SYNOPSIS
Our understanding of estrogen signaling in the nervous system has undergone a significant shift in recent years. For over three decades, the idea that all estradiol actions were explained by direct regulation of transcription held sway. Within the past decade, the idea that in addition to classical effects, membrane-initiated actions of estradiol are important has gained traction. And while several novel putative membrane estrogen receptors (ERs) have been described, a large fraction of measured responses appear to be due to membrane-localized estrogen receptor-α (ERα) and estrogen receptor-β (ERβ), the same proteins that regulate gene expression. These membrane-localized ERs participate in the regulation of the synthesis of neuroprogesterone, dorsal root ganglion (DRG) neuron excitation, and female sexual receptivity. This is achieved by the modulation of intracellular cell signaling pathways usually associated with the activation of G protein-coupled receptors (GPCRs). ERα and ERβ are themselves not GPCRs that directly activate G proteins to regulate physiological responses, but rather interact with traditional GPCRs to initiate cell signaling. This review will present results that support a direct protein-protein interaction between ERα and ERβ with metabotropic glutamate receptors (mGluRs), allowing estradiol to signal through mGluRs. This ER/mGluR hypothesis explains how estradiol can activate a wide-range of intracellular pathways and provides an underlying mechanism for the hitherto seemingly unrelated rapid membrane actions in the nervous system.
Keywords: estradiol, mGluR, lordosis, membrane, rapid actions, estrogen receptors, neurosteroids
Introduction
The importance of steroid hormones in the regulation of nervous system function cannot be overstated. Estrogens, in particular, have profound effects on a variety of neurological systems. The most obvious actions include those that are related to sexual differentiation of the brain (Gorski, 1985) and the central control of reproduction (Pfaff et al., 2000). More recently, estradiol has been shown to be critical in a number of cellular events, such as neuronal growth and restructuring, that impact cognition, long-term potentiation, neuroprotection and mood (Belcredito et al., 2001; Lee and McEwen, 2001; Smith and McMahon, 2005). While the impact of estrogens on these behaviors has been well characterized, the underlying mechanism(s) through which the steroid acts to affect many of these processes remains to be elucidated. It was once accepted that the only mechanism of estrogen action was through the binding of intracellular receptors that act as transcription factors. An important finding that has enhanced our understanding of estradiol action was the realization that estradiol can also act on estrogen receptors (ERs) localized at the cellular membrane surface. This is in distinction to the classical actions of estrogens, which typically have long time courses and involve gene transcription and protein translation. Classic actions are mediated by estrogen receptor-α (ERα) and estrogen receptor-β (ERβ). Upon steroid binding, these receptors dimerize, allowing them to functionally interact with specific parts of the genome known as estrogen response elements (EREs) to regulate gene expression. Activated estrogen receptors can also regulate transcription through the stabilization of other DNA binding proteins such as those that interact with activator protein 1 (AP-1) (Kushner et al., 2000; Paech et al., 1997; Webb et al., 1999).
Membrane-initiated Mechanisms of Estrogen Action
For many years, the principal defining feature of a non-classical, or novel effect of estradiol acting at the membrane surface was the rapid response. This criterion dates back to the initial observation of estradiol-induced accumulation of cAMP in uterine tissue by Clara Szego and June Davis (Szego and Davis, 1967). A similar time course of effects were noted in neurons by Robert Moss and Martin Kelly who showed that estradiol affected the electrical activity of preoptic and septal neurons within seconds (Kelly et al., 1976). The effects of membrane estrogen signaling can be observed within seconds, but this does not preclude more sustained action. Transmission of information from the membrane surface to the nucleus also occurs following the rapid signaling of estrogens, as illustrated by its ability to activate transcription factors such as cAMP response element binding protein (CREB) (Boulware et al., 2005; Gu et al., 1996; Szego et al., 2006; Wade and Dorsa, 2003). In addition to the time course of onset, several other hallmarks of estradiol action on the membrane have been described. Membrane estradiol signaling is typically: i) blocked by the ER antagonists; ii) activated by agonists (Boulware et al., 2005; Bulayeva et al., 2005); and iii) mimicked by membrane impermeable estrogen conjugates (Rai et al., 2005; Zheng and Ramirez, 1997). Importantly, these rapid actions cannot be induced by intracellular dialysis with of neurons with estradiol (Mermelstein et al., 1996). In 1999, overexpression of ERα and ERβ revealed that a portion of the classic ER proteins were targeted to the membrane and activated intracellular signaling (Razandi et al., 1999). This simple and elegant experiment demonstrated that the same proteins can mediate both intracellular and membrane actions of estradiol. Since this observation, studies with brain tissue have immunochemically detected a membrane localized ∼66 kDa protein that appears to be the full length ERα (Chaban et al., 2004; Dewing et al., 2007). In addition, the biological response elicited by estradiol is absent in cells that lack ERα or in cells that have been treated with siRNA directed against ERα or ERβ (Abraham et al., 2004; Marquez et al., 2006; Pedram et al., 2006; Razandi et al., 2004).
ERα and ERβ notwithstanding, there are a number of other potential membrane estradiol binding proteins, based on functional and immunochemical data (see (Hammes and Levin, 2007) for review). These include GPR30 (Filardo et al., 2007; Revankar et al., 2005), ER-X (Toran-Allerand et al., 2002) and a receptor that can be activated by the drug STX (Qiu et al., 2003). GPR30 is a relatively well characterized estrogen binding protein that has the typical GPCR seven transmembrane motif and can activate various protein kinases and modulate calcium signaling ((Filardo et al., 2000; Revankar et al., 2005; Thomas et al., 2005); but see (Otto et al., 2008)). One of the fascinating aspects of GPR30 is that its predominate localization is on the endoplasmic reticulum with no detectable expression on the cell surface (Brailoiu et al., 2007; Prossnitz et al., 2008; Revankar et al., 2005; Sakamoto et al., 2007; Wang et al., 2007). Moreover, only membrane permeable estrogenic ligands stimulated phosphoinisitide 3-kinase and calcium flux in SKBr3 breast cancer cells suggesting that an intracellular GPCR is capable of initiating cell signaling (Revankar et al., 2007). Other studies point to GPR30 as a scaffolding protein that participates in the assembly of membrane ERα or ERβ signaling complexes (Levin, 2003). Thus, GPR30 may indeed be a novel ER and mediate estradiol signaling, but it does not appear to be the predominant ER on the cell surface.
