Abstract
Very little is known about the quality and quantity of toxicants yielded by the narghile, a subject of increasing importance as this method of tobacco smoking has become popular all over the world. This study is concerned with the identification and quantification of volatile aldehydes in the gas and particle phases of mainstream narghile smoke generated using a popular type of flavored ma’ssel tobacco mixture. These compounds were analyzed based on a modified version of the Environmental Protection Agency compendium method TO-11A. Using a standardized smoking machine protocol consisting of 171 puffs, 2.6 s puff duration and 17 s inter puff interval, the average yields of formaldehyde, acetaldehyde, acrolein, propionaldehyde and methacrolein were 630, 2520, 892, 403, and 106 μg/smoking session, respectively. The results showed that none of the aldehydes identified in this study are found in the particulate phase of the smoke, except for formaldehyde for which the partitioning coefficient was estimated as Kp = 3.3 × 10-8 μg/m3. Given previously reported lung absorption fractions of circa 90% for volatile aldehydes, the yields measured in this study are sufficient to induce various diseases depending on the extent of exposure, and on the breathing patterns of the smokers.
Keywords: Aldehyde, Waterpipe, Mainstream smoke, Partitioning, Health hazards
1. Introduction
The popularity of the narghile waterpipe, also referred to as hookah, shisha or hubble-bubble (Chaaya et al., 2004; Maziak et al., 2005; Maziak and Tabbah, 2005; Nuwayhid et al., 1998; Wolfram et al., 2003) has increased tremendously during the past few decades (Chaaya et al., 2004; Maziak et al., 2005, 2004b; Tamim et al., 2007, 2001) and has spread beyond the bounds of Arab countries to other parts of the world, including Europe and America (Smith-Simone et al., 2008; Ward et al., 2007). It has been postulated that the appeal of this smoking method in part stems from its role as a way of socializing with other people and, contrary to cigarettes, is socially acceptable in the Eastern Mediterranean region, where it is common for parents to allow their sons and daughters to smoke narghile (Maziak et al., 2004a). Even though waterpipe smoking likely exposes users to high levels of various toxicants (discussed below), and that the practice may be addictive (Maziak et al., 2005), it is popularly perceived as less harmful and toxic than cigarette smoking because of the purported filtering effect of the water bubbler (Kandela, 2000; Smith-Simone et al., 2008; Ward et al., 2007). Volatile aldehydes, especially formaldehyde, are associated with a significant number of cigarette smoking diseases including chronic pulmonary disorder and cancer (IARC, 2006; MFLOHC, 1994). However, aldehydes have not been quantified for narghile smoke. This study is designed to identify and quantify aldehyde compounds in mainstream narghile smoke.
The structure of the narghile waterpipe is illustrated elsewhere (Maziak et al., 2004a; Shihadeh, 2003, 2004). An important feature distinguishing the narghile from the cigarette is the use of charcoal as a heating source in the former. Thus waterpipe smoke contains products of charcoal combustion in addition to those originating from the sweetened tobacco mixture. Despite the difference in structure between narghile and cigarettes, which include the difference in burning temperature, they both involve the consumption of tobacco. Therefore, it is expected that some of the chemical compounds identified in cigarette smoke would also be found in the smoke of a narghile, albeit at different concentrations due to the differences between the two smoking methods. While very few studies have been conducted on the chemical composition of narghile smoke, what has been found thus far suggests that this smoking method poses significant health hazards. It was found that the mainstream smoke of a single narghile smoking session contains many times the CO and nicotine found in the mainstream smoke of a single cigarette (Shihadeh and Saleh, 2005). Moreover, a single narghile smoking session yields 20 times the amount of carcinogenic polycyclic aromatic hydrocarbons (PAH) found in mainstream cigarette (Sepetdjian et al., 2008).
Aldehydes have been established as a major group of compounds emitted from cigarettes (i.e. consumption of tobacco) (Baker, 2006a; Borgerding et al., 1997; Chepiga et al., 2000; Dong and Moldoveanu, 2004; Fujioka and Shibamoto, 2006; Hatzinikolaou et al., 2006; Rustemeier et al., 2002; Stabbert et al., 2003). In their review on the chemical composition of mainstream cigarette smoke, Hoffmann et al. (2001) show that the amounts of formaldehyde, acetaldehyde and acrolein, which are primarily found in the gas phase, vary within the ranges 20-100, 400-1400 and 60-240 μg/cigarette, respectively (Hoffmann et al., 2001). Other studies show that the addition of sugars and flavors to cigarette tobacco results in an increase (up to 60%) in the amounts of aldehyde compounds produced (Baker, 2006a), and in altering the chemical composition of the smoke (Baker, 2006a).
