Abstract
A series of compounds structurally related to pramipexole were designed, synthesized and evaluated as ligands for the dopamine 3 (D3) receptor. Compound 12 has a Ki value of 0.41 nM to D3 and a selectivity of >30,000- and 800-fold over the D1-like and D2 receptors, respectively. Our in vivo functional assays showed that this compound is a partial agonist at the D3 receptor with no detectable activity at the D2 receptor.
Dopaminergic neurotransmission is mediated by five dopamine receptors (D1–D5), which can be grouped into the D1-like (D1 and D5) and D2-like (D2, D3 and D4) receptor subtypes. Recent studies have suggested that the D3 receptor is a promising therapeutic target for a variety of conditions, including drug abuse, restless legs syndrome, schizophrenia, Parkinson’s disease, and depression.1–6 Considerable effort has been devoted in recent years to the discovery and development of potent and selective D3 ligands.6–22
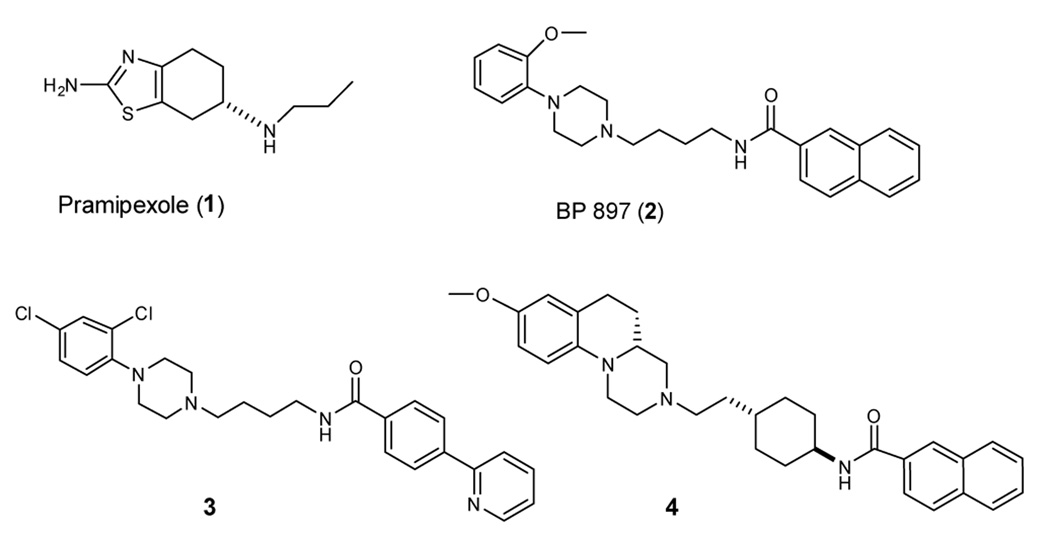
Despite intense research efforts, design of truly selective D3 ligands with good solubility and bioavailability remains a challenge. Compound 1 (pramipexole) is a potent D3–preferring agonist but has limited selectivity over the D2 receptor in vitro23 and in vivo.24,25 Compound 2 was initially reported as a D3 partial agonist and has a 67-fold selectivity over the D2 receptor.2 A number of potent and selective D3 ligands, such as 3, have been designed based upon the core structure of 2.17 Our laboratory has reported the design of 4 as a potent and selective D3 ligand using the hexahydropyrazinoquinoline as the core structure.21 Despite its relatively high affinity and excellent selectivity for D3 over other dopamine receptor subtypes, 4 has a poor aqueous solubility, which limits its in vivo evaluations. The poor aqueous solubility is also a major limitation for many recently described potent and selective D3 ligands and an obstacle for evaluation of these novel agents in behavioral models in animals and their therapeutic potential.
To overcome this major limitation, we investigated other core structures for the design of potent and selective D3 ligands. Among them, the core structure in 1 has a number of very attractive features. First, 1 itself is a very potent D3 ligand and has a Ki value of 0.78 nM to D3 in our binding assay (Table 1). Second and importantly, 1 has an excellent aqueous solubility. Third, pramipexole dihydrochloride has been approved for the treatment of Parkinson's disease and restless legs syndrome and has an excellent pharmacological and toxicological profile in humans and in animals. Hence, 1 represents a particularly attractive template for the design of potent and selective D3 ligands with desirable physiochemical and pharmacological properties. Of note, although 1 has been widely used as a D3 preferring ligand, it potently binds to the high affinity state of the D2 receptor with a Ki value of 3.1 nM in our binding assays (Table 1), thus displaying only a 4-fold selectivity for the D3 receptor over the D2 receptor.
