Abstract
Aging results in insidious decremental changes in hypothalamic, pituitary and gonadal function. The foregoing three main anatomic loci of control are regulated by intermittent time-delayed signal exchange, principally via gonadotropin-releasing hormone (GnRH), luteinizing hormone (LH) and testosterone/estradiol (Te/E2). A mathematical framework is required to embody these dynamics. The present review highlights integrative adaptations in the aging male hypothalamic-pituitary-gonadal axis, as assessed by recent objective ensemble models of the axis as a whole.
Keywords: pulses, gonadotropin, mechanism, deconvolution, approximate entropy
1. Introduction
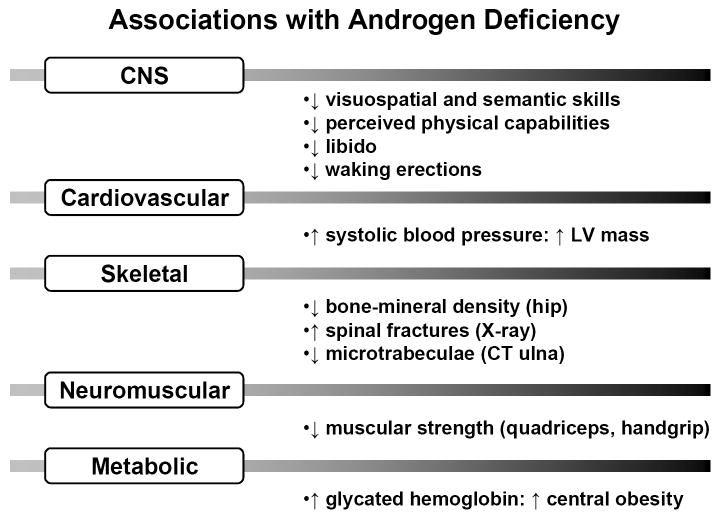
Although recognized biochemically nearly 60 years ago, the fundamental bases of androgen depletion in the aging male remain unknown (Veldhuis et al., 2004a). Testosterone (Te) deficiency has been associated epidemiologically with skeletal muscle weakness, sarcopenia, osteopenia, diminished physical stamina, erectile dysfunction, systolic hypertension, carotid artery-wall thickness, increased abdominal visceral-fat mass, insulin resistance, reduced HDL concentrations, postprandial somnolence, impaired quality of life, depressive mood, diminished working memory, and decreased executive-cognitive function (Liu et al., 2005a; Liu et al., 2004; Veldhuis et al., 2007) (Figure 1). In metaanalyses, low Te availability correlates with reduced grip strength, decreased lean-body mass and increased visceral adiposity (Bhasin et al., 2006; Isidori et al., 2005; Liverman and Blazer 2004), which are significantly reversed by Te supplementation. Whether androgen replacement is indicated in older men without frank hypogonadism is not established (Liu et al., 2004). Moreover, the mechanisms that mediate hypoandrogenemia in the elderly male are only partially understood.
Figure 1.
Epidemiological associations reported with androgen deficiency in men. CNS = central nervous system; LV = left ventricular; CT = computed tomography
2. Clinical Te deprivation in aging
Impoverished Te production in older men has been documented by (i) direct sampling of the spermatic vein, (ii) metaanalysis of cross-sectional data (Gray et al., 1991), and (iii) longitudinal investigations in healthy cohorts (Harman et al., 2001; Morley et al., 1997). Total Te concentrations fall by approximately 110 ng/dL (3.8 nmol/L) per decade in men after age 60, bioavailable (nonSHBG-bound) Te concentrations by 8-13% (0.7-1.0 nmol/L) per decade, and free Te concentrations by a similar percentage (49 pmol/nmol) SHBG per decade. Judged by free Te estimates in young men, the age-related prevalence of hypogonadal Te/SHBG ratios exceeds 20%, 30% and 50% at ages 60, 70 and 80 yr, respectively. In addition, medications, comorbidity, such as diabetes mellitus and visceral adiposity, and intercurrent illness in aging populations exacerbate androgen deficiency (Gray et al., 1991). Cross-sectional data in healthy men in Olmsted County, MN, verify age-associated decrements in total, bioavailable and free Te concentrations measured by tandem mass spectrometry (Figure 2).
Figure 2.

Decline of total, bioavailable and free Te concentrations with age in healthy men in Olmsted County, MN (unpublished cross-sectional data).
3. Need for mechanistic understanding of gonadal axis as a whole
As articulated by the U.S. Institute of Medicine and World Health Organization (Liu et al., 2004; Liverman and Blazer, 2004; World Health Organization, 2001), investigations are required to unveil the primary mechanisms mediating gradual Te depletion in the aging male. This is because both the absolute number and the proportion of men aged 60 or older will increase significantly over the next 50 years in industrialized countries. An important research goal is to identify potentially reversible mechanisms that mediate relative hypogonadism in older men. Difficult confounding factors include an age-related increase and obesity-associated decrease in SHBG concentrations and unknown alterations in hypothalamic and/or pituitary sex-steroid receptor function. Major challenges are that: (a) neuroendocrine adaptations unfold gradually and subtly in aging individuals; and (b) the gonadal axis acts as an ensemble of integrated components. In particular, hypothalamic gonadotropin-releasing hormone (GnRH) drives pituitary luteinizing hormone (LH) secretion; LH in turn stimulates testicular Te synthesis; and systemic Te concentrations feed back negatively on both GnRH and LH signaling, thus creating a dynamic system (Keenan et al., 2004). The strongly interlinked nature of the GnRH-LH-Te axis establishes that no single component of the system operates alone or, from an experimental and technical vantage, may be considered alone (Liu et al., 2005a; Veldhuis et al., 2004a). Investigations conducted under this imprimatur strongly support a thesis of multisite impairment in aging, which includes reduced hypothalamic GnRH outflow, decreased testicular responsiveness to hCG/LH and impaired androgenic negative feedback (Liu et al., 2005a; Veldhuis et al., 2007) (Figure 3).
Figure 3.
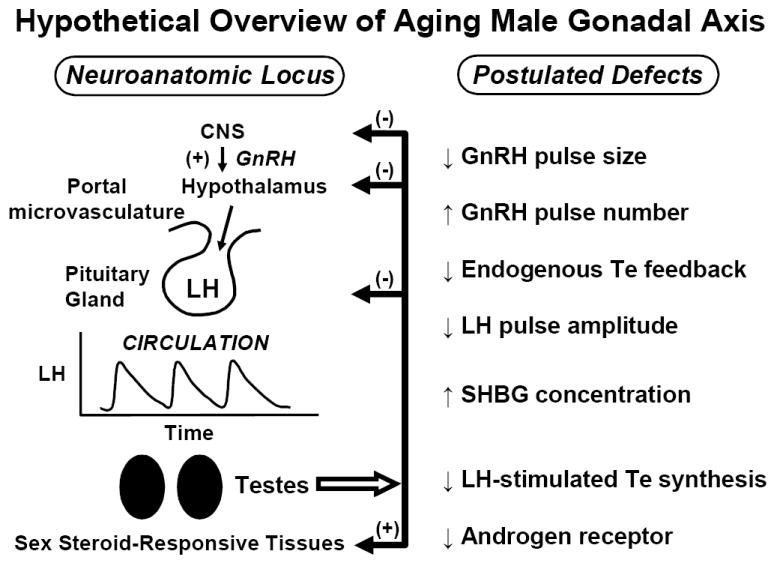
Hypothesized deficits in of aging human male gonadal axis deduced from indirect evidence. Smaller, more frequent GnRH pulses might occur under reduced negative feedback by systemic Te (or E2) resulting in low-amplitude high-frequency LH pulses. The efficacy of LH in stimulating Te secretion also appears to decline in aging individuals. Whether Te’s actions on the brain, pituitary gland and prostate via the androgen receptor (AR) are diminished is not established. Unpublished line drawing.
4. Theoretical feedback and feedforward models
Biomathematical constructs of feedback (inhibitory) and feedforward (stimulatory) dose-response pathways interlinking GnRH, LH and Te may assist in visualizing dynamic interactions that maintain euandrogenism (Keenan et al., 2003; Keenan and Veldhuis, 2001a; Keenan and Veldhuis, 2001b; Keenan and Veldhuis, 1998) (Figure 4). One analytical formalism was validated by sampling hypothalamo-pituitary, jugular and spermatic-vein hormone release in 3 mammalian species (Keenan et al., 2006; Keenan et al., 2004; Keenan and Veldhuis, 2004). Theoretical analyses based upon this integrative model indicate that: (a) isolated failure of central-neural GnRH drive would forecast inappropriately low LH pulse amplitude and attendant hypoandrogenemia; (b) deficient Leydig-cell steroidogenesis would presage low Te and elevated LH concentrations; and (c) jointly impaired LH feedforward (stimulation) and reduced Te feedback (inhibition) would predict accelerated LH pulse frequency and disorderly LH release (Keenan and Veldhuis, 2001a; Keenan and Veldhuis, 2001b; Keenan and Veldhuis, 1998; Pincus et al., 1996). However, concomitant reductions in GnRH secretion, LH-induced Te secretion, and Te negative feedback are required to explain the tripartite phenotype of low-amplitude and high-frequency LH pulses, irregular LH secretion patterns and hypoandrogenemia in healthy older men (Liu et al., 2005a; Veldhuis et al., 2004a; Veldhuis et al., 2007).
