Abstract
A plethora of in vitro and in vivo studies have supported the neuroprotective role of estrogens and their impact on the neurotransmitter systems implicated in cognition. Recent hormonal replacement therapy trials in non-demented post-menopausal women suggest a temporary positive effect (notably on verbal memory), and four meta-analyses converge to suggest a possible protective effect in relation to Alzheimer’s disease (reducing risk by 29 to 44%). However, data from the only large randomized controlled trial published to date, the Women’s Health Initiative Memory Study, did not confirm these observations and have even suggested an increase in dementia risk for women using hormonal replacement therapy compared to controls. Apart from methodological differences, one key short-coming of this trial has probably been the focus on late-onset (postmenopausal) hormonal changes, i.e. at a time when the neurodegenerative process has already begun and without taking into account individual lifetime exposure to hormone variability. Multifactorial models based on an exhaustive view of all hormonal events throughout the reproductive life (rather than on a specific exposure to a given steroid) together with other risk factors (notably genetic risk factors related to estrogen receptor polymorphisms) should be explored to clarify the role of hormonal risk factors, or protective factors for cognitive dysfunction and dementia.
Keywords: Administration, Cutaneous; Aged; Alzheimer Disease; diagnosis; drug therapy; prevention & control; Cognition Disorders; diagnosis; drug therapy; prevention & control; Estradiol; administration & dosage; therapeutic use; Estrogen Replacement Therapy; methods; Estrogens; administration & dosage; therapeutic use; Estrogens, Conjugated (USP); therapeutic use; Female; Humans; Memory Disorders; diagnosis; drug therapy; prevention & control; Middle Aged; Neuroprotective Agents; administration & dosage; therapeutic use; Progestins; therapeutic use; Randomized Controlled Trials as Topic; statistics & numerical data; Receptors, Estrogen; genetics; Research Design; standards; trends; Severity of Illness Index; Treatment Outcome
Keywords: Cognition, equine estrogens, transdermal estradiol, estrogen receptor, lifetime hormonal status, observation study, randomized controlled trial
Introduction
Over the past half-century, there has been a growing belief based on experimental and observation studies, that replacing the estrogen lost at menopause would prevent many of the manifestations of aging, including osteoporosis, cardiovascular disease, and a decline in sexual and cognitive function. This attractive view initially led to the widespread use of Hormone Replacement Therapy (HT) after the menopause, but following the recent publication of the results of the Women’s Health Initiative (WHI) study, this enthusiasm has diminished leading to new clinical recommendations. HT had potential benefits with regard to menopausal symptoms, for the prevention of osteoporotic fractures and colorectal cancer and probably also on peri-menopausal depression. On the other hand, it had potential risks for breast cancer, pulmonary embolism, stroke and possibly coronary heart disease (Anderson et al., 2004, Writing Group for the Women’s Health Initiative Investigators, 2002). In fact, these harmful effects depended on the age and health status of the women as well as on the hormonal formulation used. Estrogen Replacement Therapy (ERT) without progestin carried a risk for stroke only, and natural steroids such as transdermal estradiol or progesterone, have even lower risk.
The results concerning the potential value of HT in reducing aging-related cognitive changes and delaying the onset of dementia should be considered carefully, especially when the findings of the clinical trials are compared with those of mechanistic or observation studies. Methodological differences between studies, with regards to the type, dosage, timing and duration of HT, as well as the population group, the cognitive measures assessed and the analysis undertaken, could explain in large part the discrepant findings across studies.
In this review we argue that one of the most serious short-comings of previous studies has been their focus on hormonal changes after the menopause, without taking into account individual life-time exposure to hormonal variability. Some of the inconsistencies found between studies may be because women do not arrive at the menopause with equal risk of cognitive decline or equal susceptibility to the effects of HT. We thus present a summary of the current knowledge concerning the neuroprotective effect of exogenous (e.g. HT) or endogenous exposure to estrogens, and finally suggest an integrative model of risk based on these findings to guide future research.