Table 1.
Binding affinities at the D1-like, D2 and D3 receptors in binding assays using rat brain. Data represent the mean ± SEM of 3–5 independent determinations. For compounds producing a 2-site fit in competition with [3H]-spiperone, Ki values are presented for the high and low affinity components and are indicated by the designation “(h)” or “(l)”. All other Ki values are based on a single-site model.
| Ki ± SEM (nM) | Selectivity | ||||
|---|---|---|---|---|---|
| Ligand | D3[3H]PD128 907 | D2 [3H]Spiperone | D1-like [3H]SCH23390 | D2–like /D3 | D1–like /D3 |
| 1 | 0.78 | 3.1 ± 0.3 (h) 6400 ± 1700 (l) |
>100,000 | 4.0 | >100,000 |
| 4 | 5.7 ± 0.4 | >10000 | >50000 | >1000 | >5000 |
| 5 | 0.043 ± 0.006 | 2.7 ± 0.4 (h) 6700 ± 1500 (l) |
11,000 ± 500 | 62 | >100,000 |
| 6 | 0.40 ± 0.057 | 307 ± 38 | 3,400 ± 300 | 763 | >7,000 |
| 7 | 0.74 ± 0.083 | 55 ± 12 (h) 1300 ± 180 (l) |
5,400 ± 500 | 74 | >7,000 |
| 8 | 2.2 ± 0.10 | 345 ± 33 | 13,000 ± 1,000 | 157 | >5,000 |
| 9 | 23 ± 2.7 | 1,200 ± 170 | 4,400 ± 800 | 53 | 194 |
| 10 | 7.6 ± 0.87 | 670 ± 140 | 64,000 ± 7,000 | 88 | >8,000 |
| 11 | 0.51 ± 0.10 | 68 ± 4.6 | 4,900 ± 600 | 133 | >9,000 |
| 12 | 0.41 ± 0.031 | 330 ± 69 | 13,000 ± 1,700 | 800 | >30,000 |
Recently, the crystal structures for the human β2 adrenergic (β2AD) G-protein coupled receptor (GPCR) were solved.26,27 We have modeled the human D3 receptor structure based upon the high-resolution crystal structures of β2AD receptor since these two proteins belong to the same GPCR sub-family 28 and share close sequence homology. Because the crystal structure of β2AD receptor was solved with an inverse agonist bound to it, our modeled D3 structure likely represents the conformational state bound to either antagonists or inverse agonists. Hence, care must be taken when using the structure to model the interactions of the D3 receptor with its ligands with different intrinsic functions. Nevertheless, we reasoned the modeled human D3 structure based upon the very first human GPCR structure could be useful to guide the design of novel D3 ligands.
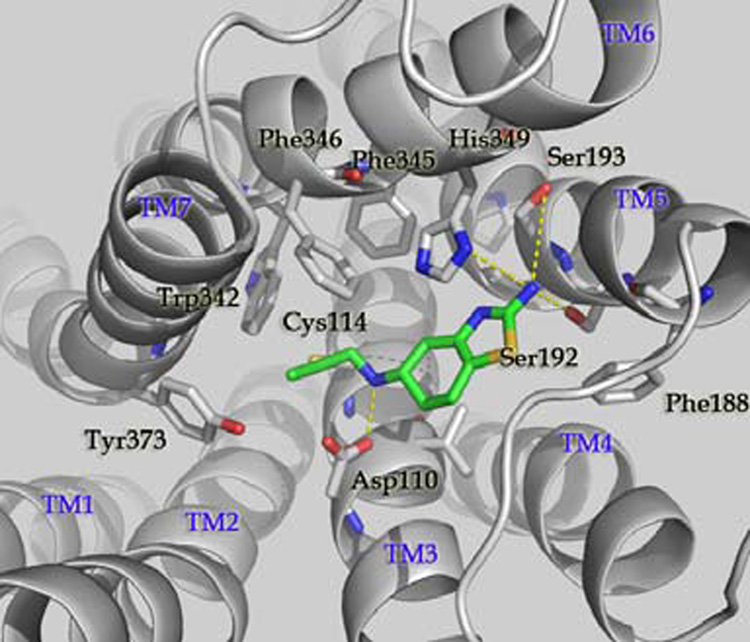
To this end, we modeled the binding of 1 to the D3 receptor structure through computational docking, followed by extensive refinement (Supporting Information). The predicted model (Figure 3) showed that the primary amino group in the thiazol ring of 1 forms a hydrogen bonding network with the hydroxyl groups of Ser192 and Ser193. The thiazol ring in 1 is parallel to the imidazole ring in His349, making favorable π-π stacking interaction. The protonated nitrogen in 1 forms a salt bridge with the negatively charged Asp110. The n-propyl group in 1 inserts into a hydrophobic channel formed by Cys114, Phe345, Phe346, Trp342 and Try373.
