Abstract
Lower molecular weight polybrominated diphenylethers (PBDEs), components of flame retardants, are found in the environment and in human and animal tissues. Toxicity studies were conducted in F344/N rats and B6C3F1 mice by administering a flame retardant containing these lower molecular weight PBDEs (BDE-47, BDE-99, BDE-100, and BDE153) by oral gavage 5 days/week for 13 weeks at doses of 0.01, 5, 50, 100 or 500 mg/kg/day. Liver was the primary target organ in rats and mice. Treatment-related increases in liver weights, liver cytochrome P450 (1A1, 1A2, 2B) and UDPGT (rats only) levels, and liver lesions were seen in both rats and mice. Hepatocyte hypertrophy and vacuolization increased in incidence and severity with treatment, and occurred at levels of 50 mg/kg and above in rats, and at 100 mg/kg and above in mice. Liver Cyp 1A1, 1A2, and 2B levels were increased at exposure levels of 50 mg/kg and above in rats and mice. In addition, treatment-related thyroid lesions occurred particularly in rats. The most sensitive parameter for PBDE toxicity was the increase in liver weights which occurred at 5 mg/kg above in rats and 50 mg/kg and above in mice. These results suggest that liver may be a target organ for carcinogenesis processes after long-term administration of PBDEs. A chronic PBDE study is currently being conducted by the National Toxicology Program.
Keywords: Flame retardant, polybrominated diphenyl ethers, liver toxicity
Introduction
The National Institute of Environmental Health Sciences/National Toxicology Program has studied the toxic and carcinogenic potential of flame retardants, chemicals added to common products (e. g. furniture, circuit boards, building materials) to suppress or inhibit the combustion process. Some of the most widely used flame retardants are the brominated flame retardants (Bromine Science and Environmental Forum, 2000), and these flame retardants can cause toxicity and/or cancer at multiple sites including liver, lung and intestine (Dunnick et al., 1997). In this paper we report our findings on the toxicity of the polybrominated diphenyl ether flame retardants.
Lower molecular weight polybrominated diphenylethers (PBDEs) are components of flame retardants, and exposure to these chemicals, including BDE-47, BDE-99, BDE-100, and BDE-153, is a health concern because these chemicals have been found in human and animal tissue (McDonald, 2005). Exposure routes to humans may include food, water, inhalation, and dermal, and the occurrence of PBDEs in house dust is prevalent (Lorber, 2007). The total daily intake of PBDEs is estimated to be 7.7 ng/kg body weight/day (Lorber, 2007).
How PBDE congeners get into the environment is a subject of continuing investigation. The low molecular weight PBDEs (i. e. BDE-47, BDE-99, BDE-100, and BDE-153) contained in flame retardants found in polyester foams, may leach from the foams when they are deposited at waste dumps (Hale et al., 2003; Hale et al., 2002; Hale et al., 2001a; Hale et al., 2001b). Microorganisms may dehalogenate higher level of brominated flame retardants (van Pee and Unversucht, 2003), and, thus, one environmental source for the lower molecular weight PBDE congeners may be from the breakdown of higher molecular weight PBDEs (Hale et al., 2003; Hale et al., 2002; Hale et al., 2001a; Hale et al., 2001b; Riess et al., 2000; Sorderstrom et al., 2004; Stapleton et al., 2004). Photolytic debromination may also occur (Sorderstrom et al., 2004)..
Studies have evaluated levels of PBDE congeners in human tissues (Petreas et al., 2003; Schecter et al., 2003). BDE-47 is the most prevalent PBDE congener found (Sjodin et al., 2004). Polybrominated diphenylethers have been reported to disrupt endocrine activity (Hamers et al., 2006; Legler and Brouwer, 2003; Meerts et al., 2001), alter thyroid hormone levels (Darnerud et al., 2007; Ellis-Hutchings et al., 2006; Zhou et al., 2002), and induce liver cytochrome p450 levels (Birnbaum and Cohen Hubal, 2006; Sanders et al., 2005; Zhou et al., 2002), activities that could contribute to disease development after long term exposures.
The objective of these PBDE flame retardant studies was to characterize liver toxicities in F344/N rats and B6C3F1 mice, the model systems used in National Toxicology Program toxicity studies.
Materials and Methods
Experimental Design
Male and female F344/N rats and B6C3F1 mice were obtained from Taconic Laboratory, Germantown, NY. At the start of the study the animals were 5 – 6 weeks of age. The animals were housed by species and sex, 2–3 male rats/cage, 5 female rats/cage, one male mouse/cage, and 5 female mice/cage. Tap water and NTP-2000 diet (Zeigler Brothers, Inc. Gardners, PA) were made available ad libitum (National Toxicology Program, 2005a; National Toxicology Program, 2005b).
The flame retardant, DE-71, was administered by oral gavage in corn oil to core groups of 10 male and female Fisher 344 rats (5 ml/kg body weight) or B6C3F1 mice (10 mg/kg body weight) to give doses of 0,0.01, 5, 50, 100 and 500 mg/kg/day for 5 days/week for 13 weeks. 10 additional rats were taken for serum collection at day 4 and 25. Tissue samples were taken from the fat and liver of male and female rats, and from the fat of male and female mice for determination of BDE levels.
Chemical
DE-71 (technical pentabromodiphenyl oxide; Cas. No. 32534-81-9) was obtained from Great Lakes Chemical Corporation (Colombus, Ohio - Lot 2550OA30A). Components of this mixture included primarily the tetra through penta PBDEs and a small component of hexabromodiphenyl ethers. The molecular weights of the diphenyl ethers increase with increasing bromination. The molecular weight for tetrabrominated diphenyl ethers is 469.6, for pentabromated diphenyl ethers, 548.5, and for hexabromo diphenyl ethers, 627.4. The DE71 bulk chemical analysis (National Toxicology Program, 2004; Sanders et al., 2005) showed a composition of: 2,2′,4,4′-tetrabromodiphenyl ether - BDE-47 (36%), 2,2′,4,4′,5-pentabromodiphenyl ether - BDE-99 (42%); 2,2′,4,4′,6-pentabromodiphenyl ether - BDE-100 (10%), 2,2′,4,4′,5,5′-hexabromodiphenyl ether - BDE-153 (3%), 2,2′,4,4′,5,6′-hexabromodiphenyl ether - BDE-154 (4%), and 2,2′,3,4,4′-pentabromodiphenyl ether - BDE-85 (2%); The remaining 3% consisted of several identified tri-heptaBDEs and some unidentified PBDEs (National Toxicology Program, 2004). This flame retardant mixture also contained approximately 66 nanograms/gram of brominated dioxins or furans (.0066% of the flame retardant). A dose of 500 mg/kg is, thus, an approximate dose of 33 nanograms/kg body weight of brominated dioxins or furans. A dose of 100 mg/kg is an approximate dose of 6.6 nanograms/kg body weight of brominated dioxins or furans (National Toxicology Program, 2004; Sanders et al., 2005).
