Abstract
Objective
Development of longitudinal 3D outcomes of inflammation and bone erosion in murine arthritis using contrast enhanced (CE) MRI and in vivo micro-CT; and in a pilot study, to determine the value of entrance criteria by age versus synovial volume in therapeutic intervention studies.
Methods
CE-MRI and in vivo micro-CT was performed on TNF-Tg and WT littermates to quantify the synovial and popliteal lymph node (LN) volumes and patella and talus bone volumes, respectively, which were validated with histology. These longitudinal outcome measures were used to assess the natural history of inflammatory-erosive arthritis. We also performed anti-TNF versus placebo efficacy studies in TNF-Tg mice in which treatment was initiated either by age (4–5 months) or synovial volume (3mm3 as detected by CE-MRI). Linear regression was performed to analyze the correlation between synovitis and focal erosion.
Results
CE-MRI demonstrated the highly variable nature of TNF-induced joint inflammation. Initiation of treatment by synovial volume produced significantly larger treatment effects on synovial volume (p=0.04) and lymph node volume (p<0.01) than initiation by age. By correlating the MRI and microCT data we were able to demonstrate a significant relationship between changes in synovial and patellar volumes (R2 =0.75; p<0.01).
Conclusion
In vivo CE-MRI and micro-CT 3D outcome measures are powerful tools that accurately demonstrate the progression of inflammatory-erosive arthritis in mice. These methods can be used to identify mice with arthritis of similar severity before intervention studies are initiated and thus minimize heterogeneity in outcome studies of chronic arthritis seen between genetically identical littermates.
Keywords: Inflammatory Arthritis, Animal Model, In vivo Imaging, 3D-MRI, Micro-CT
INTRODUCTION
Although murine models of inflammatory arthritis have significantly advanced our understanding of inflammatory-erosive arthritis (1, 2) they are limited by a lack of longitudinal-translational outcome measures of disease progression or interventional therapy. This issue presents three problems for prototypical pre-clinical investigations of drug effects on inflammation, erosion, and healing (3, 4). First, most cross-sectional studies in mice with “established arthritis” do not include an objective assessment of disease severity prior to treatment. Thus, neither rates of change nor healing responses can be assessed. Second, the commonly used endpoints (i.e. histology and ex vivo molecular analyses) require sacrifice, thus markedly increasing the number of animals needed to assess efficacy at multiple time points. Third, though the incidence and severity of arthritis varies among genetically identical littermates, there are no established scoring criteria to stratify different groups of mice based on disease activity in an intervention study. Therefore, the development of imaging techniques that could assess disease activity and progression in vivo would greatly enhance the utility of these pre-clinical studies.
Magnetic resonance imaging (MRI) has become the “gold standard” for the assessment of joint inflammation and damage in inflammatory arthritis (5–8). Several studies have demonstrated the value of MRI in the detection of synovial inflammation and bone marrow edema before irreversible joint damage occurs (9–14). While quantitative measures of these imaging biomarkers have been developed, their validation has been difficult to perform during clinical studies (15–18). Conversely, while histology of arthritis in animals models is readily available to validate imaging biomarkers, initial attempts to utilize this longitudinal in vivo imaging modality in mice have fallen short of the desired 3D quantitative outcome measure (19–22).
The purpose of the current study was to develop and validate in vivo quantitative 3D imaging biomarkers of inflammatory-erosive arthritis in mice. Although many different animal models exist (1, 2), we chose the human TNF-transgenic mouse (TNF-Tg) largely because it is a chronic model of known etiology that is completely ameliorated by anti-TNF therapy (23). Furthermore, a critical role for TNF in rheumatoid arthritis (RA) has been firmly established by the success of TNF antagonists (24). Thus, achievement of predicted outcomes in this well-established model serves as a validation of the novel 3D imaging biomarkers and has important translational value as well, given the similar histologic findings to human RA.
Using a custom designed murine MR knee coil, we have developed volumetric quantifications for two outcome measures: synovial inflammation and popliteal lymph node enlargement. We demonstrate the progression of these biomarkers in the disease, as well as the reversal of their progression after anti-TNF therapy. We also validate these measurements with histology and demonstrate significant correlations between MRI measurements and novel micro-CT measurements of bone erosion.
MATERIALS AND METHODS
Animals and anti-TNF treatment
The 3647 line of TNF-Tg mice were originally obtained from Dr. G. Kollias and are maintained as heterozygotes in a CBA x C57BL/6 background (25). Experiments are performed with sex matched TNF-Tg and WT littermate controls. The University of Rochester Committee for Animal Resources approved all animal studies.
An initial natural history study examined TNF-Tg mice and WT littermates (n=5 per group) from 2 months until 5 months of age. MRI scans were performed every half-month, except at 4 months of age because of temporary technical problems with the MR scanner. At 5 months of age, mice were sacrificed and the knee joints and popliteal lymph nodes were harvested for histology.
In the drug intervention studies, mice received either murine monoclonal anti-human TNF IgG1 antibody or an irrelevant murine IgG1 placebo control (Centocor R&D Inc., Radnor, PA) at a dose of 10mg/kg/week intraperitoneal injection as previously described (3). Two anti-TNF versus placebo studies were performed. Study 1 controlled for the age of the mice, whereas study 2 controlled for MRI-based evidence of synovitis. The age-controlled study contained four groups of 5–6 month old mice (n=4 per group) at baseline. Groups 1 and 2 were WT littermates receiving placebo or anti-TNF, respectively. Groups 3 and 4 were TNF-Tg mice receiving placebo or anti-TNF, respectively. Treatments were administered for 16 weeks. MRI scans were performed at baseline and every 4 weeks thereafter in the TNF-Tg groups, and at baseline and 16 weeks in the WT groups. At 16 weeks, mice were sacrificed and the knee joints and popliteal lymph nodes were harvested for histology.
The synovitis-controlled anti-TNF study contained two groups each containing four female TNF-Tg mice. These mice were scanned every two weeks starting at 3 months of age and entered into the study when it was determined that they had synovial volumes above 3mm3 (see MR Image Analysis below). Average age at initiation of treatment was 3.88 +/− 0.79 months. One group received weekly anti-TNF injections, while the second group was given placebo, as above. MRI scans were performed at baseline and every two weeks for 8 weeks. In vivo micro-CT scans of knees and ankles were performed at baseline and at 8 weeks. At 8 weeks, mice were sacrificed and knee joints, ankle joints, and popliteal lymph nodes were harvested for histology.
Contrast Enhanced Magnetic Resonance Imaging (CE-MRI)
MR scans were performed on a 3 Tesla Siemens Trio MRI (Siemens Medical Solutions, Erlangen, Germany). Mice were anesthetized by intraperitoneal injection of a ketamine/xylazine mixture at a dosage of 110μg/ml. Mice were then positioned on an imaging platform with the right leg inserted through the custom designed knee coil (Figure 1A). The coil is composed of a 1.5 cm diameter circular loop consisting of two parallel gauge-14 copper wires. This design was found to give optimal signal-to-noise ratio (SNR) while providing sufficient volume coverage of the joint. An imaging template assisted in reproducible positioning between scans and between animals. After a series of three 10-second localization scans, a fat-suppressed, T1-weighted high-resolution scan was performed (Sagittal T1-weighted FLASH, TR=45ms, TE=9.03ms, 192×192 pixels, 20mm×20mm FOV, 32 slices of 0.16mm slice thickness, flip angle=25°, 1 signal average, time: 8:28min). Gd-DTPA contrast agent (Omniscan, Amersham Health, Oslo, Norway) at dosage 0.500 mL/kg diluted in sterile saline was injected via the retro-orbital venous plexus. After 3 minutes to allow for Gd-DTPA to equilibrate with joint fluid, a second high-resolution scan was performed to image the knee with contrast enhancement. Imaging sessions took approximately 30 minutes per mouse.
