Abstract
Stimulation of mouse CD4+ T cells in the presence of TGF-β results in the expression of Foxp3 and induction of Treg function. Stimulation of human CD4+ T cells under similar conditions results in the expression of Foxp3, but the cells lack regulatory cell function. TGF-β expressed on the surface of Treg also induces Foxp3 expression and Treg function in responder cells. Both of these mechanisms may play a role in vivo in the induction of antigen-specific extra-thymic Treg.
Keywords: Regulatory T cells, TGF-β, Foxp3, IL-2
Introduction
I was quite skeptical by the initial reports [1-3] documenting induction of Foxp3 expression and Treg function by stimulating conventional T cells in the presence of TGF-β. My first reaction was that alternative explanations could be easily offered to explain the experimental results. Although the induced populations exhibited suppressor activity, it was unclear if the starting population was actually free of Foxp3+ cells and what percentage of cells in the induced population actually expressed Foxp3. Put simply, our interpretation was that TGF-β promoted the selective survival of Foxp3+ T cells present in the starting population. However, as soon as mAbs to Foxp3 became available, my group elected to re-examine the capacity of TGF-β to induce the differentiation of Foxp3+ T cells with regulatory function in mouse and man.
Studies with Mouse CD4+ T Cells
Culture of naïve T cells from TCR transgenic mice on a RAG-/- background (which lack Foxp3+ T cells) resulted in induction of Foxp3 expression in 85-95% of the cells [4]. In general, we stimulate the responder T cells with plate-bound anti-CD3 although similar results were obtained with soluble anti-CD3 and APC. IL-2 was found to play a non-redundant role in the induction of Foxp3 expression. The role of CD28 in the induction of Foxp3 was solely related to its capacity to enhance the endogenous production of IL-2. Foxp3 expression was stable in vitro and in vivo in the absence of IL-2.
We have performed an extensive series of in vivo and in vitro studies to test the regulatory capacity of the induced cells. They completely resemble thymic-derived Treg in vitro [4, 5]. They fail to proliferate when stimulated via the TCR alone, produce very low levels of all cytokines including IL-10, and potently suppress naïve responder cells in coculture experiments. We have examined their in vivo function in three distinct models. In a model of autoimmune gastritis when Tg T effector cells are transferred to nu/nu recipients, autoantigen-specific TGF-β induced Treg were potent inhibitors of disease induction, blocked the initial priming and expansion of effector T cells, and maintained expression of Foxp3 in vivo for > 50 days [5]. Following immunization with peptide in adjuvant, antigen-specific TGF-β- induced Treg were also capable of inhibiting the expansion of effector cells in normal mice in vivo [Davidson, T. et al, in preparation]. Lastly, a single injection of TGF-β induced polyclonal Treg at birth markedly suppressed lymphocyte expansion and autoimmune disease manifestations in scurfy mice [Huter, E. et al, in preparation]. Based on these criteria, we conclude that TGF-β induced murine Treg are the “real” thing.
It is fair to point out that some other groups [6, 7] have failed to demonstrate Treg function, stability of Foxp3 expression, or survival in vivo of TGF-β-induced Treg even though they express high levels of Foxp3 immediately after induction. From our own studies, it is clear that Foxp3 cannot be induced in T cells following an initial period of activation in vitro. One possibility to account for the differences is that the strength of the TCR signal is critical for the rapid induction of a stable Treg phenotype. Under our culture conditions, we observed induction of Foxp3 expression after 24h, prior to any cell divisions. Others [6] have only seen significant Foxp3 expression after 3 days and changes may have been induced in the responder cells downstream from Foxp3 that preclude Treg function. Lastly, trivial differences in cell culture protocols may be important. We routinely use 5ng/ml of TGF-β and 100U/ml of IL-2, while others [7] have used much higher concentrations of TGF-β and lower concentrations of IL-2. The balance between these two inductive cytokines and the strength of TCR stimulation may be critical for optimal induction of Foxp3 expression and function.
Studies with Human CD4+ T Cells
We have attempted to translate our results in the mouse to human CD4+ T cells. Considerable controversy exists regarding the regulation of Foxp3 expression in human T cells and some studies have suggested that TCR stimulation alone is sufficient to induce Foxp3 expression [8]. We found [9] that TCR stimulation alone did induce Foxp3 expression, but that the induction of Foxp3 was almost completely dependent on TGF-β present in the serum, as Foxp3 induction was markedly inhibited by the addition of anti-TGF-β to the cultures. Foxp3 expression could easily be induced in ~80% of naïve responder T cells by the addition of exogenous TGF-β. However, the induced cells were neither anergic nor suppressive as a very high percentage produced IL-2 and proliferated when restimulated via the TCR.