The importance of assessing aldehyde compounds in narghile smoke lies in the fact that these compounds are known to be toxic, carcinogenic and hazardous. Formaldehyde, for example, is classified as a group 1 carcinogen by the International Agency for Research on Cancer (IARC), causing sinonasal and nasopharyngeal cancer as well as leukemia (IARC, 2006). It is also suggested to cause irritation in the upper respiratory tract (Stabbert et al., 2003). Acetaldehyde, another volatile aldehyde present in tobacco smoke, is known as a nasal carcinogen in rodents (Stabbert et al., 2003). As for acrolein, it affects the function of the immune system, particularly T-cells, by inhibiting cytokine gene expression (Lambert et al., 2007). Furthermore, volatile aldehydes in tobacco smoke, particularly formaldehyde and acetaldehyde, are considered “minor” contributors to the occurrence of lung cancer in smokers, and “major” contributors to chronic obstructive pulmonary diseases (COPD) (Hoffmann et al., 1997).
This study identifies and quantifies major aldehyde compounds in the gas and particle phases of mainstream narghile smoke, and assesses their partitioning between the two phases.
2. Materials and methods
2.1. Materials and reagents
H30 Lp-DNPH SPE cartridges, the mixture of standard carbonyl compounds (Carb Method DNPH Mix 1) and a reverse phase C-18 Discovery high performance liquid chromatography (HPLC) column were obtained from Supelco. HPLC grade acetonitrile (ACN), CAS registry number 75-05-8, and Tetrahydrofuran, CAS registry number 109-99-9, were obtained from Acros. Powdered 2,4-dinitrophenyl hydrazine (DNPH), CAS registry number 119-26-6, was provided by Merck. Tobacco mixture with the brand name Nakhla Tobacco (Egypt) “two apples” flavor was obtained from local retail outlets, as were the Three Kings (Holland) brand quick-light charcoal disks used in this study.
2.2. Sampling of aldehyde compounds in the gas and particle phases of narghile smoke
Preparation of the narghile and all other aspects of the machine smoking regimen were identical to those presented in Shihadeh and Saleh (2005). Intrinsic air infiltration rate through the permeable leather hose used in this study was measured as specified in Saleh and Shihadeh (2007), and found to be 2.2 L/min at a waterpipe flow rate of 12.2 L/min; this infiltration rate was monitored throughout the study and found to be invariable. Because hose infiltration was found to be an important determinant of toxicant yields, it is recommended that it be routinely specified in any toxicant yield measurement with the narghile (Saleh and Shihadeh, 2007).
For the purpose of direct comparison between the amounts of aldehyde compounds found in the gas and particle phases of narghile smoke, both phases were sampled and assessed simultaneously during each full smoking session (171 puffs, 2.6 s puff duration, 17 s inter puff interval and 12.2 L/min total flow rate). A schematic diagram of the sampling system employed in this study is illustrated in Fig. 1.
Fig. 1.
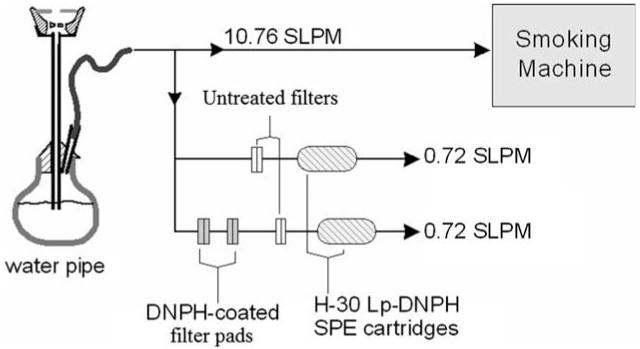
Schematic diagram of the narghile sampling system and the connections made to sample small percentages of gas and gas + particle smoke.
