Abstract
In Göttingen, Germany, circadian variations in melatonin had been determined time-macroscopically in pineal glands, blood plasma and duodenum of chicken and rats. When these data were meta-analyzed, they agreed with the results from an independent survey on tissues from rats collected in a laboratory in Pécs, Hungary. In the latter study, tissues were analyzed chemically in Bratislava, Slovakia, and numerically in Minneapolis, MN, USA, all by single- and multiple-component cosinor and parameter tests. In rats and chickens, these inferential statistical procedures clearly demonstrated a lead in phase of the 24-h cosine curves best fitting all of the duodenal vs. those best fitting all of the pineal melatonin values in each species in 2 geographic (geomagnetic) locations. The 24-h cosine curve of circulating melatonin was found to be in an intermediate phase position. Mechanisms of the phase differences and the contribution of gastrointestinal melatonin to circulating hormone concentrations are discussed.
Keywords: Blood, Chronomics, Cosinor, Duodenum, Melatonin, Sink, Sources
1. Introduction
Chronomics, the mapping of chronomes (time structures) [7,11] (cf. [8–10]) is here applied to melatonin, usually regarded as the hormone of the pineal gland, but also synthesized by various extrapineal tissues [12,15,23,25,26]. Among those, the digestive tract represents a system which contains by far the highest amount of this indoleamine [4–6,15,23]. Melatonin has properties of an antiinflammatory and antioxidant agent and is a modulator of the immune system, acid secretion and smooth muscle tone. Hence, gastrointestinal melatonin has a potential as a clinically relevant agent [4,5]. With regard to the pronounced circadian rhythmicity of melatonin released into the blood by the pineal, it also seemed worthwhile to consider the circadian aspect of secretion of gastrointestinal melatonin. In fact, already early investigations indicated the possibility of such rhythms in the digestive tract of rats [3] and pigeons [30]. But the extent of variation was consistently much lower in the gut than the pineal gland or the peripheral circulation [4,23], and sometimes rhythmicity was not readily detectable but see [30]. Since the amounts of total gastrointestinal melatonin are up to orders of magnitude higher than those found in the pineal [4–6,12,15], the contribution of this extrapineal source to the levels of circulating melatonin is of conside-rable interest. This question cannot be definitely solved by pinealectomy, since any persisting rhythms may be attributed to other extrapineal sources, such as the retina, bone marrow, pituitary and hypothalamus [10,31] or—as assumed in the pigeon [29]—the Harderian gland.
Release of melatonin from the digestive system can be a post-prandial effect [4,5] or a response to tryptophan administration [17]. These findings may account for some transient rises in melatonin, but hardly for the circadian rhythm in plasma concentration. Gastrointestinal melatonin was consistently reported to peak nocturnally, in both light-active and dark-active animals [3–5,23,30]. Therefore, melatonin rhythms in the gut are not primarily based on digestive physiology.
Since the gastrointestinal tract is reported to possess properties of both a source and a sink of melatonin [4,5,13,20,21], the functional relationships between the pineal gland, the gut and circulating melatonin deserve clarification. A thorough investigation of the phase positions of the circadian acrophases in the respective organs is highly desirable. For this reason, we meta-analyzed data obtained in the chicken and rat [18,19,22,23,32,33], and subjected them to a time-microscopic analysis by single- and multiple- component cosinor [7–9], as a suitable means for detecting any differences in timing which would otherwise not be easily apparent and which have been overlooked in the past.
2. Materials and methods
In experiments performed in Göttingen, 72 male chicken (Gallus domesticus), 21 days of age, and 66 male Wistar rats (Rattus norvegicus), 3 months of age, had been kept under similar lighting conditions in LD 15:9, with L from 04:00–19:00, supplied with food and water ad libitum. The chickens were kept at 23 °C. 70% humidity, the rats at 21 °C. 72% humidity. The rats were sampled at 08:00. 12:00. 14:00. 16:00, 18:00. 20:00. 22:00. 23:00. 00:00 and 02:00: the chicken were sampled at the same clock-hours, with one additional sampling at 04:00. Tissues were homogenized (1:10) in 10 mM K-phosphate buffer pH 7.4, extracted with chloroform; plasma was used directly. Melatonin was determined in pineal gland, blood plasma, and duodenum by RIA according to Arendt [1]. All assays were validated by serial dilution and parallel inhibition, an additional RIA procedure (scintillation proximity assay) and by HPLC with electrochemical and fluorescence detections [23,24].
