Abstract
Convection-enhanced delivery (CED) has recently entered the clinic and represents a promising new delivery option for targeted gene therapy in Parkinson’s disease (PD). The prime stereotactic target for the majority of recent gene therapy clinical trials has been the human putamen. The stereotactic delivery of therapeutic agents into putamen (or other subcortical structures) via CED remains problematic due to the difficulty in knowing what volume of therapeutic agent to deliver. Preclinical studies in non-human primates (NHP) offer a way to model treatment strategies prior to clinical trials. Understanding more accurately the volumetric differences in striatum, especially putamen, between NHP and humans is essential in predicting convective volume parameters in human clinical trials. In this study, magnetic resonance images (MRI) were obtained for volumetric measurements of striatum (putamen and caudate nucleus) and whole brain from 11 PD patients, 13 aged healthy human subjects, as well as 8 parkinsonian and 30 normal NHP. The human brain is 13–18 times larger than the monkey brain. However, this ratio is significantly smaller for striatum (5.7–6.5), caudate nucleus (4.6–6.6) and putamen (4.4–6.6). Size and species of the monkeys used for this comparative study are responsible for differences in ratios for each structure between monkeys and humans. This volumetric ratio may have important implications in the design of clinical therapies for PD and Huntington’s disease and should be considered when local therapies such as gene transfer, local protein administration or cellular replacement are translated based on non-human primate research.
Keywords: MRI, Parkinson’s disease, convection-enhanced delivery, putamen volume, non-human primates
Introduction
The putamen is a primary site for the principal neuropathology associated with PD (Aminoff, 2001; Berheimer et al., 1973) and other movement disorders, such as Multiple System Atrophy (Mark, 2001), Creutzfeldt-Jacob Disease (Maltete et al., 2006), Huntington’s disease (Berardelli et al., 1999) and Torsion Dystonia (Muller and Kupke, 1990). For this reason, our group has used the putamen as the target for an enzyme replacement gene therapy delivered via CED for PD (Bankiewicz et al., 2000; Forsayeth et al., 2006; Hadaczek et al., 2006). Similarly, other groups have also targeted the putamen with growth factors, either as recombinant protein (Lang et al., 2006) or in a gene therapy vector (Gasmi et al., 2007; Herzog et al., 2007). A challenging feature of interventional strategies is likely to be that infusion must be specifically and homogenously delivered throughout the putamen.
The aim of the current work was to establish parameters that could help define the relative volume of the striatum between NHP and humans to aid accurate translation of NHP findings into clinical application. There are few studies in the literature that give specific estimates of relative size of the basal ganglia of humans and monkeys. An extensive histological study by Hardman and colleagues (Hardman et al., 2002) showed that the relative sizes of substantia nigra (SN), external (EGP) and internal (IGP) globus pallidus, and subthalamic nucleus (STN) ranged from 2.3 (SN) to approximately 9 (IGP). Accordingly, we have conducted a more comprehensive, minimally invasive study of normal and parkinsonian brains in which MRI was used to identify and define the area of putamen, caudate nucleus and total brain volume on serial slices, and establish their volumes in both humans and non-human primates. The use of MRI rather than histology avoids some of the potential for artifacts induced by processing of post mortem tissue (Harman and Carpenter, 1950; Schroder et al., 1975).
Materials and Methods
Subjects
Data from Parkinsonian individuals were derived from MRI conducted as part of a prospective, open-label phase I clinical trial examining the safety and tolerability of putaminal gene transfer in patients with Parkinson’s disease (PD). It was reviewed and approved by the Recombinant DNA Advisory Committee of the National Institutes of Health, the United States Food and Drug Administration, and the Institutional Review Board of the University of California San Francisco. All patients underwent extensive pre-operative screening and counseling, and provided written informed consent prior to entry into the study, as well as prior to the surgical procedures. Eleven patients diagnosed with PD were all at Stages III to IV on the Hoehn and Yahr scale (5 males and 6 females), and had classic clinical features of the disorder, with no evidence of any other neurological disease. Their ages ranged from 58 to 71 years, with mean age of 64.9 years. Thirteen normal individuals (5 males and 8 females) had no neurological disease or structural lesions involving the basal ganglia. Their ages ranged from 63 to 88 years, with mean age of 72.6 years.
Eight Rhesus macaques with MPTP lesions (all male, age ranging from 5 to 8 years with mean age 6.9 years, weight 8.7–14 kg, 7 Rhesus macaques without MPTP lesions (5 male and 2 female, age ranging from 5 to 7 years with mean age 5.4 years, weight 6.9–16 kg) and 22 normal Cynomolgus (Cyno.) monkeys (16 male and 6 female, age ranging from 5 to 9 years with mean age of 6.9 years, weight 3–5 kg) were the subjects of the present study. To induce parkinsonism, monkeys (NHP) were subjected to MPTP lesioning 1–2 years before their MRI was taken. The animals were housed separately in home cages in a temperature-controlled room and exposed to 12-h light/dark cycle. They were fed twice daily in amounts appropriate for the size and age of the animals, and water was freely available. The diet was supplemented with fruit or vegetables daily. Furthermore, small pieces of fruit, cereal, or other treats were provided as part of the environmental enrichment program. Experimentation was performed according to the National Institutes of Health guidelines and to the protocols approved by the Institutional Animal Care and Use Committee at the University of California San Francisco (San Francisco, CA) and at Wincon TheraCells Biotechnologies Co. Ltd. (Nanning, China).