Figure 4.
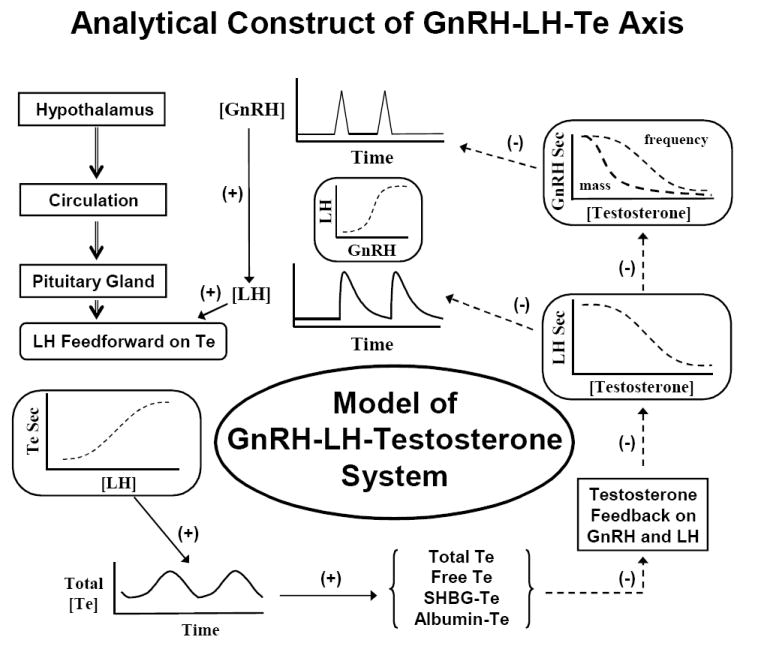
Ensemble model of GnRH, LH and testosterone (Te) signals, which are mathematically linked by nonlinear stimulatory (feedforward) and inhibitory (feedback) dose-response functions and time delays. In this ensemble structure, the hypothalamus, pituitary, gland, testis and circulation constitute anatomic loci, which communicate dynamically via intermittent signaling. Signals, such as GnRH, LH and Te, act on target sites via nonlinear (sigmoidal) dose-response functions. A goal is to estimate such functions noninvasively in vivo in aging individuals. In principle, individual or combined dose-response effects could deteriorate in aging. Adapted from (Keenan et al., 2004).
5. Experimental insights
Although multiple sites of impaired regulation probably coexist in the aging hypothalamic pituitary-gonadal axis, the following section for clarity reviews each potential locus of failure individually.
5.1 Attenuation of hypothalamic GnRH secretion in the aging male
The long-held intuition that hypothalamic outflow of GnRH is reduced in older men is difficult to establish or refute (Vermeulen et al., 1989a; Winters and Troen, 1982). The hypothesis is congruent with laboratory observations in the male rodent. Salient data include: (i) attenuated castration, anesthesia and restraint stress-induced LH release with preserved acute responses to exogenous GnRH (Clarke and Cummins, 1987;Gruenewald et al., 1994; Haji et al., 1980; Huang et al., 1987; Jarjour et al., 1986; Kaler and Critchlow, 1984; Mulligan et al., 1999; Veldhuis et al., 2005a; Vermeulen et al., 1989a; Witkin, 1987); (ii) diminished LH pulse amplitude in vivo and GnRH release by hypothalamic tissue in vitro (Gruenewald et al., 1994; Mitchell et al., 1995; Vermeulen et al., 1989b; Winters and Troen, 1982); (iii) decreased density of GnRH neuronal synapses (Witkin, 1987); and (iv) restoration of sexual activity in the impotent aged male rat after hypothalamic implantation of fetal GnRH neurons (Huang et al., 1987). Indirect clinical data are also consistent with the hypothesis that reduced GnRH secretion contributes to small LH and Te pulses in older men: Table 1. Nonetheless, occasional studies have inferred that aging blunts gonadotrope responses to exogenous GnRH, increases LH pulse size and/or decreases in vitro LH bioactivity. Varying contributions of primary and secondary hypogonadal mechanisms in any given cohort studied could account for some of the reported variability.
Table 1.
Indirect evidence for impaired GnRH outflow in older men
|
Adapted from (Veldhuis et al., 2007).
5.2 Noninvasive estimation of GnRH secretion in vivo
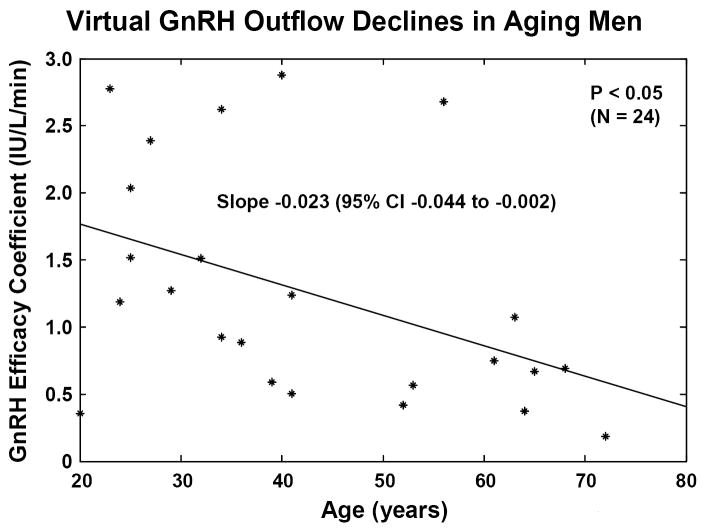
One recent strategy to reconstruct unobserved hypothalamic GnRH outflow exploits an interconnected GnRH-LH-Te feedback model and an experimental paradigm of graded competitive GnRH-receptor blockade to lower Te concentrations to four separate feedback levels (Keenan et al., 2006). The rationale is that less GnRH secretion, if operative in aging individuals, would heighten inhibition by any fixed plasma concentration of the GnRH-receptor antagonist. Analyses using this paradigm revealed a 2-fold greater sensitivity to inhibition, thereby predicting a 50% decrement GnRH outflow driving LH pulses under controlled Te feedback in healthy older men (Figure 5A). Although direct in vivo evidence that portal-venous GnRH concentrations decline in the older male animal is still needed, these data and inferences in Table 1 motivate further study of specific mechanisms that induce and restrain GnRH secretion in aging individuals.
Figure 5.
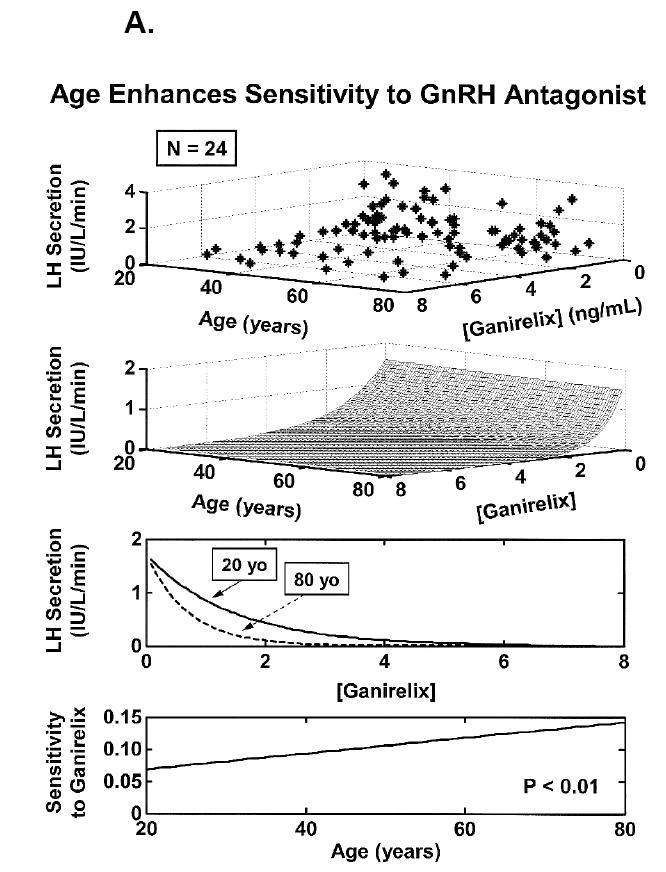
Concomitant decline of endogenous GnRH outflow and efficacy of LH drive with age. Panel A. Analytical reconstruction of LH secretion (vertical axis) as a joint function of GnRH-receptor antagonist (ganirelix) concentrations (horizontal axis) and age (oblique axis). Greater sensitivity of LH secretion to ganirelix inhibition in older men forecasts less opposition by endogenous GnRH drive. Panel B. Analogous 3-dimensional surface plot for LH → Te feedforward dose-response curves as a function of age (top). Data are from 24 healthy men ages 20 to 72 yr each studied under a 4-strata ganirelix clamp. Boxed curves are extrapolated by regression (bottom). Adapted from (Keenan et al., 2006).