The sampled mainstream smoke was split into two parallel streams, each flowing at 0.72 L/min as shown in Fig. 1. One stream was used to trap only the gas phase aldehydes, while the other was used to trap both gas and particle phase aldehydes. The proportion of gas phase to total aldehydes was computed as the ratio of the aldehydes trapped in the two streams. Small fractions of the smoke were sampled, as shown in Fig. 1, due to the limited capacity of the SPE cartridges employed (643 μg/cartridge). Sampling both phases was accomplished by collecting and derivatizing aldehyde compounds on two DNPH-coated 47 mm filter pads connected in series. The second DNPH-coated filter pad was used to ensure the derivatization of all aldehyde compounds in the sampled smoke, in case the capacity of the first DNPH-coated filter was exceeded. The two DNPH-coated filters were succeeded by a blank filter (non DNPH-coated) and an H-30 DNPH cartridge used for derivatizing any unreacted aldehyde compounds in the gas phase of the sampled smoke (Fig. 1). Meanwhile, aldehyde compounds in the gas phase of the smoke were sampled on a single DNPH cartridge preceded by a blank filter pad used for trapping the particulate phase. The flow rates through each stream were measured before and after each run to ensure the consistency of the smoking system.
2.3. Filter preparation and extraction
Aldehyde compounds in the particle phase of mainstream smoke were collected and derivatized on DNPH-coated filter pads based on the method proposed by Dong and Moldoveanu (2004) for cigarette smoke. Glass fiber filter pads (47 mm, Pall Gelman Type A/E) were manually soaked in a freshly prepared DNPH solution. This 0.015 g/ml solution was prepared by dissolving 1.5 g of recrystallized DNPH in 100 ml of acetonitrile containing 200 μl of 70% percholric acid. The acid plays a role in promoting the derivatization reaction (Dong and Moldoveanu, 2004). Filter pads soaked in this solution were then dried and stored at 4 °C for a maximum of three days prior to sampling. Following sample collection, filters were extracted by sonication in 10 ml of the extraction solution (2% pyridine, 98% ACN). The extracted solution was then filtered and delivered into ambered HPLC vials. The extraction efficiency of the filters was calculated using various sonication and drying times. The results showed that the optimal drying time is 30 min under vacuum, and that the most efficient extraction is achieved upon 10 min sonication.
Aldehydes in the gas phase of the smoke were sampled on H-30 SPE cartridges (Lp-DNPH, Supelco©). After collection, these cartridges were eluted with 10 ml of ACN, filtered, and delivered into ambered vials for HPLC analysis.
It should be noted that both filters and cartridges were wrapped tightly with aluminum foil and stored at low temperatures (4 °C) after sampling, in order to inhibit thermal and photochemical decomposition of the sampled compounds. A maximum of three days was allowed between sample collection and analysis.
2.4. Chromatography
All prepared samples of standards and narghile smoke were analyzed using HPLC (Agilent 1100) equipped with a photodiode array detector at λ = 360 nm, and at a flow rate of 1 ml/min. The analytes were identified upon comparing their HPLC retention times with those of the standards (Fig. 2).
Fig. 2.
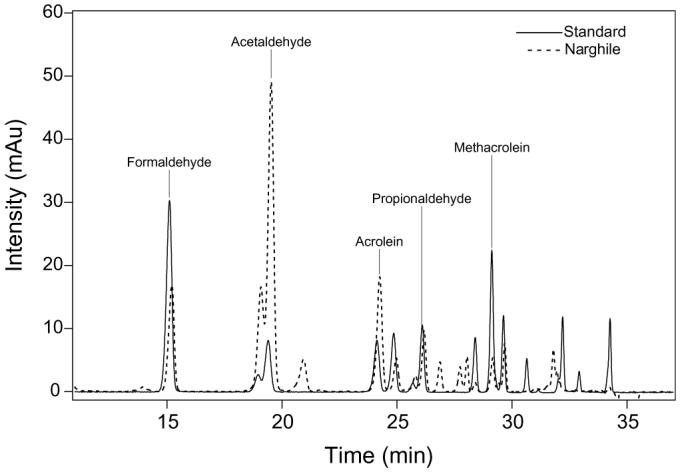
Overlayed chromatograms of 2 ppm of aldehyde standard solution and 2.75% of the mainstream narghile smoke.