We concluded from these studies that Foxp3 expression is not sufficient to confer a regulatory phenotype in human CD4+ T cells. What is the difference between mouse and man? One possibility is that CD4+CD45RA+ T cells in the peripheral blood of normal adults are not truly naïve, but similar results were observed with CD4+ naïve T cells from cord blood. It is also possible that the level of Foxp3 expression is not sufficient to confer a Treg phenotype. Although the TGF-β-induced cells expressed lower levels of Foxp3 than similarly activated thymic-derived Treg, the level of Foxp3 expression in the induced cells was always higher than in freshly explanted Treg, yet the latter are completely anergic to TCR stimulation. Foxp3 expression was also maintained for at least 30 days in culture. It is always possible, as illustrated in the mouse studies, that we did not find the optimal culture conditions that would permit Foxp3 expression coupled with Treg function. We used different APC populations, different concentrations of TGF-β, rapamycin, as well as different concentrations of anti-CD3 and/or anti-CD28 with or without IL-2, but failed to induce Foxp3+ T cells with regulatory properties. It still is unclear whether the responsiveness of naïve human T cells to stimulation to TGF-β is fundamentally different from that of mouse CD4+ T cells, or if we have just not hit on the right conditions for induction of a Treg phenotype.
In our hands, repeated restimulation in the presence or absence of TGF-β also failed to induce anergy and suppressive function. As pointed out in the accompanying article by David Horwitz, [10], it is possible that multiple cycles of stimulation will induce Treg function, but one must be cautious in the interpretation of his results. Repeated stimulation of T cells in culture may result in an anergic phenotype as the stimulated cells may lose their capacity to produce IL-2. One must also be very cautious in the interpretation of data from the standard T suppression co-culture assays, as addition of highly activated, IL-2-dependent T cells may result in “artifactual” suppression secondary to IL-2 consumption by the activated T cells. In any case, our group and the Horwitz group agree that after one round of stimulation in the presence of TGF-β, the Foxp3+ T cells do not have Treg function. If a similar phenomenon occurs in vivo at sites of inflammation where TGF-β concentrations are high, Foxp3 expression may be induced in the absence of Treg function. Such “pseudo” Treg would express high levels of CD25 and would be difficult to distinguish from “real” Treg and may account for some of the purported defects in Treg function observed with highly purified CD25hi T cells from patients with autoimmune diseases. We are presently attempting to define cell surface and molecular markers that would distinguish the “real” from the “pseudo” Treg. In addition, alternative assays for human Treg function are desperately needed including in vivo studies in humanized mice.
What is the function of Treg-produced TGF-β?
A major controversy has existed in the Treg field regarding the contribution of TGF-β, either secreted or present on the cell surface of Treg, to their suppressive function [11,12]. Our previous studies failed to define a role for Treg-produced TGF-β in our standard in vitro suppression assay. Foxp3+ Tregs from TGF-β deficient mice were also capable of suppressing the induction of colitis by Foxp3- effector cells in vivo [13]. In view of the studies in the mouse demonstrating that TGF-β is a potent inducer of Foxp3+ Treg, it remained possible that some of the purported suppressive effects of Treg-produced TGF-β were secondary to its capacity to induce Treg cells de novo and not secondary to its suppressor effector functions.
Several studies have suggested that Foxp3+ Treg selectively express TGF-β or TGF-β coupled to latency associated peptide (LAP) on their cell surface [14]. We have been unable to confirm these observations on freshly explanted Foxp3+ human or mouse Treg, but can detect LAP only on the cell surface of activated human or mouse Treg, but not T effector cells. What is the function of this cell surface expressed propeptide of LAP-TGF-β? In a co-culture model (Andersson, J. et al, in preparation), activated Foxp3+ Tregs were capable of inducing Foxp3 expression in 10-30% of naïve responder T cells in the presence of exogenous IL-2, so that suppression is partially abrogated. The induction of Foxp3 by activated Treg was TGF-β-dependent, cell contact-dependent, and required that the Treg were capable of producing active TGF-β. The Treg-induced Treg express a fully functional suppressor phenotype both in vitro and in vivo. It is as yet unknown how the Treg expressed TGF-β-LAP is activated although it does not appear to require DC or αV integrins [15].
