Abstract
Individuals suffering from systemic lupus erythematosus (SLE) are predisposed to accelerate cardiovascular disease. Our laboratory has recently developed an animal model of SLE-accelerated atherosclerosis. We have shown that, following 8 weeks feeding high fat Western diet, radiation chimeras consisting of SLE-derived haematopoietic cells transferred to low-density lipoprotein (LDL)r−/− mice (LDLr.Sle) have increased atherosclerosis compared with C57Bl/6 bone marrow recipients (LDLr.B6). However, this feeding regimen resulted in significant mortality in SLE-susceptible mice compared with controls with surviving animals having extremely elevated serum cholesterol (>500 mg/dL) and increased serum markers of kidney pathology. To test the hypothesis that SLE-associated autoimmune dysregulation can exacerbate atherosclerosis under more mild serum cholesterol conditions (approximately 200 mg/dL), we examined SLE and lesion development in radiation chimeras fed either a normal chow or high fat Western diet for 8 weeks. High fat fed LDLr.Sle mice exhibited increased mortality and were significantly more hypertensive. LDLr.Sle mice had greater titres of antibodies against dsDNA, oxLDL and phospholipid compared with controls. Lupus-susceptibility increased the atherosclerotic lesions and the percentage of CD4+ T cells in the lesions of proximal aortas, independent of diet. These data show that increased dyslipidemia resulting from high-fat feeding can exacerbate autoimmunity and associated vascular complications. Conversely, they also show that autoimmune dysregulation can accelerate atherosclerosis in LDLr-deficient animals independent of feeding high fat diet. Collectively this study provides additional evidence that the accelerated atherosclerosis observed in SLE is autoimmune associated.
Keywords: atherosclerosis, autoimmunity, high fat diet, lupus
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune inflammatory disease characterized by the production of a wide range of autoantibodies. Clinical complications because of SLE usually result in end-organ disease such as glomerulonephritis, arthritis, vasculitis and various neurological disorders.1 First recognized as a serious complication in lupus over 30 years ago, atherosclerosis has gained interest as a major cause of mortality in patients with lupus.1–4 In fact, with all other risk factors being equal, including hypertension, hyperlipidemia, diabetes and obesity, the risk of coronary events in patients with SLE is approximately eight times greater when compared with non-SLE controls and approximately 30% of deaths in SLE are atherosclerosis related.3,4 Therefore, understanding how the presence of SLE exacerbates the atherosclerotic condition is essential to optimize risk reduction for cardiovascular disease (CVD) while treating the SLE-associated inflammation.
Atherosclerosis, like SLE, is a disease involving many cellular processes, and has classically been associated with hypercholesterolemia. A large body of evidence also supports inflammation and immunity in the pathogenesis of CVD. It is well known that macrophages and T cells are present in all stages of atherosclerotic lesions and promote inflammation by producing various cytokines, attracting smooth muscle cells and other lymphocytes and increasing plaque vulnerability.5 B-cell responses are also thought to be involved in the pathogenesis of atherosclerosis and for the most part are thought to be protective. Although the involvement of acquired immunity in atherosclerosis is strongly supported by these studies, mechanisms appear to be cell type dependent and multifaceted.
A recent study by our laboratory described the development of an animal model for accelerated atherosclerosis in the face of SLE. We made low-density lipoprotein (LDL)r−/− mice susceptible to SLE by transferring haematopoietic cells from the congenic B6.Sle1.2.3 mouse strain. This unique animal model of human SLE was developed by placing three lupus-susceptibility gene intervals identified in NZM2410 mouse strain on the C57Bl/6 background.6 Using this approach, we showed that making LDLr−/− mice susceptible to lupus increased atherosclerosis in the aortic root and increased inflammatory cell accumulation in lesions. However, in general, patients with lupus do not suffer from the severe hypercholesterolemia observed in LDLr−/− mice fed a high fat diet (e.g., cholesterol levels >500 mg/dL). Therefore, the current study was conducted to show that exacerbation of atherosclerosis in lupus-susceptible mice occurs under conditions of more moderate dyslipidemia as that observed in LDLr−/− mice on a normal chow diet (total cholesterol of approximately 200 mg/dL) and that overt accumulation of atherogenic lipoproteins (i.e., VLDL and LDL) can enhance SLE disease.
Methods
Mice
All mice used in these studies have been backcrossed onto the C57Bl/6 background. C57Bl/6 and LDLr-deficient mice were originally obtained from The Jackson Laboratory and are maintained in our colony. The lupus congenic B6.Sle1.2.3 strain has been described and characterized extensively.6–13 The B6.Sle1.2.3 mice are essentially 97% genetically homologous to the C57Bl/6 strain with the NZM2410-derived lupus susceptibility loci accounting for approximately 3% of the genome. All mice are maintained in microisolator cages and used according to the guidelines and the approval of the Vanderbilt University Institutional Animal Care and Use Committee.
Production of radiation chimeras
Transfer of the wild type or lupus-susceptible bone marrow has been previously described.14
Atherosclerosis studies
LDLr-deficient animals received either C57Bl/6 or B6.Sle1.2.3 bone marrow. Sixteen weeks following transplantation, one half of the animals in each group were started on a high fat Western diet (21% milk fat and 0.15% cholesterol) for 8 weeks. The remaining mice were kept on chow diet for the same period of time. At the end of this time, animals were killed and analysed for the extent of atherosclerosis and the presence and severity of symptoms of SLE.
Immunohistochemistry
Staining for macrophages (Moma-2) and CD4+ T cells was performed as previously described.14 CD11c staining for dendritic cells was conducted using a rat anti-CD11c primary antibody (BD Biosciences, San Jose, CA USA) followed by incubation with Texas red-conjugated anti-rat IgG (Vector Labs, Burlingame, CA, USA). Cells were visualized by fluorescent microscopy and quantified by counting the number of positive cells in lesions.
ELISAs
Serum titres for antibodies against oxLDL and dsDNA were conducted as previously described.14 ELISAs for antibodies against β2-glycoprotein I (β2-GPI) was performed by coating a 96-well Maxisorb plate with 10 µg/mL of purified β2-GPI in PBS. Plates were blocked and mouse serum was added at a dilution between 1:1000 and 1:5000 and incubated overnight at 4 °C. Plates were washed with 0.5% Tween/PBS and incubated with HRP conjugated goat anti-mouse IgG (Promega, Madison, WI, USA) for 1 h at RT. Reactions were developed using the TMB substrate (BD Biosciences).