The analysis method was adopted from the California Air Resources Board (CARB) method (SOP MLD 022) as well as the Environmental Protection Agency (EPA) method (TO-11A) (EPA, 1999), with some modifications in the time program of elution for better separation. Gradient elution on a reverse phase C-18 column (25 cm × 4.6 mm, 5 μm) was performed. The solvents used were (A) water/ACN/THF (6:3:1 v/v/v), (B) water/ACN (2:3 v/v), and (C) ACN. The elution profile varied linearly in time from pure A at t = 0 min to 25:75 A:B at t = 20 min and finally to pure C at t = 35 min.
Aldehyde compounds were identified based on their individual retention times as compared to the calibration standards. Their concentrations were determined using recovered standard calibration curves which accounted for any losses in the amounts of hydrazones during extraction. The filter pads were soaked with ACN, spiked with particular volumes of the standard solution, dried for 30 min under vacuum, and then extracted via sonication in 10 ml ACN. Four standard solutions ranging in concentration between 0.5 and 10 ppm were prepared using a 20 ppm commercial standard solution consisting of 13 standard aldehyde-DNPH compounds in the form of hydrazones. Recoveries ranged between 90% and 102%. In addition, three injections from each prepared standard concentration were performed in order to check the reproducibility of the HPLC system. The maximum relative standard deviation (% RSD) value obtained was 1.16%, indicating a high degree of repeatability in the HPLC system. Calibration curves were highly linear with R2 values of 0.999, 0.998, 0.998, 0.998, and 0.999 for formaldehyde, acetaldehyde, acrolein, propionaldehyde and methacrolein, respectively. Blank cartridges were also analyzed in order to account for the background of the SPE cartridges. This background was later subtracted from the smoke sample chromatograms.
3. Results and discussion
Aldehyde yields for six repeated smoking sessions are summarized in Table 1. To account for the inherent variability of the smoking method (see Shihadeh, 2003), the results shown in Table 1 are normalized by the amount of tobacco mixture consumed in a given session.
Table 1.
Aldehyde yields in the whole narghile smoke (μg/g of tobacco mixture consumed) and fraction accounted for in the gas phase
| N = 6 | Total yield (μg/g) | Fraction in gas phase |
|---|---|---|
| Formaldehyde | 102.7 (16.3) | 0.61 (0.06) |
| Acetaldehyde | 412.2 (58.6) | 0.98 (0.02) |
| Acrolein | 145.5 (18.7) | 0.95 (0.02) |
| Propionaldehyde | 65.6 (10.3) | 1.06 (0.11) |
| Methaccrolein | 17.3 (2.6) | 1.01 (0.07) |
Yields are reported as mean and standard deviations are shown in parentheses.
The results show that among the aldehyde target compounds, only formaldehyde is found in significant proportions in the particle phase of the smoke. This may be attributed to the higher solubility of formaldehyde in water as compared to the other aldehyde compounds, in light of the fact that the narghile aerosol particles are rich in water (circa 40% water by mass; see Shihadeh, 2003). Henry’s constant is reported to be 3000 mol/Latm for formaldehyde, whereas that of acetaldehyde is 11.4 mol/Latm (Finlayson-Pitts and Pitts, 2000). Hence, the gas-particle partitioning coefficient (Kp) of formaldehyde, defined as (Jang et al., 1997a,b):
where F and A are the concentrations of a particular compound in the particle and gas phases, respectively (ng/m3), and TSP is the concentration of total suspended particulate matter (μg/m3) (Jang et al., 1997a,b), is determined to be Kp = 3.3 × 10-8 m3 μg-1 (logKp = -7.49). This figure is compared to the aqueous/gas partitioning constant of formaldehyde, which is calculated under the assumption that the mainstream TSP emitted from a narghile is purely aqueous. Therefore,
where [HCHO]aq and [HCHO]g are the concentrations of formaldehyde in the aqueous and gas phases, respectively (ng/m3), and H is the intrinsic Henry’s constant of formaldehyde (1.4 mol/Latm) (Finlayson-Pitts and Pitts, 2000). Hence, an upper limit for partitioning of formaldehyde is Kp = 1.9 × 10-6 m3 μg-1 (logKp = -5.7).