Tissue level determinations
Liver (rats only) and fat (rats and mice) samples were taken on day 93 for determination of BDE-47, BDE-99, and BDE-153 levels in rats and mice. Separation and identification of these chemicals was as previously described (National Toxicology Program, 2007a; National Toxicology Program, 2007b).
Cytochrome p450s
At day 93 a sample of liver from up to 10 male and female rats and mice were taken for enzyme analysis. CYP1A1 was determined by a spectrofloruometric method described by Change and Waxman 1999; CYP1A2 was determined by an acetanilide-4-hydroxylase assay as described by Hamm et al 1998 (Hamm et al., 1998); CYP2B1 was determined by a spectrofluorometric method of Lubet et al 1985 (Lubet et al., 1985), and UDPGT was determined by a spectrophotometric method described by Winsnes (Winsnes, 1969) National Toxicology Program, 2005a; National Toxicology Program, 2005b).
Thyroid Hormone levels
Male and female rats were anesthetized with CO2/O2 on day 4, 25, and 93 and blood was collected from up to 10 male and female rats from the retro-orbital sinus into tubes containing no anticoagulant. Hormone levels were determined using the T4 radioimmunonoassay kit (Diagnostic Products Corp., Los Angelos, CA, and the TSH radioimmunassay double-antibody kit (ICN Diagnostics)(National Toxicology Program, 2005a; National Toxicology Program, 2005b).
Pathology
Complete necropsies were performed on all animals. Tissues were preserved in 10% neutral buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. The following tissues were examined microscopically from male and/or female animals: gross lesions and tissue masses, adrenal gland, bone with marrow, brain, clitoral gland, esophagus, heart, large intestine (cecum, colon, rectum), small intestine (duodenum, jejunum, ileum), kidney, liver, lung, lymph nodes (mandibular and mesenteric), mammary gland (except male mice), nose, ovary, pancreas, pancreatic islets, parathyroid gland, pituitary gland, preputial gland, prostate gland, salivary gland, skin, spleen, stomach (forestomach and glandular), testis with epididymis and seminal vesicle, thymus, thyroid gland, trachea, urinary bladder, and uterus. Following completion of the studies, the accuracy of the histopathologic diagnosis was determined by microscopic reviews of neoplasms and target organs by quality assessment from a pathology working group (Boorman and Eustis, 1986; Hardisty and Boorman, 1986). Liver, right kidney, heart, lung, thymus, and right testes were weighed at necropsy.
Statistics
Tests of significance included two-sided pairwise comparisons between exposed groups and controls (Gart et al., 1979).
Results
Rats
All rats survived the DE-71 subchronic treatment (Table 1), but final mean body weights of male and female rats at 500 mg/kg were 76% and 69% of controls, respectively. The absolute liver weights relative to controls for the 0, 0.01, 5, 50, 100 or 500 mg/kg groups were: 111%, 120%, 159%, 170%, and 199% for male rats; and 106%, 116%, 157%, 177%, and 219% for female rats, respectively (Table 2). Small increases in kidney weights and decreases in thymus weights were seen in treated groups of male and females.
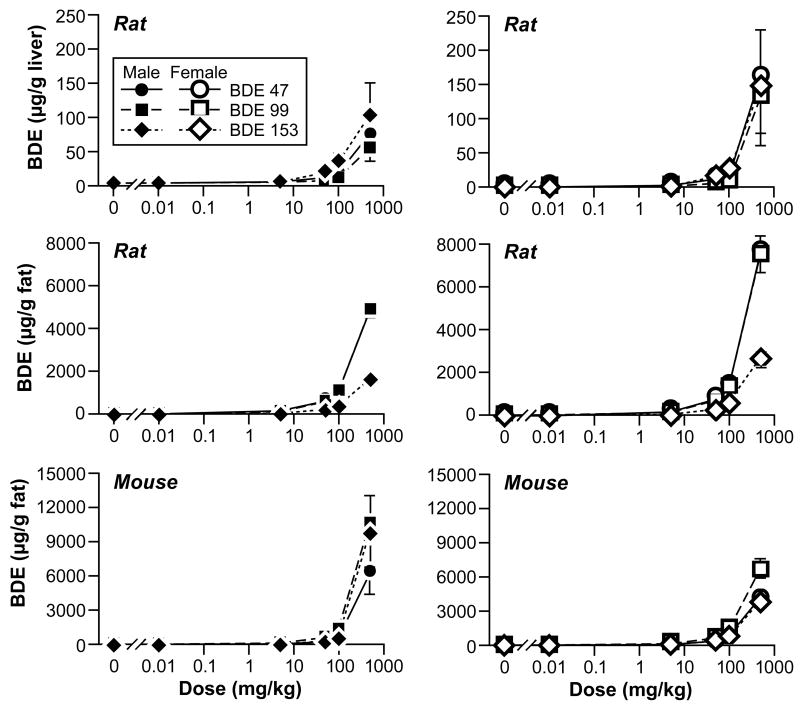
Cyp1A1, Cyp1A2, Cy2B and UDPGT (Table 2) were elevated in the 50, 100, and 500 mg/kg groups of males and females. T4 levels were decreased at the top three exposure levels in males and female rats (Table 3), while TSH levels were in general not as dramatically affected (data not shown). BDE-47, BDE-99, and BDE-153 levels were elevated in liver and fat of male and females (Figure 1), but deposited to a greater extent in fat than in liver.