Serum lipoprotein analyses
Total serum cholesterol and triglyceride were measured in fasted mice using a colorometric assay as previously described.14 Fast performance liquid chromatography (FPLC) was conducted by separating lipoproteins on a Superose 6 column (Amersham Promega, Piscataway, NJ, USA) followed by cholesterol measurement in each fraction as described.14,15
Measurement of systolic blood pressure
Systolic blood pressure was measured using a tail cuff BP-2000 instrument (Visitech Systems, Apex, NC, USA) on conscious, preconditioned mice as described.14
Purification and activation of CD4+ T cells
CD4+ T cells from the spleens of C57Bl/6 and B6.Sle1.2.3 congenic mice were isolated by positive selection using magnetic beads conjugated to anti-CD4 antibodies according to the manufacture’s protocol (Miltenyi Biotec, Auburn, CA,USA). Cells were then stimulated with Phorbol myristate acid (PMA) and ionomycin (1µg/mL) for 2 h at 37 °C and 5% CO2. Cells were then washed, stained with anti-CD40L antibody (BD Biosciences) and analysed by flow cytometry.
Statistical analyses
Statistical analyses were conducted using PRISM 5.0 software (GraphPad Software Inc., Laclolla, CA, USA). For data showing a normal distribution, significant differences were calculated using an ANOVA with a Bonferroni post-test. For data that require a nonparametric test, a Kruskal–Wallis test was conducted with a Dunns post-test.
Results
High fat Western diet increases dsDNA antibody titres and increases mortality in lupus-susceptible LDLr−/− mice
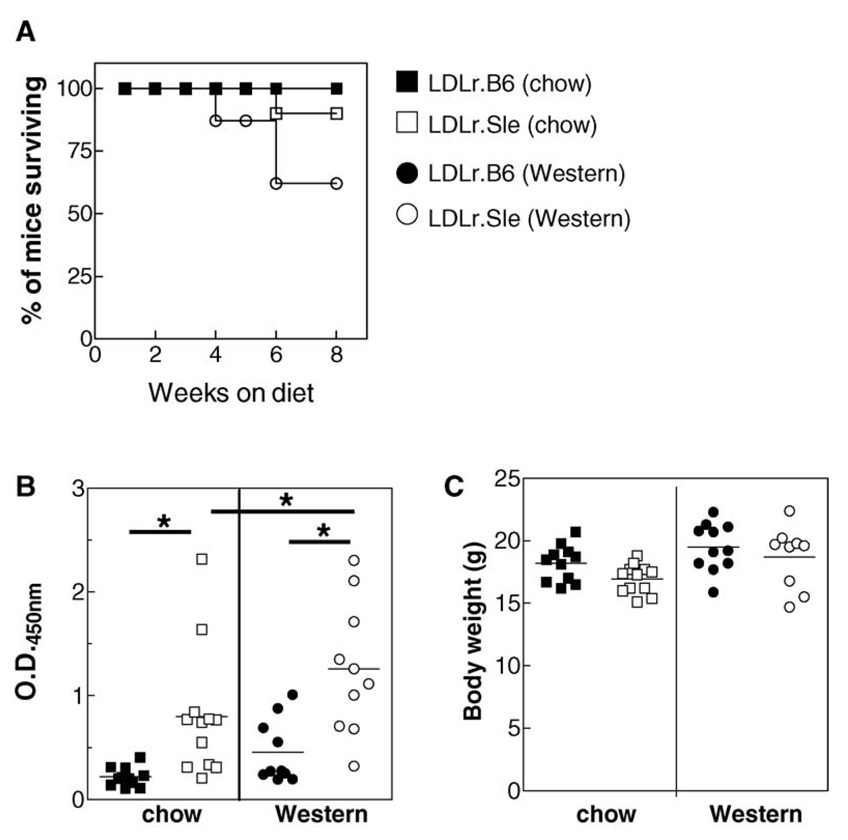
Lethally irradiated LDLr−/− mice received either C57Bl/6 or lupus-susceptible B6.Sle1.2.3 congenic bone marrow. Recipient mice are hereafter referred to as LDLr.B6 and LDLr.Sle, respectively. Sixteen weeks following transplantation, mice were either fed a high fat Western diet for eight additional weeks or left on normal rodent chow. LDLr.Sle mice fed with high fat Western diet had a 37% mortality rate, whereas LDLr.Sle mice on a chow diet only showed a 10% mortality rate (Figure 1A). Control LDLr.B6 mice tolerated both diets with a 100% survival rate at the end of the 24-week study. At the time of killing, the LDLr.Sle mice had significantly higher titres of anti–double stranded DNA (anti-dsDNA) serum antibodies compared with LDLr.B6 controls (Figure 1B). This was evident independent of diet. In addition, the LDLr.Sle mice on a Western diet had significantly increased anti-dsDNA antibodies compared with chow fed LDLr.Sle mice. Body weight at the time of killing did not differ between the LDLr.B6 and LDLr.Sle mice in either diet group (Figure 1C). The data confirm that the SLE phenotype was transferred to the LDLr-deficient mice. In addition, they show that feeding lupus-susceptible mice a high fat Western diet increases mortality and disease severity as determined by dsDNA autoantibody titre.
Figure 1. Severe dyslipidemia increases mortality and serum titres of dsDNA antibodies.
(A) Percentage of mice (total of 9–13 mice per group) surviving following feeding chow diet (squares) or Western diet (circles) for 8 weeks. (B) Serum titres for anti-dsDNA antibodies in LDLr.B6 (solid symbols) and LDLr.Sle (open symbols) mice fed a chow or Western diet. (C) Body weights for LDLr.B6 (solid symbols) and LDLr.Sle (open symbols) mice fed a chow or Western diet. *P < 0.05 as determined by one-way ANOVA. Data represent two identical experiments.