Summary: Treg and TGF-β
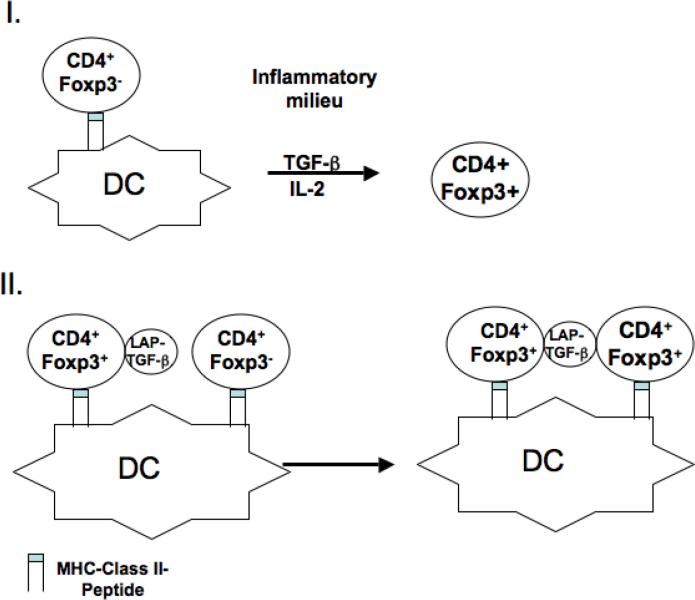
Our studies on the role of TGF-β in Treg function over the past 8 years have failed to identify a major contribution of TGF-β to the suppressor effector function of Foxp3+ Treg. On the other hand, the role of TGF-β as an inducer of Foxp3+ Treg, at least in the mouse, is now clear. We propose two pathways by which TGF-β play a critical role in the induction of Treg in vivo (Fig.1). In pathway one, Foxp3+ Treg would be induced by stimulation of naïve T cells via the TCR in the presence of TGF-β in solution. The TGF-β could be produced by any cell type or might be selectively produced by a subset of DC that also present the cognate antigen to the Treg, for example in the gut mucosa. The second pathway (Fig. 1) involves induction of TGF-β-mediated infectious tolerance by antigen-specific Treg that are activated by their cognate antigen on DC to express TGF-β-LAP on their cell surface. The activated Treg would then induce Foxp3 in T cells specific for the same antigen or a different antigen presented by the same DC in a cell contact-dependent manner. This pathway would represent a carefully regulated mechanism for the expansion of antigen-specific Treg and may be critical in protecting the host from pathogen-induced autoimmune diseases such as colitis[15, 16].
Figure 1.
Model I. Foxp3 expression is induced in naïve T cells by TCR stimulation in the presence of TGF-β present in an inflammatory infiltrate. Model II. Foxp3 expression is induced in naïve T cells by TCR stimulation and TGF-β released from the surface of activated thymic-derived Treg which have been stimulated by their cognate ligand on the surface of the same DC as the naïve T cells.
Abbreviations
- LAP
latency associated peptide
References
- 1.Chen W, et al. J. Exp. Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fantini MC, et al. J. Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 3.Zheng SG, et al. J. Immunol. 2004;172:153–1539. doi: 10.4049/jimmunol.172.3.1531. [DOI] [PubMed] [Google Scholar]
- 4.Davidson TS, et al. J. Immunol. 2007;178:4022–4026. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- 5.DiPaolo RJ, et al. J. Immunol. 2007;179:4685–4693. doi: 10.4049/jimmunol.179.7.4685. [DOI] [PubMed] [Google Scholar]
- 6.Selveraj RK, et al. J. Immunol. 2007;178:7667–7677. doi: 10.4049/jimmunol.178.12.7667. [DOI] [PubMed] [Google Scholar]
- 7.Hill JA, et al. Immunity. 2007;27:786–800. doi: 10.1016/j.immuni.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Walker MR, et al. J. Clin. Inves. 2003;112:1437–1443. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tran DQ, et al. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horwitz DA, et al. Eur. J. Immunol. 2008;37:???. doi: 10.1002/eji.200738109. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura K, et al. J. Exp. Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piccirillo CA, et al. J. Exp. Med. 2002;196:237–245. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kullberg MC, et al. Eur. J. Immunol. 2005:2886–2895. doi: 10.1002/eji.200526106. [DOI] [PubMed] [Google Scholar]
- 14.Oida T, et al. J. Immunol. 2006;177:2331–2339. doi: 10.4049/jimmunol.177.4.2331. [DOI] [PubMed] [Google Scholar]
- 15.Travis MA, et al. Nature. 2007;449:361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li MO, et al. Immunity. 2007;26:579–591. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]