Two other putative membrane ERs have been described: ER-X (Toran-Allerand et al., 2002), and an estrogen-responsive GPCR that is activated by the drug STX (Qiu et al., 2003). ER-X, which appears to be preferentially activated by 17α-estradiol (as opposed to the gonadally-derived 17β isoform (Toran-Allerand et al., 2005)), is functionally coupled to MAPK signaling (Toran-Allerand et al., 2002). In the cortex, ER-X is expressed during development, but can be up-regulated following tissue injury. To date, the amino acid composition of ER-X has yet to be determined, although based on its cross reactivity with ERα antibodies, it is of similar size and composition to this classical ER. The effects of STX, which shares structural features with estradiol, have been observed in hypothalamic neurons and linked to regulation of homeostasis (Qiu et al., 2003; Qiu et al., 2006). As with ER-X, the molecular composition of this estrogen binding protein is currently unknown, but it has been hypothesized that the STX-activated protein is a GPCR, since downstream effects are sensitive to G protein modulation.
While little doubt remains regarding the existence of membrane ERα or ERβ and that the consequences of their activation can be observed both in vitro and in vivo, their specific roles in the nervous system have remained somewhat difficult to place in a physiological context. This review will concentrate on several physiological events in which rapid, membrane-initiated estradiol signaling require ERα or ERβ. Specifically, we focus on the synthesis of neuroprogesterone involved in positive feedback of the LH surge, the modulation of putative-nociceptive signaling in dorsal root ganglion (DRG) neurons and the control of sexual receptivity. We conclude by providing a model, delineated in hippocampal tissue, which explains the proximal signaling of membrane ERα or ERβ. In these systems, we have found that membrane associated ERα and ERβ rely upon mGluRs in order to activate cell signaling events (Boulware et al., 2005).
Neuroprogesterone synthesis
Circulating estradiol regulates the hypothalamic-pituitary-gonadal (HPG) axis through negative and positive feedback mechanisms. Estrogen positive feedback that is responsible for the LH surge depends on high physiological concentrations of estradiol that stimulate both the expression of progesterone receptors as well as progesterone synthesis in the brain (i.e. neuroprogesterone; reviewed in (Micevych et al., 2008)). Estradiol facilitates neuroprogesterone synthesis in astrocytes (Micevych et al., 2008). The mechanism of neuroprogesterone synthesis in adult hypothalamic astrocytes involves estradiol-induced release of calcium from intracellular stores (Chaban et al., 2004). This calcium flux is dependent on activation of the phospholipase C/inositol trisphosphate (PLC/IP3) pathway, and is blocked by inhibiting the IP3 receptor (Chaban et al., 2004). Calcium flux in astrocytes is stereospecific, activated by 17β-estradiol and not 17α-estradiol. It is also dependent on membrane ERs, as constrained estradiol coupled to bovine serum albumin (E-6-BSA) mimicked free estradiol action. Further, thapsigargin release of IP3-sensitve calcium stores stimulates neuroprogesterone synthesis in the absence of estradiol. This increase in neuroprogesterone synthesis in the hypothalamus on the afternoon of proestrus is necessary for estradiol-induced LH surge, the event underlying ovulation (Micevych and Sinchak, 2008). According to this model, as circulating levels of estradiol from the ovary rise, they initially induce the transcription of progesterone receptors in neurons. When estradiol levels peak on proestrus, they stimulate the synthesis of neuroprogesterone in astrocytes. The locally produced neuroprogesterone then activates progesterone receptors permitting the sensory, metabolic and circadian inputs to stimulate a network that induces a surge release of GnRH, then in turn LH, inducing ovulation.
ATP signaling in DRG neurons
Estradiol also rapidly modulates cell signaling in primary afferent sensory neurons whose cell bodies are located in dorsal root ganglia (DRG). DRG neurons transmit information about chemical or mechanical stimulation from the periphery to the spinal cord. ATP is a putative signal for visceral pain and is released by distention of the viscera and tissue damage (Burnstock, 2001). ATP transmits noxious stimuli by activating purinergic, ATP-gated P2X receptors on primary afferent fibers (Dunn et al., 2001). Opening of P2X channels results in membrane depolarization sufficient to trigger action potentials and calcium influx through voltage-gated calcium channels (VGCC) associated with nociception (Koshimizu et al., 2000).
In primary DRG cultures, the ATP-mediated calcium flux was inhibited by estradiol in 85% of the small neurons. Estradiol action was stereospecific, and inhibited by ER antagonists tamoxifen and ICI 182,780. The cellular calcium response initiated by ATP has two components: the initial ion flux is through activated P2X channels and the secondary response is the opening of voltage-gated calcium channels (VGCCs) in response to membrane depolarization (Koshimizu et al., 2000). The entire free cytoplasmic calcium ([Ca2+]i) flux was blocked with the purine receptor antagonist PPADS, but the calcium response was only partially inhibited by estradiol, suggesting that estradiol did not directly antagonize P2X receptors. Nifedipine, a L-type voltage-gated calcium channel blocker was shown to mimic the effect of estradiol. This result is consistent with estradiol blockade of L-type calcium channels in PC-12 cells (Kim et al., 2000), neostriatal (Kurata et al., 2001; Mermelstein et al., 1996) and hippocampal neurons (Kurata et al., 2001; Mermelstein et al., 1996). ERα is needed for the estradiol attenuation of ATP-induced calcium influx as demonstrated by a lack of response to estradiol in DRG neurons from ERα knockout (ERαKO) mice. The estradiol response is retained in ERβ knockout (ERβKO) mice (Chaban and Micevych, 2005). Thus, the rapid estradiol actions in DRG neurons require a membrane ERα.
