Abstract
A procedure for the detection of homocysteine (Hcy) in blood plasma is described. A commercially available chromogen is added to the plasma sample. The plasma solution turns from yellow to blue upon heating for 4 min when a detectable threshold level of Hcy is present. Chromatographic separations and immunogenic materials are not needed. The protocol takes ~ 30 min.
INTRODUCTION
Biological thiols are challenging analytes. They posses similar structures, exhibit complex redox chemistry and are transparent in the visible spectral region. Over 50 thiol-reactive reagents are currently sold1. These typically contain universal electrophilic alkylating groups and are therefore nonselective for specific thiols. Many of these reagents must be handled with caution to prevent unwanted degradation and side-product formation. For instance, monobromobimane is unstable in H2O (ref. 2), iodoacetamides are cross-reactive with histidine, tyrosine and methionine1, o-phtha-laldehyde adducts exhibit high photoinstability3 and maleimide labeling produces unwanted rearrangements of conjugates4. Due to the difficulties involved in thiol detection, determinations using colorimetric or fluorimetric reagents must be carried out in conjunction with separations5. There are numerous other procedures, which include chromatographic separations, immunoassays and enzymatic assays, and electrochemical, mass spectrometric and flow-injection technologies6,7.
Elevated plasma levels (>12–15 μM) of the thiol amino-acid homocysteine (Hcy) have been associated with increased risk of myocardial infarction, stroke and venous thromboembolism. Hcy has also been linked to increased risk of Alzheimer’s disease, neural tube defects, complications during pregnancy, inflammatory bowel disease and osteoporosis7–10. In addition to analytical HPLC with a derivatization step (vide supra), gas chromatography/mass spectrometry (GC-MS) and commercial immunoassays (e.g., the fluorescence polarization immunoassay run on Abbott’s Imx and AxSYM platforms11,12) are often used to monitor Hcy. GC-MS requires instrumentation that is not typically applied at point-of-care. The immunoassays require enzymes, antibodies and biomolecules that are expensive and intrinsically unstable; therefore, careful attention to storage conditions is required.
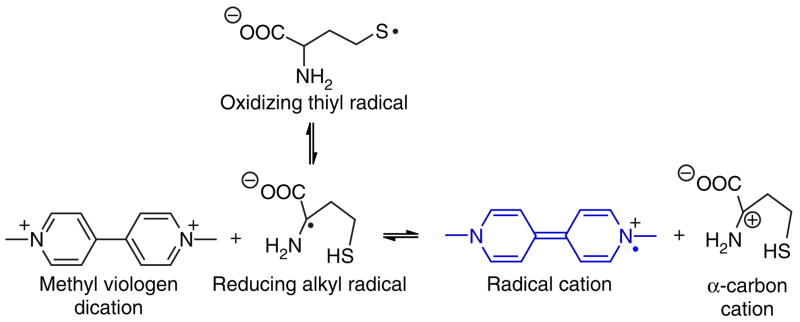
The protocol described here is based on the kinetically favored formation of the alpha-amino carbon-centered radical of Hcy, which allows for the selective reduction of methyl viologen dication to its corresponding radical cation13–15 (Fig. 1). This characteristic reduction mechanism has proven useful in promoting the selective development of chromogen solution color by Hcy. Other commonly occurring thiols (such as cysteine and glutathione) as well as other amino acids do not produce interference14,15. The method should be suitable for those who require a highly selective and simple off-to-on colorimetric test for detecting threshold levels of Hcy in its reduced (i.e., thiol rather than disulfide) form. Protection from air is not necessary during the assay; we believe that this is because dissolved O2, which would quench the radical reactions involved (Fig. 1), is eliminated upon heating. All reagents are commercially available and relatively inexpensive. The protocol is illustrated using commercial reconstituted human blood plasma as a matrix. This method should also be suitable for monitoring reduced Hcy in other biological matrices, such as urine. Changes in reagent and/or buffer concentration allow for adjustment to higher Hcy threshold detection limits15 than those shown here.
Figure 1.
Hcy undergoes a thiyl radical to carbon radical rearrangement more readily than other biothiols. This is attributed to the favored formation of a five-membered ring (H-atom transfer) transition state, as opposed to four-membered and nine-membered ring transition states as in the cases of cysteine and glutathione, respectively. Captodative stabilization of the alpha-amino carbon-centered radical renders the alpha C–H bond weaker than the S–H bond by ~ 4 kcal/mol. Alpha-amino-acid carbon radicals are well-known strong reductants. Favored formation of the reducing alpha-amino radical in the case of Hcy allows for the selective reduction of weakly oxidizing chromogens, such as methyl viologen dication at neutral pH.