Figure 1.
Tissue levels of polybrominated diphenyl ethers in rats and mice.
Treatment-related lesions were seen in the liver, stomach, and thyroid of males and females. Hepatocytic hypertrophy was observed in all female dose groups. Males treated with 5, 50, 100, and 500 mg/kg groups were affected. In both sexes the severity of the lesion increased with dosage. In males, the midzonal hepatocytes appeared to have been affected first, whereas in females, the centrilobular hepatocytes appeared to have been first affected. Hepatocyte hypertrophy consisted of enlarged hepatocytes that were up to three times their normal size (Table 1, Figure 2). Occasionally, the nuclei of these hypertrophic cells were slightly enlarged. The hepatocytes contained variable amounts of fine granular pale eosinophilic (ground glass appearing) cytoplasmic material containing basophilic clumps of chromatin material arranged in a reticular pattern in the nucleus. If less than 10% of the hepatocytes in section were affected, the lesion was graded as minimal; 10% to less than 50% of hepatocytes affected was graded as mild; 50% to less than 70% of hepatocytes affected was graded as moderate; 70% or more of hepatocytes affected was graded as marked.
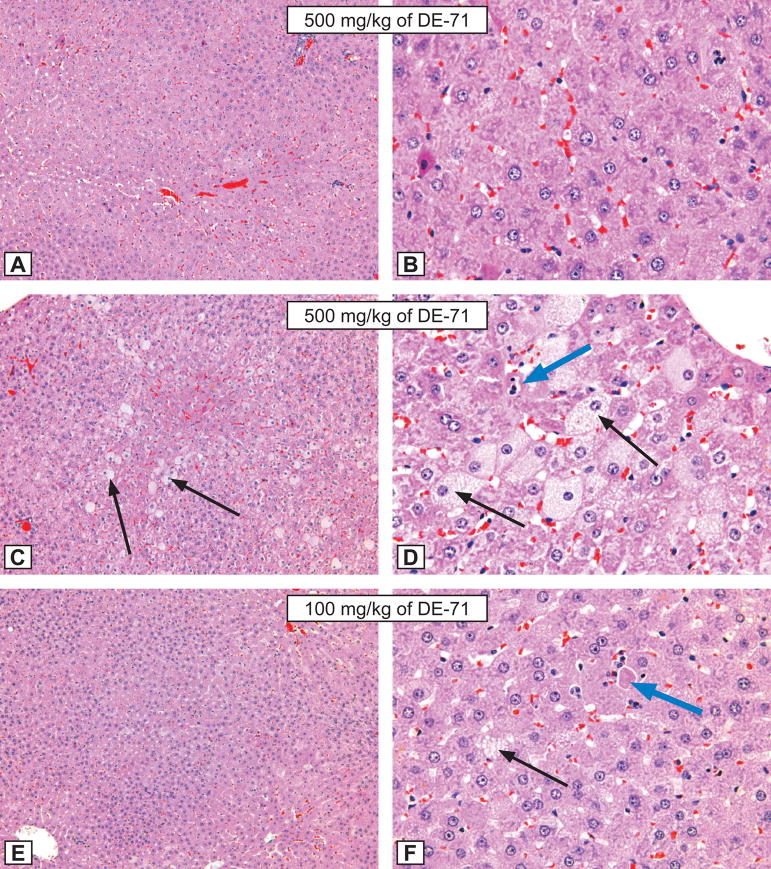
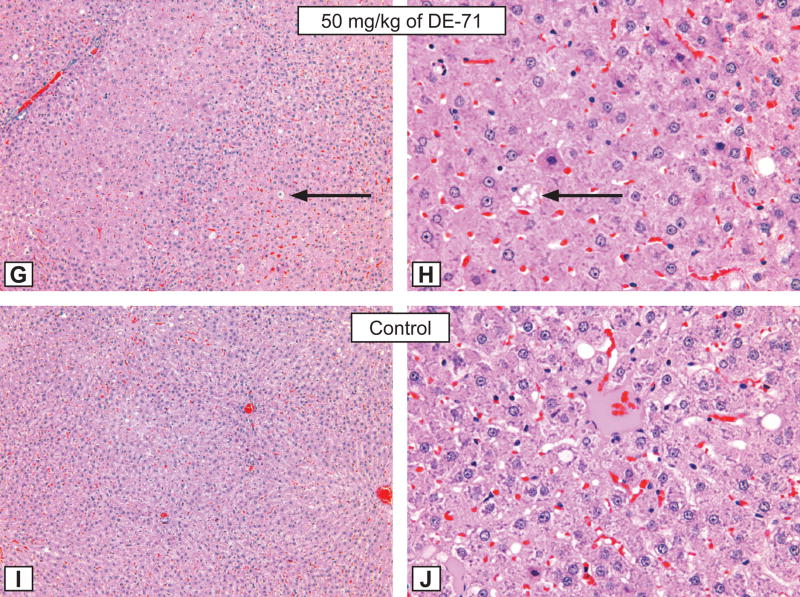
Figure 2. Rat liver lesions.
Figures A and B: Hepatocytic hypertrophy grade 3 in a male rat treated for 3 months by gavage with 500 mg/kg of DE-71. A - X12.5; B - X50. Compare with control animal, Figure I and J, respectively. H&E.
Figures C and D: Hepatocytic hypertrophy grade 4 in a male rat treated for 3 months by gavage with 500 mg/kg of DE-71. C - X12.5; D - X50. Note the presence of hepatocytic fatty degeneration (black arrows), and single hepatocytic necrosis (blue arrow). Compare with control animal, Figure I and J, respectively. H&E.
Figures E and F: Hepatocytic hypertrophy grade 3 in a male rat treated for 3 months by gavage with 100 mg/kg of DE-71. E - X12.5; F - X50. Note the presence of hepatocytic fatty degeneration (black arrows), and single hepatocytic necrosis (blue arrow). Compare with control animal, Figure I and J, respectively. H&E.