Sexual receptivity
In the female rat, estradiol acts on a limbic-hypothalamic circuit that changes the salience of information about the environment (and cutaneous sensory input) to permit the expression of the stereotypic sexually receptive behavior, the lordosis reflex (Micevych and Ulibarri, 1992; Sinchak and Micevych, 2003). In intact rodents, the rising levels of estradiol, which stimulate the lordosis regulating circuit, are facilitated by progesterone to achieve maximal sexual receptivity. To a first approximation, estradiol induction of female sexually receptive behavior has a time course that strongly implicates transcriptional actions. Indeed, studies over the years (Gorski and Yanase, 1981; Rainbow et al., 1980) have demonstrated that estradiol initiation of lordosis behavior requires transcription and translation of new proteins. With the advent of immunohistochemistry, radioimmunoassay and in situ hybridization, it was shown that reproductively relevant neuropeptide levels were increased by estradiol (e.g., (Bale et al., 1995; Crowley et al., 1995; Micevych et al., 1994; Priest et al., 1995; Priest et al., 1995; Romano et al., 1990; Wilcox and Roberts, 1985)).
Endogenous opioid peptides are among the best studied mediators of lordosis regulating circuits in the CNS. One of these endogenous opioids is β-endorphin (β-END) that participates in an arcuate nucleus to medial preoptic nucleus projection that regulates lordosis behavior (Mills et al., 2004). A signature of estradiol activation of this circuit is the rapid internalization of μ-opioid receptors (MOR) in the medial preoptic nucleus. MOR internalization is tightly correlated with the display of lordosis behavior (Eckersell et al., 1998; Sinchak and Micevych, 2001; Torii and Kubo, 1994). While the effects of estradiol on MOR internalization can be observed within minutes of steroid treatment, it is in fact critically important for the display of lordosis 30-48 hours later. This finding strongly suggests that rapid estradiol signaling is also important for the central control of lordosis (Sinchak et al., 1997). Moreover, the effect of estradiol on MOR internalization is mimicked by a membrane-constrained estradiol conjugate (i.e. E-6-biotin) injected directly into the arcuate nucleus, implying a membrane-initiated estrogen signaling mechanism within this brain region. The putative role of membrane initiated estrogen signaling was directly demonstrated by systemically injecting rats with another membrane-constrained conjugate E-6-BSA followed by a systemic injection of free estradiol. E-6-BSA was found to augment the response to free estradiol (Kow and Pfaff, 2004). Thus it appears membrane-initiated, rapid estradiol signaling facilitates nuclear ER-stimulated transcriptional events, suggesting cooperation between nuclear and membrane initiated actions of estradiol.
ER signaling through mGluRs
In each of the three physiological systems described, the response to estradiol was rapid and mediated by membrane ERα and/or ERβ; established hallmarks for membrane-initiated signaling. What was not immediately clear was how classical ERs, known to be transcription factors, with a structure what does not resemble membrane receptors, initiate cell signaling. Initially, membrane mediated estradiol actions were seen to affect G protein-dependent cell signaling pathways suggesting that membrane ERs were a GPCR (Levin, 2005; Vasudevan and Pfaff, 2007). Yet, this hypothesis does not explain how ERα or ERβ can initiate G-protein signaling. We have recently provided an alternative explanation to the “ER as GPCR hypothesis.” It is our working theory that ERs activate mGluRs through a direct protein-protein interaction. Upon estradiol binding to the ER, the ER promotes transactivation of the mGluR, initiating mGluR signaling without the requirement of glutamate. In effect, the ER alters the confirmation of the mGluR, resulting in activation of the downstream G-proteins and initiating second messenger signaling. This hypothesis is partially based on previous precedent, as in nonneuronal tissue, ERs have been found to directly bind tyrosine kinase receptors (Stefanova et al., 1991), which are in turn activated following estrogen application (Kahlert et al., 2000). This common mechanism of ERs activating surface receptors may account for the novel actions of estradiol outside of the brain, as mGluR expression is nervous system specific. Notably, this does not preclude the converse possibility, i.e. that ERs interact with tyrosine kinase receptors in the CNS as well. Indeed, these interactions occur in the hypothalamus to influence reproduction (Etgen et al., 2001) as well as in the substantia nigra where it appears to mediate neuroprotection (Quesada and Micevych, 2004). In terms of evidence for this putative mechanism, co-immunoprecipitation experiments suggest that ERs can directly interact with mGluRs (Dewing et al., 2007) (Figure 1A). Experiments utilizing an mGluR agonist and an ER antagonist strongly suggest this putative protein-protein interaction can alter the function of mGluR signaling. Figure 1B examines CREB phosphorylation following activation of mGluR1 with DHPG. Activation of CREB by DHPG is attenuated following application of the ER antagonist. This action of ICI 182,780 does not appear due to a direct effect on mGluR1, as in cultures from male animals, which lack an estrogen response, ICI does not influence the actions of DHPG. Future research will need to more definitively determine whether this hypothesis of ER to mGluR signaling is accurate.
Figure 1.
Direct ER/mGluR interactions may underlie estradiol-induced activation of mGluRs. (A) Co-immunoprecipitation of ERα with mGluR1a. (B) Antagonism of ERs with ICI 182,780 attenuate CREB phosphorylation following application of the group I mGluR agonist DHPG. (C) ICI 182,780 has no effect on CREB phosphorylation in cultures generated from male tissue, which lack the mGluR-mediated estrogen response (from (Micevych and Mermelstein, 2008)).