Magnetic resonance image (MRI)
MR images of normal human subjects were acquired on a 1.5T Siemens Magnetom Avanto (Siemens AG, Munich, Germany). Three-dimensional rapid gradient echo (MP-RAGE) images were obtained with repetition time (TR) = 2110 ms, echo time (TE) = 3.6 ms, and a flip angle of 15°. The number of excitations (NEX) = 1 (repeated 3 times), matrix = 240 × 240, field of view (FOV) = 240 × 240 × 240, and the slice thickness = 1 mm. These parameters resulted in a 1-mm3 voxel volume. The scanning time was approximately 9 min.
MR images in Parkinson’s disease patients were acquired on a 1.5T Philips Intera scanner (Philips Medical Systems, Best, The Netherlands) with a rigid head coil. Axial inversion recovery (IR) sequences were obtained with TR = 3000 ms, TE = 40 ms, and an inversion time (TI) = 200 ms. The NEX = 3, matrix = 304 × 195, FOV = 260 × 222 and the slice thickness = 2 mm. These parameters resulted in a voxel size of 0.86 × 1.14 mm. Scanning time was approximately 12 minutes.
MR images of NHP brain were acquired on a 1.5-T Sigma LX scanner (GE Medical Systems, Waukesha, WI) with a 5-inch surface coil on the subject’s head, parallel to the floor. Spoiled gradient echo (SPGR) images were T1-weighted and obtained with a spoil grass sequence, a TR = 2170 ms, a TE = 3.8 ms, and a flip angle of 15°. The NEX = 4, matrix = 256 × 192, FOV = 16 cm × 12 cm, slice thickness = 1 mm. These parameters resulted in a 0.391 mm3 voxel volume. Scanning time was approximately 20 min. NHP were sedated with a mixture of ketamine (Ketaset, 7 mg/kg, IM) and xylazine (Rompun, 3 mg/kg, IM). After sedation, each animal was placed in a MRI-compatible stereotactic frame. The ear-bar and eye-bar measurements were recorded, and an intravenous line was established. MRI data was then obtained and animals were allowed to recover under close observation until able to right themselves in their home cages.
Volumetric quantification of putamen, caudate nucleus and total brain of human and NHP
The volume of right and left putamen, caudate nucleus and brain of each subject was quantified on an Apple Macintosh G4 computer with OsiriX® Medical Image Software (v2.5.1). OsiriX software reads all data specifications from DICOM (digital imaging and communications in medicine) formatted MR images obtained via local picture archiving and communication system (PACS). Regions-of-interest (ROI’s) derived from putamen, caudate nucleus, and brain were manually defined, and the software then calculated the area from each MR image, and established the volume of the ROI, based on area defined multiplied by slice thickness (PACS volume). The boundaries of each structure were defined in the same manner in the series of MRI sections. The sum of the PACS ROI volumes (number of MRI slices evaluated) for the particular structure being analyzed determined the measured structure volume. The defined ROI volumes allowed for three-dimensional (3D) image reconstruction with BrainLAB software (BrainLAB, Heimstetten, Germany). MRI’s were evaluated and ROI volumes determined by two independent observers blind as to whether images were taken from normal or parkinsonian individuals. In a preliminary comparison of putaminal volumes calculated by the two observers in seven Rhesus monkeys, there was no significant difference between the mean values obtained.
Induction of parkinsonism in Rhesus monkeys
Eight Rhesus monkeys (8.7–14 kg) were lesioned with 1-methy-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) as previously described (Bankiewicz et al., 1986). Briefly, MPTP lesioning consisted of unilateral (right-side) intracarotid infusion of 2.5–3.5 mg of MPTP-HCI plus four intravenous administrations of 0.3 mg/kg doses of MPTP-HCI, which produces a nearly complete dopaminergic lesion on the side of carotid artery infusion (ipsilateral side) and a partial lesion on the other side of the brain (contralateral side). Neurological assessment and clinical rating scale (CRS) was performed to confirm parkinsonism in these monkeys. After MPTP administration, animals developed signs of PD manifested by general slowness, bradykinesia, rigidity, balance disturbance, and flexed posture. The left arm was less frequently used than the right in all monkeys; all showed signs of tremor.
Statistical Analysis
Volumes of putamen, caudate nucleus and striatum were compared across subject groups by Student’s t-test. The criterion for statistical significance for all tests was p < 0.05.