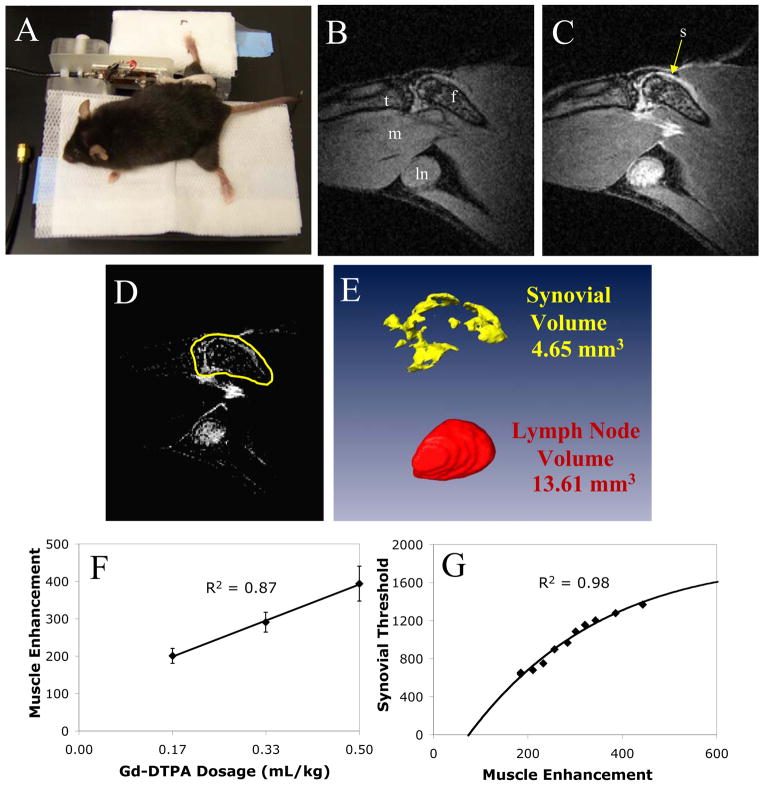
Figure 1. CE-MRI of the mouse knee.
An anesthetized mouse positioned in the knee surface coil is shown prior to MRI scan (A). A pre-contrast (B), and post-contrast (C), image from a 5-month-old TNF-Tg mouse is shown demonstrating the high-resolution of the tibia (t), femur (f), synovium (s), popliteal lymph node (ln), and gastrocnemius muscle (m) from a sagital MR view. For 3D imaging and volumetric quantitation with Amira 3.1, the pre-contrast image is first registered to and subtracted from the post-contrast image (D). Then a limit line surrounding the synovium is manually drawn around the region of interest (ROI) on the post-contrast image, and copied to the subtracted image (D, yellow). The lymph node is manually segmented as it is clearly visualized on post-contrast images. This is performed on all slices encompassing the knee and lymph node and the segmented labels are reconstructed as volumes (E) for visualization and quantification. The threshold value to quantify synovial volume in the ROI is determined from the delivered dosage of Gd-DTPA. A dosage study was performed to establish a direct linear relationship between dosage and gastrocnemius muscle contrast enhancement (F). At constant synovial volume, the threshold used to segment synovial volume was curve fit to muscle contrast enhancement (G). This curve is used to threshold and segment enhanced synovial area in the drawn ROI from the surrounding tissues using muscle as a normalization tissue to determine delivered dosage of contrast agent.
MR imaging analysis
Amira 3.1 (TGS, Mercury Computer Systems, Inc, San Diego, CA) was used for image segmentation and volume computations of synovium and popliteal lymph nodes. The 3D stacks of images for the pre-contrast (Figure 1B) and post-contrast (Figure 1C) scans were loaded into the software. An automatic registration module (Registration -> LineSearch -> Correlation) was used to align the images in 3D. The pre-contrast scan was then subtracted from the post-contrast scan using the Arithmetic module resulting in a 3D stack of images of contrast enhancement (Figure 1D).
A segmentation and threshold procedure using the Amira Segmentation Editor was used to determine synovial and lymph node volumes. The editor allows the user to create 3D “labels” of each tissue of interest. First, limit lines corresponding to the region of interests (ROI) enclosing the synovium, but excluding enhancing tissues such as the subcutaneous layer and the popliteal vessels, were drawn on each slice. For visualization purposes these lines were drawn on the post-contrast image stack and the labels were copied onto the subtracted image. Next, a section (>15mm3) of muscle tissue was labeled on the subtracted image stack. The TissueStatistics module was used to determine the contrast enhancement of the muscle as an estimate of the delivered dosage of Gd-DTPA. Based on the level of muscle enhancement, a threshold value corresponding to synovial enhancement was found using the equation determined by a dosage study (see next section). All voxels above the threshold value within the limit lines were labeled as synovium. Enhanced voxels above this threshold that were within the bone marrow were then subtracted from the label. The lymph node, which is much easier to visualize than synovium, was segmented by manually drawing ROIs on post contrast images and thresholded based on signal intensity ≥ 1500 arbitrary units (AU) to define the boundary between the lymph node and the fat pad surrounding the node. These labels were also copied onto the subtracted image. 3D rendering (Figure 1E) was performed by applying the SurfaceGen module to reconstruct the tissue labels into volumes and the SurfaceView module was used for visualization. The TissueStatistics module was used to quantify the volumes of the tissues. Total time for image analysis was around 20 minutes per mouse for an experienced operator.
In order to determine the threshold parameters for longitudinal analyses of synovial volume, we performed a dose response study in which four TNF-Tg mice (3 to 5 months old) were scanned on three consecutive days with incremental dosages of Gd-DTPA (Dose 1 = 0.167 mL/kg, Dose 2 = 0.333 mL/kg, Dose 3 = 0.500 mL/kg). The concentrations of contrast agent in saline were adjusted such that the bolus injected into each animal was at constant volume between doses. No trace of contrast agent was detected in the animals after 24 hours in the pre-contrast scans on Day 2 or Day 3. A strong linear relationship between muscle enhancement and dosage was found using a linear mixed-effects regression model (R2 = 0.87, Figure 1F). Data from Dose 2 were analyzed with the above described method, however an initial synovial threshold equal to muscle enhancement + 1000 AU was used to generate a synovial volume. The data from Day 1 and Day 3 were then analyzed and the threshold value to generate the same synovial volume was recorded. The threshold values versus muscle enhancement values for all four mice at all three doses fit the increasing form of a two-phase exponential decay curve (Figure 1G) with R2 = 0.98. The values from this curve were used to determine synovial threshold values that are adjusted for Gd-DTPA dosage as measured by contrast enhancement of muscle.
Reproducibility for synovial and lymph node volume measurements was assessed for intra-reader, inter-reader, and inter-MRI variation. For intra-reader variation 10 CE-MRI scans (5 TNF-Tg and 5 WT) were analyzed by the same operator (STP) on two different days in random order. The same 10 scans were analyzed by a second operator (MOP) after a training period of 2 hours to determine inter-reader variation. For inter-MRI variation, 5 TNF-Tg mice of varying severity were scanned twice within 48 hours and the same operator analyzed the scans in random order.
Volumetric assessment of bone erosion via micro-CT
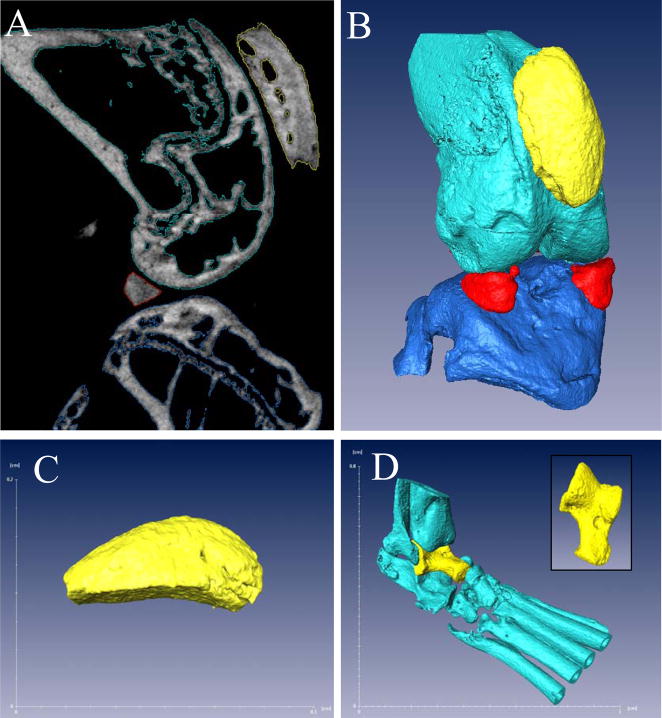
High-resolution in vivo micro computed tomography (micro-CT) was used to scan knee and ankle joints of the mice (VivaCT 40, Scanco USA, Inc). Animals were placed under anesthesia with isofluorine. Each joint was scanned at an isotropic resolution of 17.5μm in a custom sample holder at 55 kEv, with conebeam mode. The data were reconstructed via ScanCo software into DICOM files for analysis. Amira 3.1 was used to segment and visualize the bones of the knee and ankle from micro-CT scans. For the knee, the Segmentation Editor was used to label femur, tibia, patella, and menisci (Figure 2A). A density threshold higher than 11,000 AU was set as “bone” and the labels were reconstructed using the SurfaceGen module to visualize the joint (Figure 2B). The threshold was kept constant throughout the study. Since the entire patella is scanned, the volume of this bone determined from the TissueStatistics module was used as a quantitative measure of bone erosion for the knee (Figure 2C). For the ankle, the talus was specifically labeled and the volume of this bone is used as the measure of erosion in this joint (Figure 2D). Analysis typically takes around 15 minutes per joint for an experienced operator.
