Abstract
Flattening the diurnal corticosterone rhythm prevented the stimulating action of l-NAME (a nitric oxide synthase, NOS, inhibitor) on progenitor cell proliferation in the dentate gyrus in Lister-Hooded adult male rats. The increased expression of brain-derived neurotrophic factor (BDNF) and trkB mRNA in the dentate gyrus which otherwise occurred after l-NAME was also prevented by clamping the corticoid rhythm in adrenalectomized rats, but was restored by daily additional injections of corticosterone (which replicates the diurnal rhythm). Unilateral infusions of BDNF into the lateral ventricle increased proliferation in the dentate gyrus on the side of the infusion, but this was not observed following implantation of subcutaneous corticosterone, which flattened the diurnal corticosterone rhythm. 5HT1A mRNA in the dentate gyrus was increased on both sides of the brain by unilateral BDNF infusions, but this was also prevented by subcutaneous corticosterone pellets. These results show that the diurnal rhythm of corticosterone regulates the stimulating action of NOS inhibitors on BDNF as well as on neurogenesis in the dentate gyrus, and that BDNF becomes ineffective on both proliferation rates and 5HT1A expression in the absence of a rhythm in corticosterone. This, together with our previous findings, suggests that corticoid rhythms permit both serotonin and NO access to BDNF, and the latter to regulate progenitor cell activity.
Keywords: brain-derived neurotrophic factor, corticosterone, dentate gyrus, neurogenesis, nitric oxide, serotonin
Introduction
Neurogenesis in the dentate gyrus of the adult hippocampus is highly labile. Much of this lability is due to the exquisite sensitivity of the progenitor cells to glucocorticoids (Gould et al., 1992; Wong & Herbert, 2004). Glucocorticoids have two principal roles: absolute levels regulate the proliferation rate of the progenitor cells; and the presence of an intact diurnal corticoid rhythm is an essential requirement for the action of two further controlling factors, namely serotonin and nitric oxide (NO). The stimulating actions of both the selective serotonin-reuptake inhibitor (SSRI) fluoxetine and the nitric oxide synthase (NOS) inhibitor l-NAME are prevented if the diurnal rhythm of corticosterone is flattened by either implanting a pellet of corticosterone into intact rats, or maintaining adrenalectomized rats on a constant supply of corticosterone (Huang & Herbert, 2006; Pinnock et al., 2007). Restoring the diurnal rhythm in the latter by an additional daily injection of corticosterone also reinstates sensitivity of their progenitor cells to either drug (Huang & Herbert, 2006; Pinnock et al., 2007).
Brain-derived neurotrophic factor (BDNF) has an established role in the regulation of proliferation in the dentate gyrus. BDNF has high levels of expression in the adult dentate gyrus (Nibuya et al., 1995; Berchtold et al., 1999). The action of BDNF is mediated by the specific tyrosine kinase receptor TrkB, which is also widely expressed in the dentate gyrus (Barbacid, 1994). l-NAME as well as markedly stimulating neurogenesis has also been found to increase BDNF in the dentate gyrus (Cheng et al., 2003). BDNF is also sensitive to other treatments that increase neurogenesis, such as SSRIs (Nibuya et al., 1995; Duman et al., 1997; Nakagawa et al., 2002). Chronic but not acute SSRI treatment upregulates BDNF mRNA and its receptors in the hippocampus (Nibuya et al., 1995). Furthermore, infusions of BDNF into the hippocampus and midbrain area produce antidepressant-like behavioural effects in rats (Shirayama et al., 2002) and transgenic mice with reduced BDNF signalling in the brain are insensitive to antidepressants in behavioural tests (Sairanen et al., 2005). Activation of 5HT1A receptors by 8-hydroxy-2-(di-n-propylamino)tertraline (8-OH-DPAT) increased progenitor cell mitosis (Huang & Herbert, 2005), but whether these receptors are required for the action of SSRIs on progenitor cell proliferation is uncertain (Santarelli et al., 2003; Holick et al., 2008).