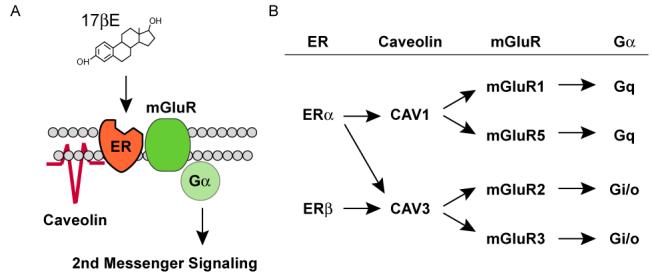
In hippocampal neurons, estradiol activation of ERs can lead to the phosphorylation of CREB via stimulation of both group I (Gq-coupled) and group II (Gi/o-coupled) mGluRs (Figure 1C) (Boulware et al., 2005). This action of estradiol in hippocampal tissue was only found in neurons generated from female and not male tissue. In a follow-up report, we determined ER interactions with mGluRs to be dependent upon caveolin proteins (Boulware et al., 2007), known to be essential for the trafficking and clustering of signaling molecules. The importance of caveolin proteins in the trafficking of ERα to the membrane was first demonstrated outside of the nervous system (Razandi et al., 2003), and in which palmitoylation of the ER was also required (Acconcia et al., 2005; Pedram et al., 2007).
ER interactions with caveolins and mGluRs are not random. In hippocampal neurons, ERα interaction with either mGluR1 or mGluR2/3 was dependent upon caveolin 1 (CAV1) or caveolin 3 (CAV3), respectively. Conversely, ERβ only interacted with mGluR2/3 via CAV3. The pairing of particular ERs with mGluRs is observed in various other brain regions (Figure 2) (Dewing et al., 2007). In striatal neurons, we have found that ERα via CAV1 activates mGluR5-based (Grove-Strawser and Mermelstein, 2007). This is of particular interest because striatal neurons express both group I mGluRs, mGluR1 and mGluR5. In striatal neurons, mGluR1 signaling was unaffected by estradiol. It remains to be determined why across different neuronal populations, ERs would be paired with discrete types of mGluRs that are known activate the same second messenger systems.
Figure 2.

ER activation of mGluR signaling through interactions with caveolin proteins. (A) Model framework of estradiol-induced activation of mGluRs via caveolin-based caveolae. (B) Summary of previous findings demonstrating ER activation of group I (mGluR1/5) and group II (mGluR2/3) metabotropic glutamate receptors is mediated by caveolin 1 and caveolin 3, respectively (from (Micevych and Mermelstein, 2008)).
Functional isolation of different ERs with mGluRs suggests a diverse array of potential estrogen-sensitive signaling pathways at the disposal of individual cells (Figure 2). The generation of specific ER/mGluR pairs via caveolin function may eventually be found to be responsible for many of the diverse observations of novel estrogen signaling in the central nervous system.
The physiology of membrane estrogen receptors can be explained by interacting with mGluRs
Each of the rapid estradiol actions: neuroprogesterone synthesis, attenuation of ATP signaling in DRG neurons and sexual receptivity are dependent on membrane ERs interacting with mGluR1s. In hypothalamic astrocyte cultures, in which estradiol stimulated calcium flux and neuroprogesterone synthesis, blocking mGluR1a with the selective antagonist, LY367385, prevented the estradiol-induced calcium flux (Kuo, Submitted). Further evidence of such an interaction is the co-immunoprecipitation of ERα and mGluR1a in the membrane fraction of astrocytes. Interestingly, separate activation by estradiol or DHPG, a selective mGluR1a agonist, produced robust calcium flux, but alone neither were as effective at stimulating calcium flux as they are together, indicating that for maximal signaling in astrocytes both glutamate and estradiol need to be present. These results suggest that estradiol may act most effectively on astrocytes that are in proximity to active glutamatergic terminals (Figure 3).
Figure 3.
Proposed mechanism through which estradiol signaling in astrocytes is integrated with local neuronal activity involved in the synthesis of neuroprogesterone. Estradiol (E2), typically of ovarian origin, binds to membrane ERα and activates the mGluR1a. This increases levels of free cytoplasmic calcium (Ca2+) through the inositol trisphosphate (IP3) receptor-mediated release of intracellular stores of calcium. Elevated levels of intracellular Ca2+ are needed for neuroprogesterone (P4) synthesis in astrocytes. Studies in vitro demonstrate that E2 alone or an mGluR1a agonist alone increase intracellular calcium levels. However, when both an mGluR1a agonist and E2 are applied to astrocytes, the resulting Ca2+ flux is significantly greater, suggesting that P4 synthesis is also augmented. We propose that in vivo when E2-stimulated astrocytes are in the proximity of active nerve terminals, the released glutamate (Glu) activates astrocyte mGluR1a, resulting in significantly greater Ca2+ responses. This elevated Ca2+ response is hypothesized to produce a greater P4 synthesis in astrocytes (from (Micevych and Mermelstein, 2008)).
As predicted by the ER/mGluR hypothesis, estradiol stimulation of mGluR2/3 should block calcium flux through L-type VGCC (Boulware et al., 2005). In DRG neurons, membrane ERα attenuated the ATP-induced calcium flux suggesting that ERα interacts, in a tissue-specific manner, with mGluR2/3 (Boulware et al., 2007; Boulware et al., 2005). To test this possibility, DRG neurons were treated with estradiol or estradiol + LY341495, an mGluR2/3 inhibitor before ATP stimulation to induce a calcium flux (Li et al., 2008). As predicted, estradiol attenuated ATP-induced calcium flux, an effect blocked by LY341495. Thus, the estradiol action on DRG neurons requires the interaction of ERα with the mGluR2/3.
