Abstract
Evidence from many human and rodent studies has established that T lymphocytes enhance inflammation in atherosclerotic plaques, and contribute to lesion progression and remodeling. Recent work also indicates that regulatory T cells are important in limiting pro-atherogenic T cell responses. Given the important role of T cells in atherosclerosis, there is a need to fully understand how pro-atherogenic T cells are activated and regulated. Antigen-dependent activation of both naïve T cells leading to clonal expansion and effector T cell differentiation, as well as activation of effector and memory T cells is enhanced by signals provided by costimulatory molecules expressed by antigen presenting cells, which bind to receptors on the T cells. In addition, T cell responses to antigen are negatively regulated by coinhibitory molecules expressed by antigen presenting cells, which bind to receptors on T cells. Two major families of costimulatory molecules include the B7 and the TNF families. These molecules bind to receptors on T cells belonging to the CD28 or TNF receptor families, respectively. The best defined coinhibitors and their receptors belong to the B7 and CD28 families. Recent work has begun to define how these T cell costimulatory and coinhibitory pathways influence atherosclerosis, largely in mouse models of the disease. Profound effects are attributable to molecules in both the B7/CD28 (B7-1/2, ICOS and PDL-1/2) and the TNF/TNF receptor (CD40, Ox40 and CD137) families. One emerging theme is that both pathogenic effector T cell responses and regulatory T cells are influenced by overlapping sets of costimulators and coinhibitors. These complexities must be considered as immunotherapeutic approaches for atherosclerotic disease are developed.
Keywords: atherosclerosis, costimulation, coinhibition, T lymphocytes
Introduction
The chronic inflammatory nature of atherosclerotic disease is now widely appreciated. The identification of molecular and cellular pathways that promote or inhibit arterial wall inflammation is a promising first step in development of immunomodulatory therapy for atherosclerosis. T cell costimulatory and coinhibitory pathways are engaged at the same time as T cell antigen recognition pathways, and are of fundamental importance to the regulation of adaptive immunity and to diseases caused by dysregulated adaptive immune responses. In this review, we will first provide a background on the role of T lymphocyte in atherosclerosis, and the regulation of T cell responses by costimulatory and coinhibitory molecules. We will then review the evidence that T cell costimulators and coinhibitors influence atherosclerotic disease.
T lymphocytes and atherosclerosis lesions
The pathogenesis of atherosclerosis involves the deposition and modification of lipids within arterial walls, as well as a chronic inflammatory response to these modified lipids 1. The inflammatory response is mediated by components of the innate immune system including macrophages and dendritic cells 2,3, and by components of the adaptive immune system, including T lymphocytes 1,4 (Figure 1). T lymphocytes are present at all stages of lesion development 5 and mouse models of atherosclerosis, especially low density lipoprotein receptor deficient mice (Ldlr−/−) and apolipoprotein E deficient mice (ApoE−/−), have established the pivotal role of T cells and their cytokines in the atherosclerotic process 6–8. In at least some studies with Ldlr−/− and ApoE−/− mice deficient in all lymphocytes (due to Rag or SCID gene mutations), there is a significant reduction of the atherosclerotic burden compared to immunocompetent controls 6 and transfer of CD4+ T-cells into SCID- ApoE−/− mice enhances atherosclerosis 7. The majority of T lymphocytes in mouse and human atherosclerotic lesions are CD4+ T-helper cells that express the αβ T cell antigen receptor (TCR) and have a T-helper 1 (Th1) phenotype. Th1 cells are derived from naïve CD4+ T cell precursors by antigen and costimulators in the presence of certain cytokines, including IL-12 and IFNγ. The defining feature of Th1 cells is their production of IFNγ, a pro-inflammatory cytokine that activates macrophages, as well as several other cell types. There is a significant presence of CD8+ T cells in human lesions, but little is known about their specificities. In addition to αβ TCR T cells, there are smaller numbers of T cells expressing invariant TCRs, including γδ T cells and iNKT cell. Studies of iNKT-deficient mice support the role for these lipid-antigen specific cells in both promoting and limiting atherosclerosis 9.
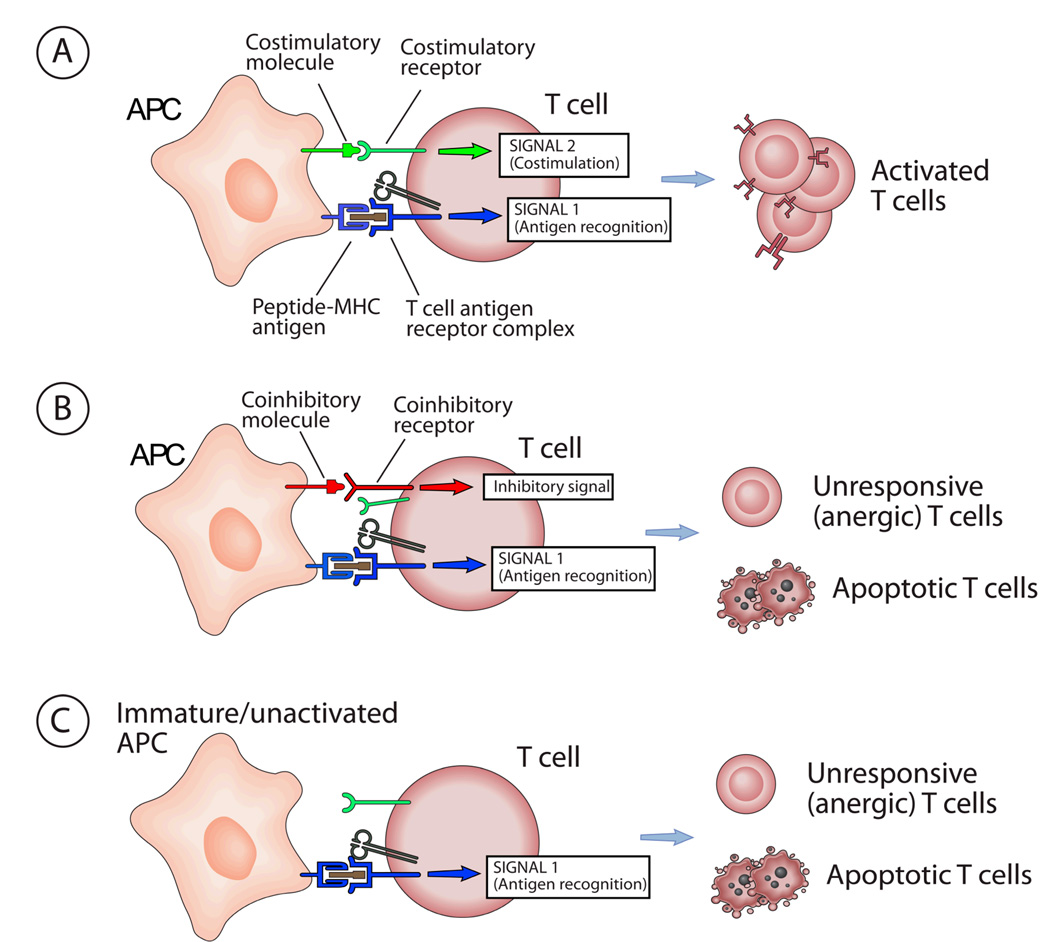
Figure 1. Influence of costimulation and coinhibition on T cells.
A. When T cells are activated by peptide-MHC antigen, the T cell antigen receptor (TCR) delivers activating signals, often called Signal 1. Mature activated dendritic cells (DCs) or other antigen presenting cells (APCs) express costimulatory molecules which bind to receptors on the T cell simultaneously with antigen recognition. The costimulatory receptors deliver signals, called Signal 2, which cooperate with TCR signals to activate the T cell. Thus the combination of antigen recognition and costimulation (Signal 1 + Signal 2) leads to T cell activation. In the case of naive T cells, activation results in clonal expansion and differentiation into effector T cells. In the case of already differentiated effector T cells or memory T cells, activation will result in performance of effector functions (e.g. cytokine secretion, target cell killing). B. APCs may express molecules which bind to coinhibitory receptors on the T cell, and deliver signals which block the activating signals delivered by the T cell antigen receptor (Signal 1 plus coinhibitory signals). The net result will be either functional inactivation of the T cells (called anergy) or T cell apoptosis. In some cases, the same molecules on the APC can bind to both coinhibitory receptors and costimulatory receptors (e.g. B7-1 and B7-2 bind to both CD28 and CTLA-4). Coinhibition may occur even in the presence of costimulators, if the coinhibitory receptor signals dominate or the coinhibitory receptors compete for binding of the costimulatory molecules. C. When immature DCs or other cells that do not express costimulatory molecules present antigen to T cells, (Signal 1 alone), T cell anergy or apoptosis may result. This is a putative mechanism for tolerance induction.
Candidate atherosclerosis-related T cell antigens
Most T cells recognize peptides bound to cell surface major histocompatibility (MHC) molecules; CD4+ T cells recognize peptides displayed by class II MHC, and CD8+ T cells recognize peptides displayed by class I MHC molecules. iNKT cells recognize lipids bound to the class I MHC-like CD1 molecules, and γδ T cells may recognize nonpeptide antigens. The relevant peptide antigen-specificities of plaque CD4 T cells have not been definitively established, but evidence implicates that many of these T cells recognize antigens generated by oxidative modification of low density lipoproteins (Ox-LDL). Ox-LDL-specific T cells can be detected in atherosclerotic animals and patients, and can be recovered from atherosclerotic lesions 10–12. ox-LDL-specific IgG antibodies, whose isotypes indicate T cell-dependent help of the B cell producing them, are also detectable in mice and humans with atherosclerosis 13. Moreover, adoptive transfer of ox-LDL antigen-specific T cells exacerbates atherosclerosis 14. A variety of studies of human and mouse lesions also indicate that T cells specific for heat shock protein 60/65 (Hsp 60/65) contribute to the inflammation in atherosclerotic arteries 15. In addition to endogenously generated autoantigens, T cell may recognize antigens produced by infectious organisms that reside in plaques. Perhaps the most extensively studied microbe putatively implicated in the pathogenesis of atherosclerosis in Chlamydia pneumoniae (Cpn) 16. T cells specific for CpN Hsp65 have been isolated from human lesions, and CpN HSP65 has been detected in the majority of human lesions analyzed 17,18. Nonetheless, studies have failed to show that antibiotic therapy reduces atherosclerosis-related clinical events in patients at risk 16,18. Another candidate antigens that has also been implicated in the pathogenesis of atherosclerosis is β-glycoprotein Ib (β-GPI) 19–21, which is expressed by platelets.