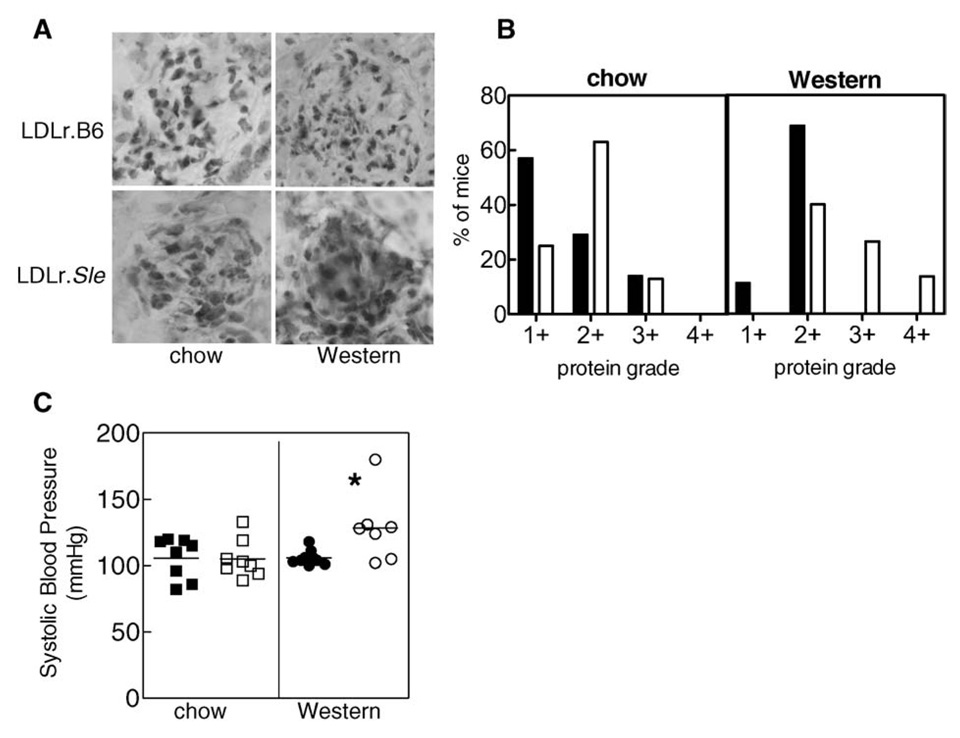
Increased immunoglobulin deposition in kidneys correlated to the dsDNA antibody titre in that LDLr.B6mice on chow diet had the least staining and LDLr.Sle mice on Western diet had the most staining (Figure 2A). Similarly, urine protein was the greatest in LDLr.Sle mice on Western diet (Figure 2B). Finally, systolic blood pressure in LDLr.Sle mice on a Western diet was significantly greater than LDLr.Sle mice on chow or LDLr.B6 mice on either Western or chow diet (Figure 2C). Collectively, these data suggest that renal function was decreased in LDLr.Sle mice on high fat diet.
Figure 2. Immune complex deposition, urine protein and blood pressure are increased in LDLr.Sle mice fed high fat diet.
(A) LDLr.Sle mice fed high fat diet exhibit increased immune complex deposition in glomeruli as detected by immunohistochemistry. Shown is one representative mouse per group. Kidney sections from a total of five mice per group were analysed with similar results. (B) Urine protein grade in mice (3–7 mice per group) was determined by Chemstix at the time of killing. LDLr.Sle mice (open bars) have increase urine protein compared with LDLr.B6 controls (closed bars). (C) LDLr.Sle mice (open circles) on Western diet have increased systolic blood pressure compared with LDLr.Sle mice on chow diet (open squares) and LDLr.B6 mice fed chow (closed squares) or Western diet (closed circles). *P < 0.05 as determined by one-way ANOVA. Data represent two identical experiments.
Lupus-susceptibility increases atherosclerosis in LDLr-deficient mice in the absence of overt dyslipidemia
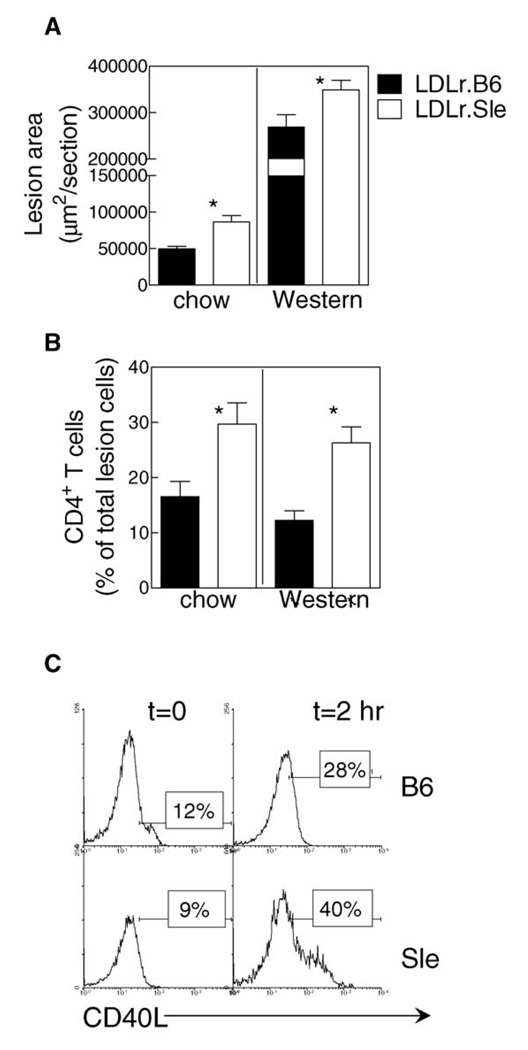
Our previous study showed that transfer of lupus susceptibility increases atherosclerosis in LDLr-deficient mice on a Western diet.14 However, feeding high fat diet in this animal model results in extreme elevations in circulating cholesterol; often greater than 1000 mg/dL. This level of cholesterol would be considered physiologically irrelevant to the human patient with lupus. We hypothesized that SLE could exacerbate atherosclerosis under more physiologic levels of serum lipoproteins (i.e., approximately 200 mg/dL). Measurement of atherosclerotic lesions in the proximal aorta showed that LDLr.Sle mice had increased atherosclerosis compared with LDLr.B6 controls independent of diet (Figure 3A). In fact, the increase in atherosclerosis in LDLr.Sle mice on chow compared with controls was actually greater (approximately 2.0-fold) than the difference between the mice-fed Western type diet (approximately 1.3-fold). Analysis of the cellular composition of plaques by immunohistochemistry showed similar macrophage (Moma-2) and dendritic cell (CD11c) content among all groups of mice (data not shown). However, CD4+ T-cell content was increased approximately threefold in the LDLr.Sle mice compared with LDLr.B6 animals (Figure 3B). This increase was independent of diet because both chow and Western diet fed animals showed similar percentages of CD4+ T cells in lesions. Stimulation with PMA and ionomycin showed an increase in CD40L expression in Sle primary CD4+ T cells compared with wild type C57Bl/6 mice (Figure 3C).
