bally MB. Identification of vesicle properties that enhance the antitumor activity of liposomal vincristine against murine L1210 leukemia. Cancer Chemother Pharmacol. 1993;33:17–24. doi: 10.1007/BF00686017. [DOI] [PubMed] [Google Scholar]
- 26.Boman NL, Bally MB, Cullis PR, Mayer LD, Webb MS. Encapsulation of vincristine in liposomes reduces its toxicity and improves its anti-tumor efficacy. J Liposome Res. 1995;5:523–541. [Google Scholar]
- 27.Webb MS, Harasym TO, Masin D, Bally MB, Mayer LD. Sphingomyelin-cholesterol liposomes significantly enhance the pharmacokinetic and therapeutic properties of vincristine in murine and human tumor models. Br J Cancer. 1995;72:896–904. doi: 10.1038/bjc.1995.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lum AFH, Borden MA, Dayton PA, Kruse DE, Simon SI, Ferrara KW. Ultrasound radiation forces enables targeted deposition of model drug carriers loaded on microbubbles. J Control Release. 2006;111:128–134. doi: 10.1016/j.jconrel.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papahadjopoulos D, Allen TM, Gabizon A, Mayhew E, Matthay K, Huang SK, Lee KD, Woodle MC, Lasic DD, Redemann C, et al. sterically stailized liposomes: improvements in pharmacokinetics and anti-tumor therapeutic efficacy. Proc Natl Acad Sci U S A. 1991;88(24):11460–11464. doi: 10.1073/pnas.88.24.11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klibanov Al, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990;268(1):235–237. doi: 10.1016/0014-5793(90)81016-h. [DOI] [PubMed] [Google Scholar]
- 31.Allen TM, Hansen C, Martin F, Redemann C, Yau-Young A. Liposomes containing synthetic lipid derivatives of poly(ethylene glycol) show prolonged circulation half-lives in vivo. Biochim Biophys Acta. 1991;1066(1):29–36. doi: 10.1016/0005-2736(91)90246-5. [DOI] [PubMed] [Google Scholar]
- 32.Ishida T, Harada M, Wang XY, Ichihara M, Irimura K, Kiwada H. Accelerated blood clearance of PEGylated liposomes following proceeding liposome injection: effects of lipid dose and PEG surface density and chain length of the first-dose liposomes. J Control Release. 2005;105(3):305–317. doi: 10.1016/j.jconrel.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Bayer EA, Wilchek M. The use of the avidin-biotin complex as a tool in molecular biology. Meth Biochem Anal. 1980;26:1–45. doi: 10.1002/9780470110461.ch1. [DOI] [PubMed] [Google Scholar]
- 34.Borden MA, Martinez GV, Ricker J, Tsvetkova N, Longo M, Gillies RJ, Dayton PA, Ferrara KW. Lateral phase separation in lipid-coated microbubbles. Langmuir. 2006;22:4291–4297. doi: 10.1021/la052841v. [DOI] [PubMed] [Google Scholar]
- 35.Huang C, Mason JT. Geometric packing constraints in egg phosphatidylcholine vesicles. Proc Natl Acad Sci USA. 1978;75(1):308–310. doi: 10.1073/pnas.75.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kucerka N, Kiselev MA, Balgavy P. Determination of bilayer thickness and lipid surface area in unilamellar dimyristoylphosphatidylcholine vesicles from small-angle neutron scattering curves: a comparison of evaluation methods. Eur Biophys J. 2004;33:328–334. doi: 10.1007/s00249-003-0349-0. [DOI] [PubMed] [Google Scholar]
- 37.Dayton PA, Allen JS, Ferrara KW. The magnitude of radiation force on ultrasound contrast agents. J Acoust Soc Am. 2002;112(5):2183–2192. doi: 10.1121/1.1509428. [DOI] [PubMed] [Google Scholar]
- 38.Kitano H, Kato N, Ise N. Mutual recognition between polymerized liposomes. III. Association process between avidin and biotin on polymerized liposome surfaces. Biotechnol Appl Biochem. 1991;14(2):192–201. [PubMed] [Google Scholar]
- 39.Photos PJ, Bacakova L, Discher B, Bates FS, Discher DE. Polymer vesicles in vivo: correlations with PEG molecular weight. J Control Release. 2003;90:323–334. doi: 10.1016/s0168-3659(03)00201-3. [DOI] [PubMed] [Google Scholar]
- 40.Johnson M, Edwards K. Liposomes, Disks, and spherical micelles: aggregate structure in mixtures of gel phase phosphatidylcholines and poly(ethylene glycol)-phospholipids. Biophys J. 2003;85:3839–3847. doi: 10.1016/S0006-3495(03)74798-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chomas JE, Dayton P, May D, Ferrara K. Threshold of fragmentation for ultrasonic contrast agents. J Biomed Optics. 2001;6(2):141–150. doi: 10.1117/1.1352752. [DOI] [PubMed] [Google Scholar]
- 42.Bentz J, Düzgünes N. Fusogenic capacities of divalent cations and effect of liposome size. Biochemistry. 1985;24:5436–5443. doi: 10.1021/bi00341a023. [DOI] [PubMed] [Google Scholar]
- 43.Kheirolomoom A, Satpathy GR, Török Z, Banerjee M, Bali R, Novaes RC, Little E, Manning DM, Dwyre DM, Tablin F, Crowe Jh, Tsvetkova NM. Phospholipid vesicles increase the survival of freeze-dried human red blood cells. Cryobiology. 2005;51:290–305. doi: 10.1016/j.cryobiol.2005.08.003. [DOI] [PubMed] [Google Scholar]