Pathogenic role of T cells in atherosclerosis
Th1 cells are involved in two distinct pathophysiologically relevant phases of atherosclerotic disease. First, the T cells contribute, over many years, to the inflammatory processes that enhance accumulation of macrophage foam cells and smooth muscle cells, which comprise the bulk of the cellular mass of lesions. This pathogenic role of T cells in atherosclerosis appears to fit the description of a chronic Th1-mediated delayed type hypersensitivity (DTH) response. DTH responses involve cognate interaction of Th1 cells with antigen-presenting macrophages, and the bidirectional activation of each cell type. The two most important effector molecules produced by the activated Th1 cell in this context are membrane-bound CD40-ligand, which binds to CD40 on the macrophage, and IFNγ, which binds to the IFNγ receptor on the macrophage. Signals from both CD40 and IFNγR synergistically induce the expression of multiple proinflammatory genes in the macrophage. This type of activation promotes further inflammation by elaboration of cytokines such as IL-1 and TNF, and causes tissue destruction by upregulation of iNOS and phagocyte oxidase activity and the release of reactive oxygen species. Several laboratories have established that IFNγ has pro-atherogenic effects, as shown by increased lesion development in hypercholesterolemic mice given IFNγ 22, and decreased lesion development in mice when IFNγ or its receptor are deficient. 8,23. T-bet is a transcription factor required for Th1 differentiation. T-bet−/−Ldlr−/− mice develop less atherosclerosis than controls, and they have an impaired Th1 response to Ox-LDL 24. A second pathogenic role of Th1 cells is their ability to stimulate the release of matrix degrading enzymes, including matrix metalloproteinases (MMPs), from lesional macrophages. These enzymes can reduce the collagen content of fibrous caps and render the plaques more likely to rupture and acutely precipitate intralumenal thrombus formation and ischemic damage of downstream tissues 25.
Regulatory T cells and atherosclerosis
Regulatory T cells (Treg) are generally defined as T cells that actively suppress activation or effector function of other T cells, either by direct effects on these T cells or through effects on APCs. There are several different subsets of T cells defined operationally by T cell suppressive functions and by cell surface molecule, cytokine and transcription factor expression. An abundant literature has established that one or more of these Treg subsets contribute to the maintenance of self tolerance and to the regulation of immune responses. The best characterized Treg are identified by a CD4+CD25+CD127lo surface phenotype 26, and expression of FoxP3, a forkhead family transcription factor. FoxP3 is a lineage specification factor for these Treg and has a crucial role in their suppressive function 27. These FoxP3+ Treg comprise 5%–10% of peripheral CD4+ T cells 28, and are generated during thymic development as well as in the periphery concomitantly with the generation of effector T cells from naïve T cell precursors. Treg cells are antigen-specific but in a permanent state of activation, enabling them to regulate other activated T cells by contact inhibition and/or through the secretion of anti-inflammatory cytokines IL-10 and TGFβ.
Recent data suggest that Treg are important in the regulation of proatherogenic T cell responses 29. In mouse models, deficiency of Treg is associated with increased atherogenesis and lesion inflammation 30–32. A large proportion of the T cells in the atherosclerotic plaque are Th1 cells secreting pro-inflammatory cytokines, and this is counter balanced and suppressed by Treg secreting anti-inflammatory cytokines. Indeed, Th1 pro-inflammatory cytokines such as IL-12 and IFN-γ have a key role in promoting atherogenesis 8,33 while the anti-inflammatory cytokines produced by Treg, IL-10 and TGFβ, have been shown to attenuate atherosclerosis 34–37. Treg may influence atherosclerosis by suppressing naïve T cell activation in lymphoid tissues and also by suppressing effector T cell activation in lesions, but the relative importance of these two sites of action is not known. It has become clear that a comprehensive understanding of the contribution of T cell immunity to atherosclerosis must include elucidating how Treg influence effector T cells responses to atherosclerosis-associated antigens.
T cell costimulation
T cell mediated immune responses begin when naïve T cells in lymphoid tissues are activated by antigen (e.g. from a microbe) to proliferate and differentiate into effector T cells. The effector T cells may then cooperate with B cells in the lymphoid tissues to promote an antibody response, or they may migrate to peripheral tissue and interact with infected cells at these sites. A small number of T cells will survive as long-lived memory cells after an acute T cell response wanes, and these cell may be reactivated by subsequent exposure to the same antigen. At each of these stages, the T cells are activated by the combination of two different types of signals, both delivered by antigen presenting cells (APCs). Dendritic cells (DCs) are the APCs that initiate T cell immune responses by transporting protein antigens from tissue sites to lymph nodes, processing the proteins into MHC-binding peptides, and displaying peptide-MHC complexes for recognition by naïve T cells that enter the lymph node from the circulation. The recognition of antigen by a naïve T cell generates Signal 1. The second signal is generated by other proteins, called costimulatory molecules, which are expressed on the APC plasma membrane and bind to receptors on the T cells, simultaneously with the recognition of antigen (Figure 1). Costimulator expression on DCs is induced and/or upregulated by microbial products, such as bacterial LPS, which signal through innate pattern recognition receptors, including Toll-like receptors. Therefore naïve T cell activation will occur only when a DC displays an antigen and concurrently displays another molecule indicating infection or cell stress. In this way, costimulation serves to promote T cell responses only to appropriate antigens and limits unwanted responses to innocuous antigens including self proteins.
Costimulatory pathways are not only necessary for naïve T cell activation, but their absence at the time of presentation of antigen to a naive T cell can lead to functional inactivation of the T cell, called anergy, or may cause death of the T cell by apoptosis (Figure 1). These consequences of costimulator deficiency are considered to be important for maintenance of self tolerance, because the peptide antigens most likely to be presented in the absence of costimulation are derived from normal self proteins. Blockade of costimulators is therefore a major investigational strategy for the therapeutic induction of tolerance to antigens that drive immune/inflammatory diseases.
In addition to naive T cell activation, costimulation of T cells can enhance responses of effector and memory T cells that are being reactivated by other types of APCs either in lymphoid tissues (e.g. B cells) or in peripheral tissues (e.g. macrophages). This concept first emerged when it was discovered that some costimulatory molecules are induced by various inflammatory stimuli on APCs other than DCs, and that receptors for some of these costimulatory molecules are only expressed after T cell activation, as discussed below. Although costimulation is now understood to regulate different stages of T cell responses, the relative importance or the specific effects of costimulation do vary among different T cell subsets. For example, activation of naïve CD4+ T cells is stringently dependent on costimulation. Activation of effector CD8+ cytotoxic T lymphocytes is perhaps least dependent on costimulation as compared to other T cell subsets.
Families of costimulatory molecules and their receptors
There are many different proteins with costimulatory activity (Table 1); the following discussion will focus on those with well documented in vivo significance and/or those that have been studied in the context of atherosclerotic disease. The best defined, and perhaps biologically most significant costimulatory molecules belong to the B7 family 38. B7-family molecules bind to members of the CD28 family of receptors expressed on T cells. The prototypical members of the B7-family are B7-1 (CD80) and B7-2 (CD86), which are expressed by dendritic cells, macrophages, B cells, and T cells. Both these proteins bind to CD28, which is constitutively expressed on virtually all mature murine T cells, 95% of human CD4+ T cells, and about 45% of human CD8+ T cells. CD28 signaling involves phosphorylation of a tyrosine in its cytoplasmic tail, binding of the Grb2 adapter protein, and activation of the PI3K/AKT pathway 49. This pathway, together with TCR signaling, promotes IL-2 gene expression and cellular proliferation, as well as expression of anti-apoptotic genes. Although these effects are physiologically only seen with concomitant antigen-receptor signaling, superagonist anti-CD28 antibodies have caused unanticipated polyclonal T cell activation in humans, presumably in the absence of TCR-signaling.
Table 1.