3. Results
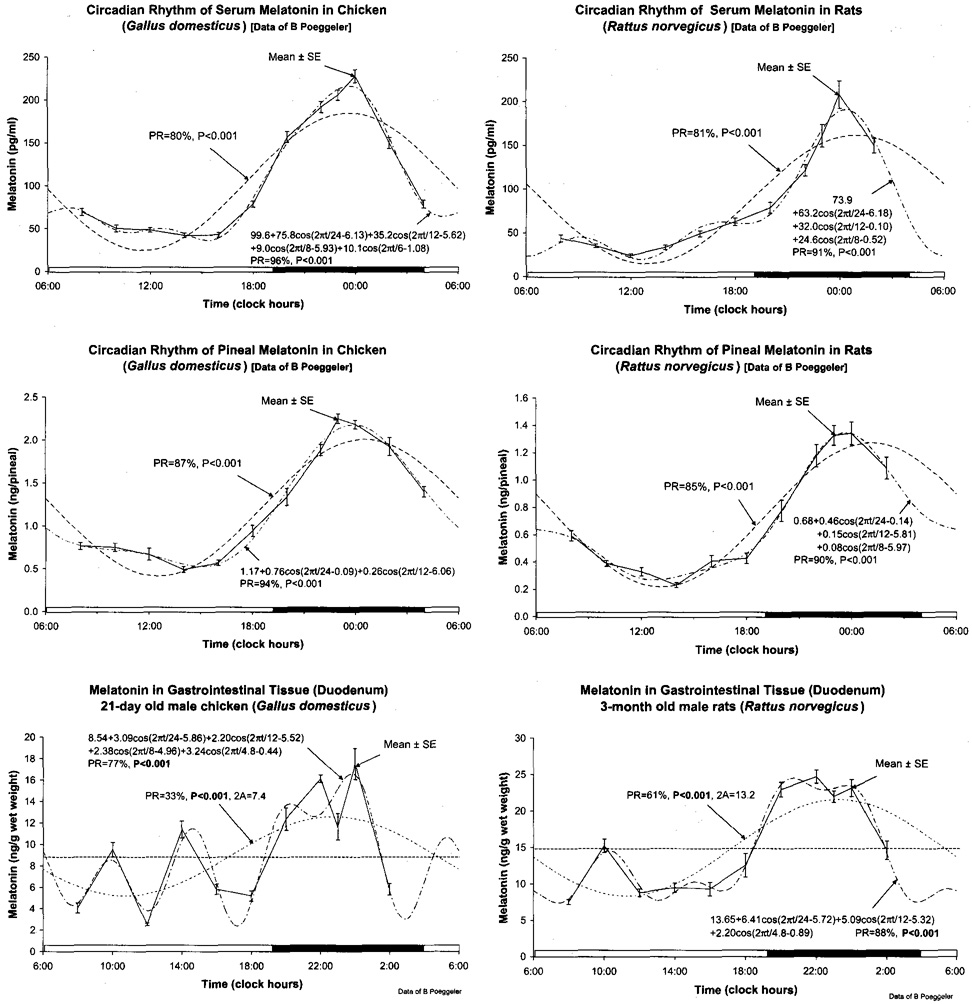
Fig. 1 shows the cosinor models fitted to the original data in chicken (left) and rats (right) for circulating (top), pineal (middle) and duodenal (bottom) melatonin. Cosinor methodology also applied to the logl0-transformed data quantifies statistically significant differences in the 24-h acrophases among melatonin in gut, plasma and pineal, in this order of occurrence (Fig. 2). The findings on acrophase relations are highly consistent between the two species. They are also in very good agreement with results from another study on rats done in an independent laboratory, reported elsewhere in this supplement [19] (cf. [32,33]).
Fig. 1.
All data from Göttingen [23] are shown along the 24-h scale, connected by a continuous line, with the cosinor model fitted to them as a dashed curve, for melatonin in serum (top), pineal (middle) and duodenum (bottom), on chickens (left) and rats (right). Without the models fitted it seems subjective to determine leads or lags in phase, unless one relies on peaks. Peak values, however, are not necessarily representative of the curve as a whole; hence the need for analyses by appropriate models, shown herein while the corresponding characteristics of timing are summarized in Fig. 2. © Halberg.