Gender differences and biological plausibility for hormone neuroprotective effects
A significantly higher incidence of Alzheimer’s disease (AD) observed in post-menopausal women in a number of studies (Gao et al., 1998), as well as the observed neuroprotective effects of estrogen in vitro and in vivo, has led to an increasing interest in the role of gonadal hormones, and in particular estrogen (markedly decreased levels after the menopause) in cognitive functioning or dementia. In fact, a lot of experimental data supports a positive role of estrogen, notably on neuronal functioning and neurotransmitter systems (mainly due to an increase in acetylcholine and brain catecholamines) or due to interactions with neurotrophic factors (see for review (Behl, 2002)). In particular, this could have a global effect on cognitive functions such as attentional and mnesic functions. Estrogens can also decrease the accumulation of neurotoxic glutamate or β amyloid peptide, and possess antioxidant activity; they can inhibit neuronal apoptosis and also modulate Apolipoprotein E gene expression. All of these properties could play a role in the neuroprotective effect of estrogen against cognitive dysfunction, mild cognitive impairment (MCI) or AD.
Effect of HT on cognitive functioning in non-demented menopausal women
Various epidemiological studies have investigated the neuroprotective effects of HT on cognitive function and on the risk and course of dementia and MCI. A meta-analysis of 17 randomized controlled trials (RCTs) which examined cognitive function in non-demented women (Hogervorst et al., 2002a) indicated a positive effect of HT on short-term verbal memory and some domains of executive function (mainly reasoning or psychomotor speed) with no consistent effects on other verbal tasks or on visuo-spatial performance. In fact, positive effects of HT were essentially observed in relatively young women and when the natural estrogen, estradiol (associated or not with progestin), was used, but very rarely with conjugated equine estrogens (CEE) (Fig. 1). Recent results of the WHI Memory Study (WHIMS) indicate a negative impact of CEE + medroxyprogesterone acetate (MPA) on verbal memory and a trend towards a positive impact on figural memory over time compared with placebo, while other cognitive domains were not affected (Resnick et al., 2006). Although the clinical relevance of these data are still under examination, they are in agreement with those of observational studies which also suggest some benefits on verbal memory, attention, some areas of executive function, and mental status. Presently, none of the analyses have determined an optimal duration, formulation or dose for a neuroprotective effect.
Fig. 1. Effects of HT on specific cognitive function in non-demented menopausal women as a function of age and type of HT.
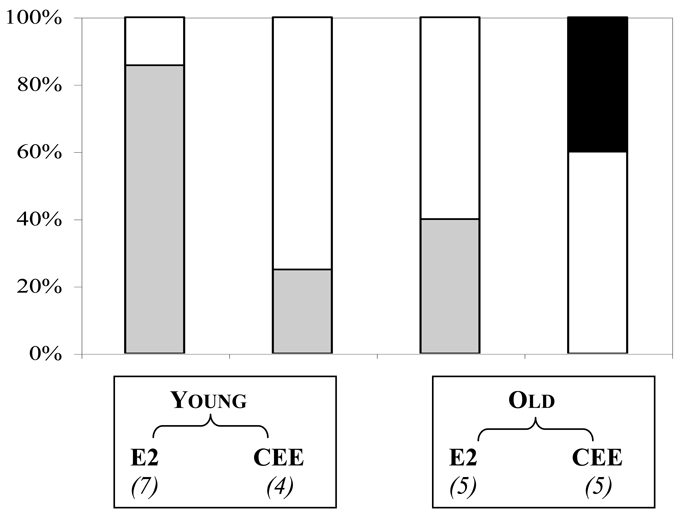
Twenty one RCTs were performed over the last 20 years using estradiol (E2) or CEE opposed or not with progestins and administered either to “young” menopausal women (i.e. younger man 65) or to “old” women of 65 years and more. The number of RCTs under each condition is indicated between brackets. Black, grey, and white bars correspond respectively to the % of RCTs where HT was found to have a negative, a positive, or no effect, on at least one cognitive task. The studies on global cognitive functioning (MMSE,…) or those with ultra-low-dose estradiol were not considered.
