Summary
Background
The main associations of body-mass index (BMI) with overall and cause-specific mortality can best be assessed by long-term prospective follow-up of large numbers of people. The Prospective Studies Collaboration aimed to investigate these associations by sharing data from many studies.
Methods
Collaborative analyses were undertaken of baseline BMI versus mortality in 57 prospective studies with 894 576 participants, mostly in western Europe and North America (61% [n=541 452] male, mean recruitment age 46 [SD 11] years, median recruitment year 1979 [IQR 1975–85], mean BMI 25 [SD 4] kg/m2). The analyses were adjusted for age, sex, smoking status, and study. To limit reverse causality, the first 5 years of follow-up were excluded, leaving 66 552 deaths of known cause during a mean of 8 (SD 6) further years of follow-up (mean age at death 67 [SD 10] years): 30 416 vascular; 2070 diabetic, renal or hepatic; 22 592 neoplastic; 3770 respiratory; 7704 other.
Findings
In both sexes, mortality was lowest at about 22·5–25 kg/m2. Above this range, positive associations were recorded for several specific causes and inverse associations for none, the absolute excess risks for higher BMI and smoking were roughly additive, and each 5 kg/m2 higher BMI was on average associated with about 30% higher overall mortality (hazard ratio per 5 kg/m2 [HR] 1·29 [95% CI 1·27–1·32]): 40% for vascular mortality (HR 1·41 [1·37–1·45]); 60–120% for diabetic, renal, and hepatic mortality (HRs 2·16 [1·89–2·46], 1·59 [1·27–1·99], and 1·82 [1·59–2·09], respectively); 10% for neoplastic mortality (HR 1·10 [1·06–1·15]); and 20% for respiratory and for all other mortality (HRs 1·20 [1·07–1·34] and 1·20 [1·16–1·25], respectively). Below the range 22·5–25 kg/m2, BMI was associated inversely with overall mortality, mainly because of strong inverse associations with respiratory disease and lung cancer. These inverse associations were much stronger for smokers than for non-smokers, despite cigarette consumption per smoker varying little with BMI.
Interpretation
Although other anthropometric measures (eg, waist circumference, waist-to-hip ratio) could well add extra information to BMI, and BMI to them, BMI is in itself a strong predictor of overall mortality both above and below the apparent optimum of about 22·5–25 kg/m2. The progressive excess mortality above this range is due mainly to vascular disease and is probably largely causal. At 30–35 kg/m2, median survival is reduced by 2–4 years; at 40–45 kg/m2, it is reduced by 8–10 years (which is comparable with the effects of smoking). The definite excess mortality below 22·5 kg/m2 is due mainly to smoking-related diseases, and is not fully explained.
Funding
UK Medical Research Council, British Heart Foundation, Cancer Research UK, EU BIOMED programme, US National Institute on Aging, and Clinical Trial Service Unit (Oxford, UK).
Introduction
Body-mass index (BMI) is a reasonably good measure of general adiposity,1 and raised BMI is an established risk factor for several causes of death, including ischaemic heart disease,2 stroke,3 and cancers of the large intestine, kidney, endometrium, and postmenopausal breast.4,5 In many populations, the average BMI has been rising by a few percent per decade,6 fuelling concern about the effects of increased adiposity on health. Some uncertainties persist, however, about the relation between BMI and mortality, including whether some of the reported positive or inverse associations have been distorted by weight loss because of pre-existing disease (reverse causality) or by inadequate control for the effects of smoking; whether the shape and strength of associations with specific diseases differ between smokers and non-smokers; how the relative and absolute risks for BMI compare with, and also combine with, those for smoking; whether the relative risks differ much by sex or age (and whether any substantial association continues into old age7); how the absolute excess risks for vascular disease compare with those for neoplastic or respiratory disease; and the extent to which some less common causes of death, such as kidney8 or liver9 disease, are associated with BMI.
Some of these uncertainties can best be addressed by large prospective observational studies—or by large collaborative analyses of individual data from such studies, as in this report—that follow up generally healthy adults for many years and identify large numbers of deaths from specific causes. In the Prospective Studies Collaboration (PSC), the investigators of 61 prospective studies have shared individual data for a million adults.10,11 The collaboration was established chiefly to assess the relevance of blood pressure10 and blood cholesterol11 to cause-specific mortality, but for 57 of the studies information was also available for BMI (although generally not for waist circumference). This PSC report examines the relevance of BMI to cause-specific mortality 5 or more years after recruitment into these studies.
Methods
Data collection
Previous PSC reports10,11 have described the methods of study selection, data collection, and statistical analysis, and similar methods were used in this report. BMI was calculated as weight in kg divided by the square of height in m. In three studies of US health professionals, height and weight were self-reported by participants. Following WHO convention, BMI of 30 kg/m2 or more is termed obese. Individuals with missing data for age, sex, or BMI were excluded, as were those with BMI less than 15 kg/m2 (188 excluded) or 50 kg/m2 or more (297), those with a baseline history of heart disease (54 347) or stroke (4349), and those with no follow-up in the age range 35–89 years (25 949), leaving 894 576 participants. Information about blood pressure (882 032) and total cholesterol (814 109) was available for most participants, as was information about tobacco smoking (849 723) and diabetes mellitus (698 255). Information was, however, available for relatively few of the participants about alcohol drinking (297 584), HDL cholesterol (114 939), and LDL cholesterol (42 937). Of current drinkers, 78% (155 900) had information about grams of alcohol consumed per day, and of current cigarette smokers, 57% (166 724) had information about daily number of cigarettes smoked.
Generally, the underlying cause of death was obtained from the death certificate (information about contributory causes was not available), but in many studies confirmation was then sought from other sources, such as medical records and autopsy findings. The cause of death was coded to 3 digits using any of International Classifications of Diseases (ICD) 6–10.
Statistical analysis
Cross-sectional associations between BMI and risk factors were estimated by multiple linear regression or logistic regression, with adjustment for study, baseline age (in 10-year groups), and baseline smoking (three groups: current cigarette smoker [32%]; never smoked any type of tobacco regularly [35%]; and other smoker, ex-smoker of any type of tobacco, or unknown [8%, 19%, and 5%, respectively]). Any individuals with missing values of BMI or the particular risk factor were excluded. Associations between baseline BMI and mortality were estimated by Cox regression, with stratification for study, sex, age at risk (in 5-year groups), and baseline smoking (as above), but not for blood pressure, blood lipids, or diabetes (since these are mechanisms by which BMI affects vascular mortality). The resulting relative risks were not corrected for the regression dilution bias,12 since one BMI measurement is highly correlated with the long-term usual BMI (self-correlation 0·90 between baseline BMI and a re-measurement of BMI some 6 years later; webappendix p 11). In categorical analyses, the boundaries of BMI categories were always multiples of 2·5 kg/m2, and the boundaries used in particular analyses are indicated by tick marks in the figures. Values exactly on a boundary went above it. In continuous analyses, log risk was regressed on BMI as a continuous variable within the range 15–25 kg/m2 (termed the lower range), 25–50 kg/m2 (upper range), or 15–50 kg/m2 (full range), yielding in each range the hazard ratio per 5 kg/m2 higher BMI (HR). To limit effects of pre-existing disease on baseline BMI, the main analyses exclude all person-years and deaths in the first 5 years of follow-up.
Relative risks for different BMI categories are presented as floating absolute risks by multiplying all of them by a common constant to make their inverse-variance-weighted average match the uniformly age-standardised death rate per 1000 person-years at ages 35–79 years (ie, the simple mean of the nine age-specific rates at ages 35–39 years to 75–79 years) either in the PSC population, or in the European Union (EU) population in 2000 (ie, the combined population of 15 western European countries).13 Multiplication of all the relative risks by this common constant means that the floating absolute risks (and the SEs of the log risks) do not depend at all on the choice of an arbitrary reference group. Hence, an appropriate SE and CI can be assigned to the log of the floating absolute risk in each BMI category.14 This SE—described more simply as the SE of the log rate (Julian Peto, London School of Hygiene and Tropical Medicine, London, UK; personal communication)—does not depend on the common constant that was chosen, and is roughly equivalent to the coefficient of variation of the risk in that one group.
The webappendix provides further information, including details of the collaborating studies, endpoint definitions, BMI re-measurement results, and findings for specific causes of death.
Role of the funding sources
The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. GW, PS, and SL had full access to the data in the study, and the writing committee had final responsibility for the decision to submit for publication.
Results
In the 57 studies with information for BMI, individual records with information about BMI were available for 894 576 adults. 92% of these participants were in Europe, Israel, the USA, or Australia; the remaining 8% (accounting for just 3% of the deaths) were in Japan. 85% (763 274) of participants were recruited during the 1970s or 1980s. Across all studies, median year of recruitment was 1979 (IQR 1975–85), mean recruitment age was 46 (SD 11) years, and 61% (n=541 452) were male. Mean BMI was 24·8 (SD 3·8) kg/m2, but it was lower in the European and Israeli studies (24·7 [3·6] kg/m2) than in the US and Australian studies (25·6 [4·3] kg/m2), and lower still in the Japanese studies (22·8 [2·9] kg/m2).