Results
Volume of putamen in normal individuals and PD patients
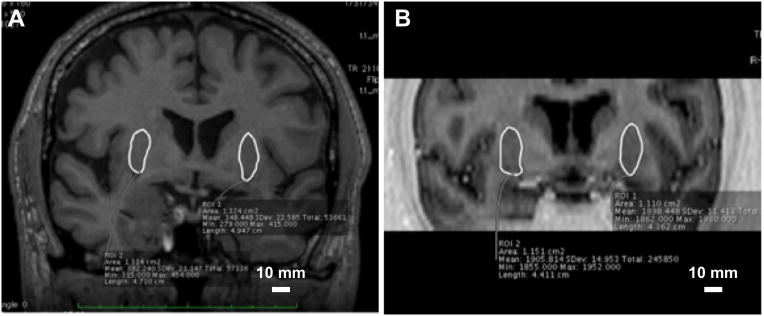
MR images were obtained from 11 PD patients (mean age 64.9 ± 1.7 years) and 13 normal individuals (mean age 72.6 ± 1.4 years). The volume of right putamen in normal individuals, ranged from 2.70 cm3 to 4.89 cm3 with mean volume of 3.60 ± 0.18 cm3, and the volume of left putamen ranged from 2.38 cm3 to 4.54 cm3 with mean volume of 3.55 ± 0.17 cm3 (Fig. 1). There was no significant difference between right and left putamen in normal individuals (p = 0.84). Pooling data from both left and right hemispheres gave a mean volume of 3.57 ± 0.12 cm3. Figure 2A shows a representative putamen from a normal individual. The volume of right putamen in PD patients ranged from 3.01 cm3 to 5.29 cm3 with mean volume of 3.94 ± 0.22 cm3, and the volume of left putamen ranged from 3.11 cm3 to 5.29 cm3 with a mean volume of 4.02 ± 0.23 cm3 (Fig. 1). There was no significant difference between right and left putamen in PD patients (p = 0.78). Pooling data from both left and right hemispheres gave a mean volume 3.98 ± 0.15 cm3. Figure 2 shows a comparison of a representative putamen from a normal subject (Panel A) and a PD patient (Panel B).
Figure 1.
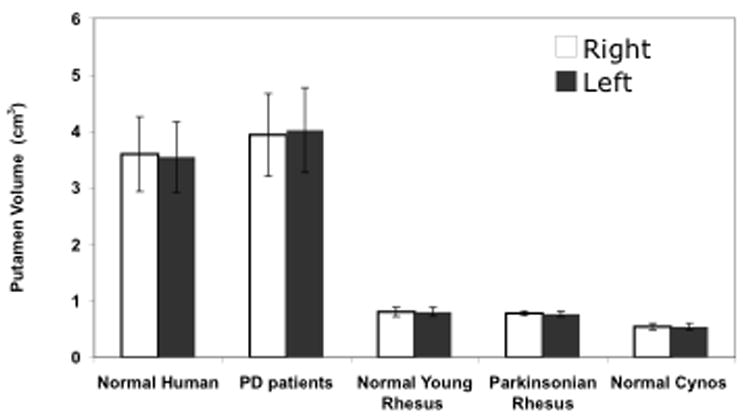
Volume of right and left putamen, caudate nucleus and striatum in normal humans, in PD patients, in parkinsonian and normal Rhesus, and in normal Cynomolgus monkeys
Figure 2.
MR images of putamen (green outline) in representative normal (A) and parkinsonian (B) Rhesus monkey
Volume of putamen in normal and parkinsonian monkeys
Seven normal aged Rhesus monkeys (mean age 5.4 ± 0.3 years) and 22 normal Cynomolgus monkeys (mean age 6.9 ± 0.2 years), as well as 8 MPTP-lesioned Rhesus monkeys (mean age 6.9 ± 0.4 years), underwent MR imaging. Parkinsonism in monkeys was confirmed by neurological and behavioral assessment (data not shown).
The volume of right putamen in normal young Rhesus monkeys without parkinsonism ranged from 0.73 cm3 to 0.89 cm3 with mean volume of 0.81 ± 0.02 cm3, and the volume of left putamen ranged from 0.72 cm3 to 0.90 cm3 with mean volume of 0.81 ± 0.02 cm3 (Fig. 1). There was no significant difference between right and left putamen in this group (p = 0.97). Overall, normal young monkeys have an average putaminal volume of 0.81 ± 0.01 cm3. Figure 3B indicates delineation of putamen in a single MRI section from a representative parkinsonian monkey. For normal Cynomolgus monkeys without parkinsonism, the volume of right putamen ranged from 0.44 cm3 to 0.65 cm3 with mean volume of 0.55 ± 0.01 cm3, and the volume of left putamen ranged between 0.45 cm3 and 0.65 cm3 with mean volume of 0.54 ± 0.01 cm3 (Fig. 1). There was no difference between right and left putamen in this group (p = 1.0). The volume of the putamen in normal aged monkeys was 0.55 ± 0.01 cm3.
Figure 3. Comparison of representative MR images of putamen (outlined in green) from human PD patient (A) and normal Rhesus monkey (B).
The monkey striatum has been enlarged relative to the human to facilitate comparison.
For parkinsonian Rhesus monkeys, the volume of right (lesioned) putamen ranged from 0.74 cm3 to 0.83 cm3 with mean volume of 0.78 ± 0.01 cm3, whereas the volume of the left (partially lesioned) putamen ranged from 0.75 cm3 to 0.84 cm3, with mean volume of 0.77 ± 0.02 cm3 (Fig. 1). There was no significant difference between the volume of right and left putamen in parkinsonian monkeys (p = 0.99). The mean volume of the putamen in parkinsonian monkeys was 0.78 ± 0.01 cm3. Figure 3 depicts a representative MRI section from a normal (panel A) and a parkinsonian (panel B) Rhesus monkey.