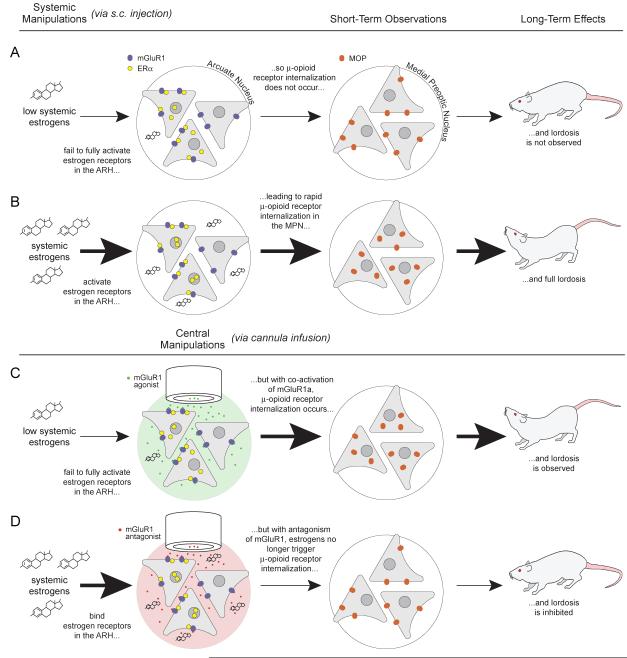
As with estrogen effects on neuroprogesterone synthesis and ATP signaling in DRG neurons, the rapid estradiol effect on MOR internalization is blocked following antagonism of the mGluR1a in the arcuate nucleus. Moreover, estradiol-induced lordosis is attenuated, but only if the mGluR1a is antagonized at the time of estradiol treatment, and not several hours after estradiol, further suggesting that mGluR1 signaling is directly related to the rapid action of estradiol. Our understanding of how the ER/mGluR interaction mediates behavior is illustrated in Figure 3. Low systemic estradiol levels are insufficient to elicit lordosis behavior. The arcuate-medial preoptic circuit is quiescent. Membrane ERs in the arcuate nucleus are not stimulated, which is reflected by the cellular distribution of MOR in the medial preoptic area. The majority of MORs are localized on the cell membrane indicating that these receptors have not been activated. In these conditions, the female rat is not sexually receptive (Figure 3A). However, when systemic estradiol levels reach levels that induce behavior, membrane ERs in the arcuate nucleus are activated, leading to MOR internalization and subsequent full lordosis behavior (Figure 3B). Accordingly, when mGluR1a is directly stimulated with an agonist under low estradiol conditions, membrane ERs can be bypassed, resulting in MOR internalization and facilitation of lordosis (Figure 4). Conversely, under high estrogenic conditions antagonizing mGluR1a blocks estrogen induced MOR internalization and attenuates sexual behavior (Figure 3D). These data are consistent with the in vitro demonstration of ERα/mGluR1a signaling in hippocampal neurons, and provided the first in vivo evidence that estradiol can signal through activation of mGluR1a. By demonstrating that lordosis behavior, a classical assay of estradiol action, has a rapid non-genomic component underscores the importance of ER/mGluR interactions in the brain (Dewing et al., 2007).
Figure 4.
ER/mGluR signaling in the arcuate nucleus (ARH) — medial preoptic nucleus (MPN) circuit regulates female sexual receptivity. When circulating levels of estradiol are low, the circuit is not activated and the rat is not sexually receptive (first row). When circulating levels of estradiol are increased, ERs in the ARH are stimulated. A population of ERs, located on the plasma membrane, induces the release of β-endorphin in the MPN from ARH projection neurons activating and internalizing MOPs, producing lordosis (second row). If mGluR1a are activated in the presence of low circulating estradiol levels, the ARH-MPN projection is activated: MOPs are activated/internalized and lordosis is displayed (third row). Conversely, when mGluR1a are antagonized in the presence of high levels of circulating estrogen, the ARH-MPN circuit is not activated: MOPs are not internalized and lordosis is attenuated (forth row) (from (Micevych and Mermelstein, 2008)).
Conclusions
Clearly, we are in the initial stages of understanding when and how rapid estradiol actions fit into the physiologic context of estradiol signaling in the nervous system. Yet, recent findings suggest a mechanism by which ERs in the membrane can elicit changes in cell signaling, and several different systems in which the actions of estradiol on membrane ERs require an interaction with mGluRs. This mechanism of ER-mGluR interactions provides a framework through which the pleiomorphic membrane-initiated estradiol actions can be understood and used to elucidate various means by which ERs play an important role in brain function.
ACKNOWLEDGEMENTS
This work was supported by NIH grants DA013185, HD042635 (PEM) and NS41302 (PGM). We appreciate the efforts of Ms Amy Christensen in the preparation of the manuscript.