For both sexes, mean BMI at baseline was greatest between 50 years and 69 years of age (webappendix p 11). For people with a re-measurement of BMI more than 5 years after baseline, the rate of change between the two measurements showed an increase in BMI in early adult life and middle age (particularly in women), a levelling off in late middle age, and a slight decrease in old age (webappendix p 11). The greatest rate of increase (about 1·5 kg/m2 per decade) was in men younger than 40 years and women younger than 50 years. Apart from these age-related trends, the changes on re-measurement were slight, and were consistent with regression to the mean having had only a minor effect that negated any even more minor tendency for usual BMI values to disperse (webappendix p 12).
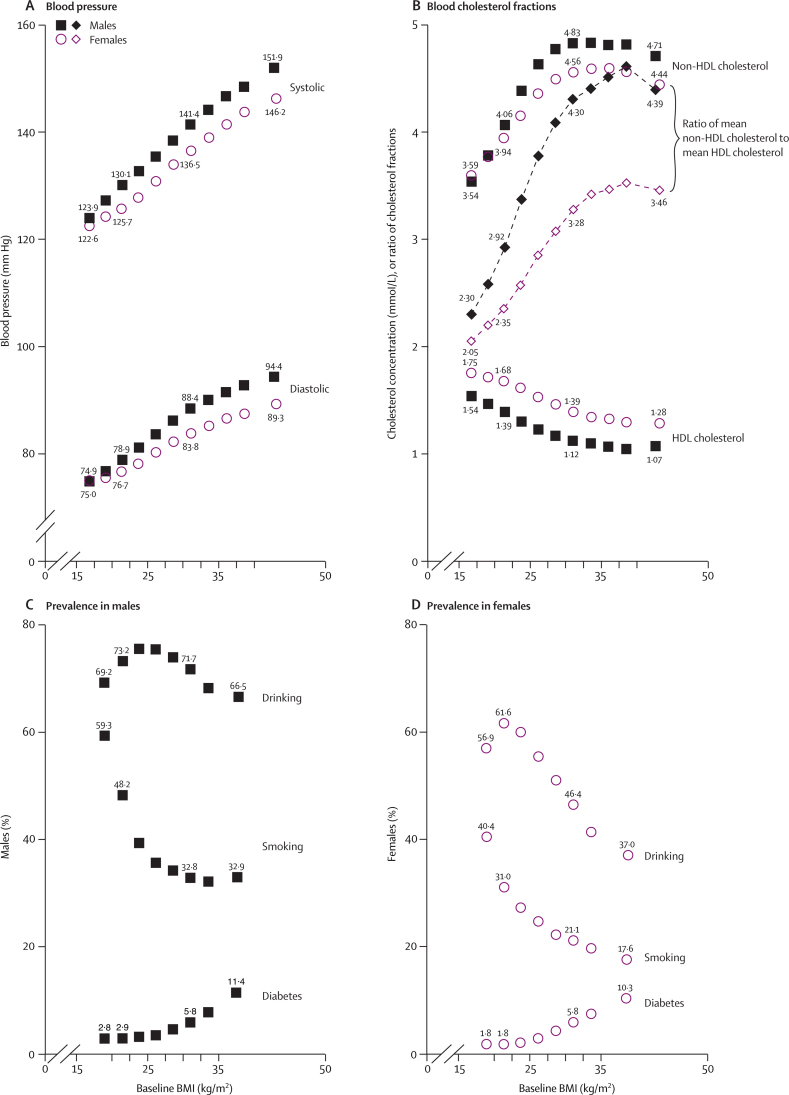
At baseline, several vascular risk factors were strongly related to BMI (figure 1). Throughout the full range (15–50 kg/m2), BMI was associated positively and nearly linearly with systolic and diastolic blood pressure (SBP, DBP; figure 1A). On average across all ages (15–89 years), every 5 kg/m2 higher BMI was associated with at least 5 mm Hg higher SBP (male 5·8 mm Hg, female 5·2 mm Hg) and about 4 mm Hg higher DBP (male 4·9 mm Hg, female 3·3 mm Hg). In the range up to 30 kg/m2, BMI was associated inversely with HDL cholesterol (male 0·16 mmol/L, female 0·14 mmol/L lower per 5 kg/m2), positively with non-HDL cholesterol (male 0·50 mmol/L, female 0·39 mmol/L higher per 5 kg/m2), and therefore strongly positively with the ratio of non-HDL to HDL cholesterol (male 0·85, female 0·54 higher ratio per 5 kg/m2, from analyses of individual ratios: figure 1). These relations with blood pressure and with the non-HDL/HDL cholesterol ratio were about a third weaker in the 2% of participants aged 70–89 years (data not shown). Above 30 kg/m2, BMI was only weakly associated with either cholesterol fraction (figure 1B). Obesity was strongly associated with diabetes (figures 1C and 1D), with the sex-specific prevalences rising more than five-fold over the full BMI range.
Figure 1.
Vascular risk factors versus BMI at baseline in the range 15–50 kg/m2
Adjusted for baseline age, baseline smoking status (apart from the smoking findings), and study. Numerical values are shown for 20–22·5 kg/m2, for 30–32·5 kg/m2, and for the extreme BMI groups. Boundaries of BMI groups are indicated by tick marks. 95% CIs are not shown, but most are narrower than the heights of the plotted symbols. (A) Blood pressure (in 533 242 males and 348 790 females). (B) Blood cholesterol fractions (in 62 364 males and 52 575 females with total and HDL cholesterol both measured); dashed line indicates the ratio of mean non-HDL cholesterol to mean HDL cholesterol (mean of the individual ratios would be about 8–12% greater). (C) Prevalences in males for alcohol drinking (168 283), cigarette smoking (334 496), and diabetes (378 854). (D) Prevalences in females for alcohol drinking (129 301), cigarette smoking (226 307), and diabetes (319 401).
Smoking and drinking may affect BMI, and, after adjustment for age and study, the mean BMI was slightly lower in current smokers than in never-smokers (male 0·3 kg/m2, female 0·9 kg/m2 lower), and in regular alcohol users than in others (male 0·1 kg/m2, female 1·2 kg/m2 lower). Hence, in both sexes the prevalences of smoking and (especially in females) drinking tended to be high in those with low BMI (figures 1C and 1D). In regular smokers or drinkers with relevant data (webappendix p 13), daily cigarette or alcohol consumption was not strongly dependent on BMI.
Of the 894 576 participants with baseline measurements of BMI, 15 996 died in the first 5 years of follow-up, and 852 824 were still alive and under observation at the start of year 5. During 6·5 million person-years of subsequent follow-up (mean 8 [SD 6] years per person), 72 749 deaths were identified. Most (90%) of this additional follow-up and more than half (58%) of these deaths were at ages 35–69 years; 9% of the follow-up and 29% of the deaths were at ages 70–79 years; and 2% and 12%, respectively, were at ages 80–89 years (table 1). Among the 72 749 who died, 54 703 (75%) were males, median year of birth was 1918 (IQR 1910–25), and, of the 62 055 deaths for which the exact year of death is available, 60 153 (97%) occurred between 1970 and 1999 (median 1986). 6197 (9%) of the deaths were from an unknown cause (6% at ages 35–69 years, 9% at 70–79 years, and 16% at 80–89 years). For the remainder, mean age at death was 67 (SD 10) years.
Table 1.