T cell costimulatory and coinhibitory molecules
| Receptor | Expression | Ligand | Expression | Effects on atherosclerosis |
|---|---|---|---|---|
| Co-stimulatory pathways-CD28/B7 families | ||||
| CD28 | T cells, constitutive | B7-1 (CD80), B7-2 (CD86) | DCs, B cells, monocytes/macrophages , T cells | B7-1/B7-2 /Ldlr KO--decreased.70Transplanted CD28 KO or B7-1/B7-2 KO bone marrow into Ldlr KO--increased.30 |
| ICOS (CD278) | Activated T cells, DCs | ICOSL (CD275) | B cells, monocytes, DCs, T cells, activated tissue cells | Immunized ApoE KO with ICOS-Ig to generate anti-ICOS antibodies--increased.73Transplanted ICOS KO bone marrow into Ldlr KO --increased.32 |
| Co-inhibitory pathways- CD28/B7 families | ||||
| CTLA4 (CD152) | Activated T cells | CD80, CD86 | DCs, B cells, monocytes/macrophages , T cells, | |
| PD1 (CD279) | Activated T cells, activated B cells, activated DCs | PDL1, (CD274 ) PDL2 (CD273) | B cells, T cells, endothelial cells (PDL1), activated monocytes, activated DCs, mast cells (PD-L1, PD-L2) | PD-L1/PD-L2/Ldlr KO-- increased. 68 |
| ? | B7H4 | DCs, B cells, monocytes/macrophages | ||
| BTLA (CD272) | T cells, B cells, DCs, myeloid cells | HVEM (TNFR super family) | T cells, B cells, NK cells, DCs, myeloid cells, inducible in somatic tissues | |
| Co-stimulatory pathways TNFR/TNF families | ||||
| 4-1BB (CD137) | Activated T cells, activated B cells, activated DCs. endothelial cells | 4-1BBL (CD137L) | Activated B cells, activated DCs, activated monocytes/macrophages , activated T cells | ApoE KO treated with agonist anti-CD137 mAb --increased. 107 |
| OX40 (CD134) | Activated T cells, activated B cells, activated DCs | OX40L (CD252) | Activated T cells, activated B cells, activated DCs, activated monocytes/macrophages , activated endothelial | OX40 KO C3H/He--decreased.90OX40 transgenic over expression in C3H/He -- increased.90 |
| cells | Polymorphic human OX40 allele--increased MI risk.90 | |||
| CD27 | T cells, activated B cells | CD70 | Activated T cells, activated B cells, activated DCs, Activated monocytes/macrophages | |
| CD30 | Activated T cells, activated B cells, activated DCs | CD30L (CD153) | Activated T cells, activated B cells, activated monocytes/macrophages | |
| CD40 | B cells, DCs | CD40L (CD154) | Activated T cells, activated DCs | Treated Ldlr KO with anti-CD40L mAB--decreased.97–99. CD40/ApoE KO--decreased.99 |
| HVEM (TNFR super family) | T cells, B cells, NK cells, DCs, myeloid cells, inducible in somatic tissues | LIGHT (CD258) | Immature DCs, monocytes, activated T cells | |
| GITR (TNFR super family) | Activated T cells, NK cells, PMN, monocytes, macrophages, B cells, DCs, mast cells. | GITRL | DC, Activated monocytes/macrophages , B cells, NK cells, PMN, , Endothelial cells, mast cells. | |
| BTLA (CD272) | T cells, B cells, DCs, myeloid cells | |||
Inducible costimulator (ICOS,CD278) is another CD28 family member, which is expressed on recently activated and effector/memory T cells, but is not present on resting naïve T cells 39. ICOS binds to ICOS-ligand (CD275), which is expressed on bone marrow-derived APCs as well as tissue cells such as endothelium. ICOS signaling involves phosphorylation of a tyrosine residue in the cytoplasmic tail, binding of the p85 subunit of PI3K, and stimulation of the PI3K/AKT pathway. In contrast to CD28 signaling, Grb2 is not recruited, and IL-2 gene expression is not enhanced. ICOS is critical for T-dependent antibody responses to protein antigens 40. Some evidence suggests a special importance for ICOS in enhancing Th2 differentiation 41. The absence of ICOS impairs differentiation of Th2-mediated anti-inflammatory responses with reduced IL-4 and IL-10 cytokine production 42,43 and antibody isotype switching 39,44. ICOS has also been implicated in regulatory T cell (Treg) function 45,46. ICOS deficiency has been shown to enhance helper T cell responses in models of autoimmune diseases including experimental autoimmune encephalitis (EAE) 39 and insulitis 45. This would suggest a prominent role for ICOS in vivo in the regulation of autoreactive T cells.
Several members of the tumor necrosis factor (TNF) family of proteins also have T cell costimulatory activity 47. These trimeric proteins are membrane bound ligands for trimeric TNF-receptor (TNFR) family proteins that are expressed on T cells. Upon ligand binding, the cytoplasmic tails of TNFR family proteins recruit TRAF proteins, leading to activation of the NF-κB and MAP kinase signaling pathways, which promote T cell survival, enhance cytokine production, and drive T cell mitotic activity. The costimulatory TNF/TNFR family ligand-receptors pairs include CD70/CD27, OX40L(CD252)/OX40(CD134), 4-1BBL/CD137(4-1BB), LIGHT/HVEM, CD30L/CD30, and GITRL/GITR (Table 1).
CD40L is a TNF family protein rapidly induced on T cells after initial activation. CD40L binds to CD40 on APCs as well as other cell types. CD40 signaling in APCs upregulates expression of CD80 and CD86, thereby enhancing the ability of the APC to costimulate T cells. In this role, CD40 is not strictly acting as a costimulator, but rather an amplifier of B7-family costimulation. However, there is evidence, largely from in vitro studies, that TNF family members expressed on T cells can activate signaling pathways in the T cell, when they bind TNFRs on APCs. This phenomenon has been called “reverse signaling” and has been documented to enhance antigen-dependent T cell proliferation and survival. CD40L, as well as for LIGHT, TRANCE, CD30L, FasL, TNF, 4-IBBL, OX40, and CD70, can all participate in reverse signaling 48.
In addition to B7/CD28 and TNF/TNFR family proteins, other proteins have costimulatory properties in vitro ( e.g. CD2/SLAM and TIM family members), but the contribution of these molecules to costimulation in vivo are only beginning to be elucidated. For example, CD2 on human T cells binds to LFA-3, and transduces signals that enhance T cell proliferation and cytokine secretion.
B7/CD28 family coinhibitors
There are at least four pathways of negative regulation of T cell responses that involve binding of B7 family molecules to CD28 family receptors on T cells (Table 1). The general importance of these negative regulatory, or co-inhibitory, pathways is evident from the autoimmune/inflammatory phenotypes of mice in which the pathways are genetically ablated 49. The outcome of a T cell encounter with antigen can be viewed as an integration of both costimulatory and coinhibitory signals.
The first T cell coinhibitory pathway to be discovered involves CTLA-4, which is a CD28 family member expressed on the surface of T cells shortly after their activation 38. CTLA-4 binds to B7-1 and B7-2, and inhibits T cell activation through mechanisms that are not yet well understood. CTLA-4 may sequester B7 ligands from CD28, compete for intracellular signaling molecules engaged by the CD28 pathway, or recruit tyrosine phosphatases, such as SHP2, which block TCR complex- mediated tyrosine kinase pathways 50.
A second T cell coinhibitory pathway involves PD1 (CD279), a CD28 family member which binds to either of two B7-family proteins, PD-L1 (B7-H1, CD274) or PD-L2 (B7-DC, CD273) 51. PD-1 expression is induced by various stimuli on a several cell types, including CD4+ T cells, CD8+ T cells, NKT cells, B cells and activated monocytes 49. The two PD-1 ligands differ in their expression with PD-L2 expression being much more restricted than PD-L1. PD-L2 is inducibly expressed on DCs, macrophages, B1 cells, and cultured bone marrow-derived mast cells. PD-L1 is expressed constitutively on T cells, B cells, DCs, macrophages, and bone marrow derived mast cells. PD-L1 expression on these cells can be further upregulated upon activation. PD-L1 is also expressed on a wide variety of nonhematopoietic cell types, including vascular endothelial cells, epithelial cells, muscle cells, and pancreatic islet cells, 49. Importantly, this tissue expression of PD-L1 has been shown to limit T cell-mediated disease in a variety of experimental models, including murine autoimmune diabetes 52 and CTL-mediated myocarditis 53. The pathways by which PD-1 exerts its inhibitory effects are incompletely understood. There is an immunoreceptor tyrosine-based switch motif (ITSM) and an immunoreceptor tyrosine-based inhibition motif (ITIM) in the PD-1 cytoplasmic tail. When PD-1 binds its ligands simultaneously with TCR binding antigen, the ITSM becomes phosphorylated. Protein tyrosine phosphatases (SHP-2 and perhaps SHP-1) may bind to the phosphorylated ITSM, and block kinase-dependent signals induced by TCR signaling 54. A role for the ITIM is not clear. PD-1 signaling can antagonize CD28-dependent expression of anti-apoptotic genes. In addition to binding to PD-1, PD-L1 can also bind to B7-1 on T cells, resulting in inhibitory reverse signaling 55. Although there are reports suggesting that PD-L1 may have a stimulatory function 56, a receptor with such a function has yet to be found.
Two additional pathways in the B7:CD28 family also can provide coinhibitory signals, B7-H4 and BTLA4/HVEM. B7-H4 is a B7 family member expressed on hematopoietically-derived APCs as well as other cell types. Its receptor on T cells is not yet known. Recombinant B7-H4-Ig fusion protein inhibits T cell activation and cytokine production and anti-B7-H4 mAb exacerbates EAE 57. BTLA is a CD28 family molecule expressed on activated T cells, and binds the TNFR family member HVEM. BTLA transduces inhibitory signals that block T cell activation by antigen 58. The cytoplasmic tail of BTLA contains ITIMs which are phosphorylated upon HVEM-ligation and recruit SHP1 and SHP2. BTLA−/− mice have increased susceptibility to autoimmune and inflammatory diseases. As noted above, HVEM on T cells transduces costimulatory signals when it binds LIGHT; thus, similar to B7-1 and B7-2, HVEM can bind a stimulatory receptor and an inhibitory receptor.
Coinhibitory pathways appear to be required for regulation of T cell responses at several stages, including the initial activation of naïve T cells and also effector and memory T cell activation. The importance of coinhibition is illustrated by the upregulation of coinhibitory receptors by viruses that cause chronic infections. This appears to be a means of viral evasion of immune eradication. For example, PD-1 is highly expressed on virus-specific CTL in mice or humans with chronic viral infections. and blocking anti-PD-1 antibodies can reactivate virus specific CTL function and promote viral clearance 51.
The effect of costimulatory and coinhibitory pathways on regulatory T cell development and function
Although the emphasis of most of the original research regarding T cell costimulatory and coinhibitory pathways has focused on their function in modulating activation of naïve and effector T lymphocytes, more recent work has established that these pathways also have profound effects on Treg development and function. Treg express costimulatory and coinhibitory molecules, including B7-1/2, CD28, CTLA-4, PD-1, PD-L1, ICOS, and OX40. The roles of these molecules in Treg biology are incompletely understood, but gene knockout and blocking studies in mice have provided compelling data supporting the importance of at least some of these proteins for Treg development and function. For example, the CD28/B7-1/B7-2 pathway is crucial for the homeostasis and survival of Treg. Targeted mutations of B7-1/B7-2 or CD28 genes or CD28/B7-1/B7-2 pathway blockade by CTLA-4Ig cause a profound reduction in Treg numbers 59,60. B7-1 and/or B7-2 also may be important on effector T cells as the target of Treg suppressive function mediated by CTLA4 61. In addition, ICOS appears to be required for optimal T reg function 32,62,63. The costimulatory molecule Ox40 inhibits the induction of Treg and Treg suppressive function 64,65. The studies cited here and many others clearly indicate that the net effect of manipulating T cell costimulation and coinhibition on atherosclerosis will reflect a balance of effects on both effector and regulatory T cells.