In comparison to a single cigarette (Table 2), one narghile smoking session is found to release greater amounts of formaldehyde, acetaldehyde, acrolein propionaldehyde and methacrolein into the mainstream smoke. For the conditions studied here, mainstream narghile smoke yields of formaldehyde and acetaldehyde are 630 and 2520 μg/narghile session, respectively. These results show that a single narghile smoking session yields in the mainstream smoke the equivalent of 17 cigarettes in formaldehyde, and five cigarettes in acetaldehyde, when compared to cigarettes with added sugar (Baker, 2006a). Such high amounts may pose substantial health risks, considering that approximately 90% of the aldehydes in both gas and particle phases of cigarette smoke are deposited in the lungs after inhalation (Baker, 2006b). The deposition percentage varies from one person to another, depending on many variables including particle diameter and growth, depth of inhalation, hold time in the lungs, puff volume and exhalation volume (Baker, 2006b; Bernstein, 2004).
Table 2.
Mean yields of aldehydes in mainstream narghile smoke for six repeated trials (standard deviations shown in parentheses) compared to reported yields of aldehydes in mainstream cigarette smoke (with and without added sugar)
| Compound | Current study (μg/narghile) | Cigarette (μg/cigarette)a | Cigarette (μg/cigarette)b | Cigarette with added sugar (μg/cigarette)c |
|---|---|---|---|---|
| Formaldehyde | 630 (133) | 22.9 | 20-100 | 38 |
| Acetaldehyde | 2520 (504) | 619.4 | 400-1400 | 526 |
| Acrolein | 892 (179) | 47.1 | 60-240 | 67 |
| Propionaldehyde | 403 (91) | 46.5 | 45 | |
| Methacrolein | 106 (22) | 23.8 | - |
Dong and Moldoveanu (2004). Results obtained for 1R4F cigarette.
Baker (2006a). These are the values reported for 5.3% by mass of added molasses.
4. Conclusion
Several conclusions may be drawn from the obtained results. First, the identified aldehyde compounds are found exclusively in the gas phase of narghile smoke, except for formaldehyde. Approximately 40% of the amount of formaldehyde found in mainstream narghile smoke is in the particulate phase. This could be explained by the high solubility of formaldehyde in the water-rich aerosol of mainstream narghile smoke, in contrast to the other identified species. The partitioning constant for formaldehyde was determined to be Kp = 3.3 × 10-8 μg/m3. Second, one narghile smoking session yields many times the formaldehyde, acetaldehyde and acrolein typically found in a cigarette, raising concern that narghile smoking may lead to many of the respiratory system ailments associated with cigarette smoking.
Acknowledgements
This project was supported by Research for International Tobacco Control (RITC), a secretariat of the International Development Research Centre, and by Grant Number R01 CA120142 from the U.S. National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the RITC, the U.S. National Cancer Institute or the U.S. National Institutes of Health. The authors would like to also thank Dr. Rima Habib and Dean Iman Nuwayhid for their assistance in evaluating the health hazards of inhaled aldehydes.
Abbreviations
- ACN
acetonitrile
- CARB
California Air Resources Board
- COPD
chronic obstructive pulmonary disease
- DNPH
dinitrophenyl hydrazine
- EPA
environmental protection agency
- HPLC
high performance liquid chromatography
- IARC
International Agency for Research on Cancer
- LPM
liters per minute
- PAH
polycylic aromatic hydrocarbons
- TSP
total suspended particulate matter
- WHO
World Health Organization
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- Baker RR. The generation of formaldehyde in cigarettes-overview and recent experiments. Food Chem. Toxicol. 2006a;44:1799–1822. doi: 10.1016/j.fct.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Baker RR. The retention of tobacco smoke constituents in the human respiratory tract. Inhal. Toxicol. 2006b;17:255–294. doi: 10.1080/08958370500444163. [DOI] [PubMed] [Google Scholar]
- Bernstein DM. A review of the influence of particle size, puff volume, and inhalation pattern on the deposition of cigarette smoke particles in the respiratory tract. Inhal. Toxicol. 2004;16:675–689. doi: 10.1080/08958370490476587. [DOI] [PubMed] [Google Scholar]