MATERIALS
REAGENTS
Certified buffer solution, pH 7, color code yellow (Fisher, cat. no. SB107)
Certified buffer solution, pH 10, color code blue (Fisher, cat. no. SB116)
L-cysteine (Sigma-Aldrich, cat. no. C-7352)
Methyl viologen dichloride hydrate (Sigma-Aldrich, cat. no. 856177)
DL-Hcy ≥95% (Sigma-Aldrich, cat. no. 193143)
Human plasma (Sigma-Aldrich, cat. no. P9523)
Hydrochloric acid (HCl) 37–38% (EMD, cat. no. HX0607-2)
Tris(hydroxymethyl)aminomethane (Sigma-Aldrich, cat. no. T87602)
EQUIPMENT
Hot plate, type 2300 (Barnstead/Thermolyne, cat. no. HP2305B)
Volumetric flask, 100 ml (Pyrex, cat. no. 5580)
pH meter (Orion, cat. no. 410A)
Glass vials, 15 × 45 mm, screw thread with rubber-lined cap (Gerresheimer, cat. no. 60910L-1)
Teflon-coated stir bar (VWR, cat. no. 58948-091)
Magnetic stirrer (Barnstead/Thermolyne, cat. no. S130815)
Plastic transfer pipettes (Samco, cat. no. 202)
Glass microsyringe, 10 μl (Hamilton, cat. no. 701)
EQUIPMENT SETUP
pH meter calibration
Turn on the pH meter. Select calibration mode from 7 to 10. Immerse the electrode into the certified buffer solution (pH 7). Wait until the reading stops blinking and a beeping sound is heard. Remove the buffer solution. Rinse the electrode with deionized H2O. Remove any excess H2O from the electrode with a paper tissue. Immerse the electrode into the certified buffer solution (pH 10). Wait until the reading stops blinking and a beep sound is heard. Remove the buffer solution. Rinse the electrode with deionized H2O. Remove the excess water with a paper tissue. Timing is 10 min.
REAGENT SETUP
Tris 0.5 M pH 7
Weigh 6.06 g Tris(hydroxymethyl)aminomethane into a 150-ml beaker. Add 90 ml deionized H2O and a Teflon-coated stir bar. Stir until all the solid is dissolved using a magnetic stirrer. Dip the pH meter electrode in the solution and keep stirring. Add concentrated HCl dropwise using a plastic transfer pipette until pH 7 is reached. Timing is 10 min. ! CAUTION HCl is corrosive. Wear gloves, apron and safety glasses, and avoid direct contact with vapors.
Plasma
Allow the bottle of lyophilized plasma to warm up to room temperature. Remove the metallic seal from the bottle and insert a needle through the rubber septum to relieve the inner pressure. Reconstitute the plasma by adding the appropriate amount of deionized water. Swirl the liquid gently until a suspension is obtained. Timing is 5 min (15 min with sample warm up).
Hcy stock solution
Weigh out 10.5 mg DL-Hcy (95%). Transfer to a 100-ml volumetric flask. Add 90 ml deionized H2O. Dissolve the solid by gently swirling the flask. Add deionized H2O until reaching the mark in the flask. Swirl the flask gently. Timing is 5 min. ▴ CRITICAL Hcy solutions should be used fresh.
Cysteine stock solution
Weigh out 272.6 mg L-cysteine. Transfer to a 100-ml volumetric flask. Add 90 ml deionized H2O. Dissolve the solid by gently swirling the flask. Add deionized H2O until reaching the mark in the flask. Swirl the flask gently. Timing is 5 min. ▴ CRITICAL Cysteine solutions should be used fresh.
PROCEDURE
Turn on the hot plate (select setting number 7) in a fume hood.
-
Weigh out 8 mg methyl viologen dichloride hydrate in a glass vial.
! CAUTION Methyl viologen dichloride hydrate is toxic. Wear gloves and safety glasses.
Add 0.1 ml Tris(hydroxymethyl)aminomethane buffer 0.5 M, pH 7 with a 1-ml plastic syringe.
Add 0.5 ml reconstituted plasma with a 1-ml plastic syringe.
Add 3.5 μl Hcy standard solution using a 10 μl glass microsyringe (the final concentration of Hcy should be 5 μM).
Close the vial tightly (using the appropriate screw cap).
Place the vial on the hot plate.
-
Shake the vial twice during the first minute of heating.
! CAUTION Handle with care as burns are possible. Wear gloves and safety glasses.
-
A faint blue color will develop in the refluxing solution after 4 min (the air stream entering the fume hood will moderately cool the walls of the vial being heated prompting solvent condensation).
▴ CRITICAL Run a replicate; be sure that heating is uniform if running samples concurrently.