All-cause mortality versus baseline BMI in the ranges 15–25 kg/m2 and 25–50 kg/m2
|
All participants |
Never smokers only |
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
15–25 kg/m2 |
25–50 kg/m2 |
15–25 kg/m2 |
25–50 kg/m2 |
||||||
| Deaths | HR (95% CI) | Deaths | HR (95% CI) | Deaths | HR (95% CI) | Deaths | HR (95% CI) | ||
| Overall | 35 256 | 0·79 (0·77–0·82) | 37 493 | 1·29 (1·27–1·32) | 7054 | 0·87 (0·81–0·94) | 9849 | 1·32 (1·28–1·36) | |
| Male | 26 720 | 0·79 (0·76–0·82) | 27 983 | 1·32 (1·29–1·36) | 3694 | 0·87 (0·78–0·97) | 4811 | 1·44 (1·36–1·53) | |
| Female | 8536 | 0·80 (0·75–0·85) | 9510 | 1·26 (1·23–1·30) | 3360 | 0·87 (0·78–0·97) | 5038 | 1·27 (1·22–1·32) | |
| Age at risk (years) | |||||||||
| 35–59 | 9333 | 0·76 (0·71–0·81) | 8386 | 1·37 (1·31–1·42) | 1665 | 0·88 (0·74–1·04) | 1667 | 1·43 (1·32–1·55) | |
| 60–69 | 11 514 | 0·77 (0·73–0·82) | 13 007 | 1·32 (1·27–1·36) | 1782 | 0·88 (0·75–1·03) | 2841 | 1·36 (1·28–1·45) | |
| 70–79 | 10 078 | 0·82 (0·77–0·87) | 11 358 | 1·27 (1·23–1·32) | 2116 | 0·93 (0·80–1·06) | 3364 | 1·33 (1·25–1·40) | |
| 80–89 | 4331 | 0·89 (0·80–0·97) | 4742 | 1·16 (1·10–1·23) | 1491 | 0·86 (0·74–1·01) | 1977 | 1·15 (1·07–1·25) | |
Hazard ratio per 5 kg/m2 higher BMI (HR). HR less than 1 if BMI inversely associated with risk. All analyses exclude the first 5 years of follow-up and adjust for study and age at risk (in 5-year groups). The overall and age-specific analyses also adjust for sex, and the all-participant analyses also adjust for baseline smoking status.
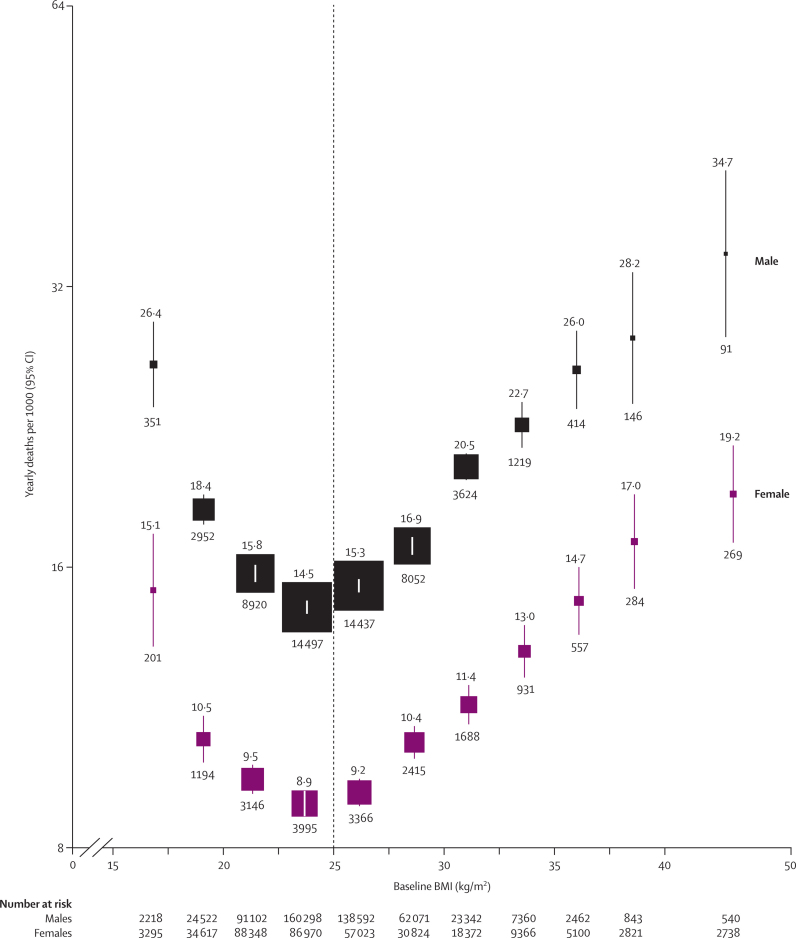
In both sexes (and at all ages: webappendix p 1), all-cause mortality was lowest at about 22·5–25 kg/m2 (figure 2). Above this minimum, mortality was on average about 30% higher for every 5 kg/m2 higher BMI. Although the proportional increase was somewhat greater at younger ages (35–59 years), each 5 kg/m2 higher BMI was still associated with almost 30% higher mortality at 70–79 years of age (table 1). In the lower BMI range (15–25 kg/m2), the inverse association of mortality with BMI (HR per 5 kg/m2 higher BMI 0·79 [95% CI 0·77–0·82]) became less extreme either when the analysis was restricted to lifelong non-smokers (0·87 [0·81–0·94]) or when a further 10 years of follow-up were excluded (0·85 [0·81–0·91]; webappendix p 14).
Figure 2.
All-cause mortality versus BMI for each sex in the range 15–50 kg/m2 (excluding the first 5 years of follow-up)
Relative risks at ages 35–89 years, adjusted for age at risk, smoking, and study, were multiplied by a common factor (ie, floated) to make the weighted average match the PSC mortality rate at ages 35–79 years. Floated mortality rates shown above each square and numbers of deaths below. Area of square is inversely proportional to the variance of the log risk. Boundaries of BMI groups are indicated by tick marks. 95% CIs for floated rates reflect uncertainty in the log risk for each single rate. Dotted vertical line indicates 25 kg/m2 (boundary between upper and lower BMI ranges in this report).
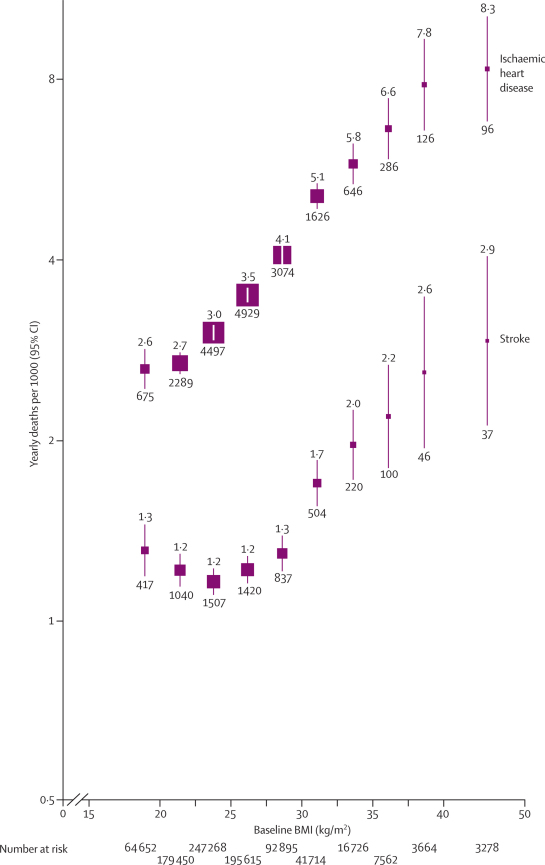
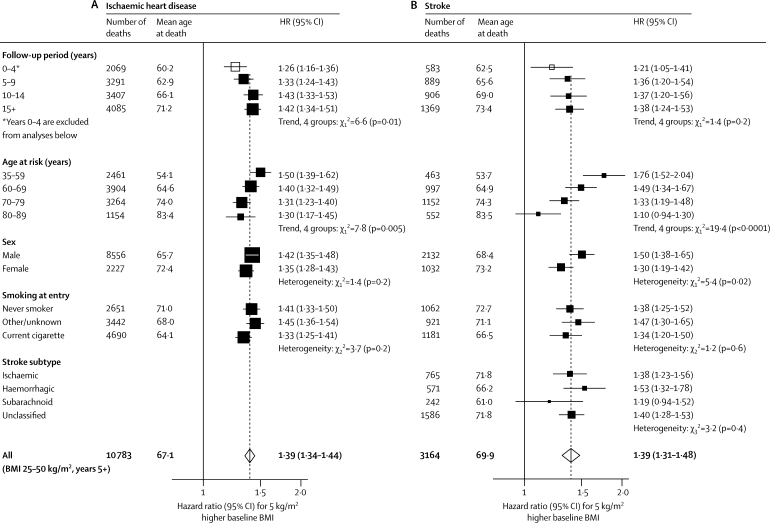
Ischaemic heart disease accounted for more than a quarter of all deaths of known cause. BMI and mortality from ischaemic heart disease were associated strongly, positively, and roughly log-linearly throughout the BMI range from 20 to 40 kg/m2, and perhaps at even greater BMI (figure 3). In the upper BMI range (25–50 kg/m2), each 5 kg/m2 higher BMI was associated with about 40% higher ischaemic heart disease mortality (figure 4, table 2). The association was a little stronger in middle than in old age, but was still definite even at ages 80–89 years (HR 1·30 [1·17–1·45]; figure 4A). In the European and Israeli studies the association was about as strong as it was in the US and Australian studies (HRs 1·38 [1·33–1·44] and 1·40 [1·32–1·50], respectively, for deaths at ages 35–89 years; only 44 deaths from this disease occurred in the Japanese studies), and there was no significant heterogeneity (p=0·13) across the HRs for the 16 larger studies (with >200 deaths from ischaemic heart disease) and the aggregated smaller studies (webappendix p 5).