Preventive and curative effect on Alzheimer’s disease or MCI
Sixteen observation studies have been published in 20 years to evaluate the effect of HT on the risk of dementia. Seven of the case-control studies, essentially the earliest ones, found no significant effect from HT use. On the other hand, while none of the studies reported a significant increased risk of dementia, six case-control studies and all three cohort studies indicated a significant reduction in the risk of developing AD. The four meta-analyses performed from the above studies concluded that there was a significant reduction from between 29 to 44% in the risk of developing dementia in the group of ever users compared to never users (Hogervorst et al., 2000, Leblanc et al., 2001, Maki and Hogervorst, 2003, Yaffe et al., 1998). Risk reduction was 51% when considering the more rigorously controlled studies performed after 1994 (Hogervorst et al., 2000). The neuroprotective effect from estradiol treatment was once again more substantial than that with CEE, and addition of MPA as the progestagen was actually detrimental. The optimal duration was variable and depended on the age at which HT began. Zandi et al. reported that past use was associated with reduced risk of AD (the strength of the association increasing with the duration of the past treatment), but without any apparent benefit for current use unless it exceeded 10 years (Zandi et al., 2002). This suggests that there is an optimal period for hormonal neuroprotection close to the peri-menopause.
There is only one RCT, the WHIMS which evaluated the preventive effect of HT on AD and MCI in post-menopausal women aged 65 years and more (Espeland et al., 2004, Rapp et al., 2003, Shumaker et al., 2004, Shumaker et al., 2003). Its design involved a daily oral administration of CEE + MPA (4532 women) or CEE alone (2947 hysterectomized women), initiated more than 12 years after menopause. The only significant result was a two-fold increase of the risk of probable dementia (of all types) in the CEE + MPA arm (but not in the CEE arm), which was significant only for vascular dementia but not for AD. The risk of MCI or of global cognitive dysfunction was also not significantly different between placebo and HT users. These results are not consistent with the findings of observation studies, which could be due to several factors, including target populations and study design. In the WHIMS, women were older than 65 and thus far from the perimenopause period and the usual clinical situation. In addition they were less healthy and with vascular risk at randomization. The type of treatment formulation as well as the high doses and the short treatment duration are also causes of concern. The neuroprotective properties of oral CEE and MPA have never been recognized and side effects, especially vascular ones or those related to breast cancer, could be extensive compared to natural steroids. Hence, the WHIMS was clearly not adapted to address the specific question of a potential neuroprotective effect of HT.
Finally, observation studies as well as RCTs suggest a very limited effect of HT when administered to postmenopausal women with AD, mainly on attention and verbal performance, but with very low clinical relevance (Hogervorst et al., 2000, Hogervorst et al., 2002b). As an adjuvant of anticholinesterasic treatment, HT was only efficient when administered before the onset of dementia (Rigaud et al., 2003, Schneider et al., 1996).
Study limitations and variability as the principal causes of inconsistencies in results
Most therapeutic trials conducted to date have been unable to conclude that HT could significantly prevent the development of AD or decrease its severity. Epidemiological studies, especially the earlier ones, have numerous limitations (see for review (Ancelin and Ritchie, 2005)). Variability in the neuropsychological tests used and their limited range, as well as the lack of standardized diagnostic criteria for dementia, are among the principal shortcomings, which make it difficult to compare studies. Studies vary widely in the age of the populations examined and in the level of control for potential confounding factors. More specifically, depressive symptomatology has rarely been evaluated and few studies have looked at ApoE-ε4 status, although it is an important genetic risk factor for AD, and an interaction has also been reported with ERT status. In prospective cohort studies on HT, there is also specific potential bias, e.g. healthy user bias, or bias by indication. Clinical therapeutic trials with no control groups raise the problem of learning effects after repetitive cognitive evaluation. RCT are the most robust studies, although they generally use smaller sample sizes and look at short-term treatment. The validity of double-blinding has also been put into question: women frequently guess the type of treatment they receive, due to bleeding or menopause symptom resolution.
Other design problems are related to the definition of menopausal status (including premenopausal women or not), the type of menopause (natural or surgical), and HT timing. The mean duration is usually less than 6 months for RCT (the longest trial being the WHIMS which lasted 5.2 years) but 13 years for observation studies. The time between the exposure window and the menopause age is highly variable (observation studies being closer to the real clinical situation than RCT, where HT is not administered during the early menopause transition). There are also numerous differences with regards to the type of HT. The more recent large RCT used only oral CEE with continuous MPA, whereas natural steroid hormones are probably more beneficial regarding neuroprotective or side-effects, due to their distinct pharmacokinetic properties or in their sequential administration. Lastly, there is also variability in the preparations used (oral vs. parenteral), which can result in different pharmacokinetic patterns (parenteral mode avoids first-pass liver effects). All of these points have to be taken into account before drawing any definitive conclusion regarding the effects of HT on cognitive functioning and dementia.