Volume of caudate nucleus, striatum and total brain in humans and monkeys
The volumes of caudate nucleus (Fig. 1 and Table 1) were 3.50 ± 0.26 cm3 for PD patients, 2.73 ± 0.06 cm3 for normal aged individuals, 0.54 ± 0.01 cm3 for parkinsonian rhesus monkey, 0.59 ± 0.02 cm3 for normal rhesus monkeys and 0.41 ± 0.02 cm3 for normal Cynomolgus monkeys. The volumes of striatum (Fig. 1) were 7.53 ± 0.84 cm3 for PD patients, 6.33 ± 0.44 cm3 for normal aged individuals, 1.32 ± 0.04 cm3 for parkinsonian Rhesus monkey, 1.40 ± 0.04 cm3 for normal Rhesus monkeys and 0.97 ± 0.04 cm3 for normal Cynomolgus monkeys. The volumes of total brain were 1470.72 ± 131.36 cm3 for PD patients, 1253.82 ± 70.90 cm3 for normal aged individuals, 104.24 ± 4.12 cm3 for parkinsonian rhesus monkey, 96.87 ± 3.00 cm3 for normal rhesus monkeys and 69.52 ± 4.56 cm3 for normal Cynomolgus monkeys.
Table 1.
Volumetric ratios of putamen, caudate nucleus and striatum vs the whole brain
| Putamen:Brain | Caudate:Brain | Striatum:Brain | |
|---|---|---|---|
| Normal Human | 1:351 | 1:459 | 1:198 |
| PD patient | 1:369 | 1:420 | 1:195 |
| Normal Rhesus | 1:120 | 1:164 | 1:69 |
| PD Rhesus | 1:134 | 1:194 | 1:77 |
| Normal Cynomolgus | 1:127 | 1:169 | 1:71 |
All three ratios are significantly smaller in NHP than humans, supporting the notion that cortex constitutes the majority of the difference in volume between human and NHP brain.
Volumetric ratios of putamen, caudate nucleus and striatum in humans and monkeys
There is a statistically significant difference in the volume of putamen, caudate nucleus and striatum between humans and monkeys. The ratio of volume of putamen, caudate nucleus and striatum in PD patients vs parkinsonian monkeys is 5.12:1 (p < 0.0001), 6.54:1 (p < 0.0001), and 5.70: 1 (p < 0.0001), respectively (Fig. 4). The ratio of volume of putamen, caudate nucleus and striatum in normal human individuals vs Rhesus monkeys is 4.43:1 (p < 0.0001), 4.62:1 (p < 0.0001) and 4.53:1 (p < 0.0001), respectively (Fig. 4). The ratio of volume of striatum in normal humans vs Cynomolgus monkeys is 6.55:1 (p < 0.0001), 6.62:1 (p < 0.0001) and 6.52:1 (p < 0.0001), respectively (Fig. 4).
Figure 4. Volume of putamen, caudate nucleus and striatum in normal human vs normal Rhesus monkeys, normal human vs Cynomolgus monkeys, and Parkinsonian patient vs Parkinsonian Rhesus monkey.
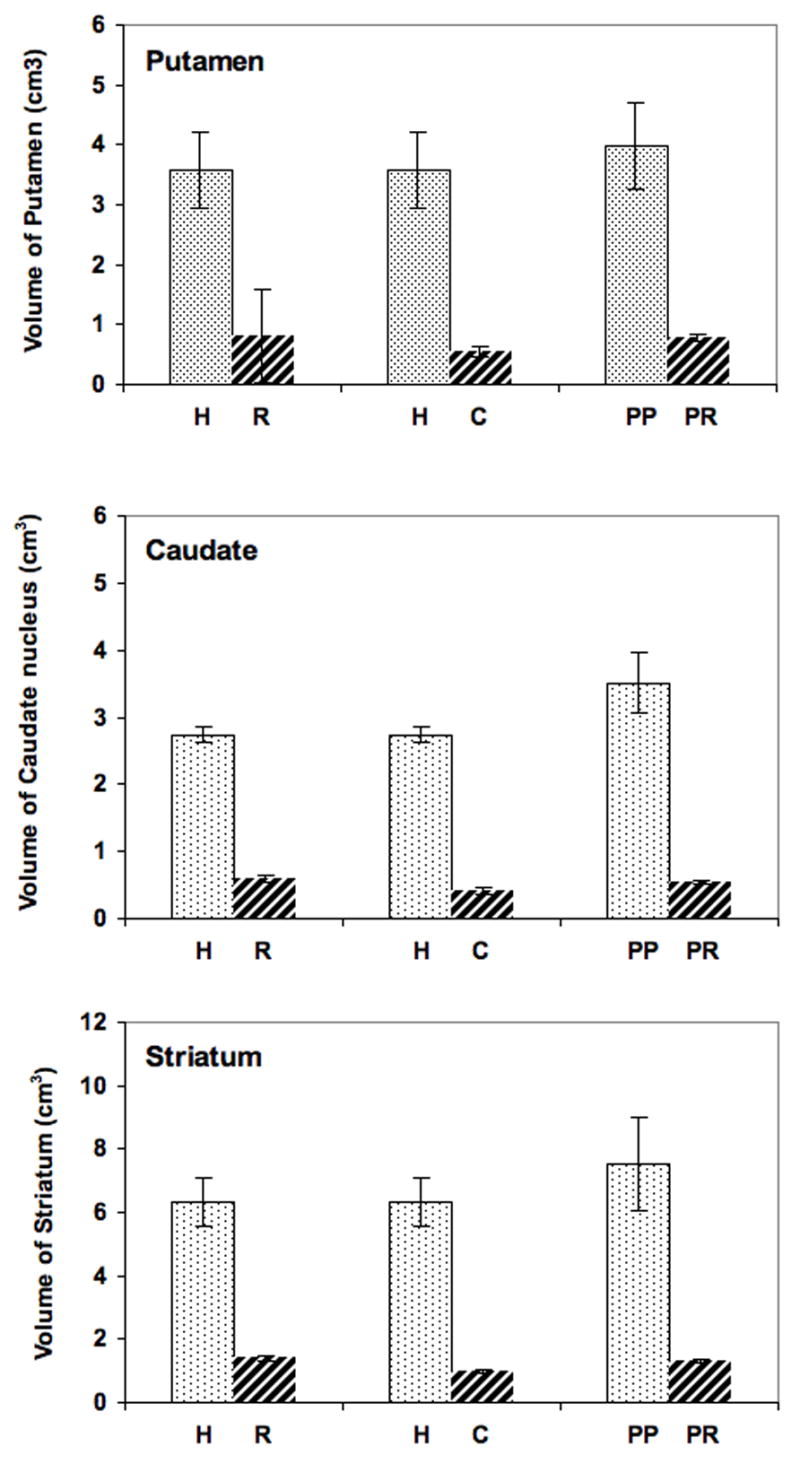
H, human; R, Rhesus; C, Cynomolgus; PP, parkinsonian patient; PR, parkinsonian Rhesus. **, p < 0.0001. Ratiometric differences are indicated above each data set.