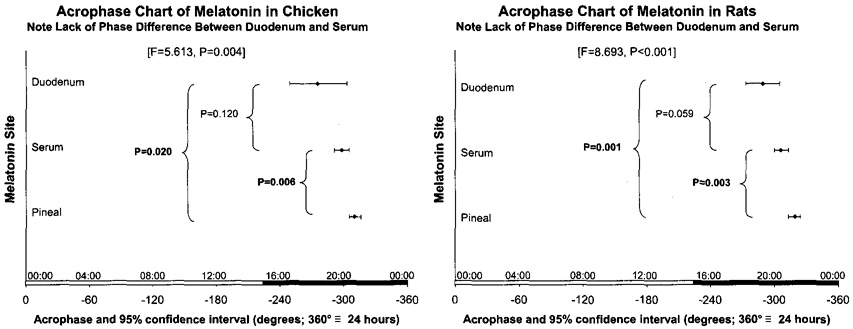
Fig. 2.
Acrophase chart shows results of parameter tests revealing a statistically significant lead in phase of a melatonin rhythm in the gut vs. that in the pineal, in chickens (P = 0.020) and in rats (P = 0.001). The multiple spectral components resolved, given with each figure, aim to quantify the waveform. © Halberg.
4. Discussion
This meta-analysis was prompted by the demonstration of Zeman et al. [32,33] in cooperative studies with Józsa et al. [18,19] of a first cosinor-validated circadian rhythm in gut melatonin and of a lead in acrophase of the circadian rhythm in melatonin concentration in gut vs. the pineal. Plasma melatonin is usually believed to result mainly from secretion by the pineal gland. Release of this indoleamine from the gastrointestinal tract in response to L-tryptophan administration [17] or to feeding [4], however, has been demonstrated. In the present study, cosinor analysis did not reveal statistically significant differences between acrophases of duodenal and plasma melatonin, but a statistically significant advance of duodenal melatonin and plasma melatonin relative to the circadian melatonin rhythm in the pineal.
In any event, data from chickens and rats agree with each other with respect to the sequence of acrophases and agree further with the sequence found independently in another laboratory. There is a consistent lead in phase of the duodenal melatonin rhythm compared to that in the pineal gland in the two species, here replicated for the rat. This conclusion would not have been possible without a time-microscopic inferential statistical analysis. Phase differences between melatonin released from the pineal and other sources, such as hypothalamus and anterior pituitary, have already been described in other studies [10,31], but the corroborated lead in phase of melatonin in the gut is the main point of this paper, awaiting scrutiny as to its degree of generality.
At first glance, our findings may be highly suggestive for assuming a substantial contribution of the gut to the rhythm of circulating melatonin. However, appropriate interpretations have to consider the complexity of sources and sinks of gastrointestinal melatonin. In the gut, melatonin seems to originate from various sources. It is obviously synthesized endogenously by the enterochromaffin cells [3,4,15,25,26]. Additionally, circulating melatonin secreted by the pineal gland is loaded to the gastrointestinal tissue [4,13,20,21]. This has been shown directly in experiments in which the hormone was administered to rats by a single injection [3] or by a continuous infusion [20,21]. In those experiments, the by far largest fraction of melatonin and melatonin metabolites appeared in the gut and was later found in the feces. The amounts of the hormone transferred to the tissues—predominantly to the gut—were remarkably high: When infused in quantities required for elevating melatonin during the day from diurnal to nocturnal levels, amounts were required as high as if the pineal gland would release its content every 2 min [ 16]—something beyond reality. Since most of the hormone was loaded to the small intestine, surpassing the quantities found in the liver by manyfold [20,21], the gut should be regarded as a high-capacity sink for melatonin, at least during the day. Moreover, these findings inevitably lead to the additional conclusion that the gastrointestinal capacity for taking up melatonin has to vary in a circadian fashion, because endogenous nocturnal values of circulating melatonin cannot disappear in the tissue at the same rate. This would also hold if one assumes a contribution of gastrointestinal melatonin to the circulating values, since, in this case, in the net balance, the gut would serve as a source rather than a sink.