Figure 3.
Predicted binding model of compound 1 to the human D3 receptor. For protein, carbon atoms of human D3 are shown in white, oxygen atoms in red, and nitrogen atoms in blue. Side chains of crucial residues in the binding site are shown as stick and labeled. Hydrogen bonds between 1 and D3 are depicted in dotted line in yellow. Figures were generated by Pymol.
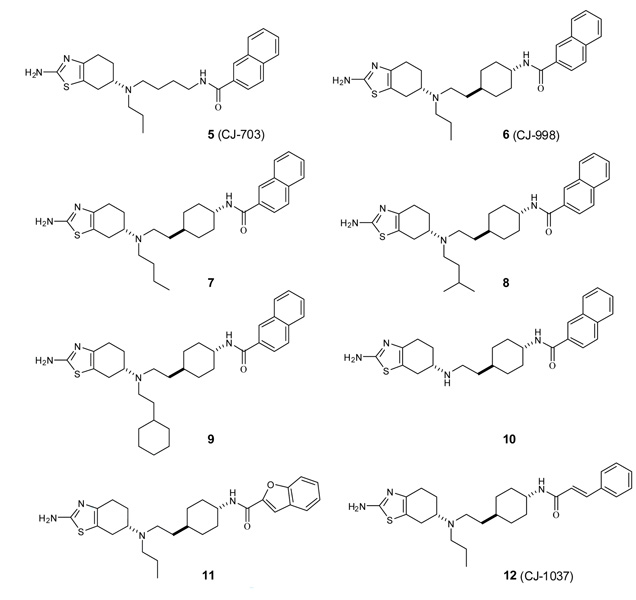
The predicted model of 1 in complex with the D3 receptor suggested that there is ample room available to accommodate a much larger hydrophobic group where the n-propyl group in 1 binds. Interestingly, in the adjacent area, there is another well-defined but smaller hydrophobic cavity formed by Cys114, Phe197 and Trp342 residues. We have thus designed and synthesized compound 5 to explore the interactions with these two pockets.
Compound 5 was tested for its binding affinities to the dopamine receptors using the same methods as described previously (Table 1).21 It was found that 5 has a Ki value of 0.043 nM to the D3 receptor, being 18-times more potent than 1. Compound 5, however, also potently binds to the high affinity state of the D2 receptor with a Ki value of 2.7 nM, thus displaying a 62-fold selectivity for the D3 receptor over the D2 receptor. Similar to 1, 5 has a weak affinity to the D1-like receptors and has a Ki value of 11,000 nM. Hence, although 5 has a very high affinity to the D3 receptor, its selectivity over the D2 receptor is modest.
In our previous design of 4, we have shown that introduction of a trans-cyclohexyl group into the linker region yielded new ligands with much improved selectivity for the D3 receptor over the D2 receptor as compared to a linear 4-carbon linker.21 We have thus designed compound 6 to investigate if introduction of this rigid cyclohexyl group into 5 may also improve the selectivity. Compound 6 binds to the D3 and D2 receptors with Ki values of 0.40 nM and 307 nM, respectively. Hence, 6 is a potent D3 ligand and displays an excellent selectivity of 763-fold for the D3 receptor over the D2 receptor.