Observational study for the detection of HT mediated neuroprotective Effects
Cognitive amelioration (essentially on verbal performance, attention and some areas of executive function) in non-demented women and prevention of MCI and dementia may be observed essentially when women were treated at the menopause transition which is the usual clinical situation. RCTs performed in healthy or cognitively impaired women far from the menopause showed no significant clinical improvement. In fact, one key shortcoming of the previous studies has probably been the focus on late-onset (postmenopausal) hormonal changes, i.e. at a time when the neurodegenerative process has already begun and without taking into account individual lifetime exposure to hormonal variability (Zandi et al., 2002). The impact of high endogenous exposure to estrogen has not been thoroughly investigated, although several recent studies indicated an association with decreased risk for cognitive impairment or AD (see (Ancelin and Ritchie, 2005), for review). A recent twin study (Rasgon et al., 2005) and RCT (Dunkin et al., 2005) also confirm this association with cognitive functioning. However, these studies have only focused on a small number of reproductive life events (generally age at menopause or/and age at menarche) without considering other endogenous factors (such as menstrual cycle history, pregnancies etc.) and exogenous factors others than HT. Although the utility of global lifetime markers of estrogen cumulative exposure has been established for other hormone dependent disorders (e.g. breast cancer and osteoporosis), little work has been done to determine whether they may contribute to our understanding of the effects of estrogens on brain functioning.
Multifactorial approach integrating genetic vulnerability
Analyses providing both detailed reproductive histories and long-term effects of natural steroids, along with adequate longitudinal data on cognitive decline and AD are lacking. Multifactorial models based on all lifetime hormonal events should be explored thoroughly to isolate the key hormonal determinants of cognitive disorders in the elderly. This could also lead to the identification of vulnerable sub-groups of hormone-sensitive women who may be at high risk of developing cognitive symptoms (on the basis of their lifetime endocrine pattern). Presently, the reasons why some women seem more vulnerable to cognitive dysfunction remains poorly understood and a multifactorial approach incorporating other predisposing factors (environmental, vascular, or genetic, factors) in addition to the hormonal ones is required. More specifically, genetic variability in estrogen receptors (ERs) could contribute to the individual susceptibility of some women to the beneficial (or harmful) effects of estrogens; a possibility which has been explored in only a few case control studies. Most of these studies have indicated a 2-fold increase in the risk of AD associated with 2 distinct single-nucleotide polymorphisms of ERα (Brandi et al., 1999, Isoe-Wada et al., 1999, Ji et al., 2000, Kravitz et al., 2006, Urakami et al., 2001), although this was not found systematically (den Heijer et al., 2004, Usui et al., 2006). An increased risk in cognitive dysfunction has also been reported in one case-control and one prospective study (Olsen et al., 2006, Yaffe et al., 2002) and polymorphisms of ERβ have been associated with the risk of AD too (Forsell et al., 2001, Lambert et al., 2001, Pirskanen et al., 2005). At this time no study has investigated the role of ER polymorphisms and genetic vulnerability in HT response.
Future Directions and perspectives: HT, cognitive functioning, AD, and MCI
Although there is insufficient evidence to recommend HT for the prevention of cognitive impairment, it remains the first-line treatment for the 75% of women experiencing menopausal symptoms at the peri-menopause. In this context, it is worthwhile evaluating the impact on cognitive functioning under normal clinical conditions, especially for women at risk of cognitive decline. Future directions could thus involve:
-
characterizing the critical period for neuroprotection both by observation studies and RCTs:
observation studies to focus on timing of initiation of HT at the peri-menopause and cognitive function later in life. In addition to the notion of a therapeutic window, this raises the question of the impact of lifelong hormonal status and estrogen exposure, which could imply that women do not arrive at menopause with equal risk of cognitive decline or equal susceptibility to the effects of HT.