REFERENCES
- -1-.Abraham IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology. 2004;145:3055–61. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- -2-.Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, Visca P, Marino M. Palmitoylation-dependent estrogen receptor alpha membrane localization: regulation by 17beta-estradiol. Mol Biol Cell. 2005;16:231–7. doi: 10.1091/mbc.E04-07-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -3-.Bale TL, Dorsa DM, Johnston CA. Oxytocin receptor mRNA expression in the ventromedial hypothalamus during the estrous cycle. J Neurosci. 1995;15:5058–64. doi: 10.1523/JNEUROSCI.15-07-05058.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -4-.Belcredito S, Vegeto E, Brusadelli A, Ghisletti S, Mussi P, Ciana P, Maggi A. Estrogen neuroprotection: the involvement of the Bcl-2 binding protein BNIP2. Brain Res Brain Res Rev. 2001;37:335–42. doi: 10.1016/s0165-0173(01)00138-2. [DOI] [PubMed] [Google Scholar]
- -5-.Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J Neurosci. 2007;27:9941–50. doi: 10.1523/JNEUROSCI.1647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -6-.Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–78. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -7-.Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193:311–21. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- -8-.Bulayeva NN, Wozniak AL, Lash LL, Watson CS. Mechanisms of membrane estrogen receptor-alpha-mediated rapid stimulation of Ca2+ levels and prolactin release in a pituitary cell line. Am J Physiol Endocrinol Metab. 2005;288:E388–97. doi: 10.1152/ajpendo.00349.2004. [DOI] [PubMed] [Google Scholar]
- -9-.Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci. 2001;22:182–8. doi: 10.1016/s0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- -10-.Chaban VV, Lakhter AJ, Micevych P. A Membrane Estrogen Receptor Mediates Intracellular Calcium Release in Astrocytes. Endocrinology. 2004;145:3788–3795. doi: 10.1210/en.2004-0149. [DOI] [PubMed] [Google Scholar]
- -11-.Chaban VV, Micevych PE. Estrogen receptor-alpha mediates estradiol attenuation of ATP-induced Ca2+ signaling in mouse dorsal root ganglion neurons. J Neurosci Res. 2005;81:31–7. doi: 10.1002/jnr.20524. [DOI] [PubMed] [Google Scholar]
- -12-.Crowley RS, Insel TR, O’Keefe JA, Kim NB, Amico JA. Increased accumulation of oxytocin messenger ribonucleic acid in the hypothalamus of the female rat: induction by long term estradiol and progesterone administration and subsequent progesterone withdrawal. Endocrinology. 1995;136:224–31. doi: 10.1210/endo.136.1.7828535. [DOI] [PubMed] [Google Scholar]
- -13-.Dewing P, Boulware MI, Sinchack K, Christensen A, Mermelstein PG, Micevych P. Membrane ERα interacts with mGluR1a to modulate female sexual receptivity. J Neurosci. 2007;27:9294–300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -14-.Dunn PM, Zhong Y, Burnstock G. P2X receptors in peripheral neurons. Prog Neurobiol. 2001;65:107–34. doi: 10.1016/s0301-0082(01)00005-3. [DOI] [PubMed] [Google Scholar]
- -15-.Eckersell CB, Popper P, Micevych PE. Estrogen-induced alteration of mu-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala. J Neurosci. 1998;18:3967–76. doi: 10.1523/JNEUROSCI.18-10-03967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -16-.Etgen AM, Ansonoff MA, Quesada A. Mechanisms of ovarian steroid regulation of norepinephrine receptor-mediated signal transduction in the hypothalamus: implications for female reproductive physiology. Horm Behav. 2001;40:169–77. doi: 10.1006/hbeh.2001.1676. [DOI] [PubMed] [Google Scholar]
- -17-.Filardo E, Quinn J, Pang Y, Graeber C, Shaw S, Dong J, Thomas P. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology. 2007;148:3236–45. doi: 10.1210/en.2006-1605. [DOI] [PubMed] [Google Scholar]
- -18-.Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–60. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- -19-.Gorski RA. Sexual dimorphisms of the brain. J Anim Sci. 1985;61(Suppl 3):38–61. doi: 10.1093/ansci/61.supplement_3.38. [DOI] [PubMed] [Google Scholar]
- -20-.Gorski RA, Yanase M. Estrogen facilitation of lordosis behavior in the female rat. Exp Brain Res. 1981;(Suppl 3):222–37. doi: 10.1007/978-3-642-45525-4_18. [DOI] [PubMed] [Google Scholar]
- -21-.Grove-Strawser D, Mermelstein PG.Estrogen receptors activate different mGluRs across distinct brain regions. Abstract 195.13/10.2007
- -22-.Gu G, Rojo AA, Zee MC, Yu J, Simerly RB. Hormonal regulation of CREB phosphorylation in the anteroventral periventricular nucleus. J Neurosci. 1996;16:3035–44. doi: 10.1523/JNEUROSCI.16-09-03035.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -23-.Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Endocr Rev. 2007;28:726–41. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- -24-.Kahlert S, Nuedling S, van Eickels M, Vetter H, Meyer R, Grohe C. Estrogen receptor alpha rapidly activates the IGF-1 receptor pathway. J Biol Chem. 2000;275:18447–53. doi: 10.1074/jbc.M910345199. [DOI] [PubMed] [Google Scholar]
- -25-.Kelly MJ, Moss RL, Dudley CA. Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Res. 1976;114:152–7. doi: 10.1016/0006-8993(76)91017-9. [DOI] [PubMed] [Google Scholar]
- -26-.Kim YJ, Hur EM, Park TJ, Kim KT. Nongenomic inhibition of catecholamine secretion by 17beta-estradiol in PC12 cells. J Neurochem. 2000;74:2490–6. doi: 10.1046/j.1471-4159.2000.0742490.x. [DOI] [PubMed] [Google Scholar]
- -27-.Koshimizu TA, Van Goor F, Tomic M, Wong AO, Tanoue A, Tsujimoto G, Stojilkovic SS. Characterization of calcium signaling by purinergic receptor-channels expressed in excitable cells. Mol Pharmacol. 2000;58:936–45. doi: 10.1124/mol.58.5.936. [DOI] [PubMed] [Google Scholar]
- -28-.Kow LM, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. Proc Natl Acad Sci USA. 2004;101:12354–7. doi: 10.1073/pnas.0404889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -29-.Kuo J, Hariri O, Bondar G, Ogi J, Micevych P. Membrane estradiol receptors interact with metabotropic glutamate receptors to mobilize intracellular calcium in hypothalamic astrocytes. Endocrinology. Submitted;
- -30-.Kurata K, Takebayashi M, Kagaya A, Morinobu S, Yamawaki S. Effect of beta-estradiol on voltage-gated Ca(2+) channels in rat hippocampal neurons: a comparison with dehydroepiandrosterone. Eur J Pharmacol. 2001;416:203–12. doi: 10.1016/s0014-2999(01)00880-9. [DOI] [PubMed] [Google Scholar]
- -31-.Kushner PJ, Agard D, Feng WJ, Lopez G, Schiau A, Uht R, Webb P, Greene G. Oestrogen receptor function at classical and alternative response elements. Novartis Found Symp. 2000;230:20–6. doi: 10.1002/0470870818.ch3.; discussion 27-40.