In fact, the complexity is even larger. For instance, melatonin undergoes enterohepatic cycling; high quantities were found in the bile fluid [28]. Large amounts of this indoleamine are present in the luminal fluid, and a fraction seems to return to the tissue [4–6]. Moreover, melatonin is taken up from the food, a process which has not only been shown by feeding nutrients high in melatonin (which still might be seen as a post-prandial effect), but also by a drop in the circulating hormone by providing a low-melatonin diet [27]. Therefore, melatonin present in the food should, at least transiently, appear in the gastrointestinal tissue. An as yet unsettled question is the contribution of bacteria present in the lower gut. Melatonin synthesis by bacteria has repeatedly been demonstrated, but not in the strictly unaerobic species making up more than 99% of the intestinal bacterial mass [13]. The possible contribution of bacterial melatonin to the circulating pool is indicated in the paper of Bubenik et al. [6]. The authors found substantial differences in melatonin concentrations of various GIT segments between the ruminant (cattle) and non-ruminant (pig) species, thus indicating bacterial production of melatonin. Another unknown parameter is the rate of gastrointestinal melatonin metabolism. There is no good reason to assume that melatonin should predominantly be hydroxylated by P450 monooxygenases in this organ system. Other pathways typical for non-hepatic tissues have to be considered, such as that of pyrrole-ring cleavage leading to 5-methoxylated kynuramines (AFMK = Nl-acetyl-N2-formyl-5-methoxykynuramine; AMK = N1-acetyl-5-methoxykynuramine) [14]. The flux through this pathway cannot be easily determined, because the highly reactive AMK is readily converted by reactive oxygen and nitrogen species.
In conclusion, the lead in phase of the duodenal melatonin rhythm can still be interpreted in different ways. It may reflect an earlier rise in local melatonin synthesis, compared to the pineal gland. In principle, this could even be triggered by a certain threshold of the circulating hormone. Alternately, it might be caused by a rhythm in intestinal uptake capacity for melatonin, provided it would attain its peak earlier than the maximum content of the pineal. As soon as the maximal storage capacity is reached, due to saturation of non-receptor binding sites, also circulating melatonin may peak before the pineal, because of reduced elimination through the sink. Phase positions of duodenal, plasma and pineal melatonin have not to be necessarily identical in different species, since rhythms of storage capacity and secretion can be different.
The main value of the time-microscopic analysis has been to direct the attention to mechanisms involved in the relationship between pineal and gastrointestinal tract, which would otherwise have been overlooked. The next step of clarification could be the determination of intestinal uptake capacity for melatonin.
References
- 1.Arendt J. Assay of melatonin and its metabolites: results in normal and unusual environments. J Neural Transm. 1986;21:11–33. [PubMed] [Google Scholar]
- 2.Bingham C, Arbogast B, Cornélissen Guillaume G, Lee JK, Halberg F. Inferential statistical methods for estimating and comparing cosinor parameters. Chronobiologia. 1982;9:397–439. [PubMed] [Google Scholar]
- 3.Bubenik GA. Localization of melatonin in the digestive tract of the rat. Effect of maturation, diurnal variation, melatonin treatment and pinealectomy. Hormone Res. 1980;12:313–323. doi: 10.1159/000179137. [DOI] [PubMed] [Google Scholar]
- 4.Bubenik GA. Localization, physiological significance and possible clinical implication of gastrointestinal melatonin. Biol Signals Recept. 2001;10:350–366. doi: 10.1159/000046903. [DOI] [PubMed] [Google Scholar]