CD28 and B7-1/B7-2 costimulatory molecules in atherosclerosis
In light of the evidence discussed above that T cell-mediated immune responses influence atherosclerosis, the importance of T cell costimulatory molecules in arterial disease becomes apparent. The first costimulatory molecules to be examined in the context of atherosclerosis were B7-1 and B7-2. These proteins were shown to be expressed in human 66 and mouse 67 atherosclerotic lesions. B7-1 and B7-2 expression was increased on splenic B cells 67 in old vs. young ApoE−/− mice, and B7-1 was increased on splenic CD11c+ DCs from cholesterol-diet fed Ldlr−/− mice compared to control diet fed Ldlr−/− mice 68. These results suggest that the systemic inflammatory responses to hypercholesterolemia activate APCs to express costimulatory molecules. However, one recent paper has reported that the amount of B7-1 and B7-2 on CD11c+CD8α− DCs isolated from hypercholesterolemic ApoE−/− mice after treatment with TLR ligands (LPS or CpG) was lower than the amount of B7-1 and B7-2 on DCs from similarly treated normocholesterolemic C57Bl/6 mice 69. Other data in that study indicated that DC maturation was impaired under hypercholesterolemic conditions. These differing results suggest that modulation of costimulatory molecules expression may vary depending on the APC population, the type of the TLR ligands or other stimuli, and perhaps the degree of hypercholesterolemia.
The contribution of B7-1 and B7-2 costimulation to pro-atherogenic immune response was more directly tested by analyzing lesions in cholesterol diet-fed B7-1/B7-2−/− Ldlr−/− mice compared to Ldlr−/− controls. The absence of B7-1 and B7-2 reduced early diet-induced atherosclerotic lesion development in the Ldlr−/− mice 70. There was also less MHC II expression in the atherosclerotic lesions. CD4+ T cells from the B7-1/B7-2−/− Ldlr−/− mice produced less IFN-γ in response to the putative athero-antigen Hsp60 in vitro. These data are consistent with an important role for the B7/CD28 pathway in the development of atherosclerotic lesions through their role in priming of antigen-specific T cells. However, different results were obtained in another study that analyzed lesion development in irradiated bone marrow chimeric Ldlr−/− mice reconstituted with B7-1−/−/B7-2−/−, CD28−/−, or control bone marrow 30. In that study, B7-1/B7-2 or CD28 deficiency in the hematopoietic compartment resulted in more atherosclerotic lesion development. This result was attributed to markedly impaired Treg development in the chimeric mice, leading to enhanced proatherogenic effector T cell responses. The opposing effects of elimination of the B7/CD28 costimulatory pathways on atherosclerosis in the two different studies cited above illustrate the complexities of these pathways, which influence the functions of both proinflammatory effector T cells and Treg suppression. In hematopoeitically intact mice, B7-1/B7-2 deficiency manifests predominantly as an effector T cell immunodeficiency 71, and can reduce susceptibility to some autoimmune diseases, such as experimental allergic encephalomyelitis 72. These findings are consistent with the reduced proatherogenic T cell responses in B7-1/B7-2−/− Ldlr−/− mice 70. Genetic deficiency or blockade of the B7/CD28 pathways results in a net enhancement of pathogenic effector T cell responses due to reduced Treg numbers and function in mice with underlying genetic susceptibility to autoimmunity, such as the Nod mouse 60, or in mice that have reconstituted their immune system after lethal irradiation and bone marrow transplantation 30. We have found similar discrepancies between the influence of ICOS deficiency on atherosclerosis in hematopoietically unmanipulated mice vs. bone marrow chimeras, which may be attributable to differences in the balance between effector T cells and Treg, as discussed below. Differences in the observable net effect of costimulatory deficiency on atherogenesis, which depend on different underlying conditions of the host, pose challenges for the design of therapeutic strategies. Nonetheless, they affirm the fundamental importance of effector T cell activation in the pathogenesis of atherosclerotic disease.
ICOS and ICOS-ligand costimulatory molecules in atherosclerosis
Two published studies have addressed the influence of the ICOS costimulatory pathway on atherosclerosis. In the first study 73, ApoE−/− mice were immunized with ICOS-human Fc chimeric protein in adjuvant, which induced the production of anti-murine ICOS antibodies. Control mice were immunized with control IgFc. Immunization with the ICOS-Fc fusion protein increased atherosclerotic lesion formation. There was a 77% increase in aortic sinus fatty streak lesion formation in a 6 week study of chow fed mice, and a more modest increase in advanced aortic sinus lesion formation in a 8 week study with a high fat diet. The findings suggested that the presence of blocking anti-ICOS antibody caused enhanced atherosclerosis. Limited immunohistochemical analyses did not detect increased macrophages or T cells in lesions, but a minor increase in IFNγ was found in the lesions of ICOS-Ig immunized mice. The results of this study are consistent with the interpretation that ICOS exerts an anti-atherogenic effect, but the experimental design leaves open the possibility that the induced anti-ICOS antibodies could act as agonists.
The influence of ICOS on atherosclerosis was also studied by the bone marrow chimeric approach in Ldlr−/− mice 32. Lethally irradiated Ldlr−/− mice were reconstituted with bone marrow from wild type or ICOS−/− mice, and after hematopoietic reconstitution, the mice were fed a cholesterol-containing diet for 10 weeks. The results indicated that ICOS on bone marrow-derived cells had an atheroprotective influence and also limited atherosclerosis-associated immune responses. Mice transplanted with ICOS−/− marrow had a significant increase in the atherosclerotic burden compared to control mice. ICOS−/− mice also had increase lesional CD4+ T cells, macrophage, smooth muscle cell, and collagen content. In vitro activated CD4+ T cells from ICOS−/− chimeras proliferated more and secreted more proinflammatory cytokines IFN-γ and TNF-α and less of the anti-inflammatory cytokine TGFβ. These data supports a suppressive effect of ICOS on atherogenesis.
Experimental evidence clearly shows that ICOS is a positive costimulatory molecule for CD4+ T cells 39. Therefore it is paradoxical that ICOS deficiency increases immune responses in vivo. One possible explanation relates to the role of ICOS in the development and/or function of Th2 cells, which could down-regulate Th1 responses 41. However, recent studies show that ICOS is also involved in Th1 differentiation 74,75. As mentioned above, ICOS also has been implicated in Treg function 45,46. Studies of ICOS−/− C57Bl/6 mice and irradiated ICOS+/+ Ldlr−/− mice reconstituted with ICOS−/− bone marrow showed that FoxP3+ regulatory T cells (Treg) constitutively express high ICOS levels 32. In vitro data demonstrated that ICOS deficiency caused impaired Treg suppressive function, and in vivo data demonstrated that ICOS−/− mice had decreased numbers of FoxP3+ Treg32. Taken together, these data suggest that ICOS has a key role in controlling atherogenesis, through its effect on regulatory T cell responses. Interestingly, no significant difference in atherosclerotic lesion development was detected in hematopoietically unmanipulated ICOS−/− Ldlr−/− mice compared to Ldlr−/− control mice (I.G., A.H.S., A.H.L.; unpublished data).
We have mentioned two examples of differences atherosclerosis and Treg development and function when a costimulatory deficiency (B7-CD28 or ICOS-ICOSL pathways) is studied in gene knock out bone marrow recipients versus hemaotopitecally unmanipulated gene knock mice. It should be pointed out that immunologic reconstitution is quite successful, and protective T cell and humoral immune functions are achieved in mice and humans, after lethal irradiation and bone marrow transplantation with genetically normal bone marrow. The mechanisms underlying variations in immune regulation observed when costimulator-deficient donor marrow is used require further study. These mechanisms may be clinically significant in the context of therapeutic bone marrow/hematopoietic stem cell transplant recipients.
PD1 and PD-L1/PD-L2 coinhibitory molecules in atherosclerosis
The importance of the PD:PD-L pathway in the maintenance of T cell self tolerance has been established in several animal models 51. The expression of PD-L1 and PD-L2 on DCs, and the wide distribution of PD-L1 on endothelium and other tissue cells suggests that both initiation of T cell responses in lymphoid tissues, and effector T cell responses in lesions may be regulated by PD-1 signaling. The influence of the PD-1/PD-L pathway on atherosclerotic disease was examined using PD-L1−/−PD-L2−/−Ldlr−/− triple knockout mice, generated by cross breeding PD-L1−/−PD-L2−/−mice with Ldlr−/− mice. The extent and phenotype of diet-induced atherosclerosis and plaque antigen-specific cell mediated responses was compared in PD-L1−/−PD-L2−/−Ldlr−/− and Ldlr−/− controls. 68. In the absence of the PD-1 ligands, there was an exaggerated systemic immune response, including lymphadenopathy, increased numbers of activated T cells in lymphoid tissues, and elevated serum levels of the pro-inflammatory cytokine TNF-α. This correlated with increased aortic atherosclerosis. After in vitro cholesterol loading, PD-L1−/−PD-L2−/− peritoneal macrophages and splenic DCs were more potent stimulators of CD4+ T cell activation than were in vitro cholesterol-loaded cells from wild type mice. APCs directly isolated from hypercholesterolemic PD-L1−/−PD-L2−/−Ldlr−/− mice stimulated stronger T cell responses to oxidized-LDL than APCs from hypercholesterolemic Ldlr−/− mice. Thus, these findings demonstrate that PD-L1 and PD-L2 exert significant anti-atherogenic and anti-inflammatory roles in hypercholesterolemic mice.