5.3 Impaired actions of LH pulses
Administration of the LH surrogate, hCG, in pharmacological amounts fails to stimulate maximal young-adult Te concentrations in many older men (Longcope, 1973; Winters and Troen, 1982). However, pharmacological hCG stimulation paradigms are difficult to interpret. Key limitations are that underlying LH concentrations differ in young and older men; the half-life of hCG (24 hr) is much longer than that of LH (1 hr), resulting in nearly continuous stimulation of Leydig cells; hCG unlike LH rapidly downregulates Leydig-cell steroidogenesis even in young men; and the dose of hCG used clinically is 300-fold greater than the lutropic activity contained in an endogenous LH pulse (Liu et al., 2005a; Veldhuis et al., 2007; Veldhuis et al., 2004a). Impairment of pharmacological LH drive will not explain declining Te secretion in older males with low or normal LH concentrations.
One approach to quantifying Leydig-cell responsiveness directly is to infuse pulses of biosynthetic LH i.v. after suppressing LH secretion overnight with a potent GnRH-receptor antagonist, ganirelix (Veldhuis and Iranmanesh, 2004). In young men, 7 consecutive i.v. pulses of recombinant human LH normalized ganirelix-suppressed LH and Te concentrations, thus validating the model (Veldhuis and Iranmanesh, 2004). The stimulation protocol elevated free and bioavailable (SHBG-unbound) Te concentrations by 50% less in older than young men (Veldhuis et al., 2005b). Extending the ganirelix clamp and i.v. LH pulses for 2 days failed to reconstitute young-adult bioavailable (nonSHBG-bound) and free (equilibrium-dialyzed) Te concentrations in aging individuals (Liu et al., 2005c). Since these studies used a single fixed LH dose, how aging alters LH→Te dose-response properties in healthy men was not determinable.
Another strategy is to mathematically reconstruct the implicit dose-response curve mediating endogenous LH’s pulsatile drive of Te secretion to ensure that physiological rather than pharmacological properties are evaluated. This can be accomplished by way of an analytical model developed for noninvasive estimation of nonlinear feedforward by secreted hormone pulses (Keenan et al., 2006; Keenan et al., 2004;Keenan and Veldhuis, 2003; Keenan 2004). Figure 5B depicts the model-based estimate of a 75% decline in the stimulatory efficacy of endogenous LH pulses between the ages of 20 and 80 yr in healthy men.
Given that hCG injections rapidly downregulate (desensitize) testicular steroidogenesis (Smals et al., 1984), the query emerges, Does the rise in LH concentrations in some older men induce gonadal desensitization? If so, higher LH concentrations (due to lower Te production) in those aging individuals could create a vicious cycle leading to progressive Leydig-cell downregulation. Implications of impaired testicular responsiveness include the design of interventions to rescue steroidogenic defects, such as by selectively antagonizing putative intragonadal repressors like cyclooxygenase type 2, cytokines and estrogen receptor-alpha (Akingbemi et al., 2003; Cao et al., 2004; Hales, 1992; Wang et al., 2005), potentiating gonadotropin action via trophic peptides like IGF-I (Saez, 1994), and directing adult bone-marrow progenitor cells to develop into steroidogenic cells (Davidoff et al., 2004).
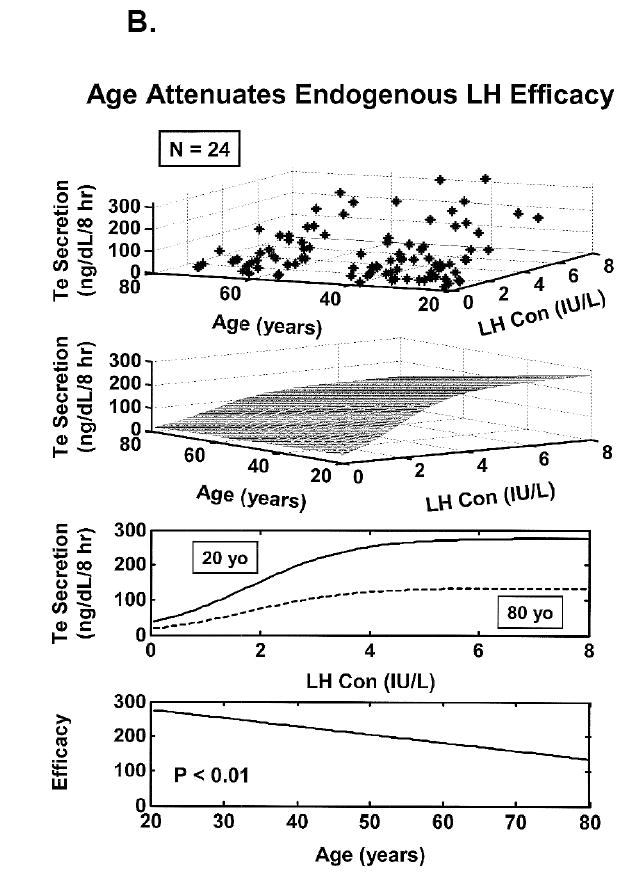
5.4 Sex steroid-mediated negative feedback in the aging male
How age affects Te-dependent negative feedback has been controversial. Three studies described excessive suppression of LH concentrations in older men by short-term (4-day) i.v. infusion of Te and longer-term (11-day and 15-month) transscrotal administration of Te or transdermal delivery of 5 alpha-dihydroTe (DHT) (Winters et al., 1984). Two other investigations reported reduced suppression of LH concentrations in older subjects after i.m. injections of high doses of Te (Gentili et al., 2002; Muta et al., 1981). Five studies inferred impaired feedback regulation of LH production by endogenous Te concentrations (Veldhuis et al., 2004b; Veldhuis et al., 2007). In addition, recent model-based analyses forecast that aging restricts negative feedback on GnRH/LH secretion by endogenous Te (Keenan et al., 2006; Keenan and Veldhuis, 2001a; Keenan and Veldhuis, 2001b; Liu et al., 2006b). Accordingly, disparate data on Te feedback might be harmonized by distinguishing exogenous (augmented) and endogenous (attenuated) negative feedback due to, respectively, pharmacologic and physiological mechanisms. Animal models would be helpful in examining this postulate further.
Histochemical data point to reduced androgen-receptor (AR) expression in the brain, pituitary gland, prostate and penis of the aged rat and in genital fibroblasts of the older human (Haji et al., 1980; Roth, 1979). AR depletion in the hypothalamo-pituitary unit could decrease the feedback efficacy of Te. Pivotal research queries are whether significant AR depletion occurs in relevant neuroregulatory regions, and whether aging diminishes local conversion of Te to E2 or DHT in the hypothalamus and pituitary gland. Broader issues are whether age limits the effects of Te on other neuroendocrine axes and/or other regions of the central nervous system. New analytical methods and experimental paradigms are needed to probe these questions noninvasively without disrupting neuroanatomic pathways. Clinical implications of demonstrating impaired feedback restraint include the design of interventions to selectively enhance androgen action in the brain.
6. Utility of paradigmatic and analytical advances
Combined paradigmatic and analytical advances have resolved some earlier controversies, and introduced new research opportunities. One noninvasive paradigm entails creating four graded strata of Te and estrogen feedback by variably suppressing endogenous GnRH action with a competitive GnRH-receptor antagonist (Liu et al., 2006a;Liu et al., 2006b) (Figure 5A). The analytical component requires estimation of a 3-dimensional dose-response surface linking GnRH outflow and LH secretion to the Te negative-feedback signal, and an LH feedforward dose-response surface linking LH pulses to Te secretion rates and age. According to this combined approach, older men exhibit significant reductions in: (a) Te feedback strength onto GnRH-dependent LH secretion (35% fall) (Figure 6); (b) endogenous GnRH outflow to the pituitary gland (50% decrease) (Figure 7); and (c) the efficacy of endogenous LH-driven Te secretion (75% decline) (Keenan et al., 2006) (Figure 5B). The deficits are selective, inasmuch as Te’s feedback onto GnRH/LH pulse frequency does not change detectably across the same age range.