We next designed and synthesized compounds 7–10 to investigate the importance of the n-propyl group in 6 for binding and selectivity. Compound 7 with an n-butyl group has a slightly weaker affinity for the D3 receptor than 6 and exhibited a 2-site competition curve at the D2 receptor, with roughly 10-fold less selectivity for the D3 receptor over the D2 receptor with the high affinity binding component. Compound 8 with an isopentyl group is 5-times less potent than 6 to the D3 receptor but has a similar binding affinity to the D2 receptor. Compound 9 with a bulky cyclohexylethyl group is 55-times less potent than 6 to the D3 receptor but is only 3-times less potent than 6 to the D2 receptor. Compound 10 with a hydrogen atom at this site has a Ki value of 7.6 nM to the D3 receptor, being 19-times less potent than 6, but their binding affinities to the D2 receptor are essentially the same. Therefore, our binding data clearly showed that the substitution on this nitrogen atom has a major effect on the binding to the D3 receptor but modest influence on the binding to the D2 receptor. Our data also showed that the n-propyl group in 6 enhances the binding affinity to the D3-receptor by 19-fold as compared to a hydrogen atom in 10.
We next investigated the influence of the naphthyl group in 6 for binding and selectivity. Compound 11, in which the naphthyl group is replaced by a 2-benzofuran, binds to the D3 receptor with the same affinity (Ki = 0.51 nM) as 6 but its selectivity over the D2 receptor is decreased to 133-fold, due to its increased binding affinity to the D2 receptor. Compound 12, in which a cinnamyl group is used to replace the naphthyl, retains a high binding affinity for the D3-receptor (Ki = 0.41 nM) and displays 800- and >30,000-fold selectivity over the D2 and D1-like receptors. These data suggested that the modifications of the naphthyl group can have a significant effect on the selectivity, and this region should be further investigated for the design of potent and selective D3 ligands.
The synthesis of compounds 5–12 is provided in the Supporting Information.
Compounds 5, 6 and 12 were found to have good aqueous solubility. For example, the dihydrochloride salt form of 6 has an aqueous solubility greater than 100 mg/ml. Their excellent aqueous solubility provided us with an opportunity to evaluate their in vivo functional profiles in animals.
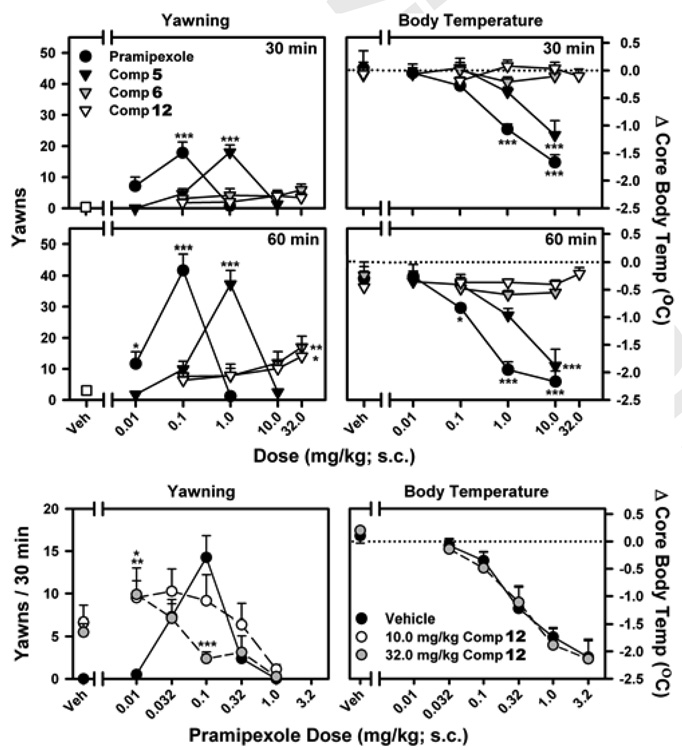
Another challenge in the development of selective D3 ligands was that the current in vitro functional assays for the D3 receptor are not predictive of the in vivo function of D3 ligands.6 Furthermore, there was also the lack of a robust in vivo functional assay for the D3 receptor. To addresses these challenges, we have recently validated in vivo functional assays for the D3 and D2 receptors.24,25 Our studies showed that yawning in rats provides a sensitive measure of in vivo agonist activity at the dopamine D3 receptor, 24,25 while the inuction of hypothermia has been shown to be mediated by agonist activity at the D2 receptor.32–33 Employing these well validated assays, we evaluated 5, 6 and 12 for their in vivo functional activity at the D3 and D2 receptors. Compound 1, a known D3 and D2 agonist, was used as a control in our evaluations. The results are shown in Figure 4.