- -32-.Lee SJ, McEwen BS. Neurotrophic and neuroprotective actions of estrogens and their therapeutic implications. Annu Rev Pharmacol Toxicol. 2001;41:569–91. doi: 10.1146/annurev.pharmtox.41.1.569. [DOI] [PubMed] [Google Scholar]
- -33-.Levin ER. Bidirectional signaling between the estrogen receptor and the epidermal growth factor receptor. Mol Endocrinol. 2003;17:309–17. doi: 10.1210/me.2002-0368. [DOI] [PubMed] [Google Scholar]
- -34-.Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19:1951–9. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -35-.Li J, Micevych P, McDonald J, Rapkin A, Chaban V. Inflammation in the uterus induces phosphorylated extracellular signal-regulated kinase and substance P immunoreactivity in dorsal root ganglia neurons innervating both uterus and colon in rats. J Neurosci Res. 2008 doi: 10.1002/jnr.21714.; In Press.
- -36-.Marquez DC, Chen HW, Curran EM, Welshons WV, Pietras RJ. Estrogen receptors in membrane lipid rafts and signal transduction in breast cancer. Mol Cell Endocrinol. 2006;246:91–100. doi: 10.1016/j.mce.2005.11.020. [DOI] [PubMed] [Google Scholar]
- -37-.Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -38-.Micevych P, Mermelstein PG. Membrane Estrogen Receptors Acting through Metabotropic Glutamate Receptors: An Emerging Mechanism of Estrogen Action in Brain. Molecular Neurobiology. 2008 doi: 10.1007/s12035-008-8034-z.; In Press.
- -39-.Micevych P, Sinchak K. Estradiol regulation of progesterone synthesis in the brain. Mol Cell Endocrinol. 2008 doi: 10.1016/j.mce.2008.04.016.; In Press.
- -40-.Micevych P, Soma KK, Sinchak K. Neuroprogesterone: Key to estrogen positive feedback? Brain Res Rev. 2008;57:470–80. doi: 10.1016/j.brainresrev.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -41-.Micevych P, Ulibarri C. Development of the limbic-hypothalamic cholecystokinin circuit: a model of sexual differentiation. Dev Neurosci. 1992;14:11–34. doi: 10.1159/000111643. [DOI] [PubMed] [Google Scholar]
- -42-.Micevych PE, Abelson L, Fok H, Ulibarri C, Priest CA. Gonadal steroid control of preprocholecystokinin mRNA expression in the limbic-hypothalamic circuit: comparison of adult with neonatal steroid treatments. J Neurosci Res. 1994;38:386–98. doi: 10.1002/jnr.490380404. [DOI] [PubMed] [Google Scholar]
- -43-.Mills RH, Sohn RK, Micevych PE. Estrogen-induced mu-opioid receptor internalization in the medial preoptic nucleus is mediated via neuropeptide Y-Y1 receptor activation in the arcuate nucleus of female rats. J Neurosci. 2004;24:947–55. doi: 10.1523/JNEUROSCI.1366-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -44-.Otto C, Rohde-Schulz B, Schwarz G, Fuchs I, Klewer M, Brittain D, Langer G, Bader B, Prelle K, Nubbemeyer R, Fritzemeier KH. GPR30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology. 2008 doi: 10.1210/en.2008-0269.; In Press.
- -45-.Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277:1508–10. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- -46-.Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol. 2006;20:1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- -47-.Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem. 2007;282:22278–88. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- -48-.Pfaff DW, Vasudevan N, Kia HK, Zhu YS, Chan J, Garey J, Morgan M, Ogawa S. Estrogens, brain and behavior: studies in fundamental neurobiology and observations related to women’s health. J Steroid Biochem Mol Biol. 2000;74:365–73. doi: 10.1016/s0960-0760(00)00114-x. [DOI] [PubMed] [Google Scholar]
- -49-.Priest CA, Eckersell CB, Micevych PE. Estrogen regulates preproenkephalin-A mRNA levels in the rat ventromedial nucleus: temporal and cellular aspects. Brain Res Mol Brain Res. 1995;28:251–62. doi: 10.1016/0169-328x(94)00213-x. [DOI] [PubMed] [Google Scholar]
- -50-.Priest CA, Vink KL, Micevych PE. Temporal regulation by estrogen of beta-preprotachykinin mRNA expression in the rat ventromedial nucleus of the hypothalamus. Brain Res Mol Brain Res. 1995;28:61–71. doi: 10.1016/0169-328x(94)00184-g. [DOI] [PubMed] [Google Scholar]
- -51-.Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol. 2008;70:165–90. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- -52-.Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–40. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -53-.Qiu J, Bosch MA, Tobias SC, Krust A, Graham SM, Murphy SJ, Korach KS, Chambon P, Scanlan TS, Ronnekleiv OK, Kelly MJ. A G-protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci. 2006;26:5649–55. doi: 10.1523/JNEUROSCI.0327-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -54-.Quesada A, Micevych PE. Estrogen interacts with the IGF-1 system to protect nigrostriatal dopamine and maintain motoric behavior after 6-hydroxdopamine lesions. J Neurosci Res. 2004;75:107–16. doi: 10.1002/jnr.10833. [DOI] [PubMed] [Google Scholar]
- -55-.Rai D, Frolova A, Frasor J, Carpenter AE, Katzenellenbogen BS. Distinctive actions of membrane-targeted versus nuclear localized estrogen receptors in breast cancer cells. Mol Endocrinol. 2005;19:1606–17. doi: 10.1210/me.2004-0468. [DOI] [PubMed] [Google Scholar]
- -56-.Rainbow TC, Davis PG, McEwen BS. Anisomycin inhibits the activation of sexual behavior by estradiol and progesterone. Brain Res. 1980;194:548–55. doi: 10.1016/0006-8993(80)91240-8. [DOI] [PubMed] [Google Scholar]