Figure 6.
Noninvasive analytical reconstruction of joint feedforward (GnRH concentrations → LH secretion) and feedback (Te concentrations → GnRH/LH secretion) response surfaces in 6 of 24 healthy men of the indicated ages. The vertical (z-axis) projection of the surface is determined jointly by GnRH outflow and free Te feedback. Impaired feedback is inferred by a lesser drop in LH secretion rates as free Te concentrations increase between 0 and 10 ng/dL at any given GnRH level in older subjects. Adapted from data in (Keenan et al., 2006).
Figure 7.
Analytical estimation of feedforward by GnRH concentrations onto LH secretion rates [defined as efficacy of GnRH drive to gonadotropes] in 24 healthy men ages 20-72 yr. Adapted from data in (Keenan et al., 2006).
6.1 Aging is marked by many small LH pulses
Three decades of investigations that were based on radioimmunoassay, infrequent blood sampling, empirical pulse-detection methods and observational data had yielded discordant reports of normal, reduced or increased LH pulse number and/or amplitude in older men [reviewed (Liu et al., 2005a; Veldhuis et al., 2004a; Veldhuis et al., 2007)]. Subsequent experiments demonstrated that blood must be sampled at 2.5 to 10-min intervals for 12-24 hr to capture the majority of LH release episodes (Veldhuis et al., 1986). Intensive blood-sampling paradigms disclose 50% more frequent and 35% smaller LH pulses in older than young men, as corroborated using 4 mathematically distinct pulse-analysis methods, 3 independent LH assays and 5 cohorts of volunteers (Keenan and Veldhuis, 2001a; Keenan and Veldhuis, 2001b; Mulligan et al., 1995; Mulligan et al., 1999; Veldhuis et al., 1992). Further analyses established that insufficiently frequent blood-sampling regimens (e.g. 30-min data) censor LH pulse number and inflate apparent LH pulse size (Veldhuis et al., 2007). Therefore, inadequate sampling regimens and unvalidated pulse-identification methods probably explain many earlier disparities in the literature.
6.2 Gonadotrope responses to pulses of GnRH are preserved in aging
Viewed mechanistically, smaller incremental LH pulses in older men could denote less hypothalamic GnRH secretion per burst, greater hypothalamo-pituitary feedback inhibition by sex steroids, and/or impaired gonadotrope responses to GnRH. The last consideration is refuted by the finding that age potentiates LH secretion stimulated by submaximal doses of exogenous GnRH (Veldhuis et al., 2005a) (Figure 8). This outcome is observed when dose-response estimates are constructed using a combined strategy of: (a) deconvolution analysis of LH secretion; (b) high-specificity robotics-automated LH assay; (c) separate-morning randomly ordered single-bolus i.v. doses of GnRH spanning a 1000-fold range; (d) nonlinear (four-parameter logistic) regression analyses to quantify gonadotrope sensitivity (slope term), GnRH potency (ED50) and GnRH efficacy (maximal LH secretory response); and (e) age comparisons in the eugonadal and Te-depleted state. Key points are that age does not alter exogenous GnRH efficacy, and increases gonadotrope sensitivity and GnRH potency primarily in a low Te milieu. The findings that age and Te availability jointly govern GnRH dose-responsiveness explain some discrepancies in the literature, since earlier studies failed to control simultaneously for Te concentrations and GnRH dose (Liu et al., 2005a; Veldhuis et al., 2004a; Veldhuis et al., 2007). Preserved GnRH responsiveness is supported by the independent observation that i.v. pulses of GnRH (100 ng/kg) administered every 90 min for 14 days sustain equivalent LH pulse amplitudes and mean (24-hr) LH concentrations in older and young men (Mulligan et al., 1999). Therefore, smaller endogenous LH pulses in older men could be explained logically by reduced hypothalamic GnRH secretion to the pituitary gland, rather than impaired pituitary responses to GnRH. This important conclusion directs research focus to factors that govern neuronal GnRH secretion in the aging male.
Figure 8.

Preservation (top, eugonadal placebo) (bottom, low-Te clamp) of GnRH dose-response properties in older (age > 60 yr) compared with young (age < 35 yr) healthy men. GnRH doses are in ng/kg. Each GnRH pulse was injected on a separate randomly ordered morning fasting. Data are the mean ± SEM. Adapted from (Veldhuis et al., 2005a).
6.3 Concept of Te feedback clamp
An unresolved mechanistic issue is whether frequent small LH pulses in the aging male represent a primary hypothalamic disturbance or constitute an expected feedback adjustment to lower systemic concentrations of Te or estradiol (E2). Data in healthy young men establish that Te and nonaromatizable androgens (e.g., DHT or fluoxymesterone) suppress LH pulse frequency, whereas ketoconazole (which blocks Te secretion) and flutamide (which inhibits androgen-receptor binding) accelerate LH pulse frequency (Urban et al., 1988; Veldhuis et al., 1997; Veldhuis et al., 2007). Other studies indicate that more frequent pulses of GnRH decrease LH peak increments (Clarke and Cummins, 1987). Accordingly, acceleration of LH pulse frequency due to low Te and E2 availability in aging might further decrease LH pulse increments (Mulligan et al., 1999; Veldhuis et al., 1992). This hypothesis has not been affirmed or rejected. What is needed is accurate quantification of LH pulsatility during imposition of a combined Te and E2 feedback ‘clamp’ achieved by blocking gonadal Te production and adding back fixed amounts of Te and E2 (Veldhuis et al., 1997; Veldhuis et al., 2005a; Veldhuis et al., 2007).
6.4 Quantifying pulsatile and nonpulsatile LH and Te secretion
Cluster analysis is one of several empirical methods to detect discrete peaks in hormone concentration profiles (Veldhuis and Johnson, 1986). This simple pooled-variance technique identifies a high frequency of low-increment LH peaks in older men (Mulligan et al., 1999). Diminutive peak increments (difference between peak maximum and prepeak nadir) imply that less LH is secreted in each burst, but only if LH clearance and distribution do not change (Veldhuis et al., 1990). This is because hormone concentrations are determined by secretion, elimination and volume of distribution.
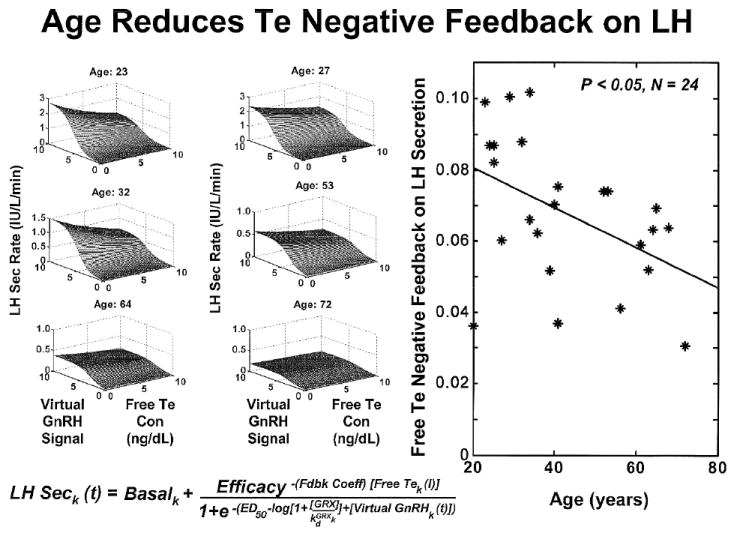
Secretion can be estimated analytically by deconvolution procedures, which quantify each of (i) the frequency [number and timing] of pulses; (ii) the amount of hormone released in each burst [mass secreted per unit distribution volume]; (iii) hormone- and subject-specific elimination half-lives; and (iv) concomitant basal (nonpulsatile) secretion. Early methods that assumed Gaussian-like symmetric secretory events identified low-amplitude and high-frequency LH secretory bursts in older men (Mulligan et al., 1995;Veldhuis et al., 1992), but could not ensure accurate simultaneous estimates of basal secretion, half-life, pulse number and mass (Veldhuis et al., 1995). A more recent deconvolution model allows for both symmetric and asymmetric secretory bursts, and achieves maximum-likelihood estimates of all parameters. The algorithm uses an image-deblurring (selective-smoothing) technique to create multiple sets of potential pulse-onset times (Keenan et al., 2005) (Figure 9A). The largest set contains all possible local nadirs (minima), and successive daughter pulse sets each contain one less peak. Inasmuch as the true pulse set is not known, deconvolution analysis is performed separately on each candidate pulse-time set. The analysis of each pulse set consists of simultaneously estimating basal secretion, the size (mass) and shape (skewness) of secretory bursts, biexponential elimination half-lives, random effects on burst mass and experimental uncertainty in the data (10 parameters): Figure 9B. The parameters for random (stochastic) variability are necessary to ensure that deterministic variables (elimination kinetics, basal secretion, mean burst size) are estimated reliably and validly. The statistically favored pulse-time set is then determined objectively from all the candidates using model-selection criteria, such as the Akaike or the Bayesian information criterion (AIC or BIC). The model is unique inasmuch as the statistical framework was verified by direct mathematical proof, and the analytical structure validated by direct venous sampling of GnRH, LH and Te secretion (Keenan et al., 2005;Keenan et al., 2004). Implementation of this deconvolution concept likewise predicts frequent and small LH secretory bursts, as well as diminutive Te pulses (but normal basal Te secretion), in the older male (Keenan et al., 2003;Keenan and Veldhuis, 2001a;Keenan and Veldhuis, 2004).