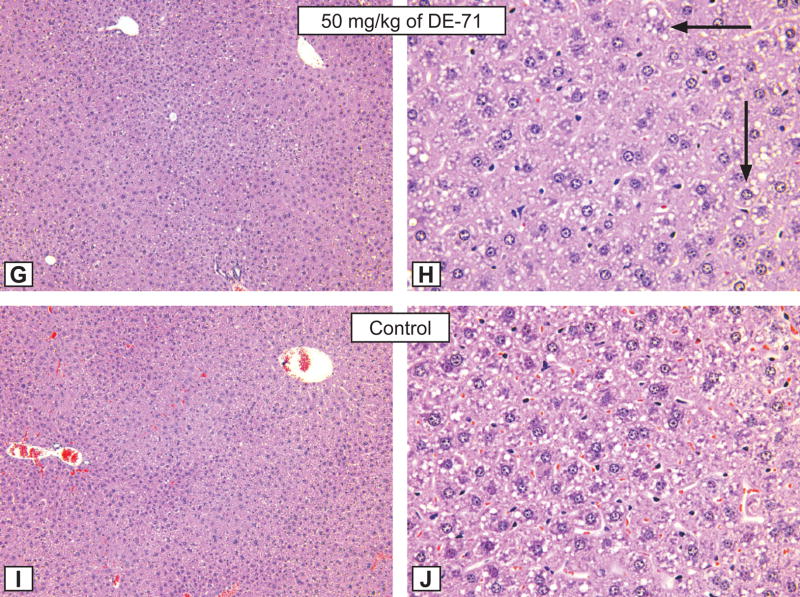
Figures G and H: Hepatocytic hypertrophy grade 3 in a male rat treated for 3 months by gavage with 50 mg/kg of DE-71. G - X12.5; H - X50. Note the presence of hepatocytic fatty degeneration (black arrows). Compare with control animal, Figure I and J, respectively. H&E.
Figures I and J: The morphological aspect of hepatocytes from a control male rat from the DE-71 study. X 12.5; B - X50. H&E.
Hepatocytic vacuolation consisted of enlarged hepatocytes with small round distinct vacuoles in the cytoplasm. Focal hepatocytic necrosis of minimal severity was noted in few males and females. This lesion was characterized by a single hepatocyte or clusters of a few hepatocytes that were shrunken with eosinophilic cytoplasm and occasional nuclear debris.
Erosion of the glandular stomach were increased in a dose-dependent manner in males (0/10, 0/10, 1/10(1.0), 2/20(1.5), 3/10(1.7), 4/10(1.5)) and was also seen in the females treated with the high dose (3/10(1.0)). This lesion was characterized by thinning of the gastric mucosal epithelium with or without necrosis.
Treatment-related increased incidence of follicular hypertrophy of the thyroid was noted in the males treated with 100 (1/10 (1.0)) and 500 (9/10 (1.0)) mg/kg and females treated with 50 (8/10 (1.0), 100 (9/10 (1.4)) and 500 (10/10(2.9)) mg/kg. The change was characterized by an increase in the number of small follicles lined by cuboidal to low columnar cells. Severity was based on the percentage of the follicles affected. In minimal grade • 30% of the follicles were affected when compared to controls; mild degree - >30% and • 60% of follicles affected when compared to controls; and moderate degree - >60% of follicles affected when compared to controls
Mice
Treatment-related mortality occurred in male and female mice at 500 mg/kg. Mean body weights of 100 mg/kg male and females were 80 and 87% of controls, respectively; mean body weights of 50 mg/kg male and females were 88% and 86% of controls, respectively. The absolute liver weights for the 0.01, 5, 50, 100, or 500 mg/kg groups relative to controls were: 95%, 109%, 130%, 160%, 298% for males; and were 85%, 85%, 117%, 142%, 212% for females, respectively (Table 2). Cyp1A1, Cyp1A2, Cyp2B, were elevated in mice at levels of 100 mg/kg and above. Thyroid hormone levels were not determined in mice. BDE-47, BDE-99, and BDE-153 levels were elevated in fat of male and females (Figure 1).
Treatment-related lesions were seen in the liver, adrenal cortex, stomach, testis, thymus, and spleen, but the major effect was seen in liver. Males treated with 5, 50, 100, and 500 mg/kg were affected. Hepatocytic hypertrophy was observed in females in the 100 and 500 mg/kg dose groups. In both sexes the severity of the lesion increased with dosage, and the centriblobular hepatocytes appeared to have been affected first and as the severity increased, the midzonal and periportal hepatocytes were affected. Hepatocytic hypertrophy consisted of enlarged hepatocytes that were up to three times their normal size (Table 1, Figure 3). Occasionally, the nuclei of these hypertrophic cells were slightly enlarged. The hepatocytes contained variable amounts of fine granular pale eosinophilic (ground glass appearing) cytoplamic material containing basophilic clumps of chromatin material arranged in a reticular pattern in the nucleus. If less than 10% of the hepatocytes in section were affected, the lesion was graded as minimal; 10% to less than 50% of hepatocytes affected was graded as mild; 50% to less than 70% of hepatocytes affected was graded as moderate; 70% or more of hepatocytes affected was graded as marked.