Figure 4.
Functional evaluations of the D3 and D2 activity of pramipexole, compounds 5, 6 and 12 in yawning and hypothermia assays in rats. Top and middle panels: Induction of yawning or hypothermia by D3 ligands. Bottom panels: Interactions between pramipexole and compound 12 in yawning and hypothermia assays.
Consistent with the data obtained in previous studies,24,25 increases in yawning were observed over low doses (0.01 to 0.1 mg/kg) of 1 with inhibition of yawning and the induction of hypothermia occurring at higher doses. These data indicate that 1 functions as a preferential D3 agonist in vivo and a D2 agonist at higher doses.
Compound 5 induced yawning and produced an inverted U-shaped dose-response curve. The maximum levels of yawning induced by 5 are very similar to that induced by 1. Furthermore, hypothermia was induced by 5 at higher doses, concurrent with deceases in yawning. These data showed that 5 functions as a full agonist at the D3 and D2 receptors in vivo, consistent with the 2-site competition curve observed in the [3H]spiperone binding assay for 1 and 5 (Supporting Information). Furthermore, the in vivo data suggested that 5 is bioavailable.
Unlike 1 and 5, the dose-response curves for 6 and 12 induced yawning were relatively flat, and failed to reach significance during the initial 30 min observation period. While significant levels of yawning induced by 6 and 12 were observed after 60 min, the dose-response curves for both compounds remained relatively flat. Moreover, 6 and 12 failed to induce changes in body temperature over the initial hour of observation, an effect that is indicative of D2 agonist activity. Together, the low levels of yawning, combined with the absence of any hypothermic effect suggested two possibilities: (1) 6 and 12 function as weak partial agonists at the D3 receptor, with no detectable agonist activity at the D2 receptor; or (2) they are simply not bioavailable.
To investigate these two possibilities, we next evaluated the ability of 12 to alter compound 1-induced yawning and hypothermia and the data are shown in Figure 4. Similar to the effects of 12 alone, but unlike the effects of D3-selecitve antagonists,24,25 low levels of yawning were observed during the initial 30 min after administration of either 10.0 or 32.0 mg/kg of 12. Interestingly, this effect appeared to persist upon administration of low doses of 1 as significant increases in yawning were observed when rats were pretreated with 12 (10.0 or 32.0 mg/kg). However, 12 resulted in a dose-dependent decrease in the amount of yawning observed following the maximally effective dose of 1 at 0.1 mg/kg. No significant effects of 12 were observed at higher doses of 1 (0.32 and 1 mg/kg). These data suggested that 12 is capable of antagonizing the D3-mediated effects of 1. However, the profile of activity for 12 is different from that observed for selective D3 antagonists, which generally produce selective rightward and/or downward shifts of the ascending limb of the yawning dose-response curve for D3-preferring agonists without increasing the amount of yawning observed at low doses.24,25 In fact, the effects of 12 alone, and in combination with 1, suggest that it is more similar to the partial agonist, aripiprazole,34 than an antagonist. Moreover, 12 failed to alter the induction of hypothermia by 1, an effect that is indicative of D2 agonist activity, which can be reliably blocked by both selective and non-selective D2 antagonists.32,33 Together, our data provide evidence that 12 is a partial agonist at the D3 receptor with no detectable agonist or antagonist activity at the D2 receptor, thus possessing a novel in vivo functional profile.
In summary, a series of enantiomerically pure pramipexole derivatives have been designed, synthesized, and evaluated for their binding and selectivity to the D3, D1-like and D2 receptor. This led to the identification of several potent and highly selective D3 ligands with excellent aqueous solubility. Our in vivo functional evaluations showed that while 5 functions as a full D3 agonist, 12 behaves as a selective D3 partial agonist with no activity at the D2 receptor. Further in vivo studies are underway to evaluate the therapeutic potential of 12 for the treatment of drug abuse and other indications. The results will be reported in due course.