Figure 9.
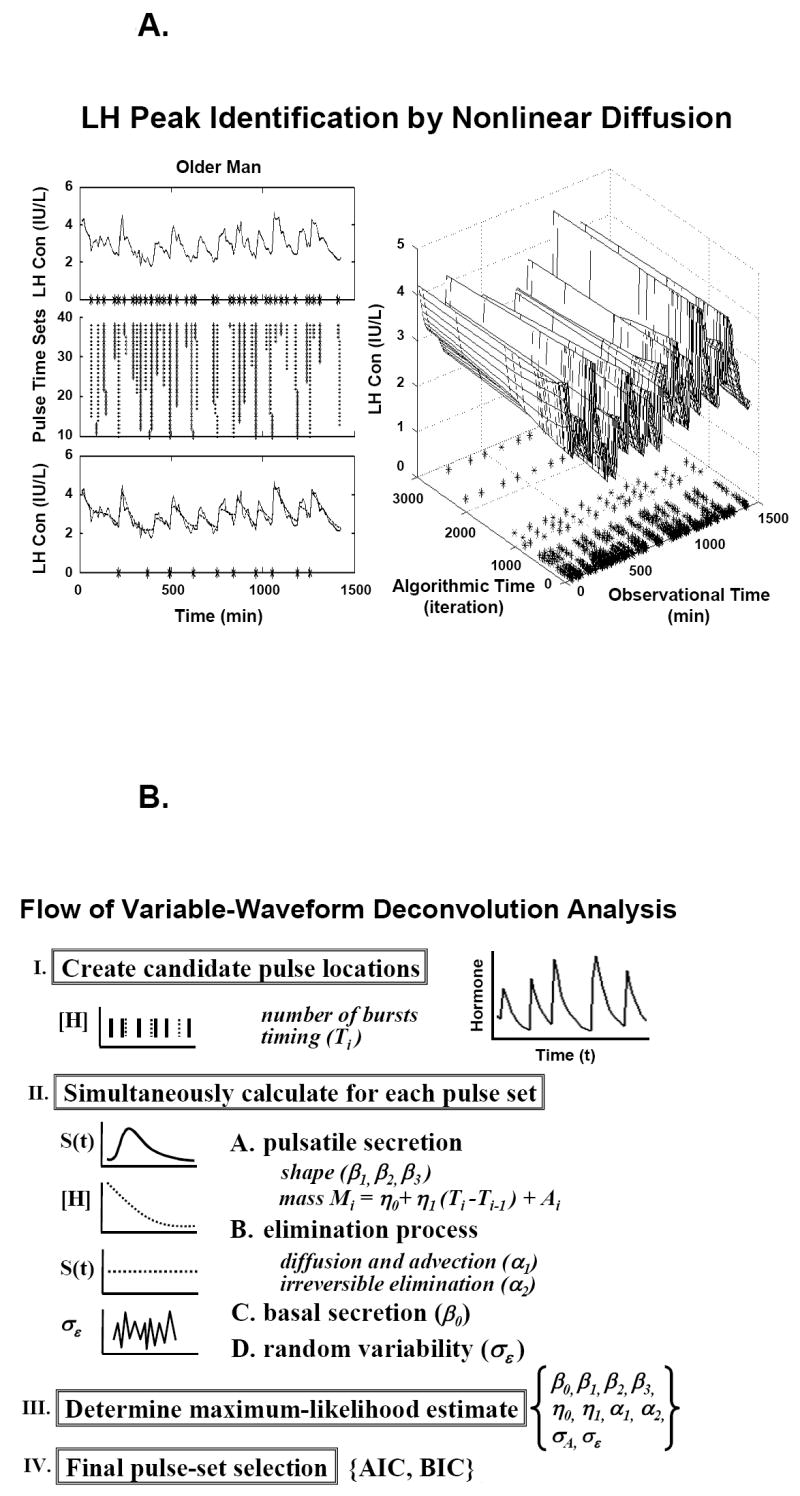
Panel A. Schema of pulse-detection phase of a third-generation flexible (Gamma) waveform deconvolution method. Asterisks denote pulse-onset times (observational time) retained after repeated incremental smoothing (algorithmic time). Panel B. Quantification of secretory-burst number, size and shape, basal secretion, biexponential elimination, stochastic variability in pulse mass, and experimental uncertainty in the data conditioned on a set of a priori candidate pulse-onset times [Panel A]. Greek symbols define parameters. AIC = Akaike and BIC = Bayesian information criteria are used to select the final pulse set. Unpublished line drawings from methods presented in (Keenan et al., 2003; Keenan et al., 2005; Keenan et al., 2006; Keenan et al., 2004).
6.5 Measuring feedback integration via a model-free ensemble statistic
Feedback control may be estimated by experimental infusion of a feedback signal and via noninvasive analytical models designed to reconstruct unobserved pathways [as discussed earlier in this article (Keenan et al., 2006; Keenan et al., 2004)]. In complementation, the model-free approximate entropy (ApEn) statistic allows one to quantify subtle feedback adaptations reflected in changes in the regularity or orderliness of hormone secretion patterns (Pincus, 1991; Pincus, 1995; Veldhuis et al., 2001a; Veldhuis et al., 2001b). ApEn for any given time series is calculated on a PC as a single positive number (between zero and the natural logarithm of 10 = 2.30). Higher ApEn values denote greater irregularity or relative randomness (less deterministic orderliness) (Figure 10A). Irregularity in turn denotes impaired feedback coordination within the network. Sensitivity and specificity of this statistic both exceed 90% (Pincus et al., 1999; Veldhuis et al., 2001a; Veldhuis et al., 2001b).
Figure 10.
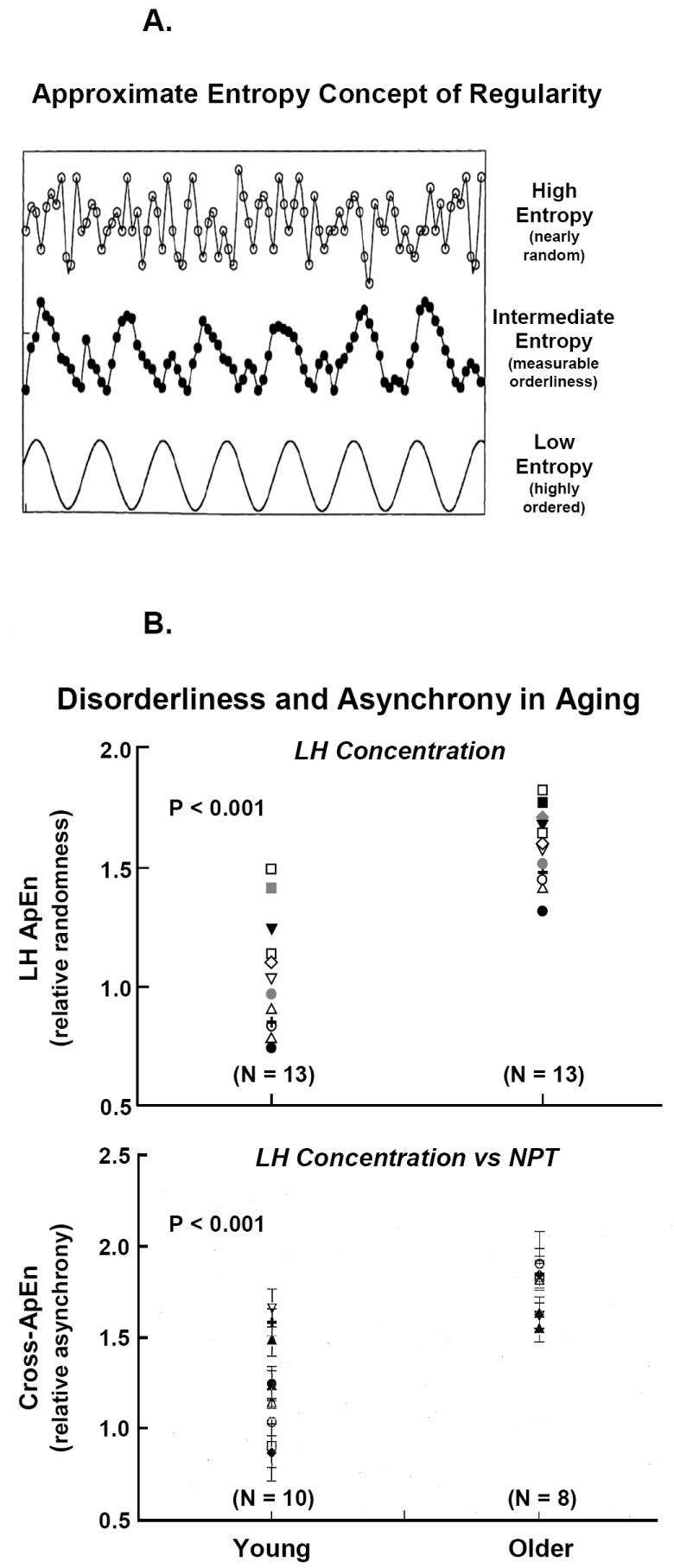
Panel A. Concept of approximate entropy (ApEn) to quantify the orderliness or regularity of hormone concentration or secretion patterns. Orderliness is a measure of feedback control in coupled systems. The bottom curve gives a cosine function (low ApEn or well ordered), the middle plot less regular data, and the top frame an irregular profile (high ApEn). Panel B. Elevated LH ApEn (top) in individual older compared with young men signifies reduced negative feedback on the secretion process. Elevated cross-ApEn (bottom) of LH outflow and nocturnal penile tumescence (NPT) oscillations denotes loss of neurohormone-outflow synchrony in aging individuals. Data are from (Veldhuis et al., 1999).