Figure 3.
Ischaemic heart disease and stroke mortality versus BMI in the range 15–50 kg/m2 (excluding the first 5 years of follow-up)
Relative risks at ages 35–89 years, adjusted for age at risk, sex, smoking, and study, were multiplied by a common factor (ie, floated) to make the weighted average match the PSC mortality rate at ages 35–79 years. Floated mortality rates shown above each square and numbers of deaths below. Area of square is inversely proportional to the variance of the log risk. Boundaries of BMI groups are indicated by tick marks. 95% CIs for floated rates reflect uncertainty in the log risk for each single rate.
Figure 4.
Ischaemic heart disease (A) and stroke mortality (B) versus BMI in the upper BMI range (25–50 kg/m2) only (excluding the first 5 years of follow-up, except as indicated)
Hazard ratios are per 5 kg/m2—eg, 30 kg/m2 versus 25 kg/m2—and are, when appropriate, adjusted for age at risk, sex, smoking, and study. Mean ages at death are given, but the dependence of the HR on mean age at death is not corrected for in analyses of factors other than age. The area of each square is inversely proportional to the variance of the log hazard ratio. White squares include the first 5 years of follow-up; black squares and white diamonds do not. Subarachnoid=subarachnoid haemorrhage (not included in haemorrhagic stroke).
Table 2.
Cause-specific mortality versus baseline BMI in the ranges 15–25 kg/m2 and 25–50 kg/m2
|
15–25 kg/m2 |
25–50 kg/m2 |
|||
|---|---|---|---|---|
| Deaths | HR (95% CI) | Deaths | HR (95% CI) | |
| Ischaemic heart disease | 7461 | 1·22 (1·13–1·32) | 10 783 | 1·39 (1·34–1·44) |
| Stroke | 2964 | 0·92 (0·82–1·03) | 3164 | 1·39 (1·31–1·48) |
| Other vascular disease | 2648 | 0·84 (0·75–0·95) | 3396 | 1·47 (1·39–1·56) |
| Diabetes | 171 | 0·96 (0·59–1·55) | 393 | 2·16 (1·89–2·46) |
| Kidney disease (non-neoplastic) | 197 | 1·14 (0·74–1·77) | 217 | 1·59 (1·27–1·99) |
| Liver disease (non-neoplastic) | 489 | 0·69 (0·52–0·91) | 603 | 1·82 (1·59–2·09) |
| Lung cancer | 2959 | 0·71 (0·63–0·79) | 2040 | 0·98 (0·88–1·09) |
| Upper aerodigestive cancer | 685 | 0·49 (0·39–0·61) | 471 | 0·98 (0·79–1·20) |
| Other specified cancer | 6134 | 0·94 (0·87–1·02) | 6190 | 1·12 (1·06–1·18) |
| Respiratory disease* | 2426 | 0·31 (0·28–0·35) | 1344 | 1·20 (1·07–1·34) |
| Other specified disease | 2049 | 0·62 (0·54–0·71) | 1823 | 1·20 (1·10–1·31) |
| External cause | 2112 | 0·82 (0·71–0·95) | 1720 | 1·19 (1·08–1·32) |
| Unknown cause† | 4961 | 0·72 (0·66–0·79) | 5349 | 1·22 (1·16–1·28) |
| All causes | 35 256 | 0·79 (0·77–0·82) | 37 493 | 1·29 (1·27–1·32) |
Hazard ratio per 5 kg/m2 higher BMI (HR). HR less than 1 if BMI inversely associated with risk. Analyses exclude the first 5 years of follow-up, and adjust for study, sex, age at risk (in 5-year groups), and baseline smoking status. For analyses restricted to those who had never smoked, see webappendix p 17.
HR 0·37 (95% CI 0·30–0·44) in the range 15–25 kg/m2 after exclusion of the first 15 years of follow-up (leaving 956 deaths).
Includes 4113 deaths from cancer of unspecified site.
Stroke accounted for a third as many deaths as ischaemic heart disease did. In the upper BMI range (25–50 kg/m2) each 5 kg/m2 higher BMI was, as for ischaemic heart disease, associated with about 40% higher mortality (table 2), largely irrespective of follow-up period (after the first 5 years), smoking status, or stroke subtype (figure 4B). As with ischaemic heart disease, there was no significant heterogeneity (p=0·64) across the HRs from the eight larger studies (with >100 stroke deaths) and the aggregated smaller studies (webappendix p 5). By contrast with ischaemic heart disease, however, the association of BMI with stroke was much stronger in middle than in old age (figure 4; for both diseases, allowance for the older age at death of women eliminates the apparent relevance of sex). Furthermore, in the lower BMI range (15–25 kg/m2) there was no evidence of a positive association between BMI and stroke (figure 3, table 2). Nor was there any evidence of a positive association in this lower range after participants who had ever smoked were excluded (HR 0·98 [0·78–1·23]), or after the analysis was restricted to haemorrhagic (0·76 [0·58–1·00]) or to ischaemic (0·87 [0·68–1·10]) stroke; the number with confirmation of subtype by imaging is, however, unknown.
For the aggregate of all other vascular causes of death (table 2), the association with BMI was similar to that for stroke. In the lower BMI range (15–25 kg/m2) there was, if anything, a slightly inverse association, but in the upper range each 5 kg/m2 higher BMI was again associated with about 40% higher mortality. Among particular other vascular causes, the associations in the upper BMI range were particularly strong for mortality attributed to heart failure (HR 1·86 [1·55–2·23]) and to hypertensive disease (2·03 [1·75–2·36]).
In the upper range (25–50 kg/m2), BMI was associated strongly and positively with mortality attributed to diabetes, to non-neoplastic kidney disease, and to non-neoplastic liver disease (table 2), which was mainly cirrhosis (HR 1·79 [1·54–2·08]).
In the range 25–50 kg/m2, neoplastic disease accounted for nearly two-thirds as many deaths as did vascular disease, but the association with BMI was much weaker: only 10% higher neoplastic mortality, compared with 40% higher vascular mortality, for each 5 kg/m2 higher BMI (neoplastic HR 1·10 [1·06–1·15]). Even across the full BMI range (15–50 kg/m2) the 95% CIs for site-specific cancers were wide (webappendix p 15), but nonetheless there were positive associations for several sites, including the liver (HR 1·47 [1·26–1.71]), kidney (1·23 [1·06–1·43]), breast (1·15 [1·02–1·31] for deaths at ages 60–89 years and, identically, for deaths at 35–59 years), endometrium (1·38 [1·08–1·77]), prostate (1·13 [1·02–1·24]), and large intestine (1·20 [1·12–1.28]; male 1·29 [1·19–1·40], female 1·05 [0·94–1·18]).
In the lower range (15–25 kg/m2), BMI was associated inversely with mortality from cancer as a whole, mainly because of steep inverse associations with the cancers most strongly related to smoking (table 2; upper aerodigestive cancer includes oesophagus cancer, which had HR 0·52 [95% CI 0·38–0·72], and cancer of the mouth, pharynx, and larynx). The inverse association of BMI with lung and upper aerodigestive cancer (combined) weakened with increasing duration of follow-up but was still definite during years 10–14 (0·65 [0·53–0·79]) and years 15 and more (0·75 [0·62–0·91]). Even among lifelong non-smokers, there was a definite inverse association for upper aerodigestive cancer (0·35 [0·16–0·74]), although not for lung cancer (0·90 [0·48–1·68]).
Respiratory disease accounted for an eighth as many deaths as did vascular disease (table 2). In the lower range, BMI was strongly and inversely associated with mortality from each main type of respiratory disease, of which the most common was chronic obstructive pulmonary disease (COPD). After exclusion of the first 5 years of follow-up, COPD mortality in this BMI range was four times higher for 5 kg/m2 lower BMI (HR 0·26 [95% CI 0·22–0·30]). In the first 5 years (excluded from all the main analyses), the inverse association at low BMI was even greater (0·11 [0·08–0·16]), since COPD can cause weight loss (ie, there is reverse causality). Exclusion of an additional 10 years further attenuated the inverse association in the lower BMI range, but a strong inverse association remained more than 15 years after the baseline BMI measurement (COPD HR 0·31 [0·24–0·40]). Relatively few deaths were attributed to tuberculosis, which can cause chronic wasting and was (in the lower BMI range) strongly inversely associated with BMI even after exclusion of the first 10 years of follow-up (HR 0·09 [95% CI 0·04–0·19]). In the upper BMI range (25–50 kg/m2), overall respiratory mortality was about 20% higher for each 5 kg/m2 higher BMI (table 2).