The volume of putamen, caudate nucleus and striatum in humans and monkeys were also compared relative to brain volume. This method enabled direct comparisons of proportional volume of the structures in relation to the volume of total brain for individuals of each species. The analysis revealed that monkeys have a larger putamen, caudate nucleus, and striatum compared with total brain volume, when matched with similar measurements in humans (Table 1). This would be expected, due to the expansion of neocortex in humans versus non-human primates. It is also instructive to compare the relative sizes of the human and Rhesus monkey brain (Fig. 6) based on the relative sizes of the striatum as calculated above. Apart from the obvious difference in size, the overall morphology of the basal ganglia is remarkably similar.
Discussion
Our MRI measurements determined the volume of the putamen to be 0.7774 ± 0.01 cm3, 0.8060 ± 0.01 cm3, and 0.5460 ± 0.01 cm3 for parkinsonian Rhesus, normal Rhesus and normal Cynomolgus monkeys, respectively, and 3.9801 ± 0.15 cm3 and 3.574 ± 0.12 cm3 for PD patients and normal healthy individuals, respectively. There were no significant differences noted between right and left hemispheres in any subjects. We also found that the volumetric ratio for the putamen was 5.12:1 for parkinsonian patients vs Rhesus monkeys, and 4.43:1 and 6.55:1 for normal human individuals vs Rhesus and Cynomolgus monkeys, respectively. Normal humans were almost 8 years of age older, on average, than the PD patients. In patients over age 60, one might expect a significant decline in brain volume. Similarly, normal aged Rhesus monkeys averaged 5.4 years of age, whereas the parkinsonian Rhesus and Cynomolgus averaged 6.9 years of age. Nevertheless, the ratios that we obtained predict a roughly 5:1 difference in volume of the human vs non-human primate putamen. As such, a putaminal Vi for CED in the non-human primate, would be multiplied by 5 for potential Vi into a human putamen, if similar CED delivery parameters are employed.
CED is being applied clinically to the treatment of neurodegenerative disorders, such as PD (Eberling et al., 2008; Gill et al., 2003) and brain malignancies (Kunwar, 2003; Mardor et al., 2001). It is essential for efficacy to cover the entire targeted treatment volume while avoiding adjacent regions of the brain or CSF pathways. It has been very difficult to predict the volume of infusion (Vi) of therapeutics delivered by CED due to a lack of knowledge of volume of distribution (Vd) under these circumstances. This is true for delivery of chemotherapeutic agents to brain tumors, and for infusion of growth factors, enzymes, and viral vectors in PD patients. MRI allows the putamen and caudate nucleus to be visualized with great precision, and enables us to understand the associated volumes of the structures to be convected (infused by CED), thereby improving the accuracy of the CED procedure, provided that the Vd and Vi for the particular therapeutic within that structure are known. Volumetric differences of brain structures between non-human primates and humans provides a basis for prediction of appropriate Vi in patients based on preclinical non-human primate data. This knowledge is also critical when calculating dose of therapeutic agents such as proteins or viral vectors to be delivered to human striatum.