Technically, ApEn is defined as the (negative logarithm of) summed conditional probabilities that subpatterns of vector length m recur upon next (m + 1) incremental comparison within a scalar tolerance range r in a data series of length N (Pincus, 1991; Pincus, 1995). Stated alternatively, greater orderliness denotes that patterns containing m similar data points more often expand to contain (m + 1) similar data points within a tolerance window of r. Tolerance r is normalized as 0.2 SD of the overall time series (for N > 30) to confer scale independence (Liu et al., 2006a;Liu et al., 2006b;Pincus et al., 1996; Pincus et al., 1999; Veldhuis et al., 2001a; Veldhuis et al., 2001b). Thus, changes in pattern orderliness, rather than absolute hormone concentrations, determine ApEn. Clinical studies unveil that secretion patterns of LH and Te as well as GH, ACTH, cortisol and insulin are more irregular (less orderly) in older than young adults (Keenan and Veldhuis, 2001b; Liu et al., 2005b; Liu et al., 2006a; Liu et al., 2006b; Meneilly et al., 1999; Mulligan et al., 1999; Pincus et al., 1996; Pincus et al., 1997; van den Berg et al., 1997; Veldhuis et al., 2007; Veldhuis et al., 1999). Elevation of LH ApEn in older men quantifies impaired feedback within the aging GnRH-LH-Te ensemble (Figure 10B top). Experimental restoration of Te feedback progressively lowers LH ApEn (increases the orderliness of LH secretion), but to a lesser degree in older than young men (Liu et al., 2006b).
Cross-ApEn is a bivariate extension of the univariate ApEn statistic that measures pattern similarities in paired time series (Keenan et al., 2001; Keenan and Veldhuis, 2001a; Liu et al., 2005b; Liu et al., 2006a; Liu et al., 2006b; Pincus et al., 1996). Cross-ApEn analyses reveal age-related erosion (higher cross-ApEn) of young adult-like pattern synchrony between LH and Te, LH and prolactin, LH and sleep-stage, and LH and nocturnal penile tumescence (NPT) oscillations (Keenan and Veldhuis, 2001a; Keenan and Veldhuis, 2001b; Pincus et al., 1996) (Figure 10Bbottom). The collective data strongly support the thesis that aging is associated with failure of integrative neurohormone outflow.
7. Conclusion and future directions
Basic research provides the foundation and clinical medicine the motivation to elucidate fundamental causes of hypoandrogenism in the older male. Future studies need to quantify control mechanisms at the systems level (full hypothalamic-pituitary-gonadal axis) using increasingly innovative experimental strategies. Two major unanswered questions are whether multiple mechanisms of regulatory failure emerge sequentially or simultaneously during the aging process, and the extent to which regulatory failure at one site exacerbates impairment at other sites.
Acknowledgments
We thank Kay Nevinger and Donna Scott for support of manuscript preparation; Ashley Bryant for data analysis and graphics; the Mayo Immunochemical Laboratory for assay assistance; and the Mayo research nursing staff for implementing the protocol. Supported in part via the Center for Translational Science Activities (CTSA) Grant Number 1 UL 1 RR024150, RR019991 from the National Center for Research Resources (Rockville, MD), and AG23133, AG029362, DK072095 and DK063609 from the National Institutes of Health (Bethesda, MD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Akingbemi BT, Ge R, Rosenfeld CS, Newton LG, Hardy DO, Catterall JF, Lubahn DB, Korach KS, Hardy MP. Estrogen receptor-alpha gene deficiency enhances androgen biosynthesis in the mouse Leydig cell. Endocrinol. 2003;144:84–93. doi: 10.1210/en.2002-220292. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Calof OM, Storer TW, Lee ML, Mazer NA, Jasuja R, Montori VM, Gao W, Dalton JT. Drug insight: Testosterone and selective androgen receptor modulators as anabolic therapies for chronic illness and aging. Nat Clin Pract Endocrinol Metab. 2006;2:146–159. doi: 10.1038/ncpendmet0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Leers-Sucheta S, Azhar S. Aging alters the functional expression of enzymatic and non-enzymatic anti-oxidant defense systems in testicular rat Leydig cells. J Steroid Biochem Mol Biol. 2004;88:61–67. doi: 10.1016/j.jsbmb.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Cummins JT. The significance of small pulses of gonadotropin-releasing hormone. J Endocrinol. 1987;113:413–418. doi: 10.1677/joe.0.1130413. [DOI] [PubMed] [Google Scholar]
- Davidoff MS, Middendorff R, Enikolopov G, Riethmacher D, Holstein AF, Muller D. Progenitor cells of the testosterone-producing Leydig cells revealed. J Cell Biol. 2004;167:935–944. doi: 10.1083/jcb.200409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentili A, Mulligan T, Godschalk M, Clore J, Patrie J, Iranmanesh A, Veldhuis JD. Unequal impact of short-term testosterone repletion on the somatotropic axis of young and older men. J Clin Endocrinol Metab. 2002;87:825–834. doi: 10.1210/jcem.87.2.8222. [DOI] [PubMed] [Google Scholar]
- Gray A, Berlin JA, McKinlay JB, Longcope C. An examination of research design effects on the association of testosterone and male aging: results of a meta-analysis. J Clin Epidemiol. 1991;44:671–684. doi: 10.1016/0895-4356(91)90028-8. [DOI] [PubMed] [Google Scholar]
- Gruenewald DA, Naai MA, Hess DL, Matsumoto AM. The Brown Norway rat as a model of male reproductive aging: evidence for both primary and secondary testicular failure. J Gerontol. 1994;49:B42–B50. doi: 10.1093/geronj/49.2.b42. [DOI] [PubMed] [Google Scholar]
- Haji M, Kato KI, Nawata H, Ibayashi H. Age-related changes in the concentrations of cytosol receptors for sex steroid hormones in the hypothalamus and pituitary gland of the rat. Brain Res. 1980;204:373–386. doi: 10.1016/0006-8993(81)90596-5. [DOI] [PubMed] [Google Scholar]
- Hales DB. Interleukin-1 inhibits Leydig cell steroidogenesis primarily by decreasing 17 alpha-hydroxylase/C17-20 lyase cytochrome P450 expression. Endocrinol. 1992;131:2165–2172. doi: 10.1210/endo.131.5.1425417. [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Huang HH, Kissane JQ, Hawrylewicz EJ. Restoration of sexual function and fertility by fetal hypothalamic transplant in impotent aged male rats. Neurobiol Aging. 1987;8:465–472. doi: 10.1016/0197-4580(87)90042-x. [DOI] [PubMed] [Google Scholar]
- Isidori AM, Giannetta E, Gianfrilli D, Greco EA, Bonifacio V, Aversa A, Isidori A, Fabbri A, Lenzi A. Effects of testosterone on sexual function in men: results of a meta-analysis. Clin Endocrinol (Oxf) 2005;63:381–394. doi: 10.1111/j.1365-2265.2005.02350.x. [DOI] [PubMed] [Google Scholar]
- Jarjour LT, Handelsman DJ, Swerdloff RS. Effects of aging on the in vitro release of gonadotropin-releasing hormone. Endocrinol. 1986;119:1113–1117. doi: 10.1210/endo-119-3-1113. [DOI] [PubMed] [Google Scholar]
- Kaler LW, Critchlow V. Anterior pituitary luteinizing hormone secretion during continuous perifusion in aging male rats. Mech Ageing Dev. 1984;25:103–115. doi: 10.1016/0047-6374(84)90133-7. [DOI] [PubMed] [Google Scholar]