Immunohistochemical analysis of the atherosclerotic lesions in PD-L1−/−PD-L2−/−Ldlr−/− revealed an enhanced inflammatory phenotype with markedly increased numbers of T cells as well as increased macrophages and smooth muscle cells 68. Both CD4+ and CD8+ T cells were much more abundant in the lesions of PD-L1−/−PD-L2−/−Ldlr−/− mice than in the lesions of Ldlr−/− controls. CD8+ T cells are relatively rare in the plaques of Ldlr−/− mice. In human lesions, CD8+ T cell are usually present but are less numerous than CD4+ T cells. Therefore the abundance of these cells in the lesions of the PD-L1−/−PD-L2−/−Ldlr mice may be an indication that CD8+ T cells specific for lesional antigens are normally under tight control by PD-1. These findings are of special interest in light of emerging evidence that chronic exposure to viral antigens can lead to upregulation of PD-1 on viral-specific CD8+ T cells and contribute to an “exhausted phenotype in which T cell proliferation and effector functions are impaired. 76,77. Chronic exposure to atherosclerosis-related antigens also may lead to upregulated PD-1 expression and inhibition of CD8+ T cells specific for these antigens. Targeting PD-1 to enhance antiviral immunity, for example in HIV infected patients, could have the complication of increased cardiovascular risks by activating proatherogenic T cell responses. Analyses of polyclonal mixtures of CD8+ T cells from hypercholesterolemic Ldlr−/− mice have not revealed increased PD-1 expression compared to normocholesterolemic controls mice (AHL, AHS unpublished results). There are no tools such as peptide-MHC tetramers currently available to identify atheroantigen-specific CD8+ T cells and determine if PD-1 is upregulated on these cells in hypercholesterolemic mice.
In the absence of infectious or other inflammatory challenges, PD-L1−/−PD-L2−/−mice do not show overt manifestations of dysregulated immunity. In the setting of an inflammatory challenge, the absence or blockade of PD-L1, PD-L2 or PD-1 result in enhanced T cell responses and acceleration or exacerbation of disease 78–81. The disease phenotype of PD-L1−/−PD-L2−/−Ldlr mice supports the concept that hypercholesterolemia is a systemic inflammatory challenge.
The site where PD-L1 or PD-L2 may be inhibiting pro-atherogenic T cell responses remains to be determined. APC’s in lymphoid tissues are likely involved since hematopoietically-derived APCs from spleen and paraaortic lymph nodes were shown to be dependent on PD-L1/PD-L2 to limit T cell activation 68. However, PD-L1 has also been shown to have an inhibitory function within tissue inflammatory sites 52,53. PD-L1 is expressed on microvascular endothelial cells 82,83 and inhibits cytotoxic T cell activation in-vitro 84 and in-vivo 53. PD-L1 is expressed on DC’s and macrophages in the neointima of atherosclerotic lesions, but has not been observed on the aortic endothelium 68. Although PD-L1 may down-regulate immune responses directly in the atherosclerotic tissue, this has yet to be established.
Ox40 and Ox40 ligand in atherosclerosis
The TNF family member Ox40 and its ligand Ox40L , a TNFR family member, provide T cell costimulatory signals 85. Ox40 is expressed on T cells, and Ox40 ligand is expressed on APCs (and endothelium). This pathway may be particularly important for sustaining T cell responses and enabling effective long-lasting T-cell responses 86. Ox40 not only is important for the development and survival of memory CD4+ T cells, but in addition inhibits T regulatory cell development and function 64,65,87. Ox40-dependent costimulation enhances autoimmune diseases such as EAE 88.
Several mouse and human studies have provided evidence that Ox40 ligand is involved in promoting atherosclerotic disease and/or its complications. In a quantitative trait locus (QTL) study to identify genes that render C57BL/6 mice more susceptible than C3H/He mice to diet-induced atherosclerosis, a locus on mouse chromosome 1 was identified that included the Ox40 ligand gene, as well as 10 other known genes 89. Ox40 ligand deficiency led to smaller lesions in high fat diet-fed C3H/He mice compared to controls, and transgenic mice over expressing Ox40 ligand had larger atherosclerotic lesions than controls 90. Furthermore, a single nucleotide polymorphism in the Ox40L gene, Tnfsf4, was found to be more frequent in patients with myocardial infarction than controls 90. In a different study, blockade of Ox40L in Ldlr−/− mice reduced atherosclerosis and this was attributed to reduced IL-4 mediated Th2 isotype switching with decreased T cell dependent anti-OxLDL IgG responses, and increased levels of anti-oxLDL IgM 91. Overall, these findings support the concept that Ox40–Ox40L dependent T cell costimulation has an important role in promoting atherosclerotic disease.
CD40 and CD40L in atherosclerosis
The CD40/CD40L pathway is not strictly a mediator of T cell costimulation, and it functions mainly to activate APCs by the T cell. Nevertheless, this pathway has a significant contribution to the indirect activation of the T cell, since CD40 signaling in the APC up regulates costimulatory molecules and thus is also important in the activation of the T cell 92. CD40L and CD40 are expressed on numerous cell types within atherosclerotic lesions including endothelial and smooth muscle cells, macrophages, and T lymphocytes 93. CD40L and CD40 interactions increase expression of pro-inflammatory cytokines, chemokines, adhesion molecules, matrix metalloproteinases and tissue factor, thus contributing to the recruitment and activation of inflammatory cells in atherosclerosis and to the instability of the atherosclerotic plaque 94. Importantly, platelets constitutively express CD40, and they express CD40L upon activation 95. A new and active area of investigation addresses how CD40 signaling in platelets contributes to proatherogenic inflammatory processes 96. CD40L is important in vivo in the development of atherosclerosis. Deficiency of CD40L by antibody blockade or targeted mutations significantly reduces atherosclerosis, including the advancement of established lesions 97–99. The relevant cell types that modulate atherosclerosis through CD40L-expression is still under investigation. Transplantation of CD40L−/− bone marrow into Ldlr−/− mice did not significantly decrease atherosclerosis, suggesting that CD40L on non-hematopoietic cells mediate proatherogenic effects 100,101. It is possible, but not proven, that blockade or deficiencies in CD40/CD40L signaling may have anti-atherogenic effects, at least in part, by reducing expression of B7-1, B7-2 and other T cell costimulators.
CD137 and CD137 ligand in atherosclerosis
CD137 (4-1BB) is TNF receptor family member, which binds CD137 ligand (CD137L). Although the cellular distribution of both the molecules is wide, CD137 expression is induced on T cells by antigen recognition, and acts as a costimulatory receptor when it binds CD137L expressed on APCs 102. Interest in CD137 as a potential therapeutic target was raised by studies in which administration of an agonistic anti-CD137 antibody ameliorated disease in mouse models of systemic lupus-like autoimmune disease 103,104, and autoimmune arthritis 105. CD137 appears to have a particularly important role in controlling CD8+ T cell responses 106. A recent study explored the relationship of the CD137/CD137L pathway in atherosclerosis 107. Significantly more CD137 mRNA was detected in human atherosclerotic arteries than normal arteries. CD137 protein was detected by immunofluorescence on T cells and endothelial cells in human atherosclerotic lesions. In vitro, CD137 was inducible on endothelial cells and vascular smooth muscle cells by proinflammatory cytokines, and crosslinking CD137 with trimeric rCD137L induced endothelial leukocyte adhesion molecule expression and reduced smooth muscle cell proliferation. When ApoE−/− mice were treated with agonist anti-CD137 antibody, this resulted in increased aortic atherosclerosis, and a marked increase in lesional inflammatory cells and cytokines. Although the expression of CD137 and CD137L on multiple cell types present in atherosclerotic arteries complicates the study of mechanisms by which these molecules may influence atherosclerotic disease, T cell costimulation is likely to play a significant role. In this regard, the CD137 agonist treatment resulted in abundant CD8+ T cell infiltration into lesions, which are otherwise unusual in mouse lesions 107. As discussed earlier, lesional CD8+ T cells were also abundant in PD-L1/L2−/−Ldlr−/− mice. Together, these findings suggest opposing roles for CD137:CD137L and PD-1:PD-L pathways in controlling CD8+ T cells in atherosclerotic lesions. Since Systemic lupus erythematosus predisposes to atherosclerotic disease, an important but unresolved question that arises is why agonist anti-CD137 antibody ameliorates lupus-like disease in mice but promotes atherosclerosis. Perhaps the opposing effects of the agonist antibody on different disease processes reflects differential effects on CD4+ and CD8+ T cells.
Conclusions and therapeutic implications
Costimulatory pathways are potentially excellent targets for therapeutic intervention in T cell-mediated diseases. Blockade of B7-CD28 interactions by CTLA-4-Ig has already been established as an effective treatment for human autoimmune diseases including rheumatoid arthritis and psoriasis. Effector T cells play an important pathogenic role in development of atherosclerosis and in destabilizing advanced lesions. Therefore inhibition of T cell activation by targeting costimulatory pathways is a sensible approach for immune modulation of arterial disease. The influence of different costimulatory pathways in atherosclerosis is likely to vary depending on the stage of disease. For example early lesion development is likely to be enhanced by priming of naïve CD4+ T cells in lymphoid tissues, which is highly dependent on CD28 signaling. In contrast, effector/memory T cells within lesions that can contribute to plaque instability may be more profoundly influenced by ICOS and PD-1 pathways. Thorough studies of the comparative roles of different costimulatory pathways in lymphoid tissues or in lesions, and at different stages of lesion progression and remodeling have not been performed but are needed in order to rationally choose the best targets.