The remaining mortality was divided into three categories (other specified diseases, external causes [mainly injury], and unknown causes), each of which had a U-shaped association with baseline BMI and a minimum at about 22·5–25 kg/m2 (table 2). For each category, 5 kg/m2 higher BMI in the 25–50 kg/m2 range was associated with about 20% higher mortality. The other specified diseases were a broad range of disorders and were not dominated by any particular causes of death. External causes of death could not be usefully analysed, since little information was available about the specific circumstances of these deaths.
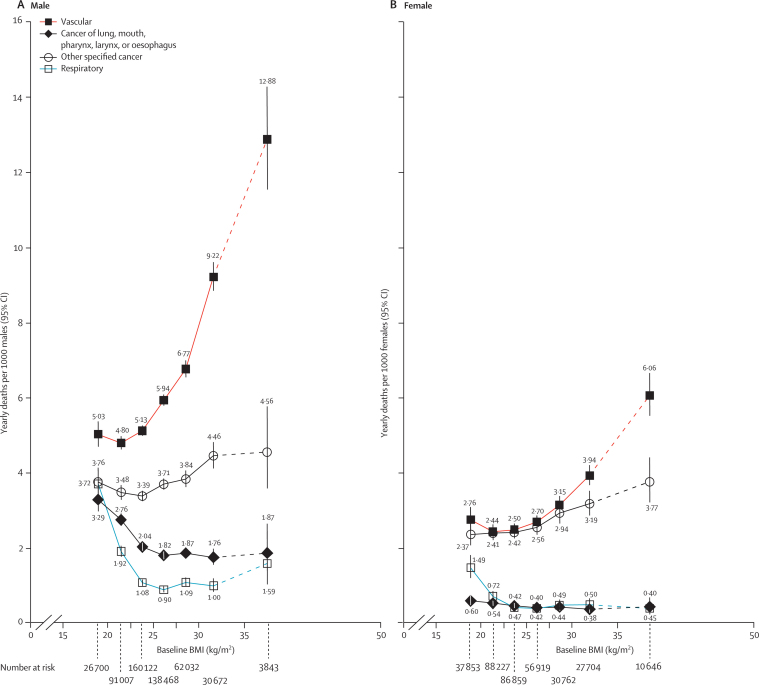
Absolute excess mortality depends not only on relative risks but also on absolute mortality rates. To indicate the absolute excess risks at different BMI levels, figure 5 is plotted on an additive scale and applies the PSC relative risks at ages 35–79 years. The absolute difference in mortality between 35–50 kg/m2 and 22·5–25 kg/m2 was five times as great for vascular as for neoplastic disease in males, and twice as great for vascular as for neoplastic disease in females. Below 22·5–25 kg/m2, there was a pronounced excess of lung cancer, upper aerodigestive cancer, and respiratory disease, particularly in males (figure 5).
Figure 5.
Mortality rates at ages 35–79 years for main disease categories versus BMI in the range 15–50 kg/m2 (excluding the first 5 years of follow-up)
Relative risks at ages 35–79 years, adjusted for age at risk, smoking, and study, were multiplied by a common factor (ie, floated) to make the weighted average match the age-standardised European Union (15 countries) mortality rate at ages 35–79 years in 2000. Neoplastic mortality is split into the types most strongly associated with smoking (cancers of the lung and upper aerodigestive tract) and all other specified types. By contrast with figures 2–4, risk is indicated on an additive rather than multiplicative scale, with floated mortality rates shown above or below each symbol. The estimates for 35–50 kg/m2 are based on limited data, so lines connecting to those estimates are dashed. Boundaries of BMI groups are indicated by tick marks. 95% CIs for floated rates reflect uncertainty in the log risk for each single rate.
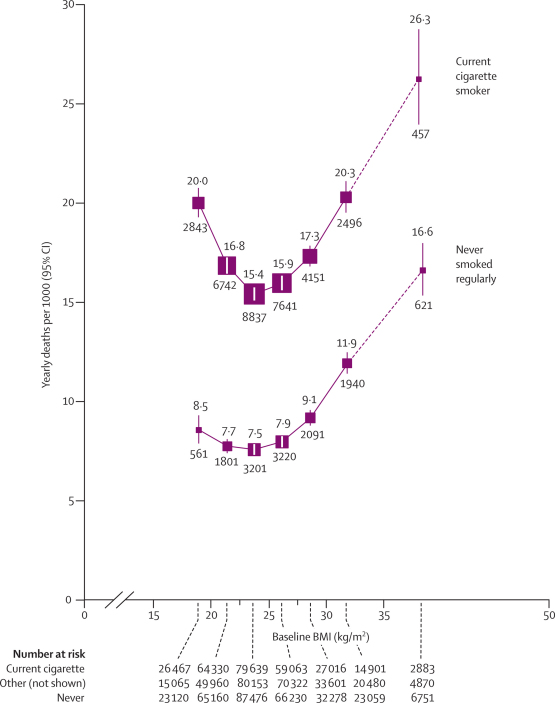
Both in current cigarette smokers and in lifelong non-smokers, overall mortality was lowest at about 22·5–25 kg/m2, but the excess mortality below this range was both relatively and absolutely much greater in smokers (figure 6, again plotted on an additive scale). In both smoking groups, the excess at 25–27·5 kg/m2 was slight, and although the excess at 30–35 kg/m2 was substantial, it was still much less than the excess attributable in this study to cigarette smoking itself (as shown in figure 6 by the vertical separation of the curves; the excess attributable to persistent cigarette smoking throughout adult life would be even greater than this). Throughout the range 25–50 kg/m2, the effects of BMI and smoking seemed to be roughly additive, rather than multiplicative, both for vascular mortality (webappendix p 8) and for all-cause mortality (figure 6).
Figure 6.
All-cause mortality at ages 35–79 years versus BMI in the range 15–50 kg/m2, by smoking status (excluding the first 5 years of follow-up)
Relative risks at ages 35–79 years, adjusted for age at risk, sex, and study, were multiplied by a common factor (ie, floated) so that the mean for all participants (including ex-smokers and anyone with missing smoking data) matches the European rate at ages 35–79 years in 2000. Results for ex-smokers and those with missing smoking data not shown (but are, taken together, only slightly above those for never smokers). Note that many smokers were at only limited risk, since they had not smoked many cigarettes during early adult life, or had stopped shortly after the baseline survey. Risk is indicated on an additive rather than multiplicative scale. The estimates for 35–50 kg/m2 are based on limited data, so lines connecting to those estimates are dashed. Floated mortality rates shown above each square and numbers of deaths below. Area of square is inversely proportional to the variance of the log risk. Boundaries of BMI groups are indicated by tick marks. 95% CIs for floated rates reflect uncertainty in the log risk for each single rate.
Discussion
In this collaborative analysis of data from almost 900 000 adults in 57 prospective studies, overall mortality was lowest at about 22·5–25 kg/m2 in both sexes and at all ages, after exclusion of early follow-up and adjustment for smoking status. Above this range, each 5 kg/m2 higher BMI was associated with about 30% higher all-cause mortality (40% for vascular; 60–120% for diabetic, renal, and hepatic; 10% for neoplastic; and 20% for respiratory and for all other mortality) and no specific cause of death was inversely associated with BMI. Below 22·5–25 kg/m2, the overall inverse association with BMI was predominantly due to strong inverse associations for smoking-related respiratory disease (including cancer), and the only clearly positive association was for ischaemic heart disease.
In laboratory studies, BMI is moderately strongly correlated (30–50%) with fat-free mass, but it is much more strongly correlated (60–90%) with fat mass.15 BMI is also strongly correlated (80–85%) with measured waist circumference;16,17 in the EPIC prospective study of 360 000 adults in Europe, for example, the two variables have about an 85% correlation, so each has a similar association with mortality.16 (In EPIC, waist-to-hip ratio was not quite as strongly related either to BMI or to mortality.) In such populations, either measurement can thus be used to help assess the causal relevance of obesity to mortality, and each could well add some predictive information to the other. Neither, however, directly measures visceral fat.
Although BMI and waist circumference are not directly causal, both are closely correlated in such populations with aspects of adiposity that directly affect blood pressure, lipoprotein particles, and diabetes (figure 1). Effective interventions for weight loss lower blood pressure, favourably affect lipoprotein particles, and increase insulin sensitivity,18 and drugs that substantially lower blood pressure19 or LDL particle numbers20 reduce vascular disease. At least some of the major adverse effects of obesity are, therefore, reversible.