Figure 2. 3D reconstruction and quantification of bone volume from micro-CT.
A representative sagittal slice from a micro-CT scan of a wild type knee is shown to demonstrate the density-based segmentation that is performed on the bone to generate labels for patella (yellow), femur (light blue), tibia (dark blue) and menisci (red), as described in Materials and Methods (A). These labels are then used to reconstruct the bones in 3D (B). The patella (C), due to its ease of reconstruction and proximity to synovitis, is used as a quantification of bone volume at the knee joint. The ankle joint (D) is reconstructed in a similar manner as the knee with the talus (yellow and inset) as measure of bone volume in this joint.
Histology
The right knee joint was removed and fixed in 10% neutral buffered formalin. The joints were then decalcified in 14% EDTA at room temperature (pH adjusted to 7.2) for 21 days. The joint was then carefully embedded in paraffin for sectioning into 3μm slices. Sections were then stained with orange G/alcian blue for histological examination. The popliteal lymph node was also harvested and prepared in a similar manner omitting the decalcification step. Lymph node area was quantified using ImageJ at the slice found to have maximal cross sectional area.
Statistical analyses
Linear mixed-effects regression models, with mouse as a random effect and time (treated as a continuous covariate) as a fixed effect, were used to assess changes over time based on longitudinal data. Similar models used age or dose instead of time to assess changes over age or linear dose-response relationships based on repeated measurements. Analyses based on cross-sectional data utilized standard linear regression models. A nonlinear mixed-effects model, with a random effect for mouse to account for the repeated measures design, was used to fit the two-phase exponential decay curve. Two-sided t-tests assuming unequal variances were used to make comparisons with micro-CT data or with CE-MRI data between groups at the same time point or age. Correlations between measures were estimated using Pearson’s correlation coefficient and tested for significance using a two-sided t-test test. Inter-reader, intra-reader, and inter-MRI reliability was estimated using coefficients of variation and intraclass correlation coefficients based on random-effects ANOVA models. All underlying assumptions of the parametric methods were checked, and no serious violations were detected. P-values less than 0.05 were considered significant and p-values less than 0.01 were considered highly significant.
RESULTS
Longitudinal CE-MRI biomarkers of TNF-induced arthritis in mice
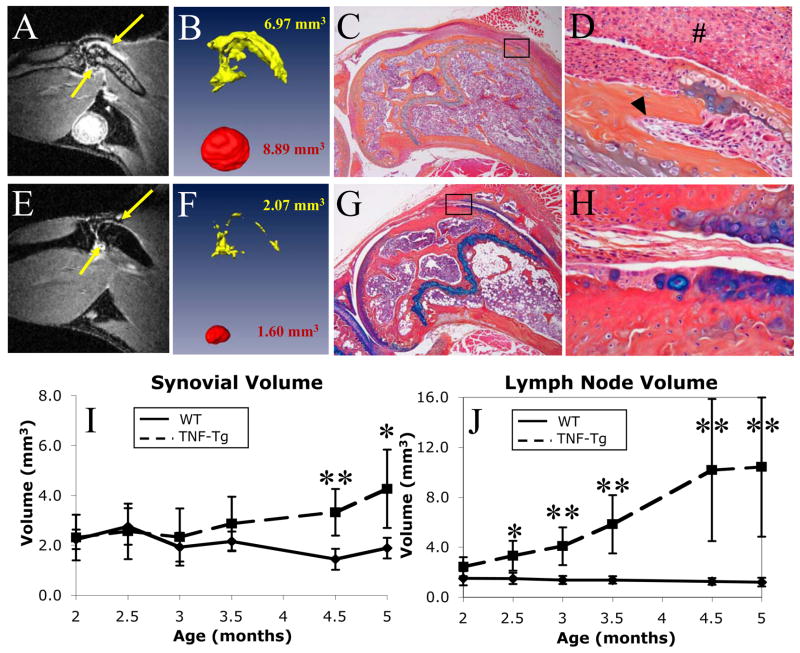
High-resolution MR images of mouse knees clearly defined contrast enhanced inflamed synovium and enlarged LN in TNF-Tg mice (Figure 3A) and permitted volumetric quantification (Figure 3B). These findings were absent in WT littermates (Figures 3E and 3F). The remarkable differences between the joints of the TNF-Tg and WT mice were confirmed by histology (Figures 3C–D and 3G–H).
Figure 3. Identification, quantification, and validation of synovial and lymph node volume as longitudinal outcome measures of inflammatory knee arthritis in mice.
Data are presented from a representative 5-month-old TNF-Tg (A–D) and a WT (E–H) mouse. The post-contrast MRI are shown with enhancing synovium denoted by yellow arrows (A and E). Also of note in these MRI scans is the bone marrow edema in the TNF-Tg mouse, as seen by the high signal intensity in the bone marrow space (A), which is absent in the WT (E). Corresponding reconstructions and calculated synovial (yellow) and popliteal lymph node (red) volumes (B and F) are presented. The dramatic differences in these quantitative imaging biomarkers are validated in corresponding orange G/alcian blue stained histology sections at 40X (C and G) and 200X (D and H). The TNF-Tg mouse displays thickened synovial lining (#) and infiltration into subchondral bone (arrow head). TNF-Tg and WT mice (n=5) were scanned bimonthly from 2 months until 5 months of age, as described in Materials and Methods. The synovial (I) and LN (J) volumes for each scan were calculated, and the data from the TNF-Tg (dashed line) and WT (solid line) are plotted as the mean +/− standard deviation. Two-sided t-tests revealed significant (*, p<0.05) and highly significant (**, p<0.01) differences between groups of the same age. Linear mixed-effects regression analysis revealed a highly significant difference in slopes for TNF-Tg vs. WT mice in both synovial volume and lymph node volumes (p<0.0001 for both comparisons).
Before we utilized these novel imaging outcome measures in prospective studies, we assessed the validity of our LN volume calculations by demonstrating correlations with the maximum LN area determined by MRI and histology (Supplemental Figure 1). Linear regression analyses demonstrated significant relationships (p<0.0001) between the 2D and 3D measurements and substantiated the validity of our volumetric CE-MRI measurements for outcomes studies.
Intra-reader, inter-reader, and inter-MRI reliability was assessed with intraclass correlation coefficient (ICC) analysis, and found to be excellent for all variables. Synovial volume measurements were found to have ICC values of 0.996 for intra-reader, 0.984 for inter-reader reliability, and 0.968 for inter-MRI reliability. Lymph node volume quantifications had ICC values of 0.999, 0.997, and 0.994, respectively. Additionally, the inter-MRI reliability was assessed with a coefficient of variation and found to be 4.53% for synovial volume and 3.95% for lymph node for n=5 TNF-Tg mice.