These experiments raise several questions: does BDNF respond to l-NAME and, if so, is this dependent on an intact diurnal rhythm of corticosterone? If this were so, it might indicate that NO acted via BDNF to increase neurogenesis, and that this interaction was corticosterone-sensitive. The second question is whether the action of BDNF itself is dependent on the corticosterone rhythm. Would direct infusions of BDNF into the brain increase neurogenesis in the absence of such a rhythm? Furthermore, given the role of 5HT1A, would the same provisos apply to this class of serotonin receptor? BDNF infusions into the brain upregulate proliferation (Scharfman et al., 2005). Fluoxetine increases BDNF mRNA expression in the dentate gyrus, but only after around 14 days of treatment, a period also required for significant increments in the number of dividing progenitor cells (Altieri et al., 2004; De Foubert et al., 2004; Sairanen et al., 2005). NO can increase both neuronal differentiation and BDNF levels in cultures of embryonic cortex (Cheng et al., 2003).
In the experiments reported here, we explore the way that BDNF interacts with corticosterone in the regulation of the rate of proliferation of progenitor cells in the dentate gyrus. First, we show that injections of l-NAME upregulate the expression of BDNF and trkB mRNA. We go on to show that l-NAME is ineffective on BDNF in adrenalectomized (ADX) rats maintained on a constant dose of cortisosterone, but that the sensitivity of BDNF mRNA expression to l-NAME was restored by giving these rats a daily injection of corticosterone, which also restores their diurnal rhythm. We also show that intra-cerebroventricular (i.c.v.) infusions of BDNF stimulated progenitor proliferation on the side of the infusion only. They also markedly increased the expression of 5HT1A receptors (on both sides). Neither increased proliferative activity nor 5HT1A expression was observed in rats implanted with a corticosterone pellet (which flatten the diurnal rhythm), thus suggesting that sensitivity of the proliferative process to this growth factor and its regulation of 5HT1A receptors is moderated by glucocorticoids.
Materials and methods
Animals
All procedures were carried out under Home Office (UK) licence. Male Lister-Hooded rats (Harlan, Oxford, UK) were used; they weighed around 200-250 g at the start of the experiment. Rats were housed in groups of three per cage in a controlled environment except for experiment 3 in which the animals were caged individually. Ambient temperature was maintained at 21 °C and humidity at 55% with ad libitum access to food and tap water (and 0.9% saline for ADX animals). Animals were kept on reversed 12/12-h light-dark cycles (lights off at 10.00 h).
Experimental manipulations
Implants of corticosterone
In-house corticosterone pellets were prepared by melting cholesterol and corticosterone together at a ratio of 70 : 30. Each pellet weighed 200 mg.
Cannula placement
Animals were anaesthetized with isofluorane, oxygen and NO and placed securely into a stereotaxic frame (David Kopf instruments, Tujunga, CA, USA). A cannula (length 5 mm, outside diameter 0.36 mm; Charles River, Margate, UK) was implanted into the right lateral ventricle. Coordinates were 1 mm posterior and 1.5 mm lateral from bregma, -3.5 mm depth from cortex (Paxinos & Watson, 1998). The cannula were fixed in place by dental cement attached to two stainless steel screws inserted into the skull and connected to an Alzet osmotic minipump (model 1007D; volume 100 μL, flow rate 0.5 μL/h; Charles River) via medical grade vinyl tubing (6 cm length). All pumps were implanted subcutaneously in the posterior upper thorax and were filled the day before surgery. The pumps and tubing were filled with either recombinant human BDNF (1 μg/μL; Invitrogen, Paisley, UK) dissolved in phosphate-buffered saline (PBS) with 0.5% rat serum albumin (RSA) or PBS. They were incubated at 37 °C overnight in a sterile saline solution to prime them before implantation. Animals received 12 μg/day of recombinant human BDNF for 7 days (Pencea et al., 2001). Animals were weighed daily. The position of each cannula was assessed post-mortem by examining its track on sections stained with cresyl violet. Animals were anaesthetized with isoflurane, oxygen and NO injecting a terminal dose of pentobarbitone sodium.
In all experiments, blood samples were collected by cardiac puncture at 10.00 h (i.e. at the start of the dark phase) into heparinized syringes within 3 min of injecting a terminal dose of pentobarbitone sodium, centrifuged and stored at -20 °C until assayed for plasma corticosterone. Brains were collected, frozen immediately on dry ice and then stored at -70 °C until required.