Figure 3. Transfer of lupus-susceptibility to LDLr-deficient mice increases atherosclerosis independent of diet.
(A) Average lesion area as determined by oil-red-O staining in LDLr.B6 (closed bars) and LDLr.Sle (open bars) mice. Lesion quantitation was performed on 9–11 mice in each group. Shown is data from two identical experiments. (B) Detection of CD4+ T cells in lesions of LDLr.B6 (closed bars) and LDLr.Sle (open bars) on chow or Western diet. Positive cells are expressed as a percent of all lesion cells as determined by DAPI staining. Four sections from 5–10 mice per group were analysed. (C) Primary CD4+ T cells were isolated from C57Bl/6 (B6) and B6.Sle1.2.3 (Sle) mice and stimulated for 2 h with PMA and ionomycin. Shown is one of three experiments with similar results.
*P < 0.05 as determined by Kruskal–Wallis test.
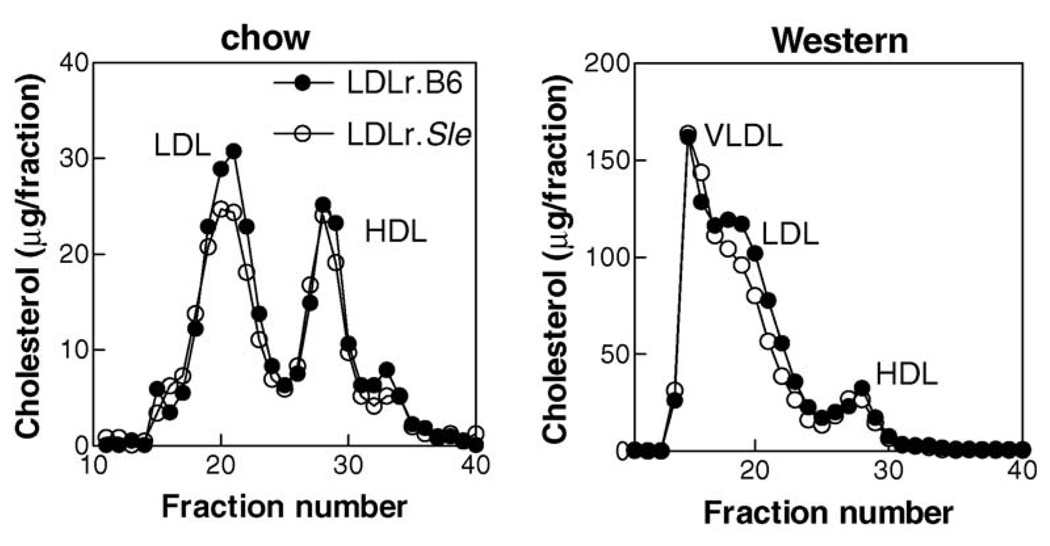
The increase in atherosclerosis in LDLr.Sle mice fed a chow diet occurred in the absence of changes in serum cholesterol or triglyceride levels (Table 1) or cholesterol-containing lipoprotein distribution as determined by FPLC (Figure 4, left panel). Mice fed a Western diet showed increased serum cholesterol and triglycerides compared with chow fed mice (Table 1). In addition, as reported in our previous study,14 serum cholesterol and triglycerides were slightly, but significantly, decreased in LDLr.Sle mice. The difference in total cholesterol was associated with decreased non–high density lipoprotein (HDL) cholesterol (Figure 4, right panel). These data show that transfer of SLE to LDLr-deficient mice can increase atherosclerosis independent of large increases in total serum cholesterol or triglycerides associated with high fat diet feeding. In addition, they show that increased numbers of CD4+ T cells in the lesions of LDLr.Sle mice is also independent of severe hyperlipidemia.
Table 1.
Serum cholesterol and triglyceride
| Group | N | Cholesterol mg/dL (±SEM) |
Triglyceride mg/dL (±SEM) |
|---|---|---|---|
| Chow diet | |||
| LDLr.B6 | 11 | 230.4 (11.3) | 76.6 (13.0) |
| LDLr.Sle | 12 | 239.4 (10.7) | 86.2 (12.5) |
| Western diet | |||
| LDLr.B6 | 10 | 971.4 (57.4) | 311.6 (10.6) |
| LDLr.Sle | 10 | 767.1 (73.3)a | 201.8 (26.6)a |
P < 0.05 compared with LDLr.B6 mice on Western diet.
Figure 4. FPLC analyses of serum cholesterol lipoprotein distribution.
Lipoproteins were separated by size-exclusion chromatography and assayed for cholesterol as described in ‘Methods’. Serum from LDLr.B6 (closed circles) and LDLr.Sle (open circles) was pooled before undergoing separation and analysis (9–11 mice per group). Similar profiles were obtained from individual mice.
Lupus susceptibility increases oxLDL and phospholipid antibodies in LDLr-deficient mice
To examine the effect of diet on production of immunoglobulin against modified LDL and phospholipid, we measured titres of oxLDL- and β2-glycoprotein I (β2-GPI)–specific antibodies in sera of control and SLE-susceptible LDLr-deficient mice. Table 2 contains the anti-oxLDL total antibody and IgG isotype titres from chow and Western diet fed mice. In general, the LDLr.Sle mice had higher total antibody, IgG1 and IgG2a titres in serum compared with LDLr.B6 mice. This increase in oxLDL-specific IgG was independent of diet feeding (Table 2). In addition, chow fed LDLr.Sle mice had significantly higher anti-oxLDL IgM levels compared with LDLr.B6 mice. The LDLr.Sle mice fed a Western diet had increased, but not significantly higher, levels of anti-oxLDL IgM.
Table 2.