In our first longitudinal study of 3D CE-MRI biomarkers of inflammatory arthritis in mice, we assessed the progression of disease in 2 month-old TNF-Tg vs. WT littermates over a 3-month time period. Figure 3I shows that TNF-induced knee synovitis initiates at ~3 months of age and steadily increases thereafter (slope = 0.60 mm3/month, p<0.0001). In contrast, the synovial volume of WT mice significantly decreased over time (slope = −0.26 mm3/month, p=0.01), likely due to growth effects. This resulted in a highly significant difference in slope for TNF-Tg versus WT mice (p<0.0001). While a highly significant difference in synovial volume between TNF-Tg and WT mice was observed at 4.5 months (3.32+/−0.93mm3 vs. 1.44+/−0.42mm3, mean+/−standard deviation; p<0.01), the significance of this difference decreased at 5 months due to the increased variability in the TNF-Tg group (4.26+/−1.57mm3 versus 1.89+/−0.41mm3; p<0.05). Interestingly, TNF-induced changes in popliteal LN volumes preceded knee synovitis (Figure 3J), as the steady-significant increase (slope = 2.95 mm3/month, p<0.0001) in TNF-Tg animals commenced at 2 months (2.43+/−0.76mm3) of age until it plateaued at 4.5 months (10.17+/−5.69mm3). Despite the large variability at the end of the study, we still observed a highly significant tenfold difference in LN volume between the TNF-Tg vs. WT mice at 5 months (10.41+/−5.57mm3 vs. 1.20+/−0.34mm3; p<0.01). As expected, the popliteal LN volume of WT mice did not change throughout the study (slope = −0.10 mm3/month, p=0.71). However, a highly significant difference in slope for TNF-Tg versus WT mice was detected (p<0.0001).
Changes in longitudinal 3D-biomarkers of inflammatory-erosive arthritis following effective anti-TNF therapy
In order to validate the CE-MRI and micro-CT 3D outcome measures as in vivo biomarkers of inflammatory-erosive arthritis in an intervention study, we utilized a model of known etiology (TNF-Tg) (23, 25), and a proven treatment (anti-TNF) whose efficacy in this model has been demonstrated by several groups (3, 23, 26). Since we observed that the onset of knee synovitis in TNF-Tg mice varies from 3 to 5 months of age (Fig. 3I), we aimed to determine if entering the animals into the study based on synovial volume rather than age would improve the statistical outcome of a small number of subjects.
Initiation of therapy based on age
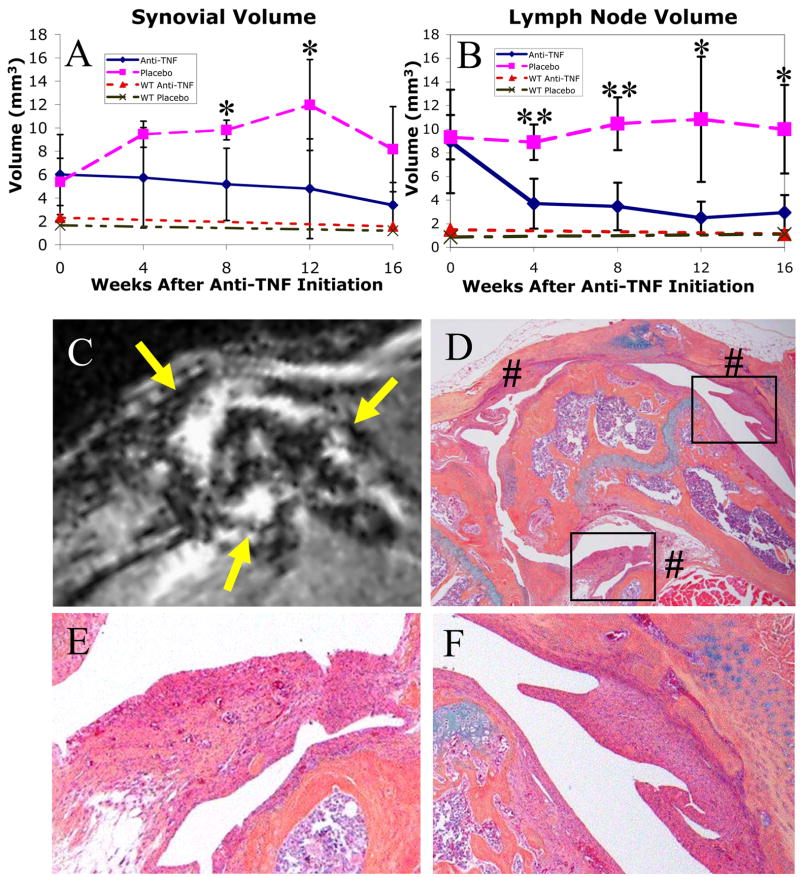
In a traditional intervention study based on age, we randomized 5 to 6 month-old TNF-Tg and WT mice to anti-TNF and placebo groups (Figure 4). The baseline CE-MRI data of the TNF-Tg mice demonstrated remarkable variability in synovial volume (5.66+/−2.46mm3) and LN volume (9.14+/−3.12mm3). Due to this variability, we did not observe a significant decrease in synovial volume with anti-TNF treatment (slope = −0.17 mm3/week, p=0.06), despite a 44% reduction from baseline values at 16 weeks (Figure 4A). However, when compared to placebo (slope = 0.20 mm3/week, p=0.02), the difference in slope is highly significant (p<0.01). In contrast, we observed a significant difference in LN volume at the first time point following treatment (anti-TNF 3.71+/−2.11mm3 versus placebo 8.91+/−1.50mm3; p<0.01). Overall, anti-TNF therapy demonstrated a significant 67% (slope = −0.33 mm3/week, p<0.0001) reduction from baseline by the end of the study (Figure 4B). Consistent with the LN volume plateau we observed in the natural history study after 4.5 months (Figure 3H), no changes were observed in TNF-Tg placebo mice over the course of this study (slope = 0.08 mm3/week, p=0.30), but slopes for placebo vs. anti-TNF treatment were highly significantly different (p<0.001). No changes or drug effects were observed in the WT groups. One surprising outcome of this study was the synovial volume peak at 12 weeks (122% increase from baseline), which decreased to a +52% change from baseline at 16 weeks (Figure 4A). To better understand this we investigated the areas of decreased Gd-DTPA enhancement (Figure 4C), with corresponding histology of the knees from these mice and we found large regions of pannus fibrosis (Figures 4D–F). This fibrosis is consistent with a loss of CE-MRI signal enhancement due to the loss of vascularity.
Figure 4. Effects of anti-TNF therapy in 5–6 month-old WT and TNF-Tg mice.
WT and TNF-Tg mice were MR imaged at baseline and were randomized into anti-TNF and placebo treatment groups (n=4 per group) at 5–6 months of age. TNF-Tg mice were scanned every 4 weeks thereafter. WT mice received a further follow-up MRI 16-weeks after treatment. The synovial (A) and LN (B) volumes for each scan were calculated, and the data from the TNF-Tg placebo (pink long-dashed) vs. anti-TNF (blue solid) groups, and WT placebo (green intermittent-dashed) vs. anti-TNF (red short-dashed) groups, are plotted as the mean +/− standard deviation. Anti-TNF treatment had no effects in WT mice, and no changes in synovial or LN volume were detected in these animals after 16 weeks. In contrast, two-sided t-tests revealed significant (*, p<0.05) and highly significant (**, p<0.01) differences between anti-TNF vs. placebo TNF-Tg groups of the same time point after therapy. Linear mixed-effects regression analysis revealed a highly significant difference in slopes for anti-TNF versus placebo in both synovial volume (p<0.01) and LN volume (p<0.001). The linear progression of inflammatory arthritis (note decrease in synovial volume from 12 to 16 weeks in placebo group) was limited by tissue fibrosis, as shown by non-enhancing synovial regions on MRI (C, yellow arrows) and corresponding 40X histology (D, indicated by #) of a representative placebo TNF-Tg mouse. The degeneration of the pannus in the end-stage of arthritis in this animal is further illustrated by the decreased cellularity of the synovium (boxes in D) shown at 200X (E and F).