For ischaemic heart disease, the magnitude of the positive association with BMI in this study can be largely accounted for by blood pressure, lipoprotein particles, and diabetes. The associations of baseline BMI with baseline measurements of SBP and of the non-HDL/HDL cholesterol ratio (figure 1) can be taken as the associations of BMI with the usual levels of these variables over the past and next few years, so they would predict at least a doubling of mortality from ischaemic heart disease between 20 kg/m2 and 30 kg/m2 (if the combined effects of SBP and the ratio of cholesterol fractions were approximately additive10,11), which is what was observed. (Merely adjusting regression analyses for single measurements of blood pressure and total cholesterol would underestimate the mediating effects of blood pressure and, especially, of lipoprotein particles.21,22) Above 30 kg/m2, further increases in BMI have little further effect on the ratio of cholesterol fractions (figure 1B), but could be associated with other adverse changes in lipoprotein particles that cannot be inferred from cholesterol fractions (eg, an increase in the number of small dense LDL particles). Diabetes becomes particularly important at BMI greater than 30 kg/m2 (figures 1C and 1D). Other hypothesised intermediate factors (eg, fibrinogen, C-reactive protein, obstructive sleep apnoea) were not assessed.
Confounding by diet, physical activity, or socioeconomic status could have somewhat affected the ischaemic heart disease results. The cardioprotective effects of physical activity might not be due solely to reduced adiposity,23 so variation in physical activity could have caused the independent effects of adiposity to be somewhat overestimated. Confounding by socioeconomic status could have caused the independent effects to be either overestimated or underestimated. In the three prospective studies of US health professionals, however, there would have been relatively little socioeconomic confounding, yet for all-cause mortality in the upper BMI range (there were too few deaths to subdivide by cause), the association seemed to be broadly similar across these three studies to that in the PSC as a whole (webappendix p 14).
The weakening of the association between BMI and mortality from ischaemic heart disease above age 70 years is probably a result of the weaker associations at older ages of blood pressure and cholesterol with risk,10,11 and the slightly weaker associations of BMI with these intermediate variables. (At older ages, BMI might depend increasingly on muscle loss.24)
For stroke, the findings in the upper and lower BMI ranges were quite different from each other. In the upper range, BMI was associated positively with ischaemic, haemorrhagic, and total stroke, and each of these associations can be largely accounted for by the effects of BMI on blood pressure. In the lower BMI range, however, there was no evidence of a positive association for ischaemic, haemorrhagic, or total stroke, despite the strong positive association between BMI and blood pressure. (For a specific blood pressure, therefore, BMI in this lower range would actually be inversely related to stroke.) These findings for stroke in the lower BMI range were not materially affected by exclusion of participants who had ever smoked (by contrast with the findings reported from a large Chinese prospective study25). The evidence from previous large studies of BMI and stroke subtype is not as consistent as might be expected,1,3,26–28 but generally suggests that the association of BMI with stroke risk is strongly positive at BMI greater than 25 kg/m2 for both ischaemic and haemorrhagic stroke, and, less definitely, that at BMI less than 25 kg/m2 it is still positive for ischaemic but not for haemorrhagic stroke. In the lower BMI range, however, the PSC found no evidence of an association for ischaemic stroke (although the possibility of a weak positive association is not excluded), and found only slight evidence of an inverse association for haemorrhagic stroke. These findings for stroke in the lower BMI range are not fully explained.
For kidney and liver disease,8,9 the positive associations with BMI could have resulted mainly from the effects of adiposity on blood pressure, diabetes, and blood lipids. Central adiposity can cause non-alcoholic fatty liver disease, which could predispose to cirrhosis or hepatocellular carcinoma (the commonest type of liver cancer).9 The positive associations of BMI with cirrhosis and liver cancer are unlikely to have been due to confounding by alcohol, since drinking was not strongly related to BMI in males and was inversely related to it in females.
For cancer, the evidence of several positive associations complements that from other million-person prospective studies (eg, the Cancer Prevention Study-II4 and the Million Women Study5). Possible mechanisms by which obesity could cause cancer at particular sites have been summarised elsewhere.29 The overall inverse association with cancer mortality in the lower BMI range (15–25 kg/m2) was mainly due to inverse associations with cancers of the lung and oesophagus. Most of the oesophageal cancer deaths occurred before the 1990s, so most are likely to have been squamous cell carcinomas,30 which are reported to be associated inversely with BMI, rather than adenocarcinomas, which are reported to be associated positively:5,31 histological subtype is, however, not available in the PSC. The inverse association for lung and upper aerodigestive cancer combined was still strongly negative even after exclusion of the first 10 years of follow-up, implying that it was not chiefly a result of reverse causality.
For COPD and other respiratory diseases, the inverse associations with BMI in the range 15–25 kg/m2 were remarkably strong. In each sex, the inverse association for respiratory mortality accounted for about 60% of the difference in all-cause mortality between 15–20 kg/m2 and 22·5–25 kg/m2 (figures 2 and 5). COPD can cause weight loss over many years, so the inverse association (even after exclusion of the first 15 years of follow-up) might have been due mainly to reverse causality (ie, to low BMI being an indicator of progressive COPD). However, some close correlate of low BMI itself could increase COPD progression and, hence, mortality.
The inverse associations with COPD, lung cancer, and upper aerodigestive cancer were much steeper in smokers than in non-smokers. Smoking is a major cause of all three diseases, and the greater steepness in smokers might have been due at least partly to uncontrolled confounding by smoking intensity. Smoking can cause weight loss,32 and if greater intensity of smoking were to cause increased weight loss, then there would be a substantially greater proportion of intensive smokers in the lower BMI categories, who would be at greater risk of these conditions (both through direct effects and, possibly, as a result of being less likely to quit). Although cigarettes smoked per day varied little with BMI in this study, other evidence (webappendix p 13) suggests that, for a specific number of cigarettes per day, leaner smokers have substantially higher blood cotinine concentrations than other smokers do (and also substantially more lung cancer, upper aerodigestive cancer, and COPD). Hence, smoking intensity might confound associations with BMI even in the absence of an association between BMI and daily cigarette consumption. Alternatively, lower BMI might somehow exacerbate the effects of smoking on respiratory cancer or other respiratory disease. The steep inverse associations for these diseases among smokers are still largely unexplained.
This study did not assess measures of central obesity, but other large epidemiological studies have done so. In the ten-country EPIC prospective study (with 12 000 deaths of known cause, of which 3000 were vascular [vs 36 000 vascular deaths in the PSC]),16 waist circumference improved the ability of BMI to predict vascular and all-cause mortality. In the 52-country INTERHEART case–control study of acute myocardial infarction (with 12 000 cases),33 a difference of 5 kg/m2 in BMI seems, for reasons that are not clear, to be of much less relevance to heart disease (odds ratio ∼1·12 [95% CI 1·08–1·16]) than it was in the PSC (HR 1·39 [1·34–1·44]) or in EPIC (HR ∼1·4), and hence to be of much less relevance than measures of central obesity are. Since case–control studies have greater potential for some types of bias, disentangling the interdependent associations of closely correlated anthropometric variables with particular diseases might need prospective studies that are even larger than this PSC study.
This report cannot quantify the effects of present levels of childhood obesity on adult mortality over the next few decades; the relevance of obesity to mortality in different ethnic groups; the substantial effects of obesity on disability, quality of life, or non-fatal disease (eg, osteoarthritis, obstructive sleep apnoea); or the positive effects of some types of adiposity on prognosis after some chronic disorders (eg, heart failure,34 respiratory disease35) have already developed. It does, however, quantify particularly reliably both the excess mortality associated with low BMI (much of which could be non-causal) and that associated with high BMI (which would be even greater if full allowance could be made for the extent to which chronic disease can cause weight loss). If the overall inverse association at low BMI is partly non-causal, then the real optimum BMI might be somewhat lower than the apparent optimum of about 23 kg/m2 or 24 kg/m2.