Serum titres of oxLDL-specific antibodies
| Group | Total Ig (±SEM)a | IgM (±SEM) | IgG1 (±SEM) | IgG2a (±SEM) | IgG1/IgG2a (±SEM) |
|---|---|---|---|---|---|
| Chow | |||||
| LDLr.B6 | 0.402 (0.104) | 0.770 (0.114) | 0.302 (0.093) | 0.431 (0.097) | 0.661 (0.020) |
| LDLr.Sle | 0.900 (0.128)b | 1.267 (0.102)b | 0.956 (0.095)b | 0.743 (0.069)b | 1.307 (0.101)b |
| Western | |||||
| LDLr.B6 | 0.398 (0.108) | 1.01 (0.180) | 0.236 (0.090) | 0.527 (0.131) | 0.399 (0.059)c |
| LDLr.Sle | 0.839 (0.090)b | 1.335 (0.069) | 1.058 (0.024)b | 0.944 (0.006)b | 1.119 (0.022)b |
Average OD450nm ± SEM.
P < 0.05 compared with LDLr.B6 mice on the same diet as determined by one-way analysis of variance.
P < 0.05 compared with LDLr.B6 mice on chow diet as determined by one-way analysis of variance.
To determine whether the antibody response to oxLDL was associated with a Th2 or a Th1 T helper phenotype, we calculated the IgG1 (Th2) to IgG2a (Th1) isotype ratio. Independent of diet, the LDLr.Sle mice had an increased IgG1/IgG2a ratio indicating that the immune response of these animals was skewed toward a Th2 phenotype. Additionally, the LDLr.B6 mice fed a Western diet appeared to have more of a Th1 type phenotype than LDLr.B6 mice on chow.
Antibodies to the phospholipid β2-GPI are present in patients with SLE and the anti-phospholipid syndrome.16,17 These antibodies are thought to be associated with increased risk of CVD. Therefore, we determined the serum titre of β2-GPI antibodies in our chow and Western diet fed animals. LDLr.Sle mice fed with chow diet had significantly higher total antibody, IgM and IgG1 specific for β2GPI compared with chow fed LDLr.B6 mice (Table 3). IgG2a showed a trend toward increased levels in LDLr.Sle mice, but did not reach statistical significance. In mice fed with Western diet, β2-GPI-specific IgG1 and IgG2a were significantly increased in LDLr.Sle mice compared with control LDLr.B6 mice. Analysis of the IgG1/IgG2a ratio showed similar trends as seen with the oxLDL antibody titres. In general, the LDLr.Sle mice had more of a Th2 phenotype compared with LDLr.B6 mice, independent of the diet fed. Additionally, LDLr.B6 mice fed a high fat diet appeared to have more of a Th1 phenotype. Collectively, these data indicate that there are quantitative and qualitative differences in the immune response to vascular disease–associated antigens between the LDLr.B6 and LDLr.Sle mice.
Table 3.
Serum titres of β2-GPI-specific antibodies
| Group | Total (±SEM)a | IgM (±SEM) | IgG1 (±SEM) | IgG2a (±SEM) | IgG1/IgG2a (±SEM) |
|---|---|---|---|---|---|
| Chow | |||||
| LDLr.B6 | 0.149 (0.033) | 0.120 (0.030) | 0.128 (0.061) | 0.158 (0.065) | 0.981 (0.187) |
| LDLr.Sle | 0.690 (0.180)b | 0.543 (0.172)b | 0.588 (0.115)b | 0.370 (0.122) | 2.040 (0.304)b |
| Western | |||||
| LDLr.B6 | 0.467 (0.186)c | 0.253 (0.101) | 0.069 (0.024) | 0.179 (0.063) | 0.474 (0.084) |
| LDLr.Sle | 0.514 (0.140) | 0.402 (0.089) | 0.722 (0.051)b | 0.635 (0.093)b | 1.270 (0.219)b |
Average OD450nm ± SEM.
P < 0.05 compared with LDLr.B6 mice on the same diet as determined by one-way analysis of variance.
P < 0.05 compared with LDLr.B6 mice on chow diet as determined by one-way analysis of variance.
Discussion
Individuals suffering with SLE are at increased risk for developing accelerated forms of atherosclerosis and vascular disease. Because many patients with SLE do not fall into the traditional risk group for atherosclerosis, the underlying aetiology for its acceleration remains largely unknown. Until recently, lupus and atherosclerosis studies have been hampered by the lack of an appropriate animal model that simultaneously develops both diseases.However, recent studies by our laboratory14 and others18–20 have reported that transfer of the lupus phenotype to atherosclerosis-susceptible mouse strains (e.g., LDLr- or apoE-deficient mice) results in dysregulated immunity, chronic inflammation and increased atherosclerotic lesions. However, we also observed that when placed on the traditional atherosclerosis-inducing Western diet, the LDLr.Sle animals exhibited increased mortality compared with the LDLr.B6 controls with the first animals dying as early as 2 weeks following diet initiation. Mortality by the end of the 8-week feeding period was approximately 40%. Whether this increased death was due to renal failure, heart failure or both is currently unknown. However, in the surviving LDLr. Sle mice, urine protein was elevated indicating the presence of some degree of kidney disease.