- Borgerding MF, Bodnar JA, Chung HL, Mangan PP, Morrison CC, Risner CH, Rogers JC, Simmons DF, Uhrig MS, Wendelboe FN, Wingate DE, Winkler LS. Chemical and biological studies of a new cigarette that primarily heats tobacco. Part 1: chemical composition of mainstream smoke. Food Chem. Toxicol. 1997;36:169–182. [PubMed] [Google Scholar]
- Chaaya M, El Roueiheb Z, Chemaitelly H, Azar G, Nasr J, Al-Sahab B. Argileh smoking among university students: a new tobacco epidemic. Nicotine Tob. Res. 2004;6:457–463. doi: 10.1080/14622200410001696628. [DOI] [PubMed] [Google Scholar]
- Chepiga TA, Morton MJ, Murphy PA, Avalos JT, Bombick BR, Doolittle DJ, Borgerding MF, Swauger JE. A comparison of the mainstream smoke chemistry and mutagenicity of a representative sample of the US cigarette market with two Kentucky reference cigarettes (K1R4F and K1R5F) Food Chem. Toxicol. 2000;38:949–962. doi: 10.1016/s0278-6915(00)00086-7. [DOI] [PubMed] [Google Scholar]
- Dong JZ, Moldoveanu SC. Gas chromatography-mass spectrometry of carbonyl compounds in cigarette mainstream smoke after derivatization with 2,4-dinitrophenyl hydrazine. J. Chromatogr. A. 2004;1027:25–35. doi: 10.1016/j.chroma.2003.08.104. [DOI] [PubMed] [Google Scholar]
- Finlayson-Pitts BJ, Pitts JN. Chemistry of the Upper and Lower Atmosphere. Academic Press; San Diego: 2000. p. 304. [Google Scholar]
- Fujioka K, Shibamoto T. Determination of toxic carbonyl compounds in cigarette smoke. Environ. Toxicol. 2006;21:47–54. doi: 10.1002/tox.20153. [DOI] [PubMed] [Google Scholar]
- Hatzinikolaou DG, Lagesson V, Stavridou AJ, Pouli AE, Lagesson-Andrasko L, Stavrides JC. Analysis of the gas phase of cigarette smoke by gas chromatography coupled with UV-diode array detection. Anal. Chem. 2006;78:4509–4516. doi: 10.1021/ac052004y. [DOI] [PubMed] [Google Scholar]
- Hoffmann D, Djordjevic MV, Hoffmann I. The changing cigarette. Prev. Med. 1997;26:427–434. doi: 10.1006/pmed.1997.0183. [DOI] [PubMed] [Google Scholar]
- Hoffmann D, Hoffmann I, El Bayoumy K. The less harmful cigarette: a controversial issue. A tribute to Ernst L. Wynder. Chem. Res. Toxicol. 2001;14:767–790. doi: 10.1021/tx000260u. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 88. Lyon: 2006. Formaldehyde, 2-Butoxyethanol and 1-tert-Butoxypropan-2-ol. [PMC free article] [PubMed] [Google Scholar]
- Jang M, Kamens RM, Leach KB, Strommen MR. A thermodynamic approach using group contribution methods to model the partitioning of semivolatile organic compounds on atmospheric particulate matter. Environ. Sci. Technol. 1997a;31:2805–2811. [Google Scholar]
- Jang M, Kamens RM, Leach KB, Strommen MR. A thermodynamic approach using group contribution methods to model the partitioning of semivolatile organic compounds on atmospheric particulate matter. Environ. Sci. Technol. 1997b;31:2805–2811. [Google Scholar]
- Kandela P. Nargile smoking keeps Arabs in wonderland. The Lancet. 2000;356.9236:1175. doi: 10.1016/s0140-6736(05)72871-3. [DOI] [PubMed] [Google Scholar]
- Lambert C, Li J, Jonscher K, Yang T, Reigan P, Quintana M, Harvey J, Freed BM. Acroleininhibits cytokine gene expression by alkylating cysteine and arginine residues in the NF-κB1 DNA binding domain. J. Biol. Chem. 2007;282:19666–19675. doi: 10.1074/jbc.M611527200. [DOI] [PubMed] [Google Scholar]
- Maziak W, Eissenberg T, Rastam S, Hammal F, Asfar T, Bachir ME, Fouad MF, Ward KD. Beliefs and attitudes related to narghile (waterpipe) smoking among university students in Syria. Ann. Epidemiol. 2004a;14:646–654. doi: 10.1016/j.annepidem.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Maziak W, Eissenberg T, Ward KD. Patterns of waterpipe use and dependence: implications for intervention development. Pharmacol. Biochem. Behav. 2005;80:173–179. doi: 10.1016/j.pbb.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Maziak W, Tabbah K. Smoking among adults in Syria: proxy reporting by 13-14 year olds. Public Health. 2005;119:578–581. doi: 10.1016/j.puhe.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Maziak W, Ward KD, Eissenberg T. Factors related to frequency of narghile (waterpipe) use: the first insights on tobacco dependence in narghile users. Drug Alcohol Depen. 2004b;76:101–106. doi: 10.1016/j.drugalcdep.2004.04.007. [DOI] [PubMed] [Google Scholar]
- MFLOHC Formaldehyde. 1994 Available from: < http://www.mflohc.mb.ca/fact_sheets_forlder/formaldehyde.html>.