Experimental groups
Experiment 1
The effect of giving l-NAME (daily injection) on the expression of BDNF and trkB mRNA in intact rats
This experiment explored the effect of the NOS inhibitor l-NAME on the expression of BDNF mRNA and trkB in the dentate gyrus. Two groups of 12 rats received daily intraperitoneal injections of either 0.9 saline (control) for 7 days or l-NAME (50 mg/kg dissolved in 0.9% saline). All animals were killed 24 h after the last injection and blood samples taken for corticosterone (10.00 h). The expression of BDNF and trkB mRNA was examined using in situ hybridisation.
Experiment 2
Effects of l-NAME on the expression of BDNF mRNA in ADX rats implanted with a 30% corticosterone pellet
This experiment tested whether BDNF mRNA expression following l-NAME was inhibited by ‘clamping’ plasma corticosterone, and whether this could be restored by adding daily corticosterone injections.
Twenty-four rats were adrenalectomised and implanted subcutaneously with a single 30% corticosterone/cholesterol pellet. The next day half of the animals received a daily injection of either corticosterone subcutaneously (2 mg/kg) or sesame oil at the beginning of the dark phase (10.00 h) for 12 days (n = 5 per group). Five days later half of each group received either a daily injection of 50 mg/kg l-NAME (dissolved in 0.9% saline) or a control injection (saline) for a further 7 days. All animals were killed 2 h after the last injection of l-NAME (10.00 h) and blood samples taken for corticosterone. Sections were stained for BDNF mRNA as above but also for glucocorticoid receptor (GR) and mineralocorticoid receptor (MR) mRNA.
Experiment 3
Effect of BDNF infusion into the lateral ventricle in intact rats treated with a 30% corticosterone pellet
One group of ten intact rats were implanted with a subcutaneous cholesterol pellet and a second group of 14 with a single 30% corticosterone/cholesterol pellet. Half of each of these two groups were also implanted with osmotic minipumps filled either with recombinant human BDNF with added 0.5% RSA or PBS with 0.5% RSA (see above). These pumps were attached to a cannula inserted into the right lateral ventricle. Seven days later all animals were killed and blood samples were taken for corticosterone assay (10.00 h); sections were stained for (1) Ki-67 or (2) 5HT1A receptor mRNA.
Brain sections
Coronal sections were taken from the entire length of the dorsal hippocampus and mounted on poly-lysine microscopic slides (BDH, Leicestershire, UK) and stored in -70 °C until required. Several series of sections, each one in six of those cut, were taken. All measurements were made on 12 sections for Ki-67, and three sections for in situ hybridization (see below for further details).
Corticosterone assay
Plasma corticosterone concentrations were measured by radioimmunoassay according to a validated procedure described previously (Chen & Herbert, 1995). The intra-assay coefficients of variation were: 5.1% for experiment 1, 6.2% for experiment 2 and 4.5% for experiment 3. The sensitivity of the assay was 0.98 ng/mL.
Immunohistochemistry
Sections for all the immunostainings were first fixed for 5 min in 4% paraformaldehyde (pH 7.4, Fisher, Loughborough, UK) then rinsed twice with K-PBS. For Ki-67 immunohistochemistry, sections were incubated in 0.01 m sodium citrate buffer (pH 6.0) for 40 min at 98 °C, rinsed, treated with hydrogen peroxide for 10 min, rinsed and then incubated overnight in K-PBS containing 1% normal horse serum, 0.5% Triton and a mouse monoclonal antibody against Ki-67 (1 : 100; Novocastra, Newcastle upon Tyne, UK). The sections were incubated with biotinylated secondary mouse IgG antibody and visualized with avidin-biotin-peroxidase complex, followed by diaminobenzidine reaction. They were then dehydrated by passing through graded alcohols and incubated in Histoclear overnight, and cover slipped under DPX for light microscopic analysis.