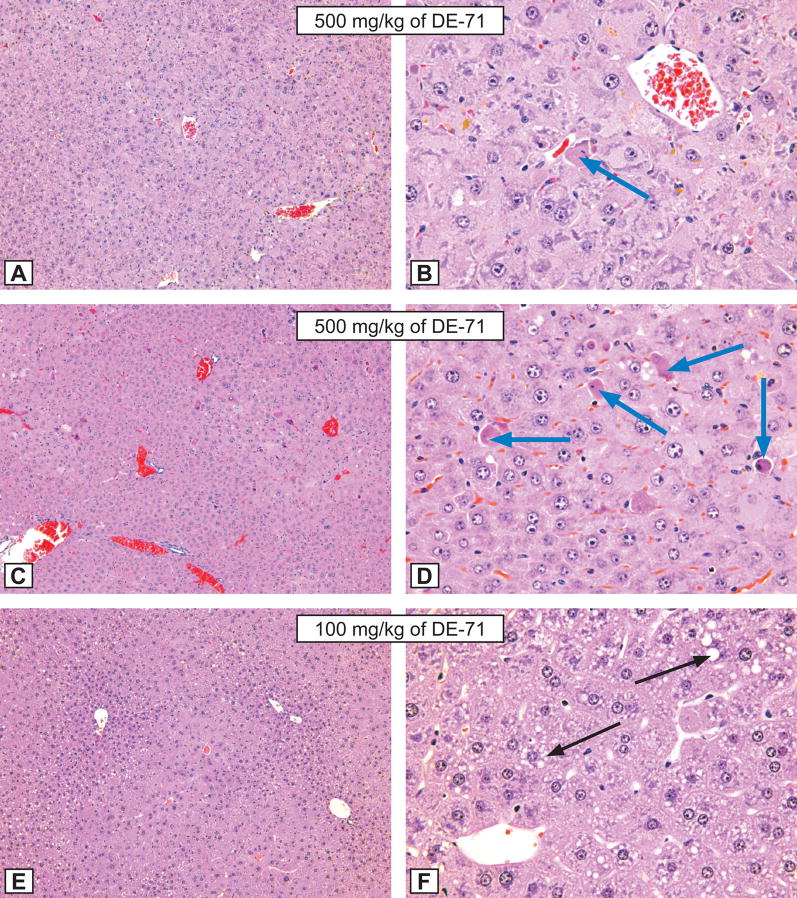
Figure 3. Mouse liver lesions.
Figures A and B: Hepatocytic hypertrophy grade 4 and hepatocytic necrosis grade 1 (blue arrows) in a male mouse treated for 3 months by gavage with 500 mg/kg of DE-71. A - X12.5; B - X50. Compare with control animal, Figure I and J, respectively. H&E.
Figures C and D: Hepatocytic hypertrophy grade 4 and hepatocytic necrosis grade 2 (blue arrows) in a male mice treated for 3 months by gavage with 500 mg/kg of DE-71. C - X12.5; D - X50. Compare with control animal, Figure I and J, respectively. H&E.
Figures E and F: Hepatocytic hypertrophy grade 3 in a male mice treated for 3 months by gavage with 100 mg/kg of DE-71. E - X12.5; F - X50. Note the presence of hepatocytic fatty degeneration (black arrows). Compare with control animal, Figure I and J, respectively. H&E.
Figures G and H: Hepatocytic hypertrophy grade 1 in a male mice treated for 3 months by gavage with 50 mg/kg of DE-71. G - X12.5; H - X50. Compare with control animal, Figure I and J, respectively. H&E.
Figures I and J: The morphological aspect of hepatocytes from a control male mice from the DE-71 study. X 12.5; B - X50. H&E.
Two types of hepatocytic necrosis were seen in male and female mice. Hepatocytic necrosis (Figure 3) was characterized by a single hepatocyte or clusters of a few hepatocytes that were shrunken with eosinophilic cytoplasm and occasionally cellular debris. The location of these necrotic hepatocytes was random.
Focal necrosis occurred in a few males at 100 or 500 mg/kg, and was characterized as foci at random, and coagulative necrosis often seen secondary to embolization of bacteria from the gastrointestinal tract (Hall et al., 1992). Hepatocytic vacuolation was observed only in male mice at 500 mg/kg and consisted of enlarged hepatocytes with small vacuoles in the cytoplasm (Figure 3).
Increased incidence of cortical cytoplasmic vacuolation (fatty change) in the adrenal cortex was noted in the 500 mg/kg (males: 4/10; females: 2/10). This change was characterized by the presence of variable sized (usually large) clear cytoplasmic vacuoles in the cytoplasm of cells in the zona fasciculate. In addition, minimal hypertrophy of the cells in the zona fascilulata was noted in the males treated with 500 mg/kg dosed group. The hypertrophy was characterized by increased cytoplasmic volume and increased cytoplasmic eosinophilia with decreased microvesculation.
Several animals at the high dose presented with erosions of the glandular stomach (males: 2/10; females: 1/10). The lesion was characterized by variable sized foci of thinning due to necrosis of the mucosa, sometimes accompanied by inflammatory cell infiltrate consisting primarily of neutrophils. A single case of mucosal erosion with secondary epithelial hyperplasia was seen in the non-glandular stomach in a male mouse treated with the 500 mg/kg, and chronic inflammation of the non-glandular stomach was seen in another male mouse from the same dose group. In the 500 mg/kg female mouse group, a non-glanudular stomach mucosal ulceration with secondary hyperplasia was seen in one female mouse, and another female mouse presented with hyperplasia of the non-glandular stomach.
Increased incidence of mild presence of abnormal residual body formation was seen in the testes of males (0/10, 0/10, 1/10, 0/10, 1/10, 5/10). This lesion was characterized by presence of large (15–35 micron), round to oval, amphophilic to eosinophilic bodies in the seminiferous tubules. These abnormal residual bodies were primarily seen free in the lumen of the tubules.
Other lesions included cortical atrophy of the thymus in six males and three females in the 500 mg/kg groups. The atrophy was characterized by reduced lymphocytes in the cortex. In addition, atrophy of the spleen was seen in three males treated with 500 mg/kg (0/10, 1/10, 1/10, 0/10, 0/10, 3/10). The atrophy was characterized by reduced lymphocytes in the cortex.
Discussion
Toxic lesions were seen primarily in the liver of rats and mice exposed to the flame retardant, DE 71. Little or no liver toxicity occurred at the low exposure (0.01 mg/kg/day), an exposure close to human exposure levels (Lorber, 2007), but more severe liver toxicity occurred at the high exposure levels (100 or 500 mg/kg/day). Toxicity was characterized by increases in liver enzyme levels, liver weights, and toxic liver and thyroid lesions (rats), and decreases in serum thyroid hormone levels (T4 – rats).
All treated rats survived the treatment, although treatment-related mortality occurred in mice at 500 mg/kg. Increased organ weight was the most sensitive endpoint for liver toxicity, and occurred at 5 mg/kg and above in rats, 50 mg/kg and above in male mice, and 100 mg/kg and above in female mice. Histopathologic liver changes were characterized by hepatocyte hypertrophy and cytoplasmic vacuolization in male and female rats and mice. Focal hepatocyte necrosis occurred in a few male rats and male and female mice at 100 or 500 mg/kg.