Initiation of therapy based on synovial volume
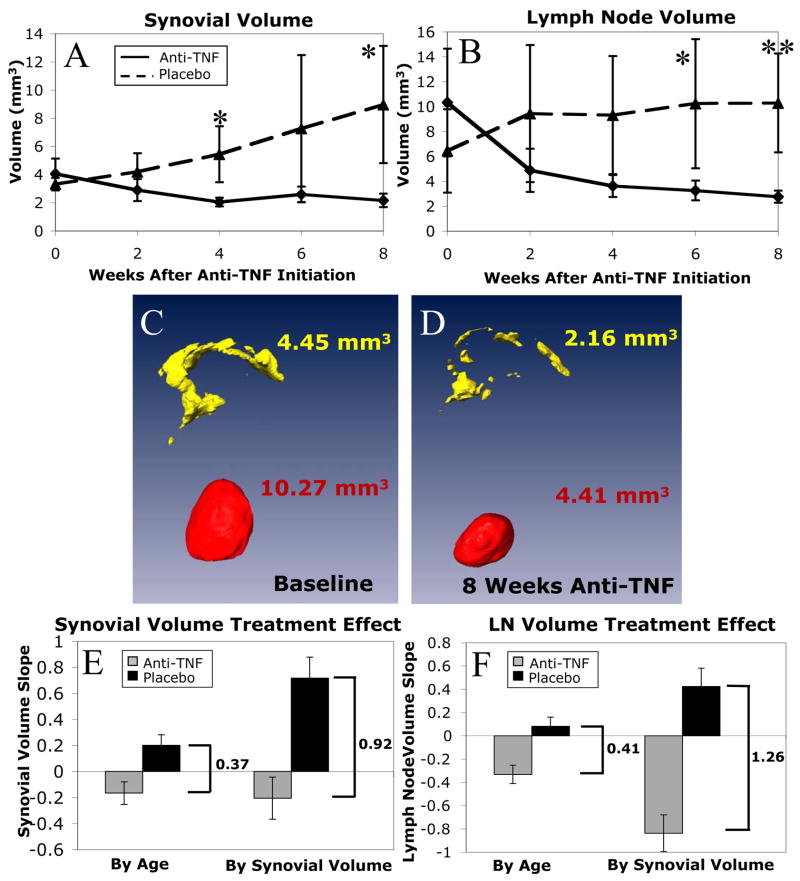
To determine if we could observe a more significant therapeutic effect of anti-TNF therapy on synovial and LN volumes in TNF-Tg mice with established arthritis, we repeated the placebo controlled study by randomizing the animals to the treatments when CE-MRI first detected a volume of knee synovium greater than 3mm3. This value represents a 50% increase over WT at 3–5 months, confirming the presence of synovitis (Figures 3I and 4A). In this study, anti-TNF therapy had dramatic effects in comparison to placebo on synovial volume (Figure 5A) and LN volume (Figure 5B) over time. Synovial volumes did not demonstrate a significant decrease during the course of the 8-week study with anti-TNF treatment (slope = −0.20 mm3/week, p=0.21). However, this was due to the rapid response in these animals to anti-TNF, reaching WT levels within 4 weeks, as visualized in volumetric reconstructions of the baseline (Figure 5C) and 4-week (Figure 5D) data from a representative animal. A significant decrease from baseline to 4 weeks was found (−49%, slope = −0.50 mm3/week, p=0.01) and there was no further change from 4 weeks to 8 weeks (+3%, slope = 0.03, p= 0.91). In contrast, the placebo mice synovial volumes showed a highly significant increase throughout the 8 weeks (+169%, slope = 0.72 mm3/week, p<0.0001). This resulted in highly significantly different slopes for placebo versus anti-TNF treatment (p<0.001). Another advantage of this study design is that no pannus fibrosis effects were seen in any of the mice. LN volume significantly decreased (−73%, slope = −0.84 mm3/week, p<0.0001) with anti-TNF therapy (Figure 5B). LN volumes also showed a highly significant increase with placebo (+60%, slope = 0.43 mm3/week, p<0.01). This resulted in highly significantly different slopes for placebo versus anti-TNF treatment (p<0.0001). Interestingly, the LN volume had wide variability in placebo mice throughout the study (Figure 5B), suggesting that synovial inflammation and LN volume are not directly linked.
Figure 5. Effects of anti-TNF therapy in TNF-Tg mice with established synovitis.
TNF-Tg mice (n=4) received bimonthly CE-MRI from 3-months of age until they reached a synovial volume above 3mm3. At this time they received an in vivo micro-CT scan and were randomized into anti-TNF and placebo treatment groups (n=4 per group). The mice were then scanned every 2 weeks until sacrifice at 8 weeks, when they received a follow-up micro-CT scan. The synovial (A) and LN (B) volumes for each scan were calculated, and the data from the placebo (dashed line) vs. anti-TNF (solid line) groups, are plotted as the mean +/− standard deviation. Two-sided t-tests revealed significant (*, p<0.05) and highly significant (**, p<0.01) differences between anti-TNF vs. placebo groups at the same time point after therapy. Linear mixed-effects regression analysis revealed a highly significant difference in slopes for anti-TNF versus placebo in both synovial (p<0.001) and LN (p<0.0001) volumes. The protective effects of anti-TNF therapy were also apparent from the Amira 3D reconstructions of pannus and LN, as shown in baseline (C) and 4 weeks (D) images from a representative mouse. Treatment effect was evaluated by a measure of the difference in slopes between anti-TNF and placebo groups. When treatment was initiated using synovial volume rather than age as the entrance criterion, there was a significantly larger treatment effect in synovial volume (E, 0.92 versus 0.37 mm3/week, p=0.04) and in lymph node volume (F, 1.26 versus 0.41 mm3/week, p=0.04).
Therapy initiated at constant synovial volume rather than age demonstrates a more significant treatment effect
The difference in slopes of volume versus time between anti-TNF and placebo groups is a measure of the treatment effect of anti-TNF therapy. Initiation of treatment by synovial volume resulted in a significantly larger treatment effect on synovial volumes than initiation by age alone (Figure 5E, difference in slopes of 0.92 versus 0.37 mm3/week, p=0.04). The treatment effect is also significantly larger on LN volume when the entrance criterion is by synovial volume as opposed to age (Figure 5F, difference in slopes of 1.26 versus 0.41 mm3/week, p<0.01).
Effect of anti-TNF therapy on bone erosion
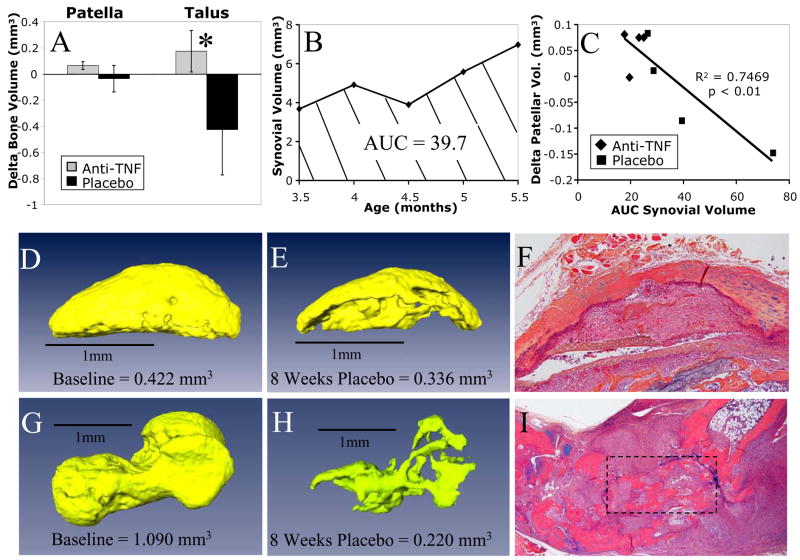
By incorporating longitudinal micro-CT analysis into this study, in which focal erosions were assessed by subtracting the baseline from the 8-week bone volumes, we were able to evaluate changes in patella and talus bone volume (Figure 6A). While half of the placebo mice had a decreased patella volume due to erosions and all of the anti-TNF mice showed an increase or no change in patellar volume, these changes were small and failed to demonstrate a significant (p=0.14) drug effect. However, all of the placebo mice had a markedly decreased talus volume, and 75% of anti-TNF treated mice showed an increase in the size of this bone, which resulted in a significant difference between these groups (p<0.02).
Figure 6. Bone erosion is arrested with anti-TNF therapy and correlates with sustained synovial inflammation during TNF-induced arthritis.