Under the best circumstances, targeting and delivery of therapeutics in the CNS can now be performed with the aid of real-time MRI imaging. These imaging modalities may markedly impact future neurosurgical treatments of neurodegenerative and neuro-oncologic diseases, by allowing more accurate, monitored delivery of therapeutics (Bobo et al., 1994; Hadaczek et al., 2006; Krauze et al., 2006; Krauze et al., 2005a; Krauze et al., 2005b; Saito et al., 2005). It has been proposed that such direct scrutiny of these treatments will increase efficacy and reduce morbidity. For the short-term, however, intra-operative MRI imaging remains a modality available to a limited number of large medical centers.
In previous reports, regional morphological changes in the brain of PD patients and healthy subjects have been studied (Duguid et al., 1986; Hutchinson and Raff, 2000; Lisanby et al., 1993; O’Neill et al., 2002; Schulz et al., 1999). However, comparison of striatal volume by MRI in non-human primates and humans has not been reported so far. By means of MR imaging of the live brain in our studies, we have avoided the artifacts of histological processing on the brain parenchyma previously noted by others (Harman and Carpenter, 1950; Schroder et al., 1975). In the present study, we employed MRI data to perform volume measurements of putamen, caudate, and total brain, and to compare the volume difference of the structures and generate volumetric ratios for these structures between non-human primates and humans. Unfortunately, MRI volumetric determinations have their own associated potential errors (Pan et al., 2007; Snell et al., 2006; Sumanaweera et al., 1994). We have minimized these volumetric errors in our study through the use of updated software on the MRI scanners to minimize scanner-induced magnetic field heterogeneous distortion or gradient field nonlinearity distortion (Sumanaweera et al., 1994). Also, since our volumetric structures are located within the brain parenchyma, without large differences in tissue magnetic susceptibility, magnetic field heterogeneous distortion induced by the imaged structure’s environment does not come into play (as it would in the measurement of structures at the skull base, where there is an air/brain/bone boundary). In addition, all of our volumetric calculations have been carried out using multiple MRI slices, which have been shown to reduce PACS-associated errors (Pan et al., 2007; Snell et al., 2006). All our volumes measured were > 0.5 cm3, making PACS volumetric measurements robust, with low associated error when combined with multiple MRI slices (Pan et al., 2007). Finally, errors present using our volumetric MRI measurements would have been similar in all of our data. While absolute volume data can be affected by error, the volumetric ratios between NHP and humans would remain valid. Despite these limitations, this is the first study to perform detailed comparative quantitative analyses of striatal structures by MRI, in preparation for additional translational designs of clinical CED therapy for PD patients, based on non-human primate studies.
In summary, the present study provides the first comparative quantitative analysis by MRI of striatal volume in humans and non-human primates, including putamen and caudate nucleus. These data have significant implications for the design of future clinical trials featuring CED of various therapeutic agents into the putamen for PD. Volumetric data developed from our translational non-human primate studies, related to Vd and Vi of various therapeutics delivered via CED into the putamen, can be scaled up to the human by a factor of 5. Although further volumetric studies and in vivo real-time MRI CED infusions will be carried out to further refine the volumetric data, we are confident in reporting these volumetric differences in the striatum of humans and non-human primates.
Figure 5. Comparison of representative MR images of from human (A) and Rhesus monkey (B) (putamen outlined in white).
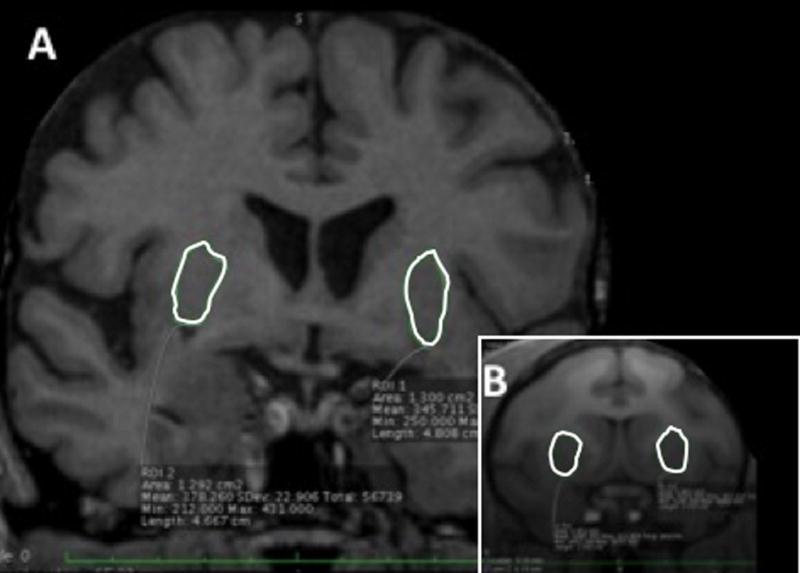
The images are scaled to illustrate the 5-fold greater striatal volume of the human striatum compared to that of the Rhesus monkey.