long RCTs with “natural” HT preparations for women with MCI or (at risk of) early-onset AD, using estradiol (especially transdermal estradiol) rather than oral CEE, and (micronized) progesterone rather than synthetic progestin, such as MPA.
evaluating the role of genetic variation, especially ER polymorphisms which could be involved in distinct vulnerability to AD (or MCI) or distinct susceptibility to HT
developing a multifactorial model which incorporates lifetime hormonal factors and other predisposing factors for neuropsychiatric disorders, such as genetic, vascular, or environmental factors
Some ongoing observation studies, for example the 1946 Birth Cohort in the U.K. (Kok et al., 2006), the ESPRIT study in France (Ancelin and Ritchie, 2005, Ritchie et al., 2004), or the REMEMBER study in Australia (MacLennan et al., 2006) as well as some RCTs, such as the Estrogen Memory Study in Canada (performed by Mary Tierney et al., personal communication, see http://www.sunnybrook.ca), or the KEEPS in the U.S. (Harman et al., 2005) will be able to address these points, notably the role of endogenous or exogenous hormone variations, such as lifetime hormonal status, ER polymorphisms, or the timing of HT initiation (“therapeutic window”) in cognitive (dys)function.
The identification of the hormonal determinants of cognitive and neurodegenerative disorders in the elderly and the characterization of sub-groups of “hormone-sensitive” subjects potentially at risk would open up the possibility of offering tailored therapies based on hormones favoring natural steroids. Obviously, recognized contraindications and health risks of HT (e.g. hormone-dependent cancer, (cardio)vascular disease (Rymer et al., 2003)) have to be taken into account (Ettinger et al., 2006). Natural formulations (micronized progesterone, transdermal 17-β-estradiol) are preferable given their probable lower side effects, especially when given at the peri-menopause (see (Farquhar et al., 2005, Skouby et al., 2005) for recent reviews) and their potentially greater impact on neuropsychiatric disorders. The public health consequences of a preventive hormonal-program could be considerable bearing in mind the projected increases in AD prevalence (14 million people worldwide may be suffering with AD by the middle of the 21st century (Marx, 1996), a number which could be underestimated considering the recent projection in dementia cases (Wimo et al., 2003)) and also the increase in menopausal women (who presently constitute around 15% of the western population) due to the aging population.
Acknowledgments
The ESPRIT Project has been financed by the Regional Government of Languedoc-Roussillon and an unconditional grant from Novartis. Joanne Ryan is the recipient of a France Alzheimer grant.
Footnotes
Conflict of interest: none
Contributors: All authors contributed to the writing of the manuscript and have approved the final version.
References
- Ancelin ML, Ritchie K. Lifelong endocrine fluctuations and related cognitive disorders. Current Pharmaceutical Design. 2005;11:4229–4252. doi: 10.2174/138161205774913228. [DOI] [PubMed] [Google Scholar]
- Anderson GL, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. The Journal of the American Medical Association. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- Behl C. Oestrogen as a neuroprotective hormone. Nature Reviews Neuroscience. 2002;3:433–442. doi: 10.1038/nrn846. [DOI] [PubMed] [Google Scholar]
- Brandi ML, et al. Association of the estrogen receptor alpha gene polymorphisms with sporadic Alzheimer’s disease. Biochemical Biophysical Research Communications. 1999;265:335–338. doi: 10.1006/bbrc.1999.1665. [DOI] [PubMed] [Google Scholar]
- den Heijer T, et al. Variations in estrogen receptor alpha gene and risk of dementia, and brain volumes on MRI. Molecular Psychiatry. 2004;9:1129–1135. doi: 10.1038/sj.mp.4001553. [DOI] [PubMed] [Google Scholar]