- -57-.Razandi M, Alton G, Pedram A, Ghonshani S, Webb P, Levin ER. Identification of a structural determinant necessary for the localization and function of estrogen receptor alpha at the plasma membrane. Mol Cell Biol. 2003;23:1633–46. doi: 10.1128/MCB.23.5.1633-1646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -58-.Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–19. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- -59-.Razandi M, Pedram A, Merchenthaler I, Greene GL, Levin ER. Plasma membrane estrogen receptors exist and functions as dimers. Mol Endocrinol. 2004;18:2854–65. doi: 10.1210/me.2004-0115. [DOI] [PubMed] [Google Scholar]
- -60-.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–30. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- -61-.Revankar CM, Mitchell HD, Field AS, Burai R, Corona C, Ramesh C, Sklar LA, Arterburn JB, Prossnitz ER. Synthetic estrogen derivatives demonstrate the functionality of intracellular GPR30. ACS Chem Biol. 2007;2:536–44. doi: 10.1021/cb700072n. [DOI] [PubMed] [Google Scholar]
- -62-.Romano GJ, Mobbs CV, Lauber A, Howells RD, Pfaff DW. Differential regulation of proenkephalin gene expression by estrogen in the ventromedial hypothalamus of male and female rats: implications for the molecular basis of a sexually differentiated behavior. Brain Res. 1990;536:63–8. doi: 10.1016/0006-8993(90)90009-z. [DOI] [PubMed] [Google Scholar]
- -63-.Sakamoto H, Matsuda K, Hosokawa K, Nishi M, Morris JF, Prossnitz ER, Kawata M. Expression of G protein-coupled receptor-30, a G protein-coupled membrane estrogen receptor, in oxytocin neurons of the rat paraventricular and supraoptic nuclei. Endocrinology. 2007;148:5842–50. doi: 10.1210/en.2007-0436. [DOI] [PubMed] [Google Scholar]
- -64-.Sinchak K, Hendricks DG, Baroudi R, Micevych PE. Orphanin FQ/nociceptin in the ventromedial nucleus facilitates lordosis in female rats. Neuroreport. 1997;8:3857–60. doi: 10.1097/00001756-199712220-00004. [DOI] [PubMed] [Google Scholar]
- -65-.Sinchak K, Micevych P. Visualizing activation of opioid circuits by internalization of G protein-coupled receptors. Mol Neurobiol. 2003;27:197–222. doi: 10.1385/MN:27:2:197. [DOI] [PubMed] [Google Scholar]
- -66-.Sinchak K, Micevych PE. Progesterone blockade of estrogen activation of muopioid receptors regulates reproductive behavior. J Neurosci. 2001;21:5723–9. doi: 10.1523/JNEUROSCI.21-15-05723.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -67-.Smith CC, McMahon LL. Estrogen-induced increase in the magnitude of long-term potentiation occurs only when the ratio of NMDA transmission to AMPA transmission is increased. J Neurosci. 2005;25:7780–91. doi: 10.1523/JNEUROSCI.0762-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -68-.Stefanova I, Horejsi V, Ansotegui IJ, Knapp W, Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science. 1991;254:1016–9. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- -69-.Szego CM, Davis JS. Adenosine 3′,5′-monophosphate in rat uterus: acute elevation by estrogen. Proc Natl Acad Sci USA. 1967;58:1711–8. doi: 10.1073/pnas.58.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -70-.Szego EM, Barabas K, Balog J, Szilagyi N, Korach KS, Juhasz G, Abraham IM. Estrogen induces estrogen receptor alpha-dependent cAMP response element-binding protein phosphorylation via mitogen activated protein kinase pathway in basal forebrain cholinergic neurons in vivo. J Neurosci. 2006;26:4104–10. doi: 10.1523/JNEUROSCI.0222-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -71-.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–32. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- -72-.Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Jr., Nethrapalli IS, Tinnikov AA. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- -73-.Toran-Allerand CD, Tinnikov AA, Singh RJ, Nethrapalli IS. 17alpha-estradiol: a brain-active estrogen? Endocrinology. 2005;146:3843–50. doi: 10.1210/en.2004-1616. [DOI] [PubMed] [Google Scholar]
- -74-.Torii M, Kubo K. The effects of intraventricular injection of beta-endorphin on initial estrogen action to induce lordosis behavior. Physiol Behav. 1994;55:157–62. doi: 10.1016/0031-9384(94)90024-8. [DOI] [PubMed] [Google Scholar]
- -75-.Vasudevan N, Pfaff DW. Membrane-initiated actions of estrogens in neuroendocrinology: emerging principles. Endocr Rev. 2007;28:1–19. doi: 10.1210/er.2005-0021. [DOI] [PubMed] [Google Scholar]
- -76-.Wade CB, Dorsa DM. Estrogen activation of cyclic adenosine 5′-monophosphate response element-mediated transcription requires the extracellularly regulated kinase/mitogen-activated protein kinase pathway. Endocrinology. 2003;144:832–8. doi: 10.1210/en.2002-220899. [DOI] [PubMed] [Google Scholar]
- -77-.Wang C, Prossnitz ER, Roy SK. Expression of G protein-coupled receptor 30 in the hamster ovary: differential regulation by gonadotropins and steroid hormones. Endocrinology. 2007;148:4853–64. doi: 10.1210/en.2007-0727. [DOI] [PubMed] [Google Scholar]
- -78-.Webb P, Nguyen P, Valentine C, Lopez GN, Kwok GR, McInerney E, Katzenellenbogen BS, Enmark E, Gustafsson JA, Nilsson S, Kushner PJ. The estrogen receptor enhances AP-1 activity by two distinct mechanisms with different requirements for receptor transactivation functions. Mol Endocrinol. 1999;13:1672–85. doi: 10.1210/mend.13.10.0357. [DOI] [PubMed] [Google Scholar]
- -79-.Wilcox JN, Roberts JL. Estrogen decreases rat hypothalamic proopiomelanocortin messenger ribonucleic acid levels. Endocrinology. 1985;117:2392–6. doi: 10.1210/endo-117-6-2392. [DOI] [PubMed] [Google Scholar]
- -80-.Zheng J, Ramirez VD. Demonstration of membrane estrogen binding proteins in rat brain by ligand blotting using a 17beta-estradiol-[125I]bovine serum albumin conjugate. J Steroid Biochem Mol Biol. 1997;62:327–36. doi: 10.1016/s0960-0760(97)00037-x. [DOI] [PubMed] [Google Scholar]