- Keenan DM, Alexander SL, Irvine CHG, Clarke IJ, Canny BJ, Scott CJ, Tilbrook AJ, Turner AI, Veldhuis JD. Reconstruction of in vivo time-evolving neuroendocrine dose-response properties unveils admixed deterministic and stochastic elements. Proc Natl Acad Sci USA. 2004;101:6740–6745. doi: 10.1073/pnas.0300619101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan DM, Chattopadhyay S, Veldhuis JD. Composite model of time-varying appearance and disappearance of neurohormone pulse signals in blood. J Theor Biol. 2005;236:242–255. doi: 10.1016/j.jtbi.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Keenan DM, Licinio J, Veldhuis JD. A feedback-controlled ensemble model of the stress-responsive hypothalamo-pituitary-adrenal axis. Proc Natl Acad Sci USA. 2001;98:4028–4033. doi: 10.1073/pnas.051624198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan DM, Roelfsema F, Biermasz N, Veldhuis JD. Physiological control of pituitary hormone secretory-burst mass, frequency and waveform: a statistical formulation and analysis. Am J Physiol. 2003;285:R664–R673. doi: 10.1152/ajpregu.00195.2003. [DOI] [PubMed] [Google Scholar]
- Keenan DM, Takahashi PY, Liu PY, Roebuck PD, Nehra AX, Iranmanesh A, Veldhuis JD. An Ensemble Model of the Male Gonadal Axis: illustrative application in aging men. Endocrinol. 2006;147:2817–2828. doi: 10.1210/en.2005-1356. [DOI] [PubMed] [Google Scholar]
- Keenan DM, Veldhuis JD. A biomathematical model of time-delayed feedback in the human male hypothalamic-pituitary-Leydig cell axis. Am J Physiol. 1998;275:E157–E176. doi: 10.1152/ajpendo.1998.275.1.E157. [DOI] [PubMed] [Google Scholar]
- Keenan DM, Veldhuis JD. Disruption of the hypothalamic luteinizing-hormone pulsing mechanism in aging men. Am J Physiol. 2001a;281:R1917–R1924. doi: 10.1152/ajpregu.2001.281.6.R1917. [DOI] [PubMed] [Google Scholar]
- Keenan DM, Veldhuis JD. Hypothesis testing of the aging male gonadal axis via a biomathematical construct. Am J Physiol. 2001b;280:R1755–R1771. doi: 10.1152/ajpregu.2001.280.6.R1755. [DOI] [PubMed] [Google Scholar]
- Keenan DM, Veldhuis JD. Cortisol feedback state governs adrenocorticotropin secretory-burst shape, frequency and mass in a dual-waveform construct: time-of-day dependent regulation. Am J Physiol. 2003;285:R950–961. doi: 10.1152/ajpregu.00299.2003. [DOI] [PubMed] [Google Scholar]
- Keenan DM, Veldhuis JD. Divergent gonadotropin-gonadal dose-responsive coupling in healthy young and aging men. Am J Physiol. 2004;286:R381–R389. doi: 10.1152/ajpregu.00376.2003. [DOI] [PubMed] [Google Scholar]
- Liu PY, Iranmanesh A, Nehra AX, Keenan DM, Veldhuis JD. Mechanisms of hypoandrogenemia in healthy aging men. Endocrinol Metab Clin N Am. 2005a;34:935–955. doi: 10.1016/j.ecl.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Liu PY, Pincus SM, Keenan DM, Roelfsema F, Veldhuis JD. Analysis of bidirectional pattern synchrony of concentration-secretion pairs: implementation in the human testicular and adrenal axes. Am J Physiol. 2005b;288:R440–R446. doi: 10.1152/ajpregu.00414.2004. [DOI] [PubMed] [Google Scholar]
- Liu PY, Pincus SM, Takahashi PY, Roebuck PD, Iranmanesh A, Keenan DM, Veldhuis JD. Aging attenuates both the regularity and joint synchrony of LH and testosterone secretion in normal men: analyses via a model of graded GnRH receptor blockade. Am J Physiol. 2006a;290:E34–E41. doi: 10.1152/ajpendo.00227.2005. [DOI] [PubMed] [Google Scholar]
- Liu PY, Swerdloff RS, Veldhuis JD. The rationale, efficacy and safety of androgen therapy in older men: future research and current practice recommendations. J Clin Endocrinol Metab. 2004;89:4789–4796. doi: 10.1210/jc.2004-0807. [DOI] [PubMed] [Google Scholar]
- Liu PY, Takahashi PY, Roebuck PD, Iranmanesh A, Veldhuis JD. Aging in healthy men impairs recombinant human LH-stimulated testosterone secretion monitored under a two-day intravenous pulsatile LH clamp. J Clin Endocrinol Metab. 2005c;90:5544–5550. doi: 10.1210/jc.2005-0909. [DOI] [PubMed] [Google Scholar]
- Liu PY, Takahashi PY, Roebuck PD, Veldhuis JD. Age or factors associated with aging attenuate testosterone’s concentration-dependent enhancement of the regularity of luteinizing hormone secretion in healthy men. J Clin Endocrinol Metab. 2006b;91:4077–4084. doi: 10.1210/jc.2005-2811. [DOI] [PubMed] [Google Scholar]
- Liverman CT, Blazer DG. Testosterone and aging: clinical research directions. Institute of Medicine, The National Academies Press; Washington, D.C: 2004. [PubMed] [Google Scholar]
- Longcope C. The effect of human chorionic gonadotropin on plasma steroid levels in young and old men. Steroids. 1973;21:583–592. doi: 10.1016/0039-128x(73)90046-9. [DOI] [PubMed] [Google Scholar]
- Meneilly GS, Veldhuis JD, Elahi D. Disruption of the pulsatile and entropic modes of insulin release during an unvarying glucose stimulus in elderly individuals. J Clin Endocrinol Metab. 1999;84:1938–1943. doi: 10.1210/jcem.84.6.5753. [DOI] [PubMed] [Google Scholar]
- Mitchell R, Hollis S, Rothwell C, Robertson WR. Age-related changes in the pituitary-testicular axis in normal men; lower serum testosterone results from decreased bioactive LH drive. Clin Endocrinol. 1995;42:501–507. doi: 10.1111/j.1365-2265.1995.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Morley JE, Kaiser FE, Perry HM, 3rd, Patrick P, Morley PM, Stauber PM, Vellas B, Baumgartner RN, Garry PJ. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metab: Clin Exp. 1997;46:410–413. doi: 10.1016/s0026-0495(97)90057-3. [DOI] [PubMed] [Google Scholar]
- Mulligan T, Iranmanesh A, Gheorghiu S, Godschalk M, Veldhuis JD. Amplified nocturnal luteinizing hormone (LH) secretory burst frequency with selective attenuation of pulsatile (but not basal) testosterone secretion in healthy aged men: possible Leydig cell desensitization to endogenous LH signaling--a clinical research center study. J Clin Endocrinol Metab. 1995;80:3025–3031. doi: 10.1210/jcem.80.10.7559891. [DOI] [PubMed] [Google Scholar]
- Mulligan T, Iranmanesh A, Kerzner R, Demers LW, Veldhuis JD. Two-week pulsatile gonadotropin releasing hormone infusion unmasks dual (hypothalamic and Leydig-cell) defects in the healthy aging male gonadotropic axis. Eur J Endocrinol. 1999;141:257–266. doi: 10.1530/eje.0.1410257. [DOI] [PubMed] [Google Scholar]
- Muta K, Kato K, Akamine Y, Ibayashi H. Age-related changes in the feedback regulation of gonadotrophin secretion by sex steroids in men. Acta Endocrinol (Copenh) 1981;96:154–162. doi: 10.1530/acta.0.0960154. [DOI] [PubMed] [Google Scholar]
- Pincus SM. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci USA. 1991;88:2297–2301. doi: 10.1073/pnas.88.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus SM. Quantifying complexity and regularity of neurobiological systems. Meth Neurosci. 1995;28:336–363. [Google Scholar]
- Pincus SM, Hartman ML, Roelfsema F, Thorner MO, Veldhuis JD. Hormone pulsatility discrimination via coarse and short time sampling. Am J Physiol. 1999;277:E948–E957. doi: 10.1152/ajpendo.1999.277.5.E948. [DOI] [PubMed] [Google Scholar]
- Pincus SM, Mulligan T, Iranmanesh A, Gheorghiu S, Godschalk M, Veldhuis JD. Older males secrete luteinizing hormone and testosterone more irregularly, and jointly more asynchronously, than younger males. Proc Natl Acad Sci USA. 1996;93:14100–14105. doi: 10.1073/pnas.93.24.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus SM, Veldhuis JD, Mulligan T, Iranmanesh A, Evans WS. Effects of age on the irregularity of LH and FSH serum concentrations in women and men. Am J Physiol. 1997;273:E989–E995. doi: 10.1152/ajpendo.1997.273.5.E989. [DOI] [PubMed] [Google Scholar]