Supplementary Material
Experimental details of computational modeling, synthesis, and in vivo characterizations of the ligands. This material is available free of charge via the Internet at http://pubs.acs.org.
Figure 1.
Chemical structures of representative D3 ligands.
Figure 2.
New analogues structurally related to pramipexole.
Acknowledgment
This work was supported by a grant from the National Institute of Drug Abuse, National Institutes of Health (R01DA020669).
Abbreviations
- D1–5
dopamine 1–5 receptor subtypes
- β2AD
the human β2 adrenergic receptor
- GPCR
G-protein coupled receptor.
References
- 1.Joyce JN. Dopamine D3 receptor as a therapeutic target for antipsychotic and antiparkinsonian drugs. Pharmacol. Ther. 2001;90:231–259. doi: 10.1016/s0163-7258(01)00139-5. [DOI] [PubMed] [Google Scholar]
- 2.Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, Schwartz J-C, Everitt BJ, Sokoloff P. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature. 1999;400:371–375. doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- 3.Koob GF, Caine SB. Cocaine addition therapy- Are we partially there? Nature Medicine. 1999;5:993–995. doi: 10.1038/12429. [DOI] [PubMed] [Google Scholar]
- 4.Montplaisir J, Nicolas A, Denesle R, Gomez-Mancilla B. Restless legs syndrome improved by pramipexole: a double-blind randomized trial. Neurology. 1999;52:938–943. doi: 10.1212/wnl.52.5.938. [DOI] [PubMed] [Google Scholar]
- 5.Levant B. The D3 dopamine receptor: neurobiology and potential clinical relevance. Pharmacol. Rev. 1997;49:231–252. [PubMed] [Google Scholar]
- 6.Newman AH, Grundt P, Nader MA. Dopamine D3 Receptor Partial Agonists and Antagonists as Potential Drug Abuse Therapeutic Agents. J. Med. Chem. 2005;48:3663–3679. doi: 10.1021/jm040190e. [DOI] [PubMed] [Google Scholar]
- 7.Grundt P, Prevatt KM, Cao J, Taylor M, Floresca CZ, Choi J-K, Jenkins BG, Luedtke RR, Newman AH. Heterocyclic Analogues of N-(4-(4-(2,3-Dichlorophenyl)piperazin-1-yl)butyl)arylcarboxamides with Functionalized Linking Chains as Novel Dopamine D3 Receptor Ligands: Potential Substance Abuse Therapeutic Agents. J. Med. Chem. 2007;50:4135–4146. doi: 10.1021/jm0704200. [DOI] [PubMed] [Google Scholar]
- 8.Wustrow DJ, Wise LD, Cody DM, MacKenzie RG, Georgic LM, Pugsley TA, Heffner TG. Studies of the active conformation of a novel series of benzamide dopamine D2 agonists. J. Med. Chem. 1994;37:4251–4257. doi: 10.1021/jm00050a022. [DOI] [PubMed] [Google Scholar]
- 9.Wustrow D, Belliotti T, Glase S, Kesten SR, Johnson D, Colbry N, Rubin R, Blackburn A, Akunne H, Corbin A, Davis MD, Georgic L, Whetzel S, Zoski K, Heffner T, Pugsley T, Wise L. Aminopyrimidines with high affinity for both serotonin and dopamine receptors. J. Med. Chem. 1998;41:760–771. doi: 10.1021/jm9707378. [DOI] [PubMed] [Google Scholar]
- 10.Belliotti TB, Kesten SR, Wustrow DJ, Geogric LM, Zoski KT, Akunne HC, Wise LD. Novel cyclohexyl amides as potent and selective D3 dopamine receptor ligands. Bioorg. Med. Chem. Letts. 1997;7:2403–2408. [Google Scholar]
- 11.Robarge MJ, Husbands SM, Kieltyka A, Brodbeck R, Thurkauf A, Newman AH. Design and synthesis of [(2,3-dichlorophenyl)piperazin-1-yl] alkylfluorenylcarboxamides as novel ligands selective for the dopamine D3 receptor subtype. J. Med. Chem. 2001;44:3175–3186. doi: 10.1021/jm010146o. [DOI] [PubMed] [Google Scholar]