Repeat Steps 1–9 for additions of 7, 10.5 and 21 μ Hcy standard solution (the final concentrations of Hcy should be 10, 15 and 30 μM, respectively). Different visual color intensities will be obtained (Fig. 1).
Run a control sample with no Hcy added.
-
Run a sample with the addition of 10 μl cysteine standard solution (the final concentration of cysteine should be 450 μM) to rule out interferences.
• TIMING 30 min for each sample if not run concurrently
• ? TROUBLESHOOTING
• TIMING
Steps 1–11: 30 min
Step 12: 30 min for each sample if not run concurrently
• ? TROUBLESHOOTING
Troubleshooting advice can be found in Table 1.
TABLE 1.
Troubleshooting table.
| Problem | Possible reasons | Solution |
|---|---|---|
| Color formation from Hcy is not observed at desired detection limit | Hcy solution is not fresh | Use sample containing reduced Hcy immediately |
| Low Hcy levels | Run replicates; concentrations of methyl viologen dichloride hydrate and/or buffer might need adjustment (e.g., see ref. 15); time and/or temperature might need adjustment | |
| Wrong buffer | Buffer must be Tris; no substitutes are acceptable | |
| Solution color fades rapidly | Methyl viologen dichloride hydrate radical cation is quenched | Sample should not be allowed to cool before visual inspection; do not move sample |
| Solution color formation is nonselective; observed with cysteine and/or other thiols | pH is too high, resulting in nonselective formation of reducing disulfide radical cation | Check buffer solution and check that the pH in the plasma sample is neutral using pH paper |
| Metal catalysis | Avoid contact with metals; use deionized water | |
| Solution turns dark greenish after 4 min | Temperature is too high or heating period is too long | Use milder heating |
| Foaming observed in vial | Leak | Make sure vials are tightly closed before heating |
ANTICIPATED RESULTS
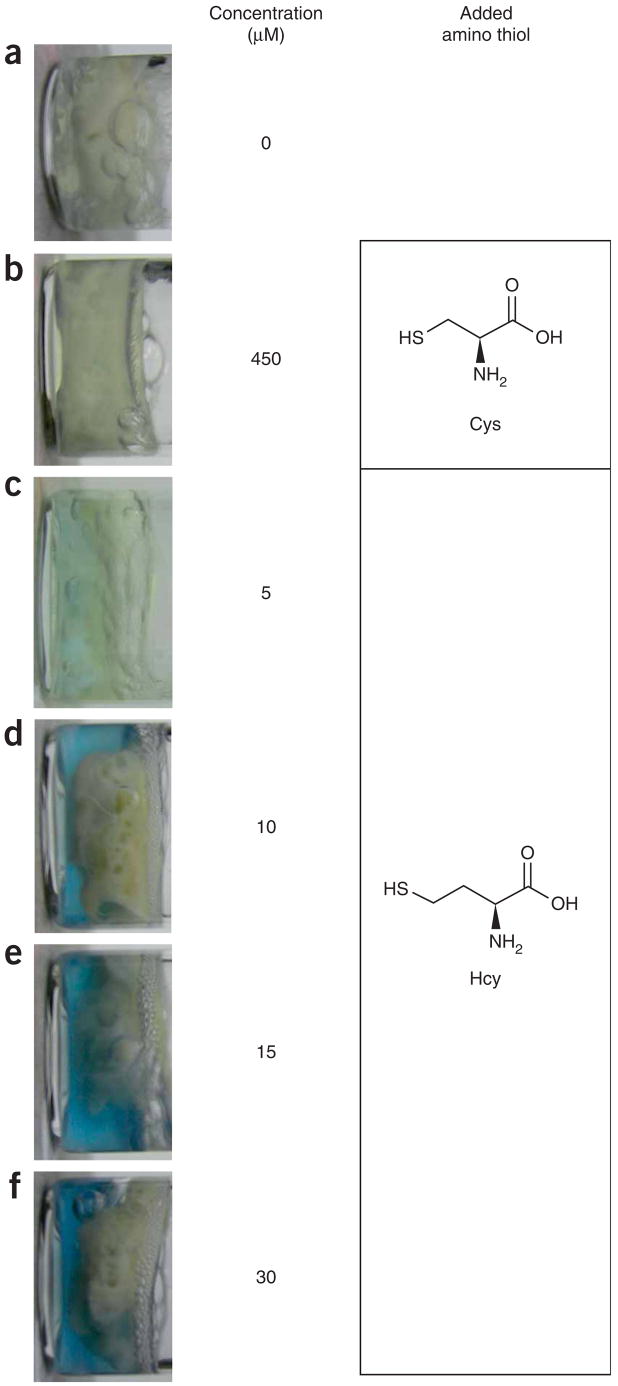
Figure 2 shows that solutions containing Hcy can be visually distinguished from the blank plasma sample and the cysteine-containing control solutions. As the concentration of Hcy is increased, the solution color intensifies.