The change in cortical bone volume determined from the baseline and 8 week micro-CT scans for the anti-TNF (gray bars) and placebo (black bars) treated mice is presented as the mean +/−SD for the patella and talus (A). While there was no significant difference in delta patellar bone volume between groups (p=0.14), anti-TNF treatment had a significant effect on talus bone loss vs. placebo (*p<0.02). The MRI synovial volume data from a representative placebo TNF-Tg mouse described in Figure 5 is graphed to demonstrate the area under the curve (AUC) measurement as a function of joint inflammation and time (B). This measurement was used to perform regression analyses of the change in patella volume vs. synovial AUC (C) of all eight mice in the study described in Figure 5. Erosion data from the representative placebo mouse in (B) are presented as micro-CT reconstructed images of the patella at baseline (D) and at 8 weeks (E) with histology (F); and of the talus at baseline (G) and 8 weeks (H) with histology (I boxed region).
Correlation of longitudinal 3D biomarkers of inflammation and bone erosion
As a final validation of our 3D biomarkers, we assessed the relationship between synovial inflammation and bone erosion using the data from the eight mice in the initiation of treatment by synovial volume study. Since the erosion data are a function of the change in bone volume over time, we first had to convert the synovial volume data to reflect the severity of synovitis over the study period. This was done by computing the area under the curve (AUC) from the synovial volume versus time plot for each animal (Figure 6B). Using this approach we found a highly significant correlation between our measures of synovial volume and patellar erosion (R2 = 0.75, p<0.01; Figure 6C). The impact of unchecked synovitis on bone is demonstrated by a longitudinal analysis of the patella (Figures 6D–E). The dramatic loss of bone quantified by micro-CT is associated with widespread synovial infiltration into the bone as visualized in the corresponding histology section (Figure 6F). An even more dramatic loss of bone was present at the talus (Figures 6G–H), in which severe synovitis as visualized by histology (Figure 6I) has reduced the volume of bone to 20% of its original size.
DISCUSSION
In vivo imaging measurements have emerged as the outcome of choice for translational research in pre-clinical studies based on their potential to objectively quantify change and their compatibility with modalities used in clinical trials (27). Since MRI and micro-CT are widely accepted as the “gold standards” to assess soft tissue and bone volumes respectively, we aimed to adapt these methods as longitudinal outcome measures of inflammatory-erosive arthritis in mice. In the case of CE-MRI for the mouse knee, several innovations were required (Figure 1). These included: i) the generation of a mouse knee specific coil that can interface with a clinical 3T MRI, ii) the establishment of pulse sequences that produce high-resolution images (105μm) with a minimal slice thickness (160μm) to reduce partial volume effects, iii) a method to normalize for the variability of Gd-DTPA administration using muscle contrast enhancement, and iv) standardization for thresholding and segmentation of biomarkers for longitudinal-quantitative 3D analyses. Although commercial small animal MRI instruments may become more popular in the future, we chose to use a clinical MRI to ensure that all of the biomarkers we identified could be used to study arthritis in humans using readily available pulse sequences. In addition, the quantification methods developed could easily be adapted to data collected with small animal scanners.
Several quantification methods have been developed to assess synovial volumes in humans using CE-MRI (28, 29). However, no quantification methods have been established for clinical trials, for which there is a great demand. The currently accepted evaluation determined by the Outcome Measures in Rheumatoid Arthritis Clinical Trails (OMERACT) task group is the RA MRI scoring system (RAMRIS) consisting of semi-quantitative global scoring system (0–3) based on synovial thickness and Gd-DTPA enhancement (30). The current gold standard for quantification of synovitis is a manual segmentation technique (14, 31). Although this technique has been validated in intervention studies, and with a correlation to an aspirated volume of synovial fluid, the length of analysis (0.75–2 hours) and technical expertise necessary has limited its use in clinical trails. To address these limitations, automated segmentation methods have been evaluated (32, 33). This two-step segmentation process consists of a limited manual segmentation to remove enhancing vessels and skin (similar to the limit lines used in the current study), and application of a threshold on subtracted images. Methods to threshold enhancing synovial tissue remain controversial. Østergaard et al. evaluated several different thresholds based on percent enhancement of synovium compared to the manual segmentation technique, and determined that a 45% enhancement threshold was optimal (32). Although this reduced the time of analysis to 20 minutes, there was increased inter-MRI variation versus manual segmentation, particularly when misalignment occurred between pre- and post-contrast scans. Palmer et al. used a threshold value based on the signal intensity differences between several regions of non-enhancing tissue (suppressed fat) and enhancing pannus (33). This method has an advantage in that the threshold is determined after each individual scan, however the time of analysis is around 45 minutes. The threshold is also sensitive to misregistration artifacts and the consistency of fat suppression.
To our knowledge, the current study is the first to use adjacent muscle as a normalization tissue to determine the synovial threshold. This approach is attractive due to the fact that muscle is an enhancing tissue that is not implicated in the pathology of inflammatory arthritis and because its tissue properties do not change significantly between scans, variations in values in muscle will reflect the signal variations that are present between scanning sessions. In small animal studies this approach is especially warranted due to the inconsistencies in dosage delivery that are inherent during administration via intravenous injection. During our dosage study, we found that muscle linearly enhanced with increasing dosage of Gd-DTPA. Even more important, a direct relationship could be derived between muscle contrast enhancement and the synovial threshold used to maintain a constant synovial volume between doses. The use of this optimized threshold, combined with the ability to minimize motion artifacts with anesthesia and standardized positioning, has allowed reproducible measurements of synovial volume in mice (4.5% coefficient of variation) that far exceed those reported in humans (22% median relative variation as measured with the manual segmentation method (34)). Whether a similar threshold approach based on muscle enhancement could be adapted to clinical studies warrants further investigation.
Considering that micro-CT has been used to examine focal erosions in murine arthritis models for several years (35), it is somewhat surprising that this approach has yet to evolve into a longitudinal-quantitative outcome measure. Based on our experience, we found that this is likely due to the difficulty in registering the baseline and outcome 3D-CT images so that the erosion as a negative change in volume could be accurately assessed. It is also clear from our work (Figure 2), that segmentation can only be readily performed on small bones that are clearly defined by soft-tissue boundaries (e.g. patella and talus). This is because minor imperfections in the registration of large bones, and subjective segmentation of bone parts (e.g. distal femur and proximal tibia) can result in significant measurement errors.
In this study we focused our attention on the synovial and LN volume as the primary outcome measures of inflammation, based on their facile segmentation and quantification from CE-MRI. Interestingly, our findings indicated that these tissues behave differently during the onset and amelioration of inflammatory arthritis following effective therapy. We found that the popliteal LN volume was the most sensitive biomarker of lower limb arthritis. This conclusion comes from the observation that LN volumes significantly increase when TNF-Tg mice are 2.5 months-old (Figure 3H), which correlates with the point at which increased TNF serum levels and changes in peripheral blood mononuclear cell (PBMC) populations are first detected in this model (36). Our finding that increases in LN volume precede knee synovitis is consistent with the function of popliteal lymph nodes in draining both the knee and ankle joints, and the fact that arthritis first occurs in the ankle in this model (23). This conclusion was corroborated by similar results using the K/BxN serum transfer model (37), in which the popliteal LN volume increased concurrently with ankle inflammation in the absence of knee synovitis (Supplemental Figure 2). Thus, the introduction of pro-inflammatory mediators into the joint alone is not sufficient for the initiation of pannus formation. Our future efforts will focus on understanding the mechanism that initiates this destructive process. This issue is paramount since synovitis correlates with joint destruction in inflammatory arthritis (Figure 6C). Additionally, many groups have generated transgenic animals that can be used to dissect these pathologies (35, 38–41), which now can be assessed with our novel longitudinal outcome measures.