- Roth GS. Hormone receptor changes during adulthood and senescence: significance for aging research. Fed Proc. 1979;38:1910–1914. [PubMed] [Google Scholar]
- Saez JM. Leydig cells: endocrine, paracrine, and autocrine regulation. Endocr Rev. 1994;15:574–626. doi: 10.1210/edrv-15-5-574. [DOI] [PubMed] [Google Scholar]
- Smals AG, Pieters GF, Boers GH, Raemakers JM, Hermus AR, Benraad TJ, Kloppenborg PW. Differential effect of single high dose and divided small dose administration of human chorionic gonadotropin on Leydig cell steroidogenic desensitization. J Clin Endocrinol Metab. 1984;58:327–331. doi: 10.1210/jcem-58-2-327. [DOI] [PubMed] [Google Scholar]
- Urban RJ, Davis MR, Rogol AD, Johnson ML, Veldhuis JD. Acute androgen receptor blockade increases luteinizing-hormone secretory activity in men. J Clin Endocrinol Metab. 1988;67:1149–1155. doi: 10.1210/jcem-67-6-1149. [DOI] [PubMed] [Google Scholar]
- van den Berg G, Pincus SM, Veldhuis JD, Frolich M, Roelfsema F. Greater disorderliness of ACTH and cortisol release accompanies pituitary-dependent Cushing’s Disease. Eur J Endocrinol. 1997;136:394–400. doi: 10.1530/eje.0.1360394. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Evans WS, Johnson ML. Complicating effects of highly correlated model variables on nonlinear least-squares estimates of unique parameter values and their statistical confidence intervals: estimating basal secretion and neurohormone half-life by deconvolution analysis. Meth Neurosci. 1995;28:130–138. [Google Scholar]
- Veldhuis JD, Evans WS, Johnson ML, Rogol AD. Physiological properties of the luteinizing hormone pulse signal: impact of intensive and extended venous sampling paradigms on their characterization in healthy men and women. J Clin Endocrinol Metab. 1986;62:881–891. doi: 10.1210/jcem-62-5-881. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Iranmanesh A. Pulsatile intravenous infusion of recombinant human luteinizing hormone under acute gonadotropin-releasing hormone receptor blockade reconstitutes testosterone secretion in young men. J Clin Endocrinol Metab. 2004;89:4474–4479. doi: 10.1210/jc.2004-0203. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Iranmanesh A, Keenan DM. An ensemble perspective of aging-related hypoandrogenemia in men. In: Winters SJ, editor. Male Hypogonadism: Basic, Clinical, and Theoretical Principles. Humana Press; Totowa, NJ: 2004a. pp. 261–284. [Google Scholar]
- Veldhuis JD, Iranmanesh A, Keenan DM. Erosion of endogenous testosterone-driven negative feedback on pulsatile LH secretion in healthy aging men. J Clin Endocrinol Metab. 2004b;89:5753–5761. doi: 10.1210/jc.2004-0399. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Iranmanesh A, Mulligan T. Age and testosterone feedback jointly control the dose-dependent actions of gonadotropin-releasing hormone in healthy men. J Clin Endocrinol Metab. 2005a;90:302–309. doi: 10.1210/jc.2004-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis JD, Iranmanesh A, Mulligan T, Pincus SM. Disruption of the young-adult synchrony between luteinizing hormone release and oscillations in follicle-stimulating hormone, prolactin, and nocturnal penile tumescence (NPT) in healthy older men. J Clin Endocrinol Metab. 1999;84:3498–3505. doi: 10.1210/jcem.84.10.6100. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Johnson ML. Cluster analysis: A simple, versatile and robust algorithm for endocrine pulse detection. Am J Physiol. 1986;250:E486–E493. doi: 10.1152/ajpendo.1986.250.4.E486. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Johnson ML, Veldhuis OL, Straume M, Pincus S. Impact of pulsatility on the ensemble orderliness (approximate entropy) of neurohormone secretion. Am J Physiol. 2001a;281:R1975–R1985. doi: 10.1152/ajpregu.2001.281.6.R1975. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Keenan DM, Iranmanesh A, Takahashi PY, Nehra AX. The ensemble male hypothalamo-pituitary-gonadal axis. In: Timiras PS, editor. Physiological Basis of Aging and Geriatrics. 4. Vol. 12. Taylor & Francis Group; LLC, Health Science Division, New York: 2007. pp. 185–203. [Google Scholar]
- Veldhuis JD, Lassiter AB, Johnson ML. Operating behavior of dual or multiple endocrine pulse generators. Am J Physiol. 1990;259:E351–E361. doi: 10.1152/ajpendo.1990.259.3.E351. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Straume M, Iranmanesh A, Mulligan T, Jaffe CA, Barkan A, Johnson ML, Pincus SM. Secretory process regularity monitors neuroendocrine feedback and feedforward signaling strength in humans. Am J Physiol. 2001b;280:R721–R729. doi: 10.1152/ajpregu.2001.280.3.R721. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Urban RJ, Lizarralde G, Johnson ML, Iranmanesh A. Attenuation of luteinizing hormone secretory burst amplitude is a proximate basis for the hypoandrogenism of healthy aging in men. J Clin Endocrinol Metab. 1992;75:52–58. doi: 10.1210/jcem.75.3.1517359. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Veldhuis NJ, Keenan DM, Iranmanesh A. Age diminishes the testicular steroidogenic response to repeated intravenous pulses of recombinant human LH during acute GnRH-receptor blockade in healthy men. Am J Physiol. 2005b;288:E775–E781. doi: 10.1152/ajpendo.00410.2004. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Zwart AD, Iranmanesh A. Neuroendocrine mechanisms by which selective Leydig-cell castration unleashes increased pulsatile LH release in the human: an experimental paradigm of short-term ketoconazole-induced hypoandrogenemia and deconvolution-estimated LH secretory enhancement. Am J Physiol. 1997;272:R464–R474. doi: 10.1152/ajpregu.1997.272.2.R464. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Deslypere JP, De Meirleir K. A new look at the andropause: altered function of the gonadotrophs. J Steroid Biochem. 1989a;32:163–165. doi: 10.1016/0022-4731(89)90158-1. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Deslypere JP, Kaufman JJ. Influence of antiopioids on luteinizing hormone pulsatility in aging men. J Clin Endocrinol Metab. 1989b;68:68–72. doi: 10.1210/jcem-68-1-68. [DOI] [PubMed] [Google Scholar]
- Wang X, Shen CL, Dyson MT, Eimerl S, Orly J, Hutson JC, Stocco DM. Cyclooxygenase-2 regulation of the age-related decline in testosterone biosynthesis. Endocrinol. 2005;146:4202–4208. doi: 10.1210/en.2005-0298. [DOI] [PubMed] [Google Scholar]
- Winters SJ, Sherins RJ, Troen P. The gonadotropin-suppressive activity of androgen is increased in elderly men. Metab. 1984;33:1052–1059. doi: 10.1016/0026-0495(84)90237-3. [DOI] [PubMed] [Google Scholar]
- Winters SJ, Troen P. Episodic luteinizing hormone (LH) secretion and the response of LH and follicle-stimulating hormone to LH-releasing hormone in aged men: evidence for coexistent primary testicular insufficiency and an impairment in gonadotropin secretion. J Clin Endocrinol Metab. 1982;55:560–565. doi: 10.1210/jcem-55-3-560. [DOI] [PubMed] [Google Scholar]
- Witkin JW. Aging changes in synaptology of luteinizing hormone-releasing hormone neurons in male rat preoptic area. Neurosci. 1987;22:1003–1013. doi: 10.1016/0306-4522(87)92976-9. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO, Ageing and Life Course Unit (report) Geneva: World Health Organization; 2001. Men, ageing and health: Achieving health across the life span. [Google Scholar]