The lesions in the adrenal cortex (rats - hypertrophy and fatty degeneration in the cytoplasm of cells in the zona fasciculate) and in the non glandular and/or glandular stomach mucosa (rats and mice - erosions and/or ulceration and inflammation) were considered to be stress related (Greaves, 2000a; Greaves, 2000b). The presence of abnormal residual body formation in the high dose mouse testes was suggestive of a primary effect on the sustentacular Sertoli cells (Melnick et al., 2007).
Proposed mechanisms for hepatocyte necrosis include extreme hypertrophy leading to reduced sinusoidal blood circulation, hypoxia and necrosis (Slauson and Cooper, 2001) and/or metabolic activation forming more toxic active metabolites (Farber, 1980). The PBDE mixture induced liver toxicity and alterations in liver enzyme levels, and, thus, metabolic activation is a likely mechanism for this toxicity. However, necrosis, secondary to the marked hypertrophy resulting in compression of the sinusoids, cannot be excluded.
Cytochrome P450 liver enzymes (CYP1A1, 1A2, 2B) were increased in rats and mice at doses of 50 mg/kg and above, and in a few cases at 5 mg/kg. PBDE exposures exhibited phenobarbital like effects including induction of cytochrome P450 2B levels, and increased liver weights and toxic liver lesions. However, there were also increases in cytochrome P450 1A1 and 1A2 levels, characteristic of dioxin-like chemicals (Sanders et al., 2005; Waxman and Azaroff, 1992). Because a mixture of polybrominated diphenyl ethers was studied, it is not possible to attribute the observed effects to one component of the mixture. Other chemicals have been shown to increase cytochrome P450 levels, but the increase in these enzyme levels were not always accompanied by hepatocyte hypertrophy as occurred in this PBDE study (Amacher et al., 1998).
PBDE exposures reduced serum T4 levels in rats throughout the course of the study. These decrease in T4 levels were consistent with the observed induction of UDPGT activity, suggesting that increased glucuronidation of T4 may be one factor contributing to a reduction in T4 serum levels (Liu et al., 1995; Zhou et al., 2001). Because of their structural similarity to thyroid hormones, polybrominated diphenyl ethers may act as endocrine disrupters via interference with the thyroid receptors (Meerts et al., 2001).
Oral absorption of BDE-47, BDE-99, or BDE-153 was approximately 70–85% in rats and mice (Chen et al., 2006; Sanders et al., 2006a; Sanders et al., 2006b), and in these oral gavage PBDE studies BDE-47, BDE-99, and BDE-153 accumulated in the liver (not measured in mice) and fat of rats and/or mice. BDE-47, BDE-99, and BDE-153 tissue levels were lower in liver than in fat, probably because of the fat solubility of polybrominated diphenyl ethers. The accumulation of BDE-153 in liver or fat was to a greater degree than expected based on the percent of BDE-153 in the mixture, and may be due to greater fat solubility or less metabolism than for BDE- 47 or BDE-99 (Sanders et al., 2006b).
Oral administration of PBDE mixture (this study) and oral administration of TCDD (National Toxicology Program, 2006) both caused treatment-related increases in cytochrome P450 levels, decreases in T4 levels, and liver lesions in female rats. A TCDD dose of 10 ng/kg body weight is approximately equilvalent to the level of brominated dioxins and furans in a dose of 100 mg PBDE mixture/kg body weight. At the end of 13 weeks of PBDE mixture exposure (100 mg/kg), the magnitude of liver toxicity in female rats was greater than in the 10 ng/kg TCDD female rat study (National Toxicology Program, 2006). After 13–14 weeks of TCDD exposure (10 ng/kg), the female rats had a 16% decrease in T4 serum levels versus a 85% decrease after PBDE mixture exposure (100 mg/kg); TCDD (10 ng/kg) gave a 220% increase in liver Cyp1A2 levels versus an 820 % increase with the PBDE mixture (100 mg/kg); and TCDD liver weight was 1.20 times control liver weight versus 2.19 with the PBDE mixture (100 mg/kg). This suggests that the liver toxicity and thyroid toxicity in PBDE mixture-treated female rats is not solely due to the presence of brominated dioxins and furans, although it should be noted that the TCDD study was conducted in Sprague-Dawley rats and the PBDE mixture study was conduced in F344/N rats.
Because induction of cytochrome enzymes may lead to the formation of oxygen radicals and/or the formation of carcinogens by forming arene oxides, diolepoxoides, and/or other electrophilic reactive species capable of causing DNA damage (Ma and Lu, 2007), studies to evaluate the cancer potential of polybrominated diphenyl ethers are needed, and the NTP is conducting these cancer studies. The high dose in the rat chronic study is 50 mg/kg, because at 100 mg/kg the liver lesions and increases in liver weights in the 13-week studies was considered to be too severe to use this dose in a long-term study. In mice, the mild/moderate hypertrophy at 100 mg/kg was not considered as severe as seen in rats, and thus, 100 mg/kg is high dose being used in the long-term study.
Acknowledgments
We thank Dr. Michael Sanders, NIEHS, and Dr. Bhanu Singh, NIEHS, for their review of this manuscript. This work was supported by the NIEHS Intramural Program. Animals were exposed to PBDEs at an NTP contract laboratory, and determinations of polybrominated diphenyl ether tissue levels were performed at another NTP Laboratory (NIH contracts N01-ES-45516, N01-ES-05456, and N01-ES-55551).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amacher DE, Schomaker SJ, Burkhardt JE. The relationship among microsomal enzyme induction, liver weight, and histologic change in rat toxicology studies. Food Chem Toxicol. 1998;36:831–39. doi: 10.1016/s0278-6915(98)00066-0. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, Cohen Hubal EA. Polybrominated diphenyl ethers: a case study for using biomonitoring data to address risk assessment questions. Environ Health Perspect. 2006;114:1770–5. doi: 10.1289/ehp.9061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman GA, Eustis SL. The pathology working roup as a means for assuring pathology quality in toxicologic studies. In: Whitmire C, Davis CL, Bristol DW, editors. Managing conduct and data quality of toxicologic studies. Princeton: Princeton Scientific Publishing; 1986. pp. 271–75. [Google Scholar]
- Bromine Science and Environmental Forum. An Introduction to Brominated Flame Retardants. 2000.