By using a validated drug therapy in our model we were able to address the major concern with all pre-clinical intervention studies of arthritis that are performed with a single cross-sectional endpoint, namely, whether or not the differences observed are due to drug effects or variability between the initiation and/or progression of disease in the individual mice. Using our previous study of anti-TNF treatment of TNF-Tg mice as an example (3), we were unable to make definitive conclusions regarding the healing effects on bone and cartilage lesions since there were no baseline assessments. When we repeated this experiment using age as the enrollment criterion (Figure 4), these concerns regarding inter-animal variability were realized based on the broad range of baseline synovitis (3.00 to 9.85 mm3). Furthermore, during this study we saw a dramatic decrease in synovial volume in the placebo group from 12 to16 weeks that was due to pannus tissue fibrosis. Thus, by modification of the study design to include entrance criterion based on synovial volume (Figure 5), we were able to make several remarkable observations including: i) demonstration that the treatment effect of anti-TNF therapy on synovial volume and LN volume is significantly greater than when entrance criterion is by age, ii) synovial volumes were significantly reduced to WT levels after only 4 weeks of anti-TNF therapy, iii) initiation of the study earlier in the disease process combined with an 8-week time course avoided the pannus tissue fibrosis effects previously observed, iv) a healing response was noted in which the mean lymph node volumes of the TNF-Tg mice treated with anti-TNF therapy were reduced but were still 2.5x higher than WT levels at the end of the study, and v) bone erosion was arrested as demonstrated by a significant difference in change in talus volume between anti-TNF and placebo animals.
In summary we found that CE-MRI and micro CT are very useful longitudinal outcome measures of the severity of inflammatory arthritis, as they demonstrated significant results with as few as four mice per group. We are also pursuing additional outcomes with the CE-MRI, including quantifications of bone marrow edema, which appears as a bright signal with this MR pulse sequence in the bones of arthritic animals versus the dark signal in WT controls (Supplemental Figure 3). This biomarker has previously been demonstrated to be a faithful predictor of focal erosions in RA (15), and its nature has recently been demonstrated to be osteitis (42). Moreover, bone marrow edema measured by CE-MRI has been effectively used to assess disease severity and response to therapy in patients with ankylosing spondylitis (43, 44). Thus, the hope is that an edema outcome measure could be developed as the long sought after surrogate of arthritic pain in animal models and/or a predictor of joint destruction.
Supplementary Material
Acknowledgments
The authors would like to thank Patricia Weber for technical assistance with the MRI, Laura Yanoso for technical assistance with the micro-CT, Colleen Hock for assisting with animal breeding and genotyping, and Krista Scorsone for technical assistance with the histology. This work was supported by research grants from Centocor Inc. and the National Institutes of Health PHS awards AR43510, AR46545, AR48697, AR51469, AR54041 and DE17096.
Footnotes
AUTHOR CONTRIBUTIONS
Dr. Schwarz and Mr. Proulx had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study design. Drs. Schwarz, Kwok, You, Shealy, Ritchlin, Awad, Boyce, Xing, and Mr. Proulx.
Acquisition of data. Drs. Kwok, You, Awad, and Mr. Proulx.
Analysis and interpretation of data. Drs. Schwarz, Kwok, Beck, Shealy, Ritchlin, Awad, Boyce, Xing, Mr. Papuga, and Mr. Proulx.
Manuscript preparation. Drs. Schwarz, Kwok, You, Beck, Shealy, Ritchlin, Awad, Boyce, Xing, Mr. Papuga and Mr. Proulx.
Statistical analysis. Drs. Schwarz, Beck and Mr. Proulx.
References
- 1.Dustin ML. In vivo imaging approaches in animal models of rheumatoid arthritis. Arthritis Res Ther. 2003;5(4):165–71. doi: 10.1186/ar768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van den Berg WB. Animal models of arthritis. What have we learned? J Rheumatol Suppl. 2005;72:7–9. [PubMed] [Google Scholar]
- 3.Shealy DJ, Wooley PH, Emmell E, Volk A, Rosenberg A, Treacy G, et al. Anti-TNF-alpha antibody allows healing of joint damage in polyarthritic transgenic mice. Arthritis Res. 2002;4(5) doi: 10.1186/ar430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li P, Schwarz EM, O’Keefe RJ, Ma L, Boyce BF, Xing L. RANK signaling is not required for TNFalpha-mediated increase in CD11(hi) osteoclast precursors but is essential for mature osteoclast formation in TNFalpha-mediated inflammatory arthritis. J Bone Miner Res. 2004;19(2):207–13. doi: 10.1359/JBMR.0301233. [DOI] [PubMed] [Google Scholar]
- 5.Conaghan PG, McQueen FM, Peterfy CG, Lassere MN, Ejbjerg B, Bird P, et al. The evidence for magnetic resonance imaging as an outcome measure in proof-of-concept rheumatoid arthritis studies. J Rheumatol. 2005;32(12):2465–9. [PubMed] [Google Scholar]
- 6.Haavardsholm EA, Ostergaard M, Ejbjerg BJ, Kvan NP, Uhlig TA, Lilleas FG, et al. Reliability and sensitivity to change of the OMERACT rheumatoid arthritis magnetic resonance imaging score in a multireader, longitudinal setting. Arthritis Rheum. 2005;52(12):3860–7. doi: 10.1002/art.21493. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Martinez MU, Cuevas-Orta E, Reyes-Vaca G, Baranda L, Gonzalez-Amaro R, Abud-Mendoza C. Magnetic resonance imaging in patients with rheumatoid arthritis with complete remission treated with disease-modifying antirheumatic drugs or anti-tumour necrosis factor alpha agents. Ann Rheum Dis. 2007;66(1):134–5. doi: 10.1136/ard.2006.056432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McQueen FM. Magnetic resonance imaging in early inflammatory arthritis: what is its role? Rheumatology (Oxford) 2000;39(7):700–6. doi: 10.1093/rheumatology/39.7.700. [DOI] [PubMed] [Google Scholar]
- 9.Kirkham BW, Lassere MN, Edmonds JP, Juhasz KM, Bird PA, Lee CS, et al. Synovial membrane cytokine expression is predictive of joint damage progression in rheumatoid arthritis: a two-year prospective study (the DAMAGE study cohort) Arthritis Rheum. 2006;54(4):1122–31. doi: 10.1002/art.21749. [DOI] [PubMed] [Google Scholar]
- 10.McQueen FM, Ostendorf B. What is MRI bone oedema in rheumatoid arthritis and why does it matter? Arthritis Res Ther. 2006;8(6):222. doi: 10.1186/ar2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dohn UM, Ejbjerg BJ, Court-Payen M, Hasselquist M, Narvestad E, Szkudlarek M, et al. Are bone erosions detected by magnetic resonance imaging and ultrasonography true erosions? A comparison with computed tomography in rheumatoid arthritis metacarpophalangeal joints. Arthritis Res Ther. 2006;8(4):R110. doi: 10.1186/ar1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benton N, Stewart N, Crabbe J, Robinson E, Yeoman S, McQueen FM. MRI of the wrist in early rheumatoid arthritis can be used to predict functional outcome at 6 years. Ann Rheum Dis. 2004;63(5):555–61. doi: 10.1136/ard.2003.011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGonagle D, Conaghan PG, O’Connor P, Gibbon W, Green M, Wakefield R, et al. The relationship between synovitis and bone changes in early untreated rheumatoid arthritis: a controlled magnetic resonance imaging study. Arthritis Rheum. 1999;42(8):1706–11. doi: 10.1002/1529-0131(199908)42:8<1706::AID-ANR20>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 14.Ostergaard M, Hansen M, Stoltenberg M, Gideon P, Klarlund M, Jensen KE, et al. Magnetic resonance imaging-determined synovial membrane volume as a marker of disease activity and a predictor of progressive joint destruction in the wrists of patients with rheumatoid arthritis. Arthritis Rheum. 1999;42(5):918–29. doi: 10.1002/1529-0131(199905)42:5<918::AID-ANR10>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 15.McQueen FM, Benton N, Perry D, Crabbe J, Robinson E, Yeoman S, et al. Bone edema scored on magnetic resonance imaging scans of the dominant carpus at presentation predicts radiographic joint damage of the hands and feet six years later in patients with rheumatoid arthritis. Arthritis Rheum. 2003;48(7):1814–27. doi: 10.1002/art.11162. [DOI] [PubMed] [Google Scholar]