- 5.Bubenik GA. Gastrointestinal melatonin: localization, function and clinical relevance. Dig Dis Sci. 2002;47:2336–2348. doi: 10.1023/a:1020107915919. [DOI] [PubMed] [Google Scholar]
- 6.Bubenik GA, Hacker RR, Brown GM, Bartos L. Melatonin concentrations in the luminal fluid, mucosa and muscularis of the bovine and porcine gastrointestinal tract. J Pineal Res. 1999;26:56–63. doi: 10.1111/j.1600-079x.1999.tb00567.x. [DOI] [PubMed] [Google Scholar]
- 7.Cornélissen G, Halberg F. Chronomedicine. In: Armitage P, Colton T, editors. Encyclopedia of biostatistics, v. 1. Chichester, UK: John Wiley & Sons Ltd.; 1998. pp. 642–649. [Google Scholar]
- 8.Halberg F. Chronobiology. Annu Rev Physiol. 1969;31:675–725. doi: 10.1146/annurev.ph.31.030169.003331. [DOI] [PubMed] [Google Scholar]
- 9.Halberg F. Chronobiology: methodological problems. Acta Med Rom. 1980;18:399–440. [Google Scholar]
- 10.Halberg F, Comélissen G, Conti A, Maestroni G, Maggioni C, Perfetto F, et al. The pineal gland and chronobiologic history: mind and spirit as feedsidewards in time structures for prehabilitation. In: Bartsch C, Bartsch H, Blask DE, Cardinali DP, Hrushesky WJM, Mecke W, editors. The pineal gland and cancer: neuroimrnunoendocrine mechanisms in malignancy. Heidelberg: Springer; 2001. pp. 66–116. [Google Scholar]
- 11.Halberg F, Cornélissen G, Otsuka K, Schwartzkopff O, Halberg J, Bakken EE. Chronomics. Biomed Pharmacother. 2001;55 Suppl. 1:153–190. [PubMed] [Google Scholar]
- 12.Hardeland R. Melatonin: Multiple functions in signaling and protection. In: Altrneyer P, Hoffmann K, Stücker M, editors. Skin Cancer and UV Radiation. Heidelberg: Springer; 1997. pp. 186–198. [Google Scholar]
- 13.Hardeland R, Poeggeler B. Non-vertebrate melatonin. J Pineal Res. 2003;34:233–241. doi: 10.1034/j.1600-079x.2003.00040.x. [DOI] [PubMed] [Google Scholar]
- 14.Hardeland R, Ressmeyer A-R, Zelosko V, Burkhardt S, Poeggeler B. Metabolites of melatonin: formation and properties of the methoxylated kynuramines AFMK and AMK. In: Haldar C, Singh SS, editors. Recent advances in endocrinology and reproduction: evolutionary, biotechnological and clinical applications. Varanasi: Banaras Hindu University; 2004. pp. 21–38. [Google Scholar]
- 15.Huether G. The contribution of extrapineal sites of melatonin synthesis to circulating melatonin levels in higher vertebrates. Experientia. 1993;49:665–670. doi: 10.1007/BF01923948. [DOI] [PubMed] [Google Scholar]
- 16.Huether G, Messner M, Rodenbeck A, Hardeland R. Effect of continuous melatonin infusions on steady-state plasma melatonin levels in rats under near physiological conditions. J Pineal Res. 1998;24:146–151. doi: 10.1111/j.1600-079x.1998.tb00527.x. [DOI] [PubMed] [Google Scholar]
- 17.Huether G, Poeggeler B, Reimer A, George A. Effect of tryptophan administration on circulating melatonin levels in chicks and rats: evidence for stimulation of melatonin synthesis and release in the gastrointestinal tract. Life Sci. 1992;51:945–953. doi: 10.1016/0024-3205(92)90402-b. [DOI] [PubMed] [Google Scholar]
- 18.Józsa R, Kaszaki J, Cornélissen G, Oláh A, Nagy G, Csernus V, et al. Extracircadian variation of endothelin-1 in murine plasma and pituitary and human blood. In: Cornélissen G, Kenner R, Fiser B, Siegelova J, editors. Proceedings symposium chronobiology in medicine. Dedicated to the 85th Anniversary of Professor Franz Halberg; Masaryk University; Brno. 2004. pp. 102–103. [Google Scholar]
- 19.Zeman M, Jozsa R, Cornelissen G, Stebelova K, Bubenik G, Olah A, Poeggeler S, Huether G, Hardeland R, Nagy G, Csernus V, Pan W, Otsuka K, Halberg F. Chronomics: circadian lead of extrapineal vs. pineal melatonin rhythms with an infradian hypothamic exploration. Biomed Pharmacother. 2005;59 Suppl 1:S213–S219. doi: 10.1016/s0753-3322(05)80034-4. [DOI] [PubMed] [Google Scholar]