- Dunkin J, et al. Reproductive events modify the effects of estrogen replacement therapy on cognition in healthy postmenopausal women. Psychoneuroendocrinology. 2005;30:284–296. doi: 10.1016/j.psyneuen.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Espeland MA, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. The Journal of the American Medical Association. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- Ettinger B, Barrett-Connor E, Hoq LA, Vader JP, Dubois RW. When is it appropriate to prescribe postmenopausal hormone therapy? Menopause. 2006;13:404–410. doi: 10.1097/01.gme.0000188735.61994.5b. [DOI] [PubMed] [Google Scholar]
- Farquhar CM, Marjoribanks J, Lethaby A, Lamberts Q, Suckling JA. Long term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database of Systematic Reviews. 2005:CD004143. doi: 10.1002/14651858.CD004143.pub2. [DOI] [PubMed] [Google Scholar]
- Forsell C, et al. Investigations of a CA repeat in the oestrogen receptor beta gene in patients with Alzheimer’s disease. European Journal of Human Genetics. 2001;9:802–804. doi: 10.1038/sj.ejhg.5200714. [DOI] [PubMed] [Google Scholar]
- Gao S, Hendrie HC, Hall KS, Hui S. The relationships between age, sex, and the incidence of dementia and Alzheimer disease: a meta-analysis. Archives of General Psychiatry. 1998;55:809–815. doi: 10.1001/archpsyc.55.9.809. [DOI] [PubMed] [Google Scholar]
- Harman SM, et al. KEEPS: The Kronos Early Estrogen Prevention Study. Climacteric. 2005;8:3–12. doi: 10.1080/13697130500042417. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Williams J, Budge M, Riedel W, Jolles J. The nature of the effect of female gonadal hormone replacement therapy on cognitive function in post-menopausal women: a meta-analysis. Neuroscience. 2000;101:485–512. doi: 10.1016/s0306-4522(00)00410-3. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Yaffe K, Richards M, Huppert F. Hormone replacement therapy for cognitive function in postmenopausal women. Cochrane Database of Systematic Reviews. 2002a:CD0031. doi: 10.1002/14651858.CD003122. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Yaffe K, Richards M, Huppert F. Hormone replacement therapy to maintain cognitive function in women with dementia (Cochrane Review) Cochrane Database of Systematic Reviews. 2002b:CD0037. doi: 10.1002/14651858.CD003799. [DOI] [PubMed] [Google Scholar]
- Isoe-Wada K, et al. Positive association between an estrogen receptor gene polymorphism and Parkinson’s disease with dementia. European Journal of Neurology. 1999;6:431–435. doi: 10.1046/j.1468-1331.1999.640431.x. [DOI] [PubMed] [Google Scholar]
- Ji Y, Urakami K, Wada-Isoe K, Adachi Y, Nakashima K. Estrogen receptor gene polymorphisms in patients with Alzheimer’s disease, vascular dementia and alcohol-associated dementia. Dementia and Geriatric Cognitive Disorders. 2000;11:119–122. doi: 10.1159/000017224. [DOI] [PubMed] [Google Scholar]
- Kok HS, et al. Cognitive function across the life course and the menopausal transition in a British birth cohort. Menopause. 2006;13:19–27. doi: 10.1097/01.gme.0000196592.36711.a0. [DOI] [PubMed] [Google Scholar]
- Kravitz HM, Meyer PM, Seeman TE, Greendale GA, Sowers MR. Cognitive functioning and sex steroid hormone gene polymorphisms in women at midlife. The American Journal of Medicine. 2006;119:S94–S102. doi: 10.1016/j.amjmed.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Lambert JC, et al. Are the estrogen receptors involved in Alzheimer’s disease? Neuroscience Letters. 2001;306:193–197. doi: 10.1016/s0304-3940(01)01806-7. [DOI] [PubMed] [Google Scholar]
- Leblanc ES, Janowsky J, Chan BK, Nelson HD. Hormone replacement therapy and cognition: systematic review and meta-analysis. The Journal of the American Medical Association. 2001;285:1489–1499. doi: 10.1001/jama.285.11.1489. [DOI] [PubMed] [Google Scholar]
- MacLennan AH, et al. Hormone therapy, timing of initiation, and cognition in women aged older than 60 years: the REMEMBER pilot study. Menopause. 2006;13:28–36. doi: 10.1097/01.gme.0000191204.38664.61. [DOI] [PubMed] [Google Scholar]
- Maki P, Hogervorst E. The menopause and HRT. HRT and cognitive decline. Best Practice & Research Clinical Endocrinology & Metabolism. 2003;17:105–122. doi: 10.1016/s1521-690x(02)00082-9. [DOI] [PubMed] [Google Scholar]