The importance of the balance of effector and regulatory T cell responses in atherosclerosis must be taken into account as such therapies are contemplated. Clearly, costimulatory blockade can impair both effector and regulatory T cell differentiation and function, and therefore modulation of these molecules could be a two edged sword. Preclinical models also have limitations in predicting responses in humans. This was evident from the results of limited trial of an agonist anti-CD28 monoclonal antibody. Although activation of CD28 by these antibodies in rats had a beneficial effect in reducing the immune response through its activation of regulatory T cell function, the antibody caused a hyper-acute systemic inflammatory response syndrome due to a pro-inflammatory cytokine storm that caused the subjects to be critically ill 108. Unanticipated effects of targeting costimulatory pathways may also arise because of the wide distribution of many of the cell surface molecules, beyond the T cells and APCs. This especially true of the TNF/TNFR family pathways. For example, antibodies specific for CD40L could pose a risk for hemostasis/thrombosis complications because CD40L is expressed on platelets 109. There is also a reasonable likelihood of T-independent effects of some B7/CD28 and TNF/TNFR family proteins on atherosclerosis, since several of the proteins are expressed on cells intrinsic to the vessel wall. Additional mouse studies of the influence of costimulatory and coinhibitory molecules on atherosclerosis in T cell-deficient mice may be helpful to define these effects.
The chronic nature of atherosclerotic disease also raises questions about the practicality of immune therapy directed at costimulatory pathways. As is the case for autoimmune diseases and allograft rejection, the ideal approach for immune therapy of atherosclerosis is to induce long lasting specific T cell tolerance to atherosclerosis-specific antigens, without global immunosuppression. One theoretical method of tolerance induction to such an antigen is delivery of a costimulatory blocking drug or a coinhibitory agonist drug, at the time as immunization with the antigen. In this way, the T cells specific for the antigen will receive signal 1 (antigen) and no signal 2, or simultaneously signal 1 plus a coinhibitory signal. The first major hurtle that must be overcome to advance this approach is the identification of the relevant antigens. Although work is in progress to achieve a better molecular understanding of the relevant T cell antigens, this remains one of the critical areas where accelerated research programs should be directed.
Acknowledgments
Source of Funding This work was supported by the following NIH grants: P50HL56985 and R01HL087282 (A.H.L); R01AI46414, R01 AI38310, and PO1AI056299 (A.H.S.).
Footnotes
Disclosure statement
None
References
- 1.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 2.Binder CJ, Chang MK, Shaw PX, Miller YI, Hartvigsen K, Dewan A, Witztum JL. Innate and acquired immunity in atherogenesis. Nat Med. 2002;8:1218–1226. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- 3.Yan ZQ, Hansson GK. Innate immunity, macrophage activation, and atherosclerosis. Immunol Rev. 2007;219:187–203. doi: 10.1111/j.1600-065X.2007.00554.x. [DOI] [PubMed] [Google Scholar]
- 4.Hansson GK. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:1876–1890. doi: 10.1161/hq1201.100220. [DOI] [PubMed] [Google Scholar]
- 5.Hansson GK, Holm J, Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989;135:169–175. [PMC free article] [PubMed] [Google Scholar]
- 6.Reardon CA, Blachowicz L, White T, Cabana V, Wang Y, Lukens J, Bluestone J, Getz GS. Effect of immune deficiency on lipoproteins and atherosclerosis in male apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:1011–1016. doi: 10.1161/01.atv.21.6.1011. [DOI] [PubMed] [Google Scholar]
- 7.Zhou X, Nicoletti A, Elhage R, Hansson GK. Transfer of CD4(+) T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation. 2000;102:2919–2922. doi: 10.1161/01.cir.102.24.2919. [DOI] [PubMed] [Google Scholar]
- 8.Buono C, Come CE, Stavrakis G, Maguire GF, Connelly PW, Lichtman AH. Influence of interferon-gamma on the extent and phenotype of diet-induced atherosclerosis in the LDLR-deficient mouse. Arterioscler Thromb Vasc Biol. 2003;23:454–460. doi: 10.1161/01.ATV.0000059419.11002.6E. [DOI] [PubMed] [Google Scholar]
- 9.Van Kaer L. NKT cells: T lymphocytes with innate effector functions. Curr Opin Immunol. 2007;19:354–364. doi: 10.1016/j.coi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Palinski W, Ord VA, Plump AS, Breslow JL, Steinberg D, Witztum JL. ApoE-deficient mice are a model of lipoprotein oxidation in atherogenesis. Demonstration of oxidation-specific epitopes in lesions and high titers of autoantibodies to malondialdehyde-lysine in serum. Arterioscler Thromb. 1994;14:605–616. doi: 10.1161/01.atv.14.4.605. [DOI] [PubMed] [Google Scholar]
- 11.Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1995;92:3893–3897. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palinski W, Horkko S, Miller E, Steinbrecher UP, Powell HC, Curtiss LK, Witztum JL. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest. 1996;98:800–814. doi: 10.1172/JCI118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palinski W, Witztum JL. Immune responses to oxidative neoepitopes on LDL and phospholipids modulate the development of atherosclerosis. J Intern Med. 2000;247:371–380. doi: 10.1046/j.1365-2796.2000.00656.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhou X, Robertson AK, Hjerpe C, Hansson GK. Adoptive transfer of CD4+ T cells reactive to modified low-density lipoprotein aggravates atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:864–870. doi: 10.1161/01.ATV.0000206122.61591.ff. [DOI] [PubMed] [Google Scholar]
- 15.Wick G, Knoflach M, Xu Q. Autoimmune and inflammatory mechanisms in atherosclerosis. Annu Rev Immunol. 2004;22:361–403. doi: 10.1146/annurev.immunol.22.012703.104644. [DOI] [PubMed] [Google Scholar]
- 16.Liu C, Waters DD. Chlamydia pneumoniae and atherosclerosis: from Koch postulates to clinical trials. Prog Cardiovasc Dis. 2005;47:230–239. doi: 10.1016/j.pcad.2005.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kol A, Sukhova GK, Lichtman AH, Libby P. Chlamydial heat shock protein 60 localizes in human atheroma and regulates macrophage tumor necrosis factor-alpha and matrix metalloproteinase expression. Circulation. 1998;98:300–307. doi: 10.1161/01.cir.98.4.300. [DOI] [PubMed] [Google Scholar]
- 18.O'Connor CM, Dunne MW, Pfeffer MA, Muhlestein JB, Yao L, Gupta S, Benner RJ, Fisher MR, Cook TD. Azithromycin for the Secondary Prevention of Coronary Heart Disease Events: The WIZARD Study: A Randomized Controlled Trial. JAMA. 2003;290:1459–1466. doi: 10.1001/jama.290.11.1459. [DOI] [PubMed] [Google Scholar]
- 19.Xu Q, Dietrich H, Steiner HJ, Gown AM, Schoel B, Mikuz G, Kaufmann SH, Wick G. Induction of arteriosclerosis in normocholesterolemic rabbits by immunization with heat shock protein 65. Arterioscler Thromb. 1992;12:789–799. doi: 10.1161/01.atv.12.7.789. [DOI] [PubMed] [Google Scholar]
- 20.George J, Shoenfeld Y, Afek A, Gilburd B, Keren P, Shaish A, Kopolovic J, Wick G, Harats D. Enhanced fatty streak formation in C57BL/6J mice by immunization with heat shock protein-65. Arterioscler Thromb Vasc Biol. 1999;19:505–510. doi: 10.1161/01.atv.19.3.505. [DOI] [PubMed] [Google Scholar]
- 21.George J, Afek A, Gilburd B, Blank M, Levy Y, Aron-Maor A, Levkovitz H, Shaish A, Goldberg I, Kopolovic J, Harats D, Shoenfeld Y. Induction of early atherosclerosis in LDL-receptor-deficient mice immunized with beta2-glycoprotein I. Circulation. 1998;98:1108–1115. doi: 10.1161/01.cir.98.11.1108. [DOI] [PubMed] [Google Scholar]
- 22.Whitman SC, Ravisankar P, Elam H, Daugherty A. Exogenous interferon-gamma enhances atherosclerosis in apolipoprotein E−/− mice. Am J Pathol. 2000;157:1819–1824. doi: 10.1016/s0002-9440(10)64820-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laurat E, Poirier B, Tupin E, Caligiuri G, Hansson GK, Bariety J, Nicoletti A. In vivo downregulation of T helper cell 1 immune responses reduces atherogenesis in apolipoprotein E-knockout mice. Circulation. 2001;104:197–202. doi: 10.1161/01.cir.104.2.197. [DOI] [PubMed] [Google Scholar]
- 24.Buono C, Binder CJ, Stavrakis G, Witztum JL, Glimcher LH, Lichtman AH. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc Natl Acad Sci U S A. 2005;102:1596–1601. doi: 10.1073/pnas.0409015102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Libby P. Atherosclerosis: disease biology affecting the coronary vasculature. Am J Cardiol. 2006;98:3Q–9Q. doi: 10.1016/j.amjcard.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 26.Liu W, Putnam AL, Xu-yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, de St. Groth BF, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 28.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 29.Mallat Z, Ait-Oufella H, Tedgui A. Regulatory T-cell immunity in atherosclerosis. Trends Cardiovasc Med. 2007;17:113–118. doi: 10.1016/j.tcm.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006 doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 31.Mor A, Luboshits G, Planer D, Keren G, George J. Altered status of CD4(+)CD25(+) regulatory T cells in patients with acute coronary syndromes. Eur Heart J. 2006;27:2530–2537. doi: 10.1093/eurheartj/ehl222. [DOI] [PubMed] [Google Scholar]