- 12.Bettinetti L, Schlotter K, Hubner H, Gmeiner P. Interactive SAR studies: rational discovery of super-potent and highly selective D3 receptor antagonistsand partial agonists. J. Med. Chem. 2002;45:4594–4597. doi: 10.1021/jm025558r. [DOI] [PubMed] [Google Scholar]
- 13.Hackling A, Ghosh R, Perachon S, Mann A, Holtje HD, Wermuth CG, Schwartz JC, Sippl W, Sokoloff P, Stark H. N-(ö-(4-(2-Methoxyphenyl)piperazin-1-yl)alkyl) carboxamides as dopamine D2 and D3 receptor ligands. J. Med. Chem. 2003;46:3883–3899. doi: 10.1021/jm030836n. [DOI] [PubMed] [Google Scholar]
- 14.Leopoldo M, Berardi F, Colabufo NA, De Giorgio P, Lacivita E, Perrone R, Tortorella V. Structure-affinity relationship study on N-[4-(4-arylpiperazin-1-yl)butyl] arylcarboxamides as potent and selective dopamine D3 receptor ligands. J. Med. Chem. 2002;45:5727–5735. doi: 10.1021/jm020952a. [DOI] [PubMed] [Google Scholar]
- 15.Campiani G, Butini S, Trotta F, Fattorusso C, Catalanotti B, Aiello F, Gemma S, Nacci V, Novellino E, Stark JA, Cagnotto A, Fumagalli E, Carnovali F, Cervo L, Mennini T. Synthesis and pharmacological evaluation of potent and highly selective D3 receptor ligands: inhibition of cocaine-seeking behavior and the role of dopamine D3/D2 receptors. J. Med. Chem. 2003;46:3822–3839. doi: 10.1021/jm0211220. [DOI] [PubMed] [Google Scholar]
- 16.Campiani G, Butini S, Fattorusso C, Catalanotti B, Gemma S, Nacci V, Morelli E, Cagnotto A, Mereghetti I, Mennini T, Carli M, Minetti P, Di Cesare MA, Mastroianni D, Scafetta N, Galletti B, Stasi MA, Castorina M, Pacifici L, Vertechy M, Serio SD, Ghirardi O, Tinti O, Carminati P. Pyrrolo[1,3]benzothiazepine-based serotonin and dopamine receptor antagonists: molecular modeling, further structure activity relationship studies, and identification of novel atypical antipsychotic agents. J. Med. Chem. 2004;47:143–157. doi: 10.1021/jm0309811. [DOI] [PubMed] [Google Scholar]
- 17.Grundt P, Carlson EE, Cao J, Bennett CJ, McElveen E, Taylor M, Luedtke RR, Newman AH. Novel Heterocyclic Trans Olefin Analogues of N-{4-[4-(2,3-Dichloro phenyl)piperazin-1-yl]butyl}arylcarboxamides as Selective Probes with High Affinity for the Dopamine D3 Receptor. J. Med. Chem. 2005;48:839–848. doi: 10.1021/jm049465g. [DOI] [PubMed] [Google Scholar]
- 18.Varady J, Wu X, Fang X, Ji M, Hu Z, Levant B, Wang S. Molecular modeling of the three-dimensional structure of dopamine 3 subtype receptor. Discovery of novel and potent D3 ligands through a hybrid pharmacophore- and structure-based database searching approach. J. Med. Chem. 2003;46:4377–4392. doi: 10.1021/jm030085p. [DOI] [PubMed] [Google Scholar]
- 19.Haadsma-Svensson SR, Cleek KA, Dinh MD, Duncan JN, Haber CL, Huff RM, Lajiness ME, Nichols NF, Smith MW, Sevensson KA, Zaya MJ, Carlsson A, Lin C-H. Dopamine D3 receptor antagonists. 1. Synthesis and structure-activity relationship of 5,6-dimethoxyl-N-alkyl- and N-alkylaryl-substituted 2-aminoindans. J. Med. Chem. 2001;44:4716–4732. doi: 10.1021/jm010145w. [DOI] [PubMed] [Google Scholar]
- 20.Ji M, Chen J, Ding K, Wu X, Varady J, Levant B, Wang S. Design, synthesis and structure-activity relationship studies of hexahydropyrazinoquinolines as a novel class of potent and selective dopamine receptor 3 (D3) ligands. Bioorg. Med. Chem. Lett. 2005;15:1701–1705. doi: 10.1016/j.bmcl.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 21.Ding K, Chen J, Ji M, Wu X, Varady J, Yang C, Lu Y, Deschamps JR, Levant B, Wang S. Enantiomerically Pure Hexahydropyrazinoquinolines as Potent and Selective Dopamine 3 Subtype Receptor Ligands. J. Med. Chem. 2005;48:3171–3181. doi: 10.1021/jm049031l. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Ding K, Levant B, Wang S. Design of Novel Hexahydropyrazinoquinolines as Potent and Selective Dopamine D3 Receptor Ligands with Improved Solubility. Bioorg. Med. Chem. Lett. 2006;16:443–446. doi: 10.1016/j.bmcl.2005.09.053. [DOI] [PubMed] [Google Scholar]