- Chen LJ, Lebetkin EH, Sanders JM, Burka LT. Metabolism and disposition of 2,2′,4,4′,5-pentabromodiphenyl ether (BDE99) following a single or repeated administration to rats or mice. Xenobiotica. 2006;36:515–34. doi: 10.1080/00498250600674477. [DOI] [PubMed] [Google Scholar]
- Darnerud PO, Aune M, Larsson L, Hallgren S. Plasma PBDE and thyroxine levels in rats exposed to Bromkal or BDE-47. Chemosphere. 2007;67:S386–92. doi: 10.1016/j.chemosphere.2006.05.133. [DOI] [PubMed] [Google Scholar]
- Dunnick JK, Heath JE, Farnell DR, Prejean JD, Haseman JK, Elwell MR. Carcinogenic activity of the flame retardant, 2,2-bis(bromomethyl)-1,3-propanediol in rodents, and comparison with the carcinogenicity of other NTP brominated chemicals. Toxicol Pathol. 1997;25:541–8. doi: 10.1177/019262339702500602. [DOI] [PubMed] [Google Scholar]
- Ellis-Hutchings RG, Cherr GN, Hanna LA, Keen CL. Polybrominated diphenyl ether (PBDE)-induced alterations in vitamin A and thyroid hormone concentrations in the rat during lactation and early postnatal development. Toxicol Appl Pharmacol. 2006;215:135–45. doi: 10.1016/j.taap.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Farber E. Toxicological significance of liver hypertrophy produced by inducers of drug-metaolizing enzyems. Excerpta Medica. 1980:261–74. doi: 10.1002/9780470720592.ch14. [DOI] [PubMed] [Google Scholar]
- Gart JJ, Chu KC, Tarone RE. Statistical issues in interpretation of chronic bioassay tests for carcinogenicity. J Natl Cancer Instiute. 1979;62:957–74. [PubMed] [Google Scholar]
- Greaves P. Histopathology of preclinical toxicity studies - Interpretation and relevance in drug safety evaluation. Amsterdam, The Netherlands: Elsevier; 2000a. Digestive System 1; pp. 312–431. [Google Scholar]
- Greaves P. Histopathology of preclinical toxicity studies - Interpretation and relevance in drug safety evaluation. Amsterdam, The Netherlands: Elsevier; 2000b. Endocrine System 1; pp. 736–822. [Google Scholar]
- Hale RC, Alaee M, Manchester-Neesvig JB, Stapleton HM, Ikonomou MG. Polybrominated diphenyl ether flame retardants in the North American environment. Environment International. 2003;29:771–79. doi: 10.1016/S0160-4120(03)00113-2. [DOI] [PubMed] [Google Scholar]
- Hale RC, La Guardia MJ, Harvey E, Mainor TM. Potential role of fire retardant-treated polyurethane foam as a source of brominated diphenyl etheres to the U. S environment. Chemosphere. 2002;46:729–35. doi: 10.1016/s0045-6535(01)00237-5. [DOI] [PubMed] [Google Scholar]
- Hale RC, La Guardia MJ, Harvey EP, Gaylor MO, Mainor TM, Duff WH. Persistant pollutants in land-applied sludges. Nature. 2001a;412:140–141. doi: 10.1038/35084130. [DOI] [PubMed] [Google Scholar]
- Hale RC, La Guardia MJ, Harvey EP, Mainor TM, duff WH, Gaylor MO. Polybrominated diphenyl ether flame retardants in Virginia freshwater fishes. Environmental Science and Technology. 2001b;35:4585–91. doi: 10.1021/es010845q. [DOI] [PubMed] [Google Scholar]
- Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Kester MH, Andersson PL, Legler J, Brouwer A. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol Sci. 2006;92:157–73. doi: 10.1093/toxsci/kfj187. [DOI] [PubMed] [Google Scholar]
- Hamm JT, Ross DG, Richardson VM, Diliberto JJ, Birnbaum LS. Methoxyresorufin: an in appropriate substrate for CYP1A2 in the mouse. Biochem Pharmacol. 1998;56:1657–60. doi: 10.1016/s0006-2952(98)00241-x. [DOI] [PubMed] [Google Scholar]
- Hardisty JG, Boorman GA. National Toxicology Program pathology quality assurrance procedures. In: Whitmire C, Davis CL, Bristol DW, editors. Managing conduct and data quality of toxicologic studies. Princeton: Princeton Publishing Company; 1986. pp. 263–69. [Google Scholar]
- Legler J, Brouwer A. Are brominated flame retardants endocrine disruptors? Environ Int. 2003;29:879–85. doi: 10.1016/S0160-4120(03)00104-1. [DOI] [PubMed] [Google Scholar]
- Liu J, Liu Y, Barter RA, Klaasen CD. Alteration of thyroid homeostatis by UDP-glucuronosyltransferase inducers in rats: a dose-response study. J Pharmacol Exp Ther. 1995;273:977–85. [PubMed] [Google Scholar]
- Lorber M. Exposure of Americans to polybrominated diphenyl ethers. J Exp Sci Environ Epidemiol. 2007:1–18. doi: 10.1038/sj.jes.7500572. epubl. [DOI] [PubMed] [Google Scholar]
- Lubet RA, Mayer RT, Cameron JW, Nims RW, Burke MD, Wolff T, Guengerich FP. Dealkylation of pentoxyresorufin: a rapid and sensitve assay for measuring induction of cytochrome(s) by phenobarbital and other xenobiotics in the rat. Arch Biochem Biophys. 1985;238:43–48. doi: 10.1016/0003-9861(85)90138-9. [DOI] [PubMed] [Google Scholar]
- Ma Q, Lu AYH. CYP1A induction and human risk assessment: an evolving take of in vitro and in vivo studies. Drug Metab Dispos. 2007;35:1009–16. doi: 10.1124/dmd.107.015826. [DOI] [PubMed] [Google Scholar]
- McDonald TA. Polybrominated diphenylether levels among United States residents: daily intake and risk of harm to the developing brain and reproductive organs. Integr Environ Assess Manag. 2005;1:343–54. [PubMed] [Google Scholar]
- Meerts IATM, Letcher RJ, Hoving S, Marsh G, Bergman A, Lemmen JG, van der Burg B, Brouwer A. In vitro estrogenicity of polybrominated diphenyl etheres, hydroxylated PBDEs, and polybrominated bisphenol A compounds. Environ Health Perspect. 2001;109:399–407. doi: 10.1289/ehp.01109399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick RL, Nyska A, Foster PM, Roycroft JH, Kissling GE. Toxicity and carcinogenicity of the water disinfection byproduct, dibromoacetic acid, in rats and mice. Toxicology. 2007;230:126–36. doi: 10.1016/j.tox.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Toxicology Program. Bulk chemical comprehensive analysis report - technical pentabromodiphenyl oxide. Battelle Report 7–173-BCCA-24, December 31, 2003 2004.