Acknowledgments
This work was supported in part by a NIH-NINDS award (U54NS045309) and a generous gift from the Kinetics Foundation. We are grateful for the technical assistance of John Bringas, Philip Pivirotto, Janine Beyer, and Feng Yue. We also acknowledge the courage and generosity of those who volunteer to become subjects in clinical studies and help to generate much useful data for the benefit of others.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aminoff MJ. Parkinson’s disease. Neurologic clinics. 2001;19:119–28. vi. doi: 10.1016/s0733-8619(05)70008-6. [DOI] [PubMed] [Google Scholar]
- Bankiewicz KS, Eberling JL, Kohutnicka M, Jagust W, Pivirotto P, Bringas J, Cunningham J, Budinger TF, Harvey-White J. Convection-enhanced delivery of AAV vector in parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Experimental neurology. 2000;164:2–14. doi: 10.1006/exnr.2000.7408. [DOI] [PubMed] [Google Scholar]
- Bankiewicz KS, Oldfield EH, Chiueh CC, Doppman JL, Jacobowitz DM, Kopin IJ. Hemiparkinsonism in monkeys after unilateral internal carotid artery infusion of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Life sciences. 1986;39:7–16. doi: 10.1016/0024-3205(86)90431-5. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Noth J, Thompson PD, Bollen EL, Curra A, Deuschl G, van Dijk JG, Topper R, Schwarz M, Roos RA. Pathophysiology of chorea and bradykinesia in Huntington’s disease. Mov Disord. 1999;14:398–403. doi: 10.1002/1531-8257(199905)14:3<398::aid-mds1003>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Berheimer H, Birkmayer W, Hornykiewics O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington- clinical, morphological and neurochemical correlations. Journal of Neurological Sciences. 1973;20:415–55. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:2076–80. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid JR, De La Paz R, DeGroot J. Magnetic resonance imaging of the midbrain in Parkinson’s disease. Annals of neurology. 1986;20:744–7. doi: 10.1002/ana.410200618. [DOI] [PubMed] [Google Scholar]
- Eberling JL, Jagust WJ, Christine CW, Starr P, Larson P, Bankiewicz KS, Aminoff MJ. Results from a phase I safety trial of hAADC gene therapy for Parkinson’s disease. Neurology. 2008 doi: 10.1212/01.wnl.0000312381.29287.ff. In Press. [DOI] [PubMed] [Google Scholar]
- Forsayeth JR, Eberling JL, Sanftner LM, Zhen Z, Pivirotto P, Bringas J, Cunningham J, Bankiewicz KS. A Dose-Ranging Study of AAV-hAADC Therapy in Parkinsonian Monkeys. Mol Ther. 2006;14:571–7. doi: 10.1016/j.ymthe.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasmi M, Brandon EP, Herzog CD, Wilson A, Bishop KM, Hofer EK, Cunningham JJ, Printz MA, Kordower JH, Bartus RT. AAV2-mediated delivery of human neurturin to the rat nigrostriatal system: Long-term efficacy and tolerability of CERE-120 for Parkinson’s disease. Neurobiology of disease. 2007;27:67–76. doi: 10.1016/j.nbd.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Gill SS, Patel NK, Hotton GR, O’Sullivan K, McCarter R, Bunnage M, Brooks DJ, Svendsen CN, Heywood P. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med. 2003;9:589–95. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- Hadaczek P, Kohutnicka M, Krauze MT, Bringas J, Pivirotto P, Cunningham J, Bankiewicz K. Convection-enhanced delivery of adeno-associated virus type 2 (AAV2) into the striatum and transport of AAV2 within monkey brain. Hum Gene Ther. 2006;17:291–302. doi: 10.1089/hum.2006.17.291. [DOI] [PubMed] [Google Scholar]
- Hardman CD, Henderson JM, Finkelstein DI, Horne MK, Paxinos G, Halliday GM. Comparison of the basal ganglia in rats, marmosets, macaques, baboons, and humans: volume and neuronal number for the output, internal relay, and striatal modulating nuclei. The Journal of comparative neurology. 2002;445:238–55. doi: 10.1002/cne.10165. [DOI] [PubMed] [Google Scholar]
- Harman PJ, Carpenter MB. Volumetric comparisons of the basal ganglia of various primates including man. The Journal of comparative neurology. 1950;93:125–37. doi: 10.1002/cne.900930107. [DOI] [PubMed] [Google Scholar]
- Herzog CD, Dass B, Holden JE, Stansell J, 3rd, Gasmi M, Tuszynski MH, Bartus RT, Kordower JH. Striatal delivery of CERE-120, an AAV2 vector encoding human neurturin, enhances activity of the dopaminergic nigrostriatal system in aged monkeys. Mov Disord. 2007 doi: 10.1002/mds.21503. [DOI] [PubMed] [Google Scholar]
- Hutchinson M, Raff U. Structural changes of the substantia nigra in Parkinson’s disease as revealed by MR imaging. Ajnr. 2000;21:697–701. [PMC free article] [PubMed] [Google Scholar]