In general, patients with lupus do not develop the severe dyslipidemia observed in LDLr-deficient mice fed with Western diet (cholesterol ≥600 mg/dL). In addition, increased serum cholesterol is not predictive of accelerated atherosclerosis in patients with lupus.21,22 Therefore, we decided to test the hypothesis that the transfer of lupus is sufficient to exacerbate atherosclerosis in the presence of more physiological levels of serum cholesterol (approximately 200 mg/dL). To test this hypothesis, we transferred lupus-susceptible or resistant bone marrow to LDLr-deficient mice and fed the mice either chow or high fat Western diet. In line with our previous study, we observed a 37% mortality rate at the end of 8 weeks in LDLr.Sle mice fed with Western diet compared with LDLr.Sle mice fed with chow diet or LDLr.B6 mice fed either diet (Figure 1A). In addition, LDLr.Sle mice fed a high fat diet had higher serum titres of dsDNA antibodies compared with LDLr.B6 mice on high fat and LDLr.Sle mice on chow diet. These data are in line with previous work reporting that lupus-susceptible NZB/W F1 mice fed a high fat diet develop increased anti-dsDNA and cardiolipin antibody titres,23,24 increased MHC class II expression on accessory cells,25 increased cytokine production and more severe lupus nephritis.26 However, the dietary effects in these studies were observed over a period of 2–9 months of feeding. In the current study, the LDLr.Sle mice were only fed diet for 2 weeks when the animals started dying. In two separate studies, Lin, et al.24,25 reported a decreased life span in the NZB/W F1 mice fed with high fat diet. The average life span of these animals was 285 days compared with the low fat fed controls, which lived for an average of 389 days. Because we were interested in measuring atherosclerosis, we did not allow the mice to proceed past 8 weeks of diet feeding. However, by 6 weeks of diet feeding, the LDLr.Sle mice on high fat diet had already suffered significant mortality compared with the other three groups indicating that the life span of these animals was greatly decreased. Because the LDLr.Sle mice fed a high fat diet had dsDNA titres even greater than the chow fed LDLr.Sle mice, we were not surprised to see that the immunoglobulin deposition, urine protein grade and systolic blood pressure were also elevated in these animals (Figure 2). Although most individuals with SLE do not exhibit total serum cholesterol levels greater than 200 mg/dL, the fact that the LDLr.Sle mice fed high fat diet have even greater disease symptoms compared with the LDLr.Sle mice on chow diet suggests that severe dyslipidemia can further exacerbate lupus disease in these animals. Certainly, these data are consistent with the clinical evidence showing that elevated serum total cholesterol in patients with SLE is associated with increased kidney pathology and death.27
Examination of atherosclerosis in the proximal aorta of the mice showed that increased atherosclerosis in LDLr.Sle mice compared with control LDLr.B6 controls was independent of high fat diet feeding (Figure 3). In fact, the LDLr.Sle mice fed with chow diet showed an even greater increase over controls compared with their high fat fed counterparts. Additionally, the average serum total cholesterol and triglyceride levels did not differ between the LDLr.B6 and LDLr.Sle mice on chow diet and the serum lipoprotein distribution of cholesterol was similar (Figure 4). However, the LDLr.Sle mice fed a high fat diet had decreased serum cholesterol and triglyceridewith decreased non-HDL cholesterol. The accumulation of CD4+ T cells in the atherosclerotic lesions of LDLr.Sle was similarly not dependent of high fat diet feeding and CD4+ T cells isolated from chow fed B6.Sle triple congenic mice show increased CD40L expression upon stimulation with PMA and ionomycin compared with C57Bl/6 controls. Collectively, these data suggest that the autoimmune dysregulation and perhaps chronic inflammation have a greater influence on atherosclerosis than serum cholesterol; a more traditional risk factor for atherosclerosis. More importantly, the data show that non-physiologically high serum cholesterol levels are not necessary to exacerbate atherosclerosis in the face of lupus.
Although the lipoprotein distribution of cholesterol did not differ between LDLr-deficient mice receiving either normal or lupus-susceptible bone marrow, we cannot exclude the possibility that the LDLr.Sle mice harbour dysfunctional HDL. HDL functions not only in reverse cholesterol transport but also acts to prevent oxidation of LDL.28 When inflammation becomes chronic, HDL appears to loose its capacity to prevent the formation of oxLDL. In SLE, it has been shown that HDL function is abnormal in 45% of patients compared with only 4% of control.29 Therefore, in the lupus mice, it will be interesting to determine whether we see similar decreases in HDL’s anti-oxidative abilities compared with normal control animals.
Patients with lupus are known to develop autoantibodies to many atherosclerosis-associated antigens, such as oxLDL, β2-glycoprotein I, cardiolipin30 and antibodies to atheroprotective proteins such as the HDL-associated apolipoprotein AI.31 A recent study by Svenungsson, et al.32 reported a strong correlation between plasma concentrations of oxLDL and anti-oxLDL antibodies in SLE patients with coronary heart disease (CHD) complications. The authors suggest using atherosclerosis-associated antibodies as a screen for identifying SLE patients with increased risk for the development of atherosclerosis. However, whether the autoantibody production in these studies was the cause of enhanced atherosclerosis or a secondary effect of other immune responses remains to be determined. In the current study, we observed an increase in the anti-oxLDL and β2-GPI serum IgG levels in LDLr.Sle mice compared with control LDLr.B6 animals. This increase appeared to be diet independent as the LDLr.Sle mice fed a Western diet did not show increased levels of antibodies compared with LDLr.Sle mice fed with chow diet. Interestingly, the LDLr.Sle mice show an IgG1 bias for both anti-oxLDL and anti-β2-GPI antibodies compared with LDLr.B6 mice independent of diet. This suggests that high fat feeding in LDLr.Sle mice skews the immune response toward a Th2-like phenotype. It has been shown in apoE-deficient mice that atherosclerotic lesion progression is accompanied by an increase in the oxLDL IgG1 serum titres33 and a bias toward Th2 immune responses. Therefore, these data support the hypothesis that the atherosclerotic disease in the LDLr.Sle mice is more advanced that those seen in the LDLr.B6 controls.
Although titres of oxLDL antibodies are shown to correlate directly with severity of disease and are often used as markers of CHD risk,34–37 their role in the initiation and/or progression of atherosclerosis is not yet conclusive. In addition, it has been shown that in patients with SLE, there is extensive antibody cross-reactivity between antiphospholipid (e.g., β2-GPI) and oxLDL. Therefore, in the current study, it cannot be concluded that the oxLDL antibodies are unique from the β2-GPI antibodies. However, a recent report by Kobayashi, et al.38 suggested that anti-β2-GPI IgG significantly increases oxLDL/β2-GPI complex binding to macrophages and uptake via Fcγ receptors and, thus, may contribute to the atherosclerotic process. Therefore, one may hypothesize that the increase in β2-GPI antibody titres seen in the LDLr.Sle mice accelerates uptake of modified atherogenic lipoproteins such as oxLDL/β2GPI complexes and potentiates foam cell formation in these animals. Interestingly, although increased anti-β2-GPI antibodies are associated with increased risk of atherosclerosis, one study in mice showed that β2-GPI reactive T cells, and not antibodies, may be pathogenic.39 The authors reported that adoptive transfer of whole splenocytes, but not T-depleted splenocytes, from mice immunized with β2-GPI increased atherosclerosis in LDLr-deficient animals in the absence of detectable antigen-specific antibody. These data would argue that antibodies against β2-GPI are a useful marker for cardiovascular risk but are not pathological. However, the current studies do not directly address either of these possibilities and ongoing studies in our laboratory are aimed at examining these hypotheses.