In-situ hybridization
Sections were allowed to air dry at room temperature and were then fixed with 4% paraformaldehyde (Sigma, Dorset, UK) for 5 min, washed in PBS and then dehydrated in 70% ethanol and 95% ethanol for 5 min before finally storing in fresh 95% ethanol. In-situ hybridization was carried out under RNAase-free conditions. The accuracy of synthetic antisense oligonucleotide probes was confirmed by blast searches on the NCBI database. A 48-base and a 45-base oligonucleotide complementary to exonic mRNA encoding BDNF and trkB mRNA (Al-Majed et al., 2000) 5′-AGTTCCAGTGCCTTTTGTCATGCCCCTGCAGCTTCCTTCGTGTAACCC-3′ and 5′-GAGAGGGCTGGCAGAGTCATCGTCGTTGCTGATGACGGAAGCTGG-3′ were used. For 5HT1A mRNA the probe was 5′-GGTTAGCGTGGGAGGAAGGGAGACTAGCTGTCTGAGCGACATACAAG-3′ (Fairchild et al., 2003). For GR the probe was 5′-AGGAGAATCCTCTGCTGCTTGGAATCTGCCTGA-3′ (McQuade et al., 2004), and for MR was 5′-TTCGGAATAGCACCGGAAACGCAGCTGACGTTGACAATCT-3′ (van Riel et al., 2003)
All probes were end-labelled with 35S-ATP as follows: 2 μL of purified oligonucleotide (5 ng/μL) was added to 1.25 μL buffer and 1.25 μL cobalt chloride (New England Biosystem, Hitchin, Hertfordshire, UK). DEPC-treated water (6.5 μL) was added, followed by 1 μL terminal 35S deoxyadenosine 5′ (α-thio) triphosphate (10 mCi/mL; Amersham, Bucks, UK) and 0.5 μL (15-20 U) terminal deoxynucleotide transferase enzyme (New England Biosystem). Probes were incubated at 37 °C for 1 h before 40 μL of DEPC was added to terminate the reaction. Purification of labelled probe from unincorporated nucleotides was accomplished by centrifugation (2092.5 g for 2 min) through a G-50 sephadex micro-column (Amersham, UK). Probes were evaluated for incorporation of radiolabel by scintillation counting. All hybridizations were carried out at 2500-5000 c.p.m./μL in hybridization buffer [50% deionized formamide, 4× SSC, 5× Denhardt’s, 100 μg/mL polyadenylic (potassium salt) acid, 200 μg/mL salmon sperm DNA, 120 μg/mL heparin (BDH), 25 mm sodium phosphate, pH 7.0, 1 mm sodium pyrophosphate, 10% (w/v) dextran sulphate in DEPC-treated water (all Sigma, Dorset, UK)]. Sections were covered with parafilm and hybridized overnight at 44 °C in a humid atmosphere. Excess unbound probe was removed using the following washes: slides were rinsed in 1× SSC (Sigma) at room temperature, washed twice for 30 min at 55 °C with 1× SSC and then rinsed at room temperature for 2 min, each in 1× SSC, 0.1× SSC, 18 Ω water, 50, 70 and 95% ethanol (BDH). Sections were thoroughly air-dried at room temperature before exposure to autoradiographic X-ray film (Amersham) for 7 days for BDNF, 14 days for trkB and 6 days for 5HT1A mRNA. Sense probes for each of the mRNAs were run as negative controls.
Quantification
Proliferating cells
All slides were randomized and coded prior to quantitative analysis. Sections were examined using a 40× objective. Ki-67-labelled stained cells were counted under experimentally ‘blind’ conditions. Only cells on the internal border of the subgranular zone were included. The data shown are the mean count per section obtained from 12 sections per animal.
mRNA expression
For quantification of mRNA, the sections and 14C-labelled standards of known radioactivity (Amersham) were placed in X-ray cassettes and then exposed to autoradiographic film. The optical density (OD) of the autoradiographic images was measured using a computerized PC-based image analysis system (NIH Image, North Carolina, USA). ODs were obtained from three consecutive sections per rat and the mean value for each rat was entered into the equation derived from the 14C standards. The final value was used to calculate group means. Sections from all groups were hybridized at the same time to avoid intrinsic variations between different in situ hybridizations.
Statistics
Results were analysed using one- or two-way anova where appropriate. Data were transformed if the variances of the means were not homogeneous. Pairwise comparisons were by made using the Bonferroni test unless otherwise indicated.
Results
The effect of l-NAME on expression of BDNF and trkB mRNAs in the dentate gyrus of intact rats
The number of Ki-67-labelled cells was markedly increased by l-NAME [F = 18.6, P < 0.001; reported previously (Pinnock et al., 2007) - data not shown]. l-NAME also significantly increased the expression of both BDNF and trkB mRNAs in the dentate gyrus (F = 467.5, P < 0.001; F = 179.4, P < 0.001; Fig. 1).