- 32.Gotsman I, Grabie N, Gupta R, Dacosta R, MacConmara M, Lederer J, Sukhova G, Witztum JL, Sharpe AH, Lichtman AH. Impaired regulatory T-cell response and enhanced atherosclerosis in the absence of inducible costimulatory molecule. Circulation. 2006;114:2047–2055. doi: 10.1161/CIRCULATIONAHA.106.633263. [DOI] [PubMed] [Google Scholar]
- 33.Lee TS, Yen HC, Pan CC, Chau LY. The role of interleukin 12 in the development of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 1999;19:734–742. doi: 10.1161/01.atv.19.3.734. [DOI] [PubMed] [Google Scholar]
- 34.Mallat Z, Besnard S, Duriez M, Deleuze V, Emmanuel F, Bureau MF, Soubrier F, Esposito B, Duez H, Fievet C, Staels B, Duverger N, Scherman D, Tedgui A. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999;85:e17–e24. doi: 10.1161/01.res.85.8.e17. [DOI] [PubMed] [Google Scholar]
- 35.Pinderski Oslund LJ, Hedrick CC, Olvera T, Hagenbaugh A, Territo M, Berliner JA, Fyfe AI. Interleukin-10 blocks atherosclerotic events in vitro and in vivo. Arterioscler Thromb Vasc Biol. 1999;19:2847–2853. doi: 10.1161/01.atv.19.12.2847. [DOI] [PubMed] [Google Scholar]
- 36.Mallat Z, Gojova A, Marchiol-Fournigault C, Esposito B, Kamate C, Merval R, Fradelizi D, Tedgui A. Inhibition of transforming growth factor-beta signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ Res. 2001;89:930–934. doi: 10.1161/hh2201.099415. [DOI] [PubMed] [Google Scholar]
- 37.Robertson AK, Rudling M, Zhou X, Gorelik L, Flavell RA, Hansson GK. Disruption of TGF-beta signaling in T cells accelerates atherosclerosis. J Clin Invest. 2003;112:1342–1350. doi: 10.1172/JCI18607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 39.Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, Flavell RA. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 40.van Berkel MEAT, Oosterwegel MA. CD28 and ICOS: Similar or separate costimulators of T cells? Immunology Letters. 2006;105:115–122. doi: 10.1016/j.imlet.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Vieira PL, Wassink L, Smith LM, Nam S, Kingsbury GA, Gutierrez-Ramos JC, Coyle AJ, Kapsenberg ML, Wierenga EA. ICOS-mediated signaling regulates cytokine production by human T cells and provides a unique signal to selectively control the clonal expansion of Th2 helper cells. Eur J Immunol. 2004;34:1282–1290. doi: 10.1002/eji.200324417. [DOI] [PubMed] [Google Scholar]
- 42.Nurieva RI, Duong J, Kishikawa H, Dianzani U, Rojo JM, Ho I, Flavell RA, Dong C. Transcriptional regulation of th2 differentiation by inducible costimulator. Immunity. 2003;18:801–811. doi: 10.1016/s1074-7613(03)00144-4. [DOI] [PubMed] [Google Scholar]
- 43.Lohning M, Hutloff A, Kallinich T, Mages HW, Bonhagen K, Radbruch A, Hamelmann E, Kroczek RA. Expression of ICOS in vivo defines CD4+ effector T cells with high inflammatory potential and a strong bias for secretion of interleukin 10. J Exp Med. 2003;197:181–193. doi: 10.1084/jem.20020632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tafuri A, Shahinian A, Bladt F, Yoshinaga SK, Jordana M, Wakeham A, Boucher LM, Bouchard D, Chan VS, Duncan G, Odermatt B, Ho A, Itie A, Horan T, Whoriskey JS, Pawson T, Penninger JM, Ohashi PS, Mak TW. ICOS is essential for effective T-helper-cell responses. Nature. 2001;409:105–109. doi: 10.1038/35051113. [DOI] [PubMed] [Google Scholar]
- 45.Herman AE, Freeman GJ, Mathis D, Benoist C. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J Exp Med. 2004;199:1479–1489. doi: 10.1084/jem.20040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kohyama M, Sugahara D, Sugiyama S, Yagita H, Okumura K, Hozumi N. Inducible costimulator-dependent IL-10 production by regulatory T cells specific for self-antigen. Proc Natl Acad Sci U S A. 2004;101:4192–4197. doi: 10.1073/pnas.0400214101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 48.Sun M, Fink PJ. A New Class of Reverse Signaling Costimulators Belongs to the TNF Family. J Immunol. 2007;179:4307–4312. doi: 10.4049/jimmunol.179.7.4307. [DOI] [PubMed] [Google Scholar]
- 49.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 50.Lee KM, Chuang E, Griffin M, Khattri R, Hong DK, Zhang W, Straus D, Samelson LE, Thompson CB, Bluestone JA. Molecular basis of T cell inactivation by CTLA-4. Science. 1998;282:2263–2266. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
- 51.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 52.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grabie N, Gotsman I, DaCosta R, Pang H, Stavrakis G, Butte MJ, Keir ME, Freeman GJ, Sharpe AH, Lichtman AH. Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T-cell mediated injury in the heart. Circulation. 2007;116:2062–2071. doi: 10.1161/CIRCULATIONAHA.107.709360. [DOI] [PubMed] [Google Scholar]
- 54.Coenen JJA, Koenen HJPM, van Rijssen E, Boon L, Joosten I, Hilbrands LB. CTLA-4 Engagement and Regulatory CD4+CD25+ T Cells Independently Control CD8+-Mediated Responses under Costimulation Blockade. J Immunol. 2006;176:5240–5246. doi: 10.4049/jimmunol.176.9.5240. [DOI] [PubMed] [Google Scholar]
- 55.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed Death-1 Ligand 1 Interacts Specifically with the B7-1 Costimulatory Molecule to Inhibit T Cell Responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kanai T, Totsuka T, Uraushihara K, Makita S, Nakamura T, Koganei K, Fukushima T, Akiba H, Yagita H, Okumura K, Machida U, Iwai H, Azuma M, Chen L, Watanabe M. Blockade of B7-H1 suppresses the development of chronic intestinal inflammation. J Immunol. 2003;171:4156–4163. doi: 10.4049/jimmunol.171.8.4156. [DOI] [PubMed] [Google Scholar]
- 57.Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, Chapoval AI, Flies DB, Bajorath J, Chen L. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18:849–861. doi: 10.1016/s1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 58.Murphy KM, Nelson CA, Sedy JR. Balancing co-stimulation and inhibition with BTLA and HVEM. 2006;6:671–681. doi: 10.1038/nri1917. [DOI] [PubMed] [Google Scholar]
- 59.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 60.Bour-Jordan H, Salomon BL, Thompson HL, Szot GL, Bernhard MR, Bluestone JA. Costimulation controls diabetes by altering the balance of pathogenic and regulatory T cells. J Clin Invest. 2004;114:979–987. doi: 10.1172/JCI20483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paust S, Lu L, McCarty N, Cantor H. Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proc Natl Acad Sci U S A. 2004;101:10398–10403. doi: 10.1073/pnas.0403342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, Berry G, DeKruyff RH, Umetsu DT. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med. 2002;8:1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- 63.Miyamoto K, Kingsley CI, Zhang X, Jabs C, Izikson L, Sobel RA, Weiner HL, Kuchroo VK, Sharpe AH. The ICOS molecule plays a crucial role in the development of mucosal tolerance. J Immunol. 2005;175:7341–7347. doi: 10.4049/jimmunol.175.11.7341. [DOI] [PubMed] [Google Scholar]
- 64.So T, Croft M. Cutting edge: OX40 inhibits TGF-beta- and antigen-driven conversion of naive CD4 T cells into CD25+Foxp3+ T cells. J Immunol. 2007;179:1427–1430. doi: 10.4049/jimmunol.179.3.1427. [DOI] [PubMed] [Google Scholar]
- 65.Kroemer A, Xiao X, Vu MD, Gao W, Minamimura K, Chen M, Maki T, Li XC. OX40 controls functionally different T cell subsets and their resistance to depletion therapy. J Immunol. 2007;179:5584–5591. doi: 10.4049/jimmunol.179.8.5584. [DOI] [PubMed] [Google Scholar]
- 66.de Boer OJ, Hirsch F, van der Wal AC, van der Loos CM, Das PK, Becker v. Costimulatory molecules in human atherosclerotic plaques: an indication of antigen specific T lymphocyte activation. Atherosclerosis. 1997;133:227–234. doi: 10.1016/s0021-9150(97)00135-4. [DOI] [PubMed] [Google Scholar]
- 67.Afek A, Harats D, Roth A, Keren G, George J. Evidence for the involvement of T cell costimulation through the B-7/CD28 pathway in atherosclerotic plaques from apolipoprotein E knockout mice. Exp Mol Pathol. 2004;76:219–223. doi: 10.1016/j.yexmp.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 68.Gotsman I, Grabie N, Dacosta R, Sukhova G, Sharpe A, Lichtman AH. Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. J Clin Invest. 2007;117:2974–2982. doi: 10.1172/JCI31344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shamshiev AT, Ampenberger F, Ernst B, Rohrer L, Marsland BJ, Kopf M. Dyslipidemia inhibits Toll-like receptor-induced activation of CD8alpha-negative dendritic cells and protective Th1 type immunity. J Exp Med. 2007;204:441–452. doi: 10.1084/jem.20061737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Buono C, Pang H, Uchida Y, Libby P, Sharpe AH, Lichtman AH. B7-1/B7-2 costimulation regulates plaque antigen-specific T-cell responses and atherogenesis in low-density lipoprotein receptor-deficient mice. Circulation. 2004;109:2009–2015. doi: 10.1161/01.CIR.0000127121.16815.F1. [DOI] [PubMed] [Google Scholar]