In conclusion, in our current study, we have reported that severe dyslipidemia, as that seen in LDLr-deficient mice fed with Western diet, can exacerbate the lupus phenotype and accelerate mortality beyond that previously reported in the NZB/W F1 mice fed with high fat diets.25 This suggests that perhaps the lipoprotein profile (i.e., increased VLDL and LDL cholesterol) can adversely affect the lupus disease process. Additionally, we also provide strong evidence that the accelerated atherosclerosis observed in LDLr.Sle mice is more closely associated with immune hyperactivity and not a secondary effect of autoimmune-mediated renal pathology associated with SLE. Planned future studies will allow us to delineate which specific immune cell type and function facilitates accelerated atherogenesis, ultimately leading to the identification of novel therapeutics that target both lupus and CVD.
Acknowledgements
We thank Adam C.Morgan for expert technical assistance. This work was supported by a grant from the Lupus Research Institute, New York, NY, the National Institutes of Health (NIH) BIRCWH Grant 5 K12 HD043483-04 (to A.S.M.), the NIH NRSA 5 T32HL07751 (year 15)Training Grant in Mechanisms of Vascular Disease (to N.A.B) and the NIH 5T32 HL007411-29 Training Grant in Cardiovascular Mechanisms: Training in Investigation (to N.S.W.).
Footnotes
References
- 1.Ward MM, Pyun E, Studenski S. Causes of death in systemic lupus erythematosus. Long-term followup of an inception cohort. Arthritis Rheum. 1995;8:1492–1499. doi: 10.1002/art.1780381016. [DOI] [PubMed] [Google Scholar]
- 2.Aranow C, Ginzle EM. Epidemiology of cardiovascular disease in systemic lupus erythematosus. Lupus. 2000;9:166–169. doi: 10.1191/096120300678828208. [DOI] [PubMed] [Google Scholar]
- 3.Lockshin MD, Salmon JE, Roman MJ. Atherosclerosis and lupus: a work in progress. Arthritis Rheum. 2001;44:2215–2217. doi: 10.1002/1529-0131(200110)44:10<2215::aid-art381>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 4.Chiu YH, Jayawardena J, Weiss A, et al. Distinct subsets of CD1d-restricted T cells recognize self-antigens loaded in different cellular compartments. J Exp Med. 1999;189:103–110. doi: 10.1084/jem.189.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glass C, Witzaum J. Atheroscleroisis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 6.Morel L, Rudofsky UH, Longmate JA, Schiffenbauer J, Wakeland EK. Polygenic control of susceptibility to murine systemic lupus erythematosus. Immunity. 1994;1:219–229. doi: 10.1016/1074-7613(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 7.Mohan C, Morel L, Yang P, Wakeland EK. Genetic dissection of systemic lupus erythematosus pathogenesis: Sle2 on murine chromosome 4 leads to B cell hyperactivity. J Immunol. 1997;159:454–465. [PubMed] [Google Scholar]
- 8.Mohan C, Alas E, Morel L, Yang P, Wakeland EK. Genetic dissection of SLE pathogenesis. Sle1 on murine chromosome 1 leads to a selective loss of tolerance to H2A/H2B/DNA subnucleosomes. J Clin Invest. 1998;101:1362–1372. doi: 10.1172/JCI728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohan C, Yu Y, Morel L, Yang P, Wakeland EK. Genetic dissection of Sle pathogenesis: Sle3 on murine chromosome 7 impacts T cell activation, differentiation, and cell death. J Immunol. 1999;162:6492–6502. [PubMed] [Google Scholar]
- 10.Morel L, Mohan C, Yu Y, et al. Functional dissection of systemic lupus erythematosus using congenic mouse strains. J Immunol. 1997;158:6019–6028. [PubMed] [Google Scholar]
- 11.Morel L, Yu Y, Blenman KR, Caldwell RA, Wakeland EK. Production of congenic mouse strains carrying genomic intervals containing SLE-susceptibility genes derived from the SLE-prone NZM2410 strain. Mamm Genome. 1996;7:335–339. doi: 10.1007/s003359900098. [DOI] [PubMed] [Google Scholar]
- 12.Sobel ES, Mohan C, Morel L, Schiffenbauer J, Wakeland EK. Genetic dissection of SLE pathogenesis: adoptive transfer of Sle1 mediates the loss of tolerance by bone marrow-derived B cells. J Immunol. 1999;162:2415–2421. [PubMed] [Google Scholar]
- 13.Sobel ES, Morel L, Baert R, Mohan C, Schiffenbauer J, Wakeland EK. Genetic dissection of systemic lupus erythematosus pathogenesis: evidence for functional expression of Sle3/5 by non-T cells. J Immunol. 2002;169:4025–4032. doi: 10.4049/jimmunol.169.7.4025. [DOI] [PubMed] [Google Scholar]
- 14.Stanic AK, Stein CM, Morgan AC, et al. Immune dysregulation accelerates atherosclerosis and modulates plaque composition in systemic lupus erythematosus. Proc Natl Acad Sci U S A. 2006;103:7018–7023. doi: 10.1073/pnas.0602311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Major AS, Fazio S, Linton MF. B-lymphocyte deficiency increases atherosclerosis in LDL receptor-null mice. Arterioscler Thromb Vasc Biol. 2002;22:1892–1898. doi: 10.1161/01.atv.0000039169.47943.ee. [DOI] [PubMed] [Google Scholar]
- 16.Delgado Alves J, Ames PR, Donohue S, et al. Antibodies to high-density lipoprotein and beta2-glycoprotein I are inversely correlated with paraoxonase activity in systemic lupus erythematosus and primary antiphospholipid syndrome. Arthritis Rheum. 2002;46:2686–2694. doi: 10.1002/art.10542. [DOI] [PubMed] [Google Scholar]
- 17.Delgado Alves J, Kumar S, Isenberg DA. Cross-reactivity between anti-cardiolipin, anti-high-density lipoprotein and anti-apolipoprotein A-I IgG antibodies in patients with systemic lupus erythematosus and primary antiphospholipid syndrome. Rheumatology (Oxford) 2003;42:893–899. doi: 10.1093/rheumatology/keg248. [DOI] [PubMed] [Google Scholar]