Figure 2.
Photographs of vials containing human blood plasma and methyl viologen dication in buffer solution containing (from upper to lower) (a) no added reduced Hcy, (b) 450 μM cysteine, (c) 5 μM Hcy, (d) 10 μM Hcy, (e) 15 μM Hcy and (f) 30 μM Hcy. The results demonstrate that the color formation and intensity are due to the Hcy levels. Threshold values denoting dangerous Hcy concentrations in human blood plasma are ≥12–15 μM. Normal urinary values might vary but are generally <9.5 μM The shades of the solutions shown are intended as a general guide, along with the protocol, to allow the user to adjust the sensitivity and detection limit. The solid material in the vials apparently comprises denatured proteins and related materials that congeal upon heating.
Footnotes
COMPETING INTERESTS STATEMENT The authors declare that they have no competing financial interests.
References
- 1.Haugland RP. Thiol-reactive probes. In: Gregory J, editor. Molecular Probes – Handbook of Fluorescent Probes and Research Products. 9. Molecular Probes, Inc; Eugene, Oregon: 2002. pp. 79–93. [Google Scholar]
- 2.Ivanov AR, Nazimov IV, Baratova LA. Qualitative and quantitative determination of biologically active low-molecular-mass thiols in human blood by reversed-phase high-performance liquid chromatography with photometry and fluorescence detection. J Chromatogr A. 2000;870:433. doi: 10.1016/s0021-9673(99)00947-4. [DOI] [PubMed] [Google Scholar]
- 3.Fermo I, et al. High-performance liquid chromatographic method for measuring total plasma homocysteine levels. J Chromatogr B. 1998;31:779. doi: 10.1016/s0378-4347(98)00405-8. [DOI] [PubMed] [Google Scholar]
- 4.Wu CW, Yarbrough LR, Wu FYH. N-(1-pyrene)maleimide: a fluorescent crosslinking reagent. Biochemistry. 1976;15:2863. doi: 10.1021/bi00658a025. [DOI] [PubMed] [Google Scholar]
- 5.Shimada K, Mitamura K. Derivatization of thiol-containing compounds. J Chromatogr B. 1994;659:227–241. doi: 10.1016/0378-4347(93)e0444-u. [DOI] [PubMed] [Google Scholar]
- 6.Nekrassova O, Lawrence NS, Compton RG. Analytical determination of homocysteine: a review. Talanta. 2003;60:1085–1095. doi: 10.1016/S0039-9140(03)00173-5. [DOI] [PubMed] [Google Scholar]
- 7.Refsum H, et al. Facts and recommendations about total homocysteine determinations: an expert opinion. Clin Chem. 2004;50:3–32. doi: 10.1373/clinchem.2003.021634. [DOI] [PubMed] [Google Scholar]
- 8.Refsum H, Ueland PM, Nygård O, Vollset SE. Homocysteine and cardiovascular disease. Annu Rev Med. 1998;49:31–62. doi: 10.1146/annurev.med.49.1.31. [DOI] [PubMed] [Google Scholar]
- 9.Seshadri S, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 10.Lentz SR, Haynes WG. Homocysteine: a clinically important cardiovascular risk factor? Cleveland Clinic J Med. 2004;71:729–734. doi: 10.3949/ccjm.71.9.729. [DOI] [PubMed] [Google Scholar]
- 11.Shipchandler MT, Moore EG. Rapid, fully automated measurement of plasma homocyst(e)ine with the abbott imx analyzer. Clin Chem. 1995;41:991–994. [PubMed] [Google Scholar]
- 12.Pernet P, Lasnier E, Vaubourdolle M. Evaluation of the AxSYM homocysteine assay and comparison with the Imx homocysteine assay. Clin Chem. 2000;46:1440–1441. [PubMed] [Google Scholar]
- 13.Zhao R, Lind J, Merényi G, Eriksen TE. Kinetics of one-electron oxidation of thiols and hydrogen abstraction by thiyl radicals from alpha-amino C-H bonds. J Am Chem Soc. 1994;116:12010–12015. [Google Scholar]
- 14.Wang W, Escobedo JO, Lawrence CM, Strongin RM. Direct detection of homocysteine. J Am Chem Soc. 2004;126:3400–3401. doi: 10.1021/ja0318838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang W, et al. Detection of homocysteine and cysteine. J Am Chem Soc. 2005;127:15949–15958. doi: 10.1021/ja054962n. [DOI] [PMC free article] [PubMed] [Google Scholar]