- 71.Borriello F, Sethna MP, Boyd SD, Schweitzer AN, Tivol EA, Jacoby D, Strom TB, Simpson EM, Freeman GJ, Sharpe AH. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303–313. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- 72.Chang TT, Jabs C, Sobel RA, Kuchroo VK, Sharpe AH. Studies in B7-deficient Mice Reveal a Critical Role for B7 Costimulation in Both Induction and Effector Phases of Experimental Autoimmune Encephalomyelitis. J. Exp. Med. 1999;190:733–740. doi: 10.1084/jem.190.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Afek A, Harats D, Roth A, Keren G, George J. A functional role for inducible costimulator (ICOS) in atherosclerosis. Atherosclerosis. 2005;183:57–63. doi: 10.1016/j.atherosclerosis.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 74.Bonhagen K, Liesenfeld OMS, AHutloff A, Erb K, Coyle A, Lipp M, Kroczek R, Kamradt T. Th cells produce distinct cytokines in different mucosal immune responses. European Journal of Immunology. 2003;33:392–401. doi: 10.1002/immu.200310013. [DOI] [PubMed] [Google Scholar]
- 75.Wassink L, Vieira PL, Smits HH, Kingsbury GA, Coyle AJ, Kapsenberg ML, Wierenga EA. ICOS Expression by Activated Human Th Cells Is Enhanced by IL-12 and IL-23: Increased ICOS Expression Enhances the Effector Function of Both Th1 and Th2 Cells. J Immunol. 2004;173:1779–1786. doi: 10.4049/jimmunol.173.3.1779. [DOI] [PubMed] [Google Scholar]
- 76.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 77.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 78.Koga N, Suzuki J, Kosuge H, Haraguchi G, Onai Y, Futamatsu H, Maejima Y, Gotoh R, Saiki H, Tsushima F, Azuma M, Isobe M. Blockade of the interaction between PD-1 and PD-L1 accelerates graft arterial disease in cardiac allografts. Arterioscler Thromb Vasc Biol. 2004;24:2057–2062. doi: 10.1161/01.ATV.0000145015.23656.e4. [DOI] [PubMed] [Google Scholar]
- 79.Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, Kuchroo VK, Freeman GJ, Sharpe AH. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci U S A. 2004;101:10691–10696. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu B, Guleria I, Khosroshahi A, Chitnis T, Imitola J, Azuma M, Yagita H, Sayegh MH, Khoury SJ. Differential role of programmed death-ligand 1 and programmed death-ligand 2 in regulating the susceptibility and chronic progression of experimental autoimmune encephalomyelitis. J Immunol. 2006;176:3480–3489. doi: 10.4049/jimmunol.176.6.3480. [DOI] [PubMed] [Google Scholar]
- 81.Salama AD, Chitnis T, Imitola J, Ansari MJ, Akiba H, Tushima F, Azuma M, Yagita H, Sayegh MH, Khoury SJ. Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J Exp Med. 2003;198:71–78. doi: 10.1084/jem.20022119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liang SC, Latchman YE, Buhlmann JE, Tomczak MF, Horwitz BH, Freeman GJ, Sharpe AH. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol. 2003;33:2706–2716. doi: 10.1002/eji.200324228. [DOI] [PubMed] [Google Scholar]
- 83.Eppihimer MJ, Gunn J, Freeman GJ, Greenfield EA, Chernova T, Erickson J, Leonard JP. Expression and regulation of the PD-L1 immunoinhibitory molecule on microvascular endothelial cells. Microcirculation. 2002;9:133–145. doi: 10.1038/sj/mn/7800123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T, Greenfield EA, Liang SC, Sharpe AH, Lichtman AH, Freeman GJ. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol. 2003;33:3117–3126. doi: 10.1002/eji.200324270. [DOI] [PubMed] [Google Scholar]
- 85.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 86.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 87.Vu MD, Clarkson MR, Yagita H, Turka LA, Sayegh MH, Li XC. Critical, but conditional, role of OX40 in memory T cell-mediated rejection. J Immunol. 2006;176:1394–1401. doi: 10.4049/jimmunol.176.3.1394. [DOI] [PubMed] [Google Scholar]
- 88.Ndhlovu LC, Ishii N, Murata K, Sato T, Sugamura K. Critical involvement of OX40 ligand signals in the T cell priming events during experimental autoimmune encephalomyelitis. J Immunol. 2001;167:2991–2999. doi: 10.4049/jimmunol.167.5.2991. [DOI] [PubMed] [Google Scholar]
- 89.Phelan SA, Beier DR, Higgins DC, Paigen B. Confirmation and high resolution mapping of an atherosclerosis susceptibility gene in mice on Chromosome 1. Mammalian Genome. 2002;13:548–553. doi: 10.1007/s00335-002-2196-1. [DOI] [PubMed] [Google Scholar]
- 90.Wang X, Ria M, Kelmenson PM, Eriksson P, Higgins DC, Samnegard A, Petros C, Rollins J, Bennet AM, Wiman B, de Faire U, Wennberg C, Olsson PG, Ishii N, Sugamura K, Hamsten A, Forsman-Semb K, Lagercrantz J, Paigen B. Positional identification of TNFSF4, encoding OX40 ligand, as a gene that influences atherosclerosis susceptibility. Nat Genet. 2005;37:365–372. doi: 10.1038/ng1524. [DOI] [PubMed] [Google Scholar]
- 91.van Wanrooij EJ, van Puijvelde GH, de Vos P, Yagita H, van Berkel TJ, Kuiper J. Interruption of the Tnfrsf4/Tnfsf4 (OX40/OX40L) pathway attenuates atherogenesis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:204–210. doi: 10.1161/01.ATV.0000251007.07648.81. [DOI] [PubMed] [Google Scholar]
- 92.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 93.Schonbeck U, Libby P. The CD40/CD154 receptor/ligand dyad. Cell Mol Life Sci. 2001;58:4–43. doi: 10.1007/PL00000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lutgens E, Lievens D, Beckers L, Donners M, Daemen M. CD40 and its ligand in atherosclerosis. Trends Cardiovasc Med. 2007;17:118–123. doi: 10.1016/j.tcm.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 95.Danese S, Fiocchi C. Platelet activation and the CD40/CD40 ligand pathway: mechanisms and implications for human disease. Crit Rev Immunol. 2005;25:103–121. doi: 10.1615/critrevimmunol.v25.i2.20. [DOI] [PubMed] [Google Scholar]
- 96.Croce K, Libby P. Intertwining of thrombosis and inflammation in atherosclerosis. Curr Opin Hematol. 2007;14:55–61. doi: 10.1097/00062752-200701000-00011. [DOI] [PubMed] [Google Scholar]
- 97.Mach F, Schonbeck U, Sukhova GK, Atkinson E, Libby P. Reduction of atherosclerosis in mice by inhibition of CD40 signalling. Nature. 1998;394:200–203. doi: 10.1038/28204. [DOI] [PubMed] [Google Scholar]
- 98.Schonbeck U, Sukhova GK, Shimizu K, Mach F, Libby P. Inhibition of CD40 signaling limits evolution of established atherosclerosis in mice. Proc Natl Acad Sci U S A. 2000;97:7458–7463. doi: 10.1073/pnas.97.13.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lutgens E, Gorelik L, Daemen MJ, de Muinck ED, Grewal IS, Koteliansky VE, Flavell RA. Requirement for CD154 in the progression of atherosclerosis. Nat Med. 1999;5:1313–1316. doi: 10.1038/15271. [DOI] [PubMed] [Google Scholar]
- 100.Bavendiek U, Zirlik A, LaClair S, MacFarlane L, Libby P, Schonbeck U. Atherogenesis in mice does not require CD40 ligand from bone marrow-derived cells. Arterioscler Thromb Vasc Biol. 2005;25:1244–1249. doi: 10.1161/01.ATV.0000161420.55482.ef. [DOI] [PubMed] [Google Scholar]
- 101.Smook ML, Heeringa P, Damoiseaux JG, Daemen MJ, de Winther MP, Gijbels MJ, Beckers L, Lutgens E, Tervaert JW. Leukocyte CD40L deficiency affects the CD25(+) CD4 T cell population but does not affect atherosclerosis. Atherosclerosis. 2005;183:275–282. doi: 10.1016/j.atherosclerosis.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 102.Vinay DS, Kwon BS. Role of 4-1BB in immune responses. Seminars in Immunology. 1998;10:481–489. doi: 10.1006/smim.1998.0157. [DOI] [PubMed] [Google Scholar]
- 103.Foell J, Strahotin S, O'Neil SP, McCausland MM, Suwyn C, Haber M, Chander PN, Bapat AS, Yan XJ, Chiorazzi N, Hoffmann MK, Mittler RS. CD137 costimulatory T cell receptor engagement reverses acute disease in lupus-prone NZB x NZW F1 mice. J Clin Invest. 2003;111:1505–1518. doi: 10.1172/JCI17662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sun Y, Chen HM, Subudhi SK, Chen J, Koka R, Chen L, Fu YX. Costimulatory molecule-targeted antibody therapy of a spontaneous autoimmune disease. Nat Med. 2002;8:1405–1413. doi: 10.1038/nm1202-796. [DOI] [PubMed] [Google Scholar]
- 105.Foell JL, Diez-Mendiondo BI, Diez OH, Holzer U, Ruck P, Bapat AS, Hoffmann MK, Mittler RS, Dannecker GE. Engagement of the CD137 (4-1BB) costimulatory molecule inhibits and reverses the autoimmune process in collagen-induced arthritis and establishes lasting disease resistance. Immunology. 2004;113:89–98. doi: 10.1111/j.1365-2567.2004.01952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dawicki W, Bertram EM, Sharpe AH, Watts TH. 4-1BB and OX40 act independently to facilitate robust CD8 and CD4 recall responses. J Immunol. 2004;173:5944–5951. doi: 10.4049/jimmunol.173.10.5944. [DOI] [PubMed] [Google Scholar]
- 107.Olofsson PS, Soderstrom LA, Wagsater D, Sheikine Y, Ocaya P, Lang F, Rabu C, Chen L, Rudling M, Aukrust P, Hedin U, Paulsson-Berne G, Sirsjo A, Hansson GK. CD137 is expressed in human atherosclerosis and promotes development of plaque inflammation in hypercholesterolemic mice. Circulation. 2008;117:1292–1301. doi: 10.1161/CIRCULATIONAHA.107.699173. [DOI] [PubMed] [Google Scholar]
- 108.Sharpe AH, Abbas AK. T-cell costimulation--biology, therapeutic potential, and challenges. N Engl J Med. 2006;355:973–975. doi: 10.1056/NEJMp068087. [DOI] [PubMed] [Google Scholar]
- 109.Nakamura M, Tanaka Y, Satoh T, Kawai M, Hirakata M, Kaburaki J, Kawakami Y, Ikeda Y, Kuwana M. Autoantibody to CD40 ligand in systemic lupus erythematosus: association with thrombocytopenia but not thromboembolism. Rheumatology (Oxford) 2006;45:150–156. doi: 10.1093/rheumatology/kei118. [DOI] [PubMed] [Google Scholar]