- 18.Aprahamian T, Bonegio R, Rizzo J, et al. Simvastatin treatment ameliorates autoimmune disease associated with accelerated atherosclerosis in a murine lupus model. J Immunol. 2006;177:3028–3034. doi: 10.4049/jimmunol.177.5.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gautier EL, Huby T, Ouzilleau B, et al. Enhanced immune system activation and arterial inflammation accelerates atherosclerosis in lupus-prone mice. Arterioscler Thromb Vasc Biol. 2007;27:1625–1631. doi: 10.1161/ATVBAHA.107.142430. [DOI] [PubMed] [Google Scholar]
- 20.Aprahamian T, Rifkin I, Bonegio R, et al. Impaired clearance of apoptotic cells promotes synergy between atherogenesis and autoimmune disease. J Exp Med. 2004;199:1121–1131. doi: 10.1084/jem.20031557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roman MJ, Shanker BA, Davis A, et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. NEngl J Med. 2003;349:2399–2406. doi: 10.1056/NEJMoa035471. [DOI] [PubMed] [Google Scholar]
- 22.Roman MJ, Salmon JE, Sobel R, et al. Prevalence and relation to risk factors of carotid atherosclerosis and left ventricular hypertrophy in systemic lupus erythematosus and antiphospholipid antibody syndrome. Am J Cardiol. 2001;87:663–666–A611. doi: 10.1016/s0002-9149(00)01453-3. [DOI] [PubMed] [Google Scholar]
- 23.Morrow WJ, Ohashi Y, Hall J, et al. Dietary fat and immune function. I. Antibody responses, lymphocyte and accessory cell function in (NZB × NZW)F1 mice. J Immunol. 1985;135:3857–3863. [PubMed] [Google Scholar]
- 24.Lin BF, Jeng SJ, Chiang BL, Huang CC. Dietary fat affects lipids and anti-cardiolipin antibody levels in autoimmune-prone NZB/WF1 mice. Br J Nutr. 1997;77:657–669. doi: 10.1079/bjn19970063. [DOI] [PubMed] [Google Scholar]
- 25.Lin BF, Huang CH, Chiang BL, Jeng SJ. Dietary fat influences Ia antigen expression, cytokines and prostaglandin E2 production of immune cells in autoimmune-prone NZB × NZW F1 mice. Br J Nutr. 1996;75:711–722. doi: 10.1079/bjn19960175. [DOI] [PubMed] [Google Scholar]
- 26.Yumura W, Hattori S, Morrow WJ, Mayes DC, Levy JA, Shirai T. Dietary fat and immune function. II. Effects on immune complex nephritis in (NZB × NZW)F1 mice. J Immunol. 1985;135:3864–3868. [PubMed] [Google Scholar]
- 27.Tisseverasinghe A, Lim S, Greenwood C, Urowitz M, Gladman D, Fortin PR. Association between serumtotal cholesterol level and renal outcome in systemic lupus erythematosus. Arthritis Rheum. 2006;54:2211–2219. doi: 10.1002/art.21929. [DOI] [PubMed] [Google Scholar]
- 28.McMahon M, Hahn BH. Atherosclerosis and systemic lupus erythematosus: mechanistic basis of the association. Curr Opin Immunol. 2007;19:633–639. doi: 10.1016/j.coi.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMahon M, Grossman J, FitzGerald J, et al. Proinflammatory high-density lipoprotein as a biomarker for atherosclerosis in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 2006;54:2541–2549. doi: 10.1002/art.21976. [DOI] [PubMed] [Google Scholar]
- 30.Thiagarajan P. Atherosclerosis, autoimmunity, and systemic lupus erythematosus. Circulation. 2001;104:1876–1877. [PubMed] [Google Scholar]
- 31.Vuilleumier N, Reber G, James R, et al. Presence of autoantibodies to apolipoprotein A-1 in patients with acute coronary syndrome further links autoimmunity to cardiovascular disease. J Autoimmun. 2004;23:353–360. doi: 10.1016/j.jaut.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Svenungsson E, Jensen-Urstad K, Heimburger M, et al. Risk factors for cardiovascular disease in systemic lupus erythematosus. Circulation. 2001;104:1887–1893. doi: 10.1161/hc4101.097518. [DOI] [PubMed] [Google Scholar]
- 33.Zhou X, Paulsson G, Stemme S, Hansson GK. Hypercholesterolemia is associated with a T helper (Th) 1/Th2 switch of the autoimmune response in atherosclerotic apoE-knockout mice. J Clin Invest. 1998;101:1717–1725. doi: 10.1172/JCI1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Geest B, Collen D. Antibodies against oxidized LDL for non-invasive diagnosis of atherosclerotic vascular disease. Eur Heart J. 2001;22:1517–1518. doi: 10.1053/euhj.2001.2659. [DOI] [PubMed] [Google Scholar]
- 35.Wu JT, Wu LL. Autoantibodies against oxidized LDL. A potential marker for atherosclerosis. Clin Lab Med. 1997;17:595–604. [PubMed] [Google Scholar]
- 36.Steinerova A, Racek J, Stozicky F, Zima T, Fialova L, Lapin A. Antibodies against oxidized LDL–theory and clinical use. Physiol Res. 2001;50:131–141. [PubMed] [Google Scholar]
- 37.Paiker JE, Raal FJ, von Arb M. Auto-antibodies against oxidized LDL as a marker of coronary artery disease in patients with familial hypercholesterolaemia. Ann Clin Biochem. 2000;37:174–178. doi: 10.1258/0004563001899177. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi K, Tada K, Itabe H, et al. Distinguished effects of anti-phospholipid antibodies and anti-oxidized LDL antibodies on oxidized LDL uptake by macrophages. Lupus. 2007;16:929–938. doi: 10.1177/0961203307084170. [DOI] [PubMed] [Google Scholar]
- 39.George J, Harats D, Gilburd B, et al. Adoptive transfer of beta(2)-glycoprotein I-reactive lymphocytes enhances early atherosclerosis in LDL receptor-deficient mice. Circulation. 2000;102:1822–1827. doi: 10.1161/01.cir.102.15.1822. [DOI] [PubMed] [Google Scholar]