- Krauze MT, Forsayeth J, Park JW, Bankiewicz KS. Real-time imaging and quantification of brain delivery of liposomes. Pharm Res. 2006;23:2493–504. doi: 10.1007/s11095-006-9103-5. [DOI] [PubMed] [Google Scholar]
- Krauze MT, McKnight TR, Yamashita Y, Bringas J, Noble CO, Saito R, Geletneky K, Forsayeth J, Berger MS, Jackson P, Park JW, Bankiewicz KS. Real-time visualization and characterization of liposomal delivery into the monkey brain by magnetic resonance imaging. Brain Res Brain Res Protoc. 2005a doi: 10.1016/j.brainresprot.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Krauze MT, Saito R, Noble C, Bringas J, Forsayeth J, McKnight TR, Park J, Bankiewicz KS. Effects of the perivascular space on convection-enhanced delivery of liposomes in primate putamen. Experimental neurology. 2005b;196:104–11. doi: 10.1016/j.expneurol.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Kunwar S. Convection enhanced delivery of IL13-PE38QQR for treatment of recurrent malignant glioma: presentation of interim findings from ongoing phase 1 studies. Acta Neurochir Suppl. 2003;88:105–11. doi: 10.1007/978-3-7091-6090-9_16. [DOI] [PubMed] [Google Scholar]
- Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, Brooks DJ, Hotton G, Moro E, Heywood P, Brodsky MA, Burchiel K, Kelly P, Dalvi A, Scott B, Stacy M, Turner D, Wooten VG, Elias WJ, Laws ER, Dhawan V, Stoessl AJ, Matcham J, Coffey RJ, Traub M. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Annals of neurology. 2006;59:459–66. doi: 10.1002/ana.20737. [DOI] [PubMed] [Google Scholar]
- Lisanby SH, McDonald WM, Massey EW, Doraiswamy PM, Rozear M, Boyko OB, Krishnan KR, Nemeroff C. Diminished subcortical nuclei volumes in Parkinson’s disease by MR imaging. Journal of neural transmission. 1993;40:13–21. [PubMed] [Google Scholar]
- Maltete D, Guyant-Marechal L, Mihout B, Hannequin D. Movement disorders and Creutzfeldt-Jakob disease: a review. Parkinsonism & related disorders. 2006;12:65–71. doi: 10.1016/j.parkreldis.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Mardor Y, Roth Y, Lidar Z, Jonas T, Pfeffer R, Maier SE, Faibel M, Nass D, Hadani M, Orenstein A, Cohen JS, Ram Z. Monitoring response to convection-enhanced taxol delivery in brain tumor patients using diffusion-weighted magnetic resonance imaging. Cancer Res. 2001;61:4971–3. [PubMed] [Google Scholar]
- Mark MH. Lumping and splitting the Parkinson Plus syndromes: dementia with Lewy bodies, multiple system atrophy, progressive supranuclear palsy, and cortical-basal ganglionic degeneration. Neurologic clinics. 2001;19:607–27. vi. doi: 10.1016/s0733-8619(05)70037-2. [DOI] [PubMed] [Google Scholar]
- Muller U, Kupke KG. The genetics of primary torsion dystonia. Human genetics. 1990;84:107–15. doi: 10.1007/BF00208922. [DOI] [PubMed] [Google Scholar]
- O’Neill J, Schuff N, Marks WJ, Jr, Feiwell R, Aminoff MJ, Weiner MW. Quantitative 1H magnetic resonance spectroscopy and MRI of Parkinson’s disease. Mov Disord. 2002;17:917–27. doi: 10.1002/mds.10214. [DOI] [PubMed] [Google Scholar]
- Pan HC, Cheng FC, Sun MH, Chen CC, Sheehan J. Prediction of volumetric data errors in patients treated with gamma knife radiosurgery. Stereotactic and functional neurosurgery. 2007;85:184–91. doi: 10.1159/000101297. [DOI] [PubMed] [Google Scholar]
- Saito R, Krauze MT, Bringas JR, Noble C, McKnight TR, Jackson P, Wendland MF, Mamot C, Drummond DC, Kirpotin DB, Hong K, Berger MS, Park JW, Bankiewicz KS. Gadolinium-loaded liposomes allow for real-time magnetic resonance imaging of convection-enhanced delivery in the primate brain. Experimental neurology. 2005 doi: 10.1016/j.expneurol.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Schroder KF, Hopf A, Lange H, Thorner G. Morphometrical-statistical structure analysis of human striatum, pallidum and subthalamic nucleus. Journal fur Hirnforschung. 1975;16:333–50. [PubMed] [Google Scholar]
- Schulz JB, Skalej M, Wedekind D, Luft AR, Abele M, Voigt K, Dichgans J, Klockgether T. Magnetic resonance imaging-based volumetry differentiates idiopathic Parkinson’s syndrome from multiple system atrophy and progressive supranuclear palsy. Annals of neurology. 1999;45:65–74. [PubMed] [Google Scholar]
- Snell JW, Sheehan J, Stroila M, Steiner L. Assessment of imaging studies used with radiosurgery: a volumetric algorithm and an estimation of its error. Technical note Journal of neurosurgery. 2006;104:157–62. doi: 10.3171/jns.2006.104.1.157. [DOI] [PubMed] [Google Scholar]
- Sumanaweera TS, Adler JR, Jr, Napel S, Glover GH. Characterization of spatial distortion in magnetic resonance imaging and its implications for stereotactic surgery. Neurosurgery. 1994;35:696–703. doi: 10.1227/00006123-199410000-00016. discussion -4. [DOI] [PubMed] [Google Scholar]