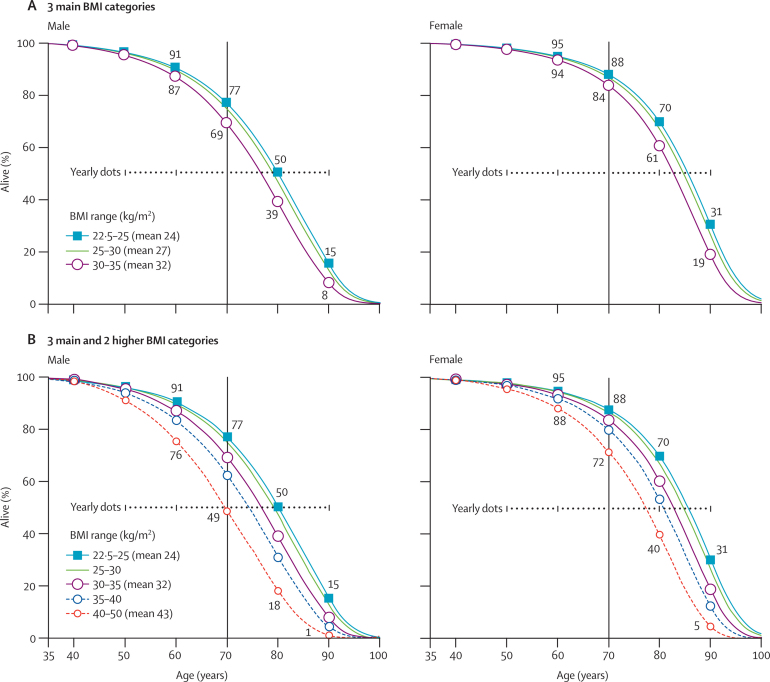
The absolute excess mortality at BMI greater than 22·5–25 kg/m2 was mainly vascular, but also partly neoplastic, and was probably largely causal (ie, due to causal factors closely associated with BMI). Figure 7 shows, for different BMI levels in middle age, estimates of the lifetime probabilities of surviving from age 35 years, which are calculated by applying the relative risks that were considered likely to be causal (webappendix p 18) to disease-specific mortality rates at ages 35–79 years from the EU in 2000.13 (The year 2000 EU probability of surviving from birth to age 35 years is 98%.) For both sexes, the median survival (figure 7A) is reduced by 0–1 year for people who would, by about age 60 years, reach a BMI of 25–27·5 kg/m2, by 1–2 years for those who would reach 27·5–30 kg/m2, and by 2–4 years for those who would become obese (30–35 kg/m2). Much less information was available for BMI greater than 35 kg/m2 (hence the dashed lines in figure 7B), but the median survival seems to be reduced by about 8–10 years in those who would become morbidly obese (40–50 kg/m2, which in the PSC is mainly 40–45 kg/m2).
Figure 7.
BMI versus lifespan in western Europe, year 2000
Estimated effects of the BMI that would be reached by about 60 years of age on survival from age 35 years, identifying European Union (EU) mortality rates in 2000 with those for BMI 25–30 kg/m2 and combining the disease-specific EU mortality rates with disease-specific relative risks (for details, see webappendix pp 18–20). The absolute differences in median survival (but probably not in survival to age 70 years) should be robust to changes in mortality rates, and therefore generalisable decades hence. (A) 3 main BMI categories. (B) 3 main and 2 higher BMI categories. (The 2 higher BMI categories account for just 2% of PSC participants, and so are indicated by dashed lines.)
The extreme reduction in survival with morbid obesity is about as great as the 10-year reduction caused by persistent cigarette smoking in male British doctors born in 1900–30, for whom the cigarette smoker versus non-smoker mortality rate ratio was about 2·5 not only at 35–69 years but also at 70–79 years of age.13,36 In the present report, the smoker versus non-smoker mortality rate ratio is slightly less than 2·5 for men aged 35–69 years, and much less than 2·5 for women aged 70–79 years (webappendix p 7). In both cases this was partly because many who were current smokers at baseline did not smoke as many cigarettes when young as the British doctors did (or, indeed, as young smokers do nowadays), and partly because many who were current smokers at baseline in the PSC would have quit during follow-up (which is taken account of in the doctors' study,36 but not in the PSC). The difference in mortality between smokers and non-smokers in figure 6 therefore underestimates the effects of smoking throughout adult life, but it could likewise underestimate the effects of becoming obese well before middle age.
These PSC relative risks for BMI, combined with recent population BMI values,37,38 suggest that in the present decade, about 29% of vascular deaths and 8% of neoplastic deaths in late middle age in the USA (where mean BMI6 at age 50 years was 28·5–29 kg/m2 in 2000) would have been attributable to having a BMI greater than 25 kg/m2; for the UK (where mean BMI38 at that age was about 1 kg/m2 lower), the corresponding proportions would have been about 23% and 6%, respectively. In both countries, as elsewhere, these proportions will probably increase if average BMI in middle age continues to rise, even if rates of vascular and neoplastic mortality continue to fall because of decreases in smoking, improvements in treatment, or other reasons. Moreover, since BMI is an imperfect measure of visceral and other adiposity, the number of vascular and other deaths attributable to all adiposity-related factors is probably appreciably greater than these calculations suggest.
In adult life, it may be easier to avoid substantial weight gain than to lose that weight once it has been gained. By avoiding a further increase from 28 kg/m2 to 32 kg/m2, a typical person in early middle age would gain about 2 years of life expectancy. Alternatively, by avoiding an increase from 24 kg/m2 to 32 kg/m2 (ie, to a third above the apparent optimum), a young adult would on average gain about 3 extra years of life.
Acknowledgments
Acknowledgments
The Prospective Studies Collaboration has been supported by the UK Medical Research Council, British Heart Foundation, Cancer Research UK, EU BIOMED programme, NIA grant P01 AG17625-01, and CTSU overheads. Merck (F Walker) helped support the 1996 meeting of collaborators. Gary Whitlock was supported by a Girdlers' Health Research Council of New Zealand Fellowship. Sarah Lewington had a British Heart Foundation Fellowship to coordinate the project. Sarah Parish supplied unpublished analyses of BMI and cotinine (webappendix).
Contributors
All members of the writing committee contributed to the collection and analysis of the data, and to the preparation of the report. All collaborators had an opportunity to contribute to the interpretation of the results and to the re-drafting of the report. The writing committee accepts full responsibility for the content of this paper.
Members of the Prospective Studies Collaboration
Writing Committee—Gary Whitlock, Sarah Lewington, Paul Sherliker, Robert Clarke, Jonathan Emberson, Jim Halsey, Nawab Qizilbash, Rory Collins, Richard Peto.
Steering Committee—S Lewington (coordinator and statistician), S MacMahon (chair), R Peto (statistician), A Aromaa, C Baigent, J Carstensen, Z Chen, R Clarke, R Collins, S Duffy, D Kromhout, J Neaton, N Qizilbash, A Rodgers, S Tominaga, S Törnberg, H Tunstall-Pedoe, G Whitlock.
PSC Collaborators—Atherosclerosis Risk in Communities (ARIC): L Chambless; Belgian Inter-university Research on Nutrition and Health (BIRNH): G De Backer, D De Bacquer, M Kornitzer; British Regional Heart Study (BRHS): P Whincup, S G Wannamethee, R Morris; British United Provident Association (BUPA): N Wald, J Morris, M Law; Busselton: M Knuiman, H Bartholomew; Caerphilly and Speedwell: G Davey Smith, P Sweetnam, P Elwood, J Yarnell; Cardiovascular Health Study (CHS): R Kronmal; CB Project: D Kromhout; Charleston: S Sutherland, J Keil; Copenhagen City Heart Study: G Jensen, P Schnohr; Evans County: C Hames (deceased), A Tyroler; Finnish Mobile Clinic Survey (FMCS): A Aromaa, P Knekt, A Reunanen; Finrisk: J Tuomilehto, P Jousilahti, E Vartiainen, P Puska; Flemish Study on Environment, Genes and Health (FLEMENGHO): T Kuznetsova, T Richart, J Staessen, L Thijs; Research Centre for Prevention and Health (Glostrup Population Studies): T Jørgensen, T Thomsen; Honolulu Heart Program: D Sharp, J D Curb; Imperial College and Oxon Epidemiology Limited: N Qizilbash; Ikawa, Noichi and Kyowa: H Iso, S Sato, A Kitamura, Y Naito; Centre d'Investigations Preventives et Cliniques (IPC), Paris: A Benetos, L Guize (deceased); Israeli Ischaemic Heart Disease Study: U Goldbourt; Japan Railways: M Tomita, Y Nishimoto, T Murayama; Lipid Research Clinics Follow-up Study (LRC): M Criqui, C Davis; Midspan Collaborative Study: C Hart, G Davey Smith, D Hole (deceased), C Gillis; Minnesota Heart Health Project (MHHP) and Minnesota Heart Survey (MHS): D Jacobs, H Blackburn, R Luepker; Multiple Risk Factor Intervention Trial (MRFIT): J Neaton, L Eberly; First National Health and Nutrition Examination Survey Epidemiologic Follow-up Study (NHEFS): C Cox; NHLBI Framingham Heart Study: D Levy, R D'Agostino, H Silbershatz; Norwegian Counties Study: A Tverdal, R Selmer; Northwick Park Heart Study (NPHS): T Meade, K Garrow, J Cooper; Nurses' Health Study: F Speizer, M Stampfer; Occupational Groups (OG), Rome: A Menotti, A Spagnolo; Ohasama: I Tsuji, Y Imai, T Ohkubo, S Hisamichi; Oslo: L Haheim, I Holme, I Hjermann, P Leren; Paris Prospective Study: P Ducimetiere, J Empana; Perth: K Jamrozik, R Broadhurst; Prospective Cardiovascular Munster Study (PROCAM): G Assmann, H Schulte; Prospective Study of Women in Gothenburg: C Bengtsson, C Björkelund, L Lissner; Puerto Rico Health Heart Program (PRHHP): P Sorlie, M Garcia-Palmieri; Rancho Bernardo: E Barrett-Connor, M Criqui, R Langer; Renfrew and Paisley study: C Hart, G Davey Smith, D Hole (deceased); Saitama Cohort Study: K Nakachi, K Imai; Seven Cities China: X Fang, S Li; Seven Countries (SC) Croatia: R Buzina; SC Finland: A Nissinen; SC Greece (Greek Islands Study): C Aravanis, A Dontas, A Kafatos; SC Italy: A Menotti; SC Japan: H Adachi, H Toshima, T Imaizumi; SC Netherlands: D Kromhout; SC Serbia: S Nedeljkovic, M Ostojic; Shanghai: Z Chen; Scottish Heart Health Study (SHHS): H Tunstall-Pedoe; Shibata: T Nakayama, N Yoshiike, T Yokoyama, C Date, H Tanaka; Tecumseh: J Keller; Tromso: K Bonaa, E Arnesen; United Kingdom Heart Disease Prevention Project (UK HDPP): H Tunstall-Pedoe; US Health Professionals Follow-up Study: E Rimm; US Physicians' Health Study: M Gaziano, J E Buring, C Hennekens; Värmland: S Törnberg, J Carstensen; Whitehall: M Shipley, D Leon, M Marmot; Clinical Trial Service Unit (CTSU): R Clarke, R Collins, J Emberson, J Halsey, S Lewington, A Palmer (deceased), S Parish, R Peto, P Sherliker, G Whitlock.