- 16.Appel H, Loddenkemper C, Grozdanovic Z, Ebhardt H, Dreimann M, Hempfing A, et al. Correlation of histopathological findings and magnetic resonance imaging in the spine of patients with ankylosing spondylitis. Arthritis Res Ther. 2006;8(5):R143. doi: 10.1186/ar2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaffney K, Cookson J, Blades S, Coumbe A, Blake D. Quantitative assessment of the rheumatoid synovial microvascular bed by gadolinium-DTPA enhanced magnetic resonance imaging. Ann Rheum Dis. 1998;57(3):152–7. doi: 10.1136/ard.57.3.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clunie G, Hall-Craggs MA, Paley MN, King A, Wilkinson ID, Ell PJ, et al. Measurement of synovial lining volume by magnetic resonance imaging of the knee in chronic synovitis. Ann Rheum Dis. 1997;56(9):526–34. doi: 10.1136/ard.56.9.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munasinghe JP, Tyler JA, Hodgson RJ, Barry MA, Gresham GA, Evans R, et al. Magnetic resonance imaging, histology, and x-ray of three stages of damage to the knees of STR/ORT mice. Invest Radiol. 1996;31(10):630–8. doi: 10.1097/00004424-199610000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Kapadia RD, Stroup GB, Badger AM, Koller B, Levin JM, Coatney RW, et al. Applications of micro-CT and MR microscopy to study pre-clinical models of osteoporosis and osteoarthritis. Technol Health Care. 1998;6(5–6):361–72. [PubMed] [Google Scholar]
- 21.Dardzinski BJ, Schmithorst VJ, Holland SK, Boivin GP, Imagawa T, Watanabe S, et al. MR imaging of murine arthritis using ultrasmall superparamagnetic iron oxide particles. Magn Reson Imaging. 2001;19(9):1209–16. doi: 10.1016/s0730-725x(01)00448-9. [DOI] [PubMed] [Google Scholar]
- 22.Saidenberg-Kermanac HN, Leclercq F, Herscovici J, Scherman D, Boissier MC. High-resolution magnetic resonance imaging of knee inflammatory arthritis in mice. Clin Exp Rheumatol. 2006;24(2):216–7. [PubMed] [Google Scholar]
- 23.Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaris E, Kioussis D, et al. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. Embo J. 1991;10(13):4025–31. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldmann M, Maini RN. Lasker Clinical Medical Research Award. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat Med. 2003;9(10):1245–50. doi: 10.1038/nm939. [DOI] [PubMed] [Google Scholar]
- 25.Li P, Schwarz EM. The TNF-alpha transgenic mouse model of inflammatory arthritis. Springer Semin Immunopathol. 2003;25(1):19–33. doi: 10.1007/s00281-003-0125-3. [DOI] [PubMed] [Google Scholar]
- 26.Redlich K, Hayer S, Maier A, Dunstan CR, Tohidast-Akrad M, Lang S, et al. Tumor necrosis factor alpha-mediated joint destruction is inhibited by targeting osteoclasts with osteoprotegerin. Arthritis Rheum. 2002;46(3):785–92. doi: 10.1002/art.10097. [DOI] [PubMed] [Google Scholar]
- 27.Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17(5):545–80. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- 28.Lassere MN, Bird P. Measurements of rheumatoid arthritis disease activity and damage using magnetic resonance imaging. Truth and discrimination: does MRI make the grade? J Rheumatol. 2001;28(5):1151–7. [PubMed] [Google Scholar]
- 29.Farrant JM, O’Connor PJ, Grainger AJ. Advanced imaging in rheumatoid arthritis : Part 1: Synovitis. Skeletal Radiol. 2007;36(4):269–79. doi: 10.1007/s00256-006-0219-9. [DOI] [PubMed] [Google Scholar]
- 30.Ostergaard M, Peterfy C, Conaghan P, McQueen F, Bird P, Ejbjerg B, et al. OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging Studies. Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system. J Rheumatol. 2003;30(6):1385–6. [PubMed] [Google Scholar]
- 31.Ostergaard M, Stoltenberg M, Lovgreen-Nielsen P, Volck B, Jensen CH, Lorenzen I. Magnetic resonance imaging-determined synovial membrane and joint effusion volumes in rheumatoid arthritis and osteoarthritis: comparison with the macroscopic and microscopic appearance of the synovium. Arthritis Rheum. 1997;40(10):1856–67. doi: 10.1002/art.1780401020. [DOI] [PubMed] [Google Scholar]
- 32.Ostergaard M. Different approaches to synovial membrane volume determination by magnetic resonance imaging: manual versus automated segmentation. Br J Rheumatol. 1997;36(11):1166–77. doi: 10.1093/rheumatology/36.11.1166. [DOI] [PubMed] [Google Scholar]
- 33.Palmer WE, Rosenthal DI, Schoenberg OI, Fischman AJ, Simon LS, Rubin RH, et al. Quantification of inflammation in the wrist with gadolinium-enhanced MR imaging and PET with 2-[F-18]-fluoro-2-deoxy-D-glucose. Radiology. 1995;196(3):647–55. doi: 10.1148/radiology.196.3.7644624. [DOI] [PubMed] [Google Scholar]
- 34.Klarlund M, Ostergaard M, Lorenzen I. Finger joint synovitis in rheumatoid arthritis: quantitative assessment by magnetic resonance imaging. Rheumatology (Oxford) 1999;38(1):66–72. doi: 10.1093/rheumatology/38.1.66. [DOI] [PubMed] [Google Scholar]
- 35.Herrak P, Gortz B, Hayer S, Redlich K, Reiter E, Gasser J, et al. Zoledronic acid protects against local and systemic bone loss in tumor necrosis factor-mediated arthritis. Arthritis Rheum. 2004;50(7):2327–37. doi: 10.1002/art.20384. [DOI] [PubMed] [Google Scholar]
- 36.Li P, Schwarz EM, O’Keefe RJ, Ma L, Looney RJ, Ritchlin CT, et al. Systemic tumor necrosis factor alpha mediates an increase in peripheral CD11bhigh osteoclast precursors in tumor necrosis factor alpha-transgenic mice. Arthritis Rheum. 2004;50(1):265–76. doi: 10.1002/art.11419. [DOI] [PubMed] [Google Scholar]
- 37.Mangialaio S, Ji H, Korganow AS, Kouskoff V, Benoist C, Mathis D. The arthritogenic T cell receptor and its ligand in a model of spontaneous arthritis. Arthritis Rheum. 1999;42(12):2517–23. doi: 10.1002/1529-0131(199912)42:12<2517::AID-ANR3>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 38.Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402(6759):304–9. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 39.Pettit AR, Ji H, von Stechow D, Muller R, Goldring SR, Choi Y, et al. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am J Pathol. 2001;159(5):1689–99. doi: 10.1016/S0002-9440(10)63016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Redlich K, Hayer S, Ricci R, David JP, Tohidast-Akrad M, Kollias G, et al. Osteoclasts are essential for TNF-alpha-mediated joint destruction. J Clin Invest. 2002;110(10):1419–27. doi: 10.1172/JCI15582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Lent PL, Grevers L, Lubberts E, de Vries TJ, Nabbe KC, Verbeek S, et al. Fcgamma receptors directly mediate cartilage, but not bone, destruction in murine antigen-induced arthritis: uncoupling of cartilage damage from bone erosion and joint inflammation. Arthritis Rheum. 2006;54(12):3868–77. doi: 10.1002/art.22253. [DOI] [PubMed] [Google Scholar]
- 42.Jimenez-Boj E, Nobauer-Huhmann I, Hanslik-Schnabel B, Dorotka R, Wanivenhaus AH, Kainberger F, et al. Bone erosions and bone marrow edema as defined by magnetic resonance imaging reflect true bone marrow inflammation in rheumatoid arthritis. Arthritis Rheum. 2007;56(4):1118–24. doi: 10.1002/art.22496. [DOI] [PubMed] [Google Scholar]
- 43.Braun J, Landewe R, Hermann KG, Han J, Yan S, Williamson P, et al. Major reduction in spinal inflammation in patients with ankylosing spondylitis after treatment with infliximab: results of a multicenter, randomized, double-blind, placebo-controlled magnetic resonance imaging study. Arthritis Rheum. 2006;54(5):1646–52. doi: 10.1002/art.21790. [DOI] [PubMed] [Google Scholar]
- 44.van der Heijde D, Han C, DeVlam K, Burmester G, van den Bosch F, Williamson P, et al. Infliximab improves productivity and reduces workday loss in patients with ankylosing spondylitis: results from a randomized, placebo-controlled trial. Arthritis Rheum. 2006;55(4):569–74. doi: 10.1002/art.22097. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.