- 23.Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin JA, Newman-Tancredi A. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes. J. Pharmacol. Exp. Ther. 2002;303:791–804. doi: 10.1124/jpet.102.039867. [DOI] [PubMed] [Google Scholar]
- 24.Collins GT, Witkin JM, Newman AH, Svensson KA, Grundt P, Cao J, Woods JH. Dopamine agonist-induced yawning in rats: a dopamine D3 receptor-mediated behavior. J. Pharmacol. Exp. Ther. 2005;314:310–319. doi: 10.1124/jpet.105.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins GT, Newman AH, Grundt P, Rice KC, Husbands SM, Chauvignac C, Chen J, Wang S, Woods JH. Yawning and hypothermia in rats: effects of dopamine D3 and D2 agonists and antagonists. Psychopharmacology (Berl) 2007;193:159–170. doi: 10.1007/s00213-007-0766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC. High resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VR, Sanishvili R, Fischetti RF, Schertler GF, Weis WI, Kobilka BK. Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature. 2007;450:383–387. doi: 10.1038/nature06325. [DOI] [PubMed] [Google Scholar]
- 28.Kenakin T. The classification of seven transmembrane receptors in recombinant expression systems. Pharmacol. Rev. 1996;48:413–463. [PubMed] [Google Scholar]
- 29.Levant B, Grigoriadis DE, De Souza EB. Characterization of [3H]quinpirole binding to D2-like dopamine receptors in rat brain. J. Pharmacol. Exp. Ther. 1992;262:929–935. [PubMed] [Google Scholar]
- 30.Bancroft GN, Morgan KA, Flietstra RJ, Levant B. Binding of [3H]PD 128907, a putatively selective ligand for the D3 dopamine receptor, in rat brain: a receptor binding and quantitative autoradiographic study. Neuropsychopharmacology. 1998;18:305–316. doi: 10.1016/S0893-133X(97)00162-0. [DOI] [PubMed] [Google Scholar]
- 31.Levant B. Characterization of dopamine receptors. In: Enna SJ, Williams M, Ferkany JW, Kenakin T, Porsolt RD, Sullivan JP, editors. Current Protocols in Pharmacology. New York: John Wiley & Sons; 1998. pp. 1.6.1–1.6.16. [Google Scholar]
- 32.Boulay D, Depoortere R, Perrault G, Borrelli E, Sanger DJ. Dopamine D2 receptor knock-out mice are insensitive to the hypolocomotor and hypothermic effects of dopamine D2/D3 receptor agonists. Neuropharmacology. 1999;38:1389–1396. doi: 10.1016/s0028-3908(99)00064-7. [DOI] [PubMed] [Google Scholar]
- 33.Chaperon F, Tricklebank MD, Unger L, Neijt HC. Evidence for regulation of body temperature in rats by dopamine D2 receptor and possible influence of D1 but not D3 and D4 receptors. Neuropharmacology. 2003;44:1047–1053. doi: 10.1016/s0028-3908(03)00113-8. [DOI] [PubMed] [Google Scholar]
- 34.Fujikawa M, Nagashima M, Inoue T, Yamada K, Furukawa T. Partial agonistic effects of OPC-14597, a potential antipsychotic agent, on yawning behavior in rats. Pharmacol. Biochem. Behav. 1996;53:903–909. doi: 10.1016/0091-3057(95)02096-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental details of computational modeling, synthesis, and in vivo characterizations of the ligands. This material is available free of charge via the Internet at http://pubs.acs.org.