Conflict of interest statement
All the writing committee (except NQ) work in the CTSU, which has a policy of staff not accepting fees, honoraria, or consultancies. The CTSU is involved in clinical trials with funding from the UK Medical Research Council, British Heart Foundation, and/or various companies (AstraZeneca, Bayer, Merck, Schering-Plough, Solvay) as research grants to (and administered by) the University of Oxford. NQ is a former Director of Epidemiology at GlaxoSmithKline and now works in Oxon Epidemiology, which has undertaken consultancy work for several pharmaceutical companies (including GlaxoSmithKline, Pfizer, Lilly, Roche, Gilead, and Grunenthal).
Web Extra Material
References
- 1.Hu F. Obesity epidemiology. Oxford University Press; Oxford: 2008. [Google Scholar]
- 2.Manson JE, Colditz GA, Stampfer MJ. A prospective study of obesity and risk of coronary heart disease in women. N Engl J Med. 1990;322:882–889. doi: 10.1056/NEJM199003293221303. [DOI] [PubMed] [Google Scholar]
- 3.Song Y-M, Sung J, Davey Smith G. Body mass index and ischemic and hemorrhagic stroke: a prospective study in Korean men. Stroke. 2004;35:831–836. doi: 10.1161/01.STR.0000119386.22691.1C. [DOI] [PubMed] [Google Scholar]
- 4.Calle EE, Rodriguez C, Walker-Thurmond K. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 5.Reeves GK, Pirie K, Beral V. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134–1139. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO Global InfoBase team . The SuRF Report 2. Surveillance of chronic disease risk factors: Country-level data and comparable estimates. World Health Organization; Geneva: 2005. [Google Scholar]
- 7.Janssen I, Mark AE. Elevated body mass index and mortality risk in the elderly. Obes Rev. 2007;8:41–59. doi: 10.1111/j.1467-789X.2006.00248.x. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Chen X, Song Y. Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int. 2008;73:19–33. doi: 10.1038/sj.ki.5002586. [DOI] [PubMed] [Google Scholar]
- 9.Gaemers IC, Groen AK. New insights in the pathogenesis of non-alcoholic fatty liver disease. Curr Opin Lipidol. 2006;17:268–273. doi: 10.1097/01.mol.0000226118.43178.98. [DOI] [PubMed] [Google Scholar]
- 10.Prospective Studies Collaboration Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 11.Prospective Studies Collaboration Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55 000 vascular deaths. Lancet. 2007;370:1829–1839. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 12.Clarke R, Shipley M, Lewington S. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol. 1999;150:341–353. doi: 10.1093/oxfordjournals.aje.a010013. [DOI] [PubMed] [Google Scholar]
- 13.Peto R, Watt J, Boreham J. Deaths from smoking. 2006. http://www.deathsfromsmoking.net/ (accessed Jan 26, 2009).
- 14.Plummer M. Improved estimates of floating absolute risk. Statist Med. 2004;23:93–104. doi: 10.1002/sim.1485. [DOI] [PubMed] [Google Scholar]
- 15.Gallagher D, Visser M, Sepúlveda D. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol. 1996;143:228–239. doi: 10.1093/oxfordjournals.aje.a008733. [DOI] [PubMed] [Google Scholar]
- 16.Pischon T, Boeing H, Koffmann K. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 17.Rexrode KM, Carey VJ, Hennekens CH. Abdominal adiposity and coronary heart disease in women. JAMA. 1998;280:1843–1848. doi: 10.1001/jama.280.21.1843. [DOI] [PubMed] [Google Scholar]
- 18.National Institutes of Health Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. Obes Res. 1998;6(suppl 2):51S–209S. [PubMed] [Google Scholar]
- 19.Blood Pressure Lowering Treatment Trialists' Collaboration Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–1535. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 20.Cholesterol Treatment Trialists' (CTT) Collaborators Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 21.Jee SH, Sull JW, Park J. Body-mass index and mortality in Korean men and women. N Engl J Med. 2006;355:779–787. doi: 10.1056/NEJMoa054017. [DOI] [PubMed] [Google Scholar]
- 22.Bogers RP, Bemelmans WJE, Hoogenveen RT. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: a meta-analysis of 21 cohort studies including more than 300 000 persons. Arch Intern Med. 2007;167:1720–1728. doi: 10.1001/archinte.167.16.1720. [DOI] [PubMed] [Google Scholar]
- 23.Li TY, Rana JS, Manson JE. Obesity as compared with physical activity in predicting risk of coronary heart disease in women. Circulation. 2006;113:499–506. doi: 10.1161/CIRCULATIONAHA.105.574087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Micozzi MS, Harris TM. Age variations in the relation of body mass indices to estimates of body fat and muscle mass. Am J Phys Anthropol. 1990;881:375–379. doi: 10.1002/ajpa.1330810307. [DOI] [PubMed] [Google Scholar]
- 25.Zhou M, Offer A, Yang G. Body mass index, blood pressure, and mortality from stroke: A nationally representative prospective study of 212 000 Chinese men. Stroke. 2008;39:753–759. doi: 10.1161/STROKEAHA.107.495374. [DOI] [PubMed] [Google Scholar]
- 26.Rexrode KM, Hennekens CH, Willett WC. A prospective study of body mass index, weight change, and risk of stroke in women. JAMA. 1997;277:1539–1545. doi: 10.1001/jama.1997.03540430051032. [DOI] [PubMed] [Google Scholar]
- 27.Kurth T, Gaziano M, Rexrode KM. Prospective study of body mass index and risk of stroke in apparently healthy women. Circulation. 2005;111:1992–1998. doi: 10.1161/01.CIR.0000161822.83163.B6. [DOI] [PubMed] [Google Scholar]
- 28.Asia Pacific Cohort Studies Collaboration Body mass index and cardiovascular disease in the Asia-Pacific Region: an overview of 33 cohorts involving 310 000 participants. Int J Epidemiol. 2004;33:751–758. doi: 10.1093/ije/dyh163. [DOI] [PubMed] [Google Scholar]
- 29.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nature Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 30.Devesa SS, Blot WJ, Fraumeni JF., Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049–2053. [PubMed] [Google Scholar]
- 31.Smith M, Zhou M, Whitlock G. Esophageal cancer and body mass index: results from a prospective study of 220 000 men in China and a meta-analysis of published studies. Int J Cancer. 2007;122:1604–1610. doi: 10.1002/ijc.23198. [DOI] [PubMed] [Google Scholar]
- 32.Williamson DF, Madans J, Anda RF. Smoking cessation and severity of weight gain in a national cohort. N Engl J Med. 1991;324:739–745. doi: 10.1056/NEJM199103143241106. [DOI] [PubMed] [Google Scholar]
- 33.Yusuf S, Hawken S, Ôunpuu S. Obesity and the risk of myocardial infarction in 27 000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 34.Curtis JP, Selter JG, Wang Y. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005;165:55–61. doi: 10.1001/archinte.165.1.55. [DOI] [PubMed] [Google Scholar]
- 35.Landbo C, Prescott E, Lange P. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:1856–1861. doi: 10.1164/ajrccm.160.6.9902115. [DOI] [PubMed] [Google Scholar]
- 36.Doll R, Peto R, Boreham J. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ. 2004;328:1519–1533. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogden CL, Carroll MD, Curtin LR. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 38.Health Survey for England. 2004. http://www.dh.gov.uk/en/Publicationsandstatistics/PublishedSurvey/HealthSurveyForEngland/index.htm (accessed Jan 26, 2009).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.