- Marx J. Searching for drugs that combat Alzheimer’s. Science. 1996;273:50–53. [PubMed] [Google Scholar]
- Olsen L, et al. Estrogen receptor alpha and risk for cognitive impairment in postmenopausal women. Psychiatric Genetics. 2006;16:85–88. doi: 10.1097/01.ypg.0000194445.27555.71. [DOI] [PubMed] [Google Scholar]
- Pirskanen M, et al. Estrogen receptor beta gene variants are associated with increased risk of Alzheimer’s disease in women. European Journal of Human Genetics. 2005;13:1000–1006. doi: 10.1038/sj.ejhg.5201447. [DOI] [PubMed] [Google Scholar]
- Rapp SR, et al. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. The Journal of the American Medical Association. 2003;289:2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- Rasgon NL, et al. Endogenous and exogenous hormone exposure and risk of cognitive impairment in Swedish twins: a preliminary study. Psychoneuroendocrinology. 2005;30:558–567. doi: 10.1016/j.psyneuen.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Resnick SM, et al. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. Journal of Clinical Endocrinology and Metabolism. 2006;91:1802–1810. doi: 10.1210/jc.2005-2097. [DOI] [PubMed] [Google Scholar]
- Rigaud AS, Andre G, Vellas B, Touchon J, Pere JJ. No additional benefit of HRT on response to rivastigmine in menopausal women with AD. Neurology. 2003;60:148–149. doi: 10.1212/wnl.60.1.148-a. [DOI] [PubMed] [Google Scholar]
- Ritchie K, et al. Prevalence of DSM-IV psychiatric disorder in the French elderly population. British Journal of Psychiatry. 2004;184:147–152. doi: 10.1192/bjp.184.2.147. [DOI] [PubMed] [Google Scholar]
- Rymer J, Wilson R, Ballard K. Making decisions about hormone replacement therapy. British Medical Journal. 2003;326:322–326. doi: 10.1136/bmj.326.7384.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider LS, Farlow MR, Henderson VW, Pogoda JM. Effects of estrogen replacement therapy on response to tacrine in patients with Alzheimer’s disease. Neurology. 1996;46:1580–1584. doi: 10.1212/wnl.46.6.1580. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. The Journal of the American Medical Association. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. The Journal of the American Medical Association. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Skouby SO, et al. Climacteric medicine: European Menopause and Andropause Society (EMAS) 2004/2005 position statements on peri- and postmenopausal hormone replacement therapy. Maturitas. 2005;51:8–14. doi: 10.1016/j.maturitas.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Urakami K, et al. [Causative genes in Alzheimer’s disease] Nippon Ronen Igakkai Zasshi. 2001;38:117–120. doi: 10.3143/geriatrics.38.117. [DOI] [PubMed] [Google Scholar]
- Usui C, et al. No Genetic Association between the Myeloperoxidase Gene -463 Polymorphism and Estrogen Receptor-alpha Gene Polymorphisms and Japanese Sporadic Alzheimer’s Disease. Dementia and Geriatric Cognitive Disorders. 2006;21:296–299. doi: 10.1159/000091437. [DOI] [PubMed] [Google Scholar]
- Wimo A, Winblad B, Aguero-Torres H, von Strauss E. The magnitude of dementia occurrence in the world. Alzheimer Diseases and Associated Disorders. 2003;17:63–67. doi: 10.1097/00002093-200304000-00002. [DOI] [PubMed] [Google Scholar]
- Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. The Journal of the American Medical Association. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lui LY, Grady D, Stone K, Morin P. Estrogen receptor 1 polymorphisms and risk of cognitive impairment in older women. Biological Psychiatry. 2002;51:677–682. doi: 10.1016/s0006-3223(01)01289-6. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Sawaya G, Lieberburg I, Grady D. Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. The Journal of the American Medical Association. 1998;279:688–695. doi: 10.1001/jama.279.9.688. [DOI] [PubMed] [Google Scholar]
- Zandi PP, et al. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. The Journal of the American Medical Association. 2002;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]