- Nuwayhid IA, Yamout B, Azar G, Al Kouatly Kambris M. Narghile (hubble-bubble) smoking, low birth weight, and other pregnancy outcomes. Am. J. Epidemiol. 1998;148:375–383. doi: 10.1093/oxfordjournals.aje.a009656. [DOI] [PubMed] [Google Scholar]
- Rustemeier K, Stabbert R, Haussmann HJ, Roemer E, Carmines EL. Evaluation of the potential effects of ingredients added to cigarettes. Part 2: chemical composition of mainstream smoke. Food Chem. Toxicol. 2002;40:93–104. doi: 10.1016/s0278-6915(01)00085-0. [DOI] [PubMed] [Google Scholar]
- Saleh R, Shihadeh A. Hygroscopic growth and evaporation in an aerosol with boundary heat and mass transfer. J. Aero. Sci. 2007;38:1–16. [Google Scholar]
- Sepetdjian E, Shihadeh A, Saliba NA. Measurement of 16 polycyclic aromatic hydrocarbons in narghile waterpipe tobacco smoke. Food Chem. Toxicol. 2008;46:1582–1590. doi: 10.1016/j.fct.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Shihadeh A. Investigation of mainstream smoke aerosol of the argileh waterpipe. Food Chem. Toxicol. 2003;41:143–152. doi: 10.1016/s0278-6915(02)00220-x. [DOI] [PubMed] [Google Scholar]
- Shihadeh A, Azar S, Antonios C, Haddad A. Towards a topographical model of narghile waterpipe cafe smoking: a pilot study in a high socioeconomic status neighborhood of Beirut, Lebanon. Pharmacol. Biochem. Behav. 2004;79:75–82. doi: 10.1016/j.pbb.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Shihadeh A, Saleh R. Polycyclic aromatic hydrocarbons, carbon monoxide, “tar”, and nicotine in the mainstream smoke aerosol of the narghile waterpipe. Food Chem. Toxicol. 2005;43:655–661. doi: 10.1016/j.fct.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Smith-Simone S, Maziak W, Ward KD, Eissenberg T. Waterpipe tobacco smoking: knowledge, attitudes, beliefs, and behavior in two US samples. Nicotine Tob. Res. 2008;10:393–398. doi: 10.1080/14622200701825023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabbert R, Voncken P, Haussmann HJ, Roemer E, Schaffernicht H, Patskan G. Toxicological evaluation of an electrically heated cigarette. Part 2: chemical composition of mainstream smoke. J. Appl. Toxicol. 2003;23:329–339. doi: 10.1002/jat.924. [DOI] [PubMed] [Google Scholar]
- Tamim H, Al-Sahab B, Akkary G, Ghanem M, Tamim N, El Roueiheb Z, Kanj M, Afifi R. Cigarette and nargileh smoking practices among school students in Beirut, Lebanon. Am. J. Health Behav. 2007;31:56–63. doi: 10.5555/ajhb.2007.31.1.56. [DOI] [PubMed] [Google Scholar]
- Tamim H, Terro A, Kassem H, Ghazi A, Abou Khamis T, Abdul Hay M, Musharrafieh U. Tobacco use by university students, Lebanon. Addiction. 2001;98:933–939. doi: 10.1046/j.1360-0443.2003.00413.x. [DOI] [PubMed] [Google Scholar]
- Ward KD, Eissenberg T, Gray J, Srinivas V, Wilson N, Maziak W. Characteristics of US waterpipe users: a preliminary report. Nicotine Tob. Res. 2007;9:1339–1346. doi: 10.1080/14622200701705019. [DOI] [PubMed] [Google Scholar]
- Wolfram RM, Chehne F, Oguogho A, Sinzinger H. Narghile (waterpipe) smoking influences platelet function and (iso-)eicosanoids. Life Sci. 2003;74:47–53. doi: 10.1016/j.lfs.2003.06.020. [DOI] [PubMed] [Google Scholar]