- 20.Messner M, Hardeland R, Rodenbeck A, Huether G. Effect of continuous melatonin infusions on steady-state plasma melatonin levels, metabolic fate and tissue retention in rats under near physiological conditions. Adv Exp Med Biol. 1999;467:303–313. doi: 10.1007/978-1-4615-4709-9_39. [DOI] [PubMed] [Google Scholar]
- 21.Messner M, Hardeland R, Rodenbeck A, Huether G. Tissue retention and subcellular distribution of continuously infused melatonin in rats under near physiological conditions. J Pineal Res. 1998;25:251–259. doi: 10.1111/j.1600-079x.1998.tb00395.x. [DOI] [PubMed] [Google Scholar]
- 22.Oláh A, Józsa R, Comélissen G, Zeman M, Nagy G, Kaszaki J, et al. Validation of exclusive daytime murine sampling on antiphasic lighting regimens by circadian rhythmic core temperature behavior. In: Cornélissen G, Kenner R, Fiser B, Siegelova J, editors. Proceedings Symposium Chronobiology in Medicine. Dedicated to the 85th Anniversary of Professor Franz Halberg; Masaryk University; Brno. 2004. pp. 100–101. [Google Scholar]
- 23.Poeggeler B. Doctoral thesis. Germany: University of Göttingen; 1992. Vergleichende Untersuchungen über Melatonin und strukturverwandte Tryptophanmetabolite. Zur Rolle yon Melatonin und 5-Methoxytryptamin bei einem Dinoflagellaten, Gonyaulax polyedra, sowie pinealen und extrapinealen 5-methoxylierten Indolaminen bei Vertebraten. [Google Scholar]
- 24.Poeggeler B, Balzer I, Hardeland R, Lerchl A. Pineal hormone melatonin oscillates also in the dinoflagellate Gonyaulax polyedra. Naturwissenschaften. 1991;78:268–269. [Google Scholar]
- 25.Raikhlin NT, Kvetnoy IM. Melatonin and enterochromaffin cells. Acta Histochim. 1976;55:19–24. doi: 10.1016/S0065-1281(76)80092-X. [DOI] [PubMed] [Google Scholar]
- 26.Raikhlin NT, Kvetnoy IM, Tolkachev VN. Melatonin may be synthesized in enterochromaffin cells. Nature. 1975;225:344–345. doi: 10.1038/255344a0. [DOI] [PubMed] [Google Scholar]
- 27.Tan D-X, Manchester LC, Hardeland R, Lopez-Burillo S, Mayo JC, Sainz RM, et al. Melatonin--a hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J Pineal Res. 2003;34:75–78. doi: 10.1034/j.1600-079x.2003.02111.x. [DOI] [PubMed] [Google Scholar]
- 28.Tan D-X, Manchester LC, Reiter RJ, Qi W, Hanes MA, Farley NJ. High physiological levels of melatonin in the bile of mammals. Life Sci. 1999;65:2523–2529. doi: 10.1016/s0024-3205(99)00519-6. [DOI] [PubMed] [Google Scholar]
- 29.Vakkuri O, Rintamäki H, Leppaluoto J. Plasma and tissue concentrations of melatonin after midnight light exposure and pinealectomy in the pigeon. J Endocrinol. 1985;105:263–268. doi: 10.1677/joe.0.1050263. [DOI] [PubMed] [Google Scholar]
- 30.Vakkuri O, Rintamäki H, Leppäluoto J. Presence of immunoreactive melatonin in different tissues of the pigeon (Columba livia) Gen Comp Endocrinol. 1985;58:69–75. doi: 10.1016/0016-6480(85)90136-4. [DOI] [PubMed] [Google Scholar]
- 31.Wetterberg L, Sanchez de la Peña S, Halberg F. Circadian rhythm of melatonin release from pineal, hypothalamus and pituitary in hypertensive rats. Prog Clin Biol Res. 1990;341 B:145–153. [PubMed] [Google Scholar]
- 32.Zeman M, Cornélissen G, Balazova K, Herichova I, Jozsa R, Olah A, et al. Circadian acrophase differences in plasma, pineal, hypothalamic and duodenal melatonin in rats—qualified in quail. In: Matsubayasi K, editor. Proceedings 5th International Workshop on Chronoastrobiology and Chronotherapy; Center for Southeast Asian Studies; Tokyo. 2004. pp. 81–84. [Abstract] [Google Scholar]
- 33.Zeman M, Cornélissen G, Balazova K, Józsa R, Oláh A, Nagy G, et al. Circadian rhythm of melatonin in rat duodenum. In: Cornélissen G, Kenner R, Fiser B, Siegelova J, editors. Proceedings Symposium Chronobiology in Medicine. Dedicated to the 85th Anniversary of Professor Franz Halberg; Masaryk University; Brno. 2004. pp. 95–97. [Google Scholar]