- National Toxicology Program. Three-month toxicity study of a pentabromodiphenyl oxide mixture (DE-71) administered by gavage to B6C3F1 mice. Southern Research Institute Report 11052.01.02, October 25, 2005 2005a.
- National Toxicology Program. Three-month toxicity study of a pentabromodiphenyl oxide mixture (DE-71) administered by gavage to Fisher-344 rats. Southern Research Institute Report 11052.01.01, December 2, 2005 2005b.
- National Toxicology Program. Toxicology and carcinogenesis studies of 2, 3, 7, 8-tertrachloridedibenxo-p-dioxin (TCDD) in female Harlan Sprague-Dawley Rats. NTP TR 521 2006; NIH Publication No. 06–4469.
- National Toxicology Program. Analysis of fat samples from a subchroinic toxicity study of technical pentabromodiphenyl oxide. Battelle Report 10–173-BSA-109, March 2, 2007 2007a.
- National Toxicology Program. Analysis of liver samples from a subchronic toxicity study of technical pentabromodiphenyl oxide. Battelle Report 10–173-BSA-100, February 20, 2007 2007b.
- Petreas M, She J, Brown FR, Winkler J, Windham G, Rogers E, Zhao G, Bhatia R, Charles MJ. High body burdens of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) in California women. Environ Health Perspect. 2003;111:1175–79. doi: 10.1289/ehp.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riess M, Ernst T, Popp R, Muller B, Thoma H, Vierle O, Wolf M, van Eldik R. Analysis of flame retardant polymers and recycling materials. Chemosphere. 2000;40:937–41. doi: 10.1016/s0045-6535(99)00336-7. [DOI] [PubMed] [Google Scholar]
- Sanders JM, Burka LT, Smith CS, Black W, James R, Cunningham ML. Differential expression of CYP1A, 2B, and 3A genes in the F344 rat following exposure to a polybrominated diphenyl ether mixture or individual components. Toxicol Sci. 2005;88:127–33. doi: 10.1093/toxsci/kfi288. [DOI] [PubMed] [Google Scholar]
- Sanders JM, Chen LJ, Lebetkin EH, Burka LT. Metabolism and disposition of 2,2′,4,4′-tetrabromodiphenyl ether following administration of single or multiple doses to rats and mice. Xenobiotica. 2006a;36:103–17. doi: 10.1080/00498250500485107. [DOI] [PubMed] [Google Scholar]
- Sanders JM, Lebetkin EH, Chen LJ, Burka LT. Disposition of 2,2′,4,4′,5,5′-hexabromodiphenyl ether (BDE153) and its interaction with other polybrominated diphenyl ethers (PBDEs) in rodents. Xenobiotica. 2006b;36:824–37. doi: 10.1080/00498250600815906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Pavuk M, Papke O, Ryan JJ, Birnbaum L, Rosen R. Polybrominated diphenyl etheres (PBDEs) in U. S. Mothers’ milk. Environ Health Perspect. 2003;111:1723–29. doi: 10.1289/ehp.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodin A, Jones RS, Focant JF, Lapeza C, Wang RY, McGahee EEI, Zhang Y, Turner WE, Slazyk B, Needham LL, Patterson DG., Jr Retrospective time trend study of polybrominate diphenyl ether and polybrominated and polychlorineated biphenyl levels in human serum form the United States. Environ Health Perspect. 2004;112:654–58. doi: 10.1289/ehp.112-1241957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slauson DO, Cooper BJ. Mechanisms of Disease - a textbook of comparative general pathology. Mosby; 2001. [Google Scholar]
- Sorderstrom G, Sellstrom U, De Wit cA, Tysklind M. Photolytic debromination of decabromodiphenyl ether (BDE 209) Envir Sci Technol. 2004;38:127–32. doi: 10.1021/es034682c. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Alaee M, Letcher RJ, Baker JE. Debromination of the flame retardant decabromodiphenyl ether by Juvenile Carp (Cyprinus carpio) following dietary exposure. Envir. Sci Technol. 2004;38:112–19. doi: 10.1021/es034746j. [DOI] [PubMed] [Google Scholar]
- van Pee KH, Unversucht S. Biological dehalogenation and halogenation reactions. Chemosphere. 2003 doi: 10.1016/S0045-6535(03)00204-2. [DOI] [PubMed] [Google Scholar]
- Waxman DJ, Azaroff L. Phenobarbital induction of cytochrome P-450 gene expression. Biochem J. 1992;281:577–92. doi: 10.1042/bj2810577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsnes A. Studies on the activation of in vitro of gucuronyltransferase. Biochem Biophys Acta. 1969;191:279–91. doi: 10.1016/0005-2744(69)90247-2. [DOI] [PubMed] [Google Scholar]
- Zhou T, Ross DG, DeVito MJ, Crofton KM. Effects of short-term in vivo exposure to polybrominated dipheyl etheres on thyroid hormones and hepatic enzyme activities in weanling rats. Toxicol Sci. 2001;61:76–82. doi: 10.1093/toxsci/61.1.76. [DOI] [PubMed] [Google Scholar]
- Zhou T, Taylor MM, DeVito MJ, Crofton KM. Developmental exposure to brominated diphenyl etheres results in thyroid hormone disruption. Toxicolog Sci. 2002;66:105–16. doi: 10.1093/toxsci/66.1.105. [DOI] [PubMed] [Google Scholar]