Fig. 1.
Expression of BDNF and trkB mRNA in the dentate gyrus of intact rats following treatment with l-NAME (50 mg/kg/day) for 7 days. Above: sections showing expression of the two mRNAs by in situ hybridization. Below: effects on optical density values in the dentate gyrus. Values are mean ± SEM. *P < 0.05, **P < 0.001 compared with control.
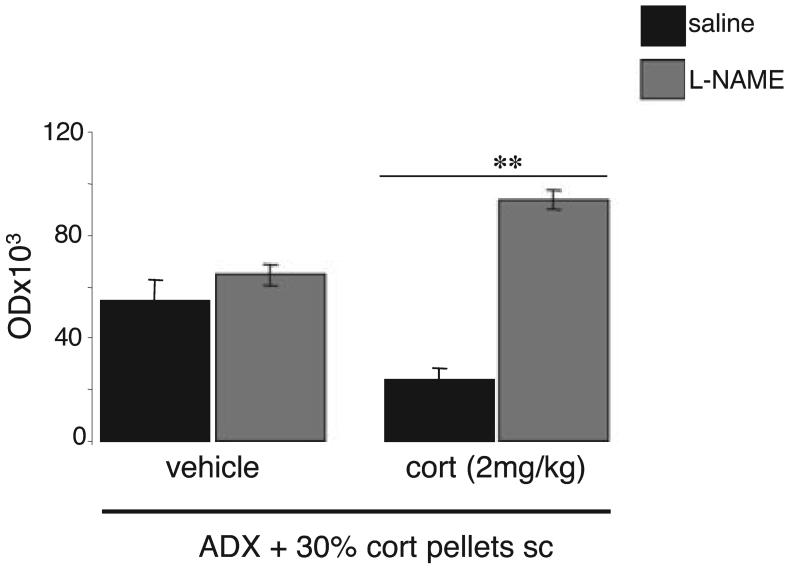
Effects of l-NAME on expression of BDNF mRNA in ADX rats implanted with 30% corticosterone pellets and given an additional daily injection of corticosterone (2 mg/kg)
The mean plasma level of corticosterone in these animals has already been reported (Pinnock et al., 2007). It should be noted that there was no significant difference between l-NAME-treated ADX rats given an extra daily dose of corticosterone and those receiving saline (Bonferroni: P > 0.05).
There was a highly significant interaction between l-NAME and corticosterone treatments (F = 35.1, P < 0.001) and further analysis, using pairwise comparisons, showed that whereas l-NAME had no effect on BDNF in ADX rats receiving a daily saline injection (Bonferonni: n.s.), there was a highly significant increase (P < 0.001) in those given daily additional corticosterone (2 mg/kg) which reconstituted the diurnal rhythm (Fig. 2).
Fig. 2.
Effect of l-NAME on BDNF mRNA expression in adrenalectomised (ADX) Lister-Hooded rats implanted with one 30% corticosterone pellet and receiving either additional (2 mg/kg/day) corticosterone or seasame oil daily at 10.00 h for 12 days. Values are mean ± SEM. **P < 0.001 compared with controls.
There was a significant interaction between additional daily injections of corticosterone and l-NAME on GR (F = 9.8, P = 0.008) but not on MR mRNA (F = 0.5, P = n.s.). Pairwise comparisons showed that MR was decreased by l-NAME (Bonferroni, P = 0.02) but not by daily additional corticosterone, whereas GR was unaffected by l-NAME but increased by daily corticosterone (P < 0.001; Table 1).
Table 1.
GR and MR mRNA in the dentate gyrus in ADX corticosterone-implanted rats treated with either l-NAME or daily injections of additional cortocosterone or both
| Treatment (in addition to ADX + 30% corticosterone pellet s.c.) | Optical density (pixels) |
|
|---|---|---|
| GR mRNA | MR mRNA | |
| Oil s.c. + saline i.p. | 112.6 ± 3.05 | 304.9 ± 32.57 |
| Oil s.c. + l-NAME (50 mg/kg) | 113.4 ± 2.60 | 244.2 ± 28.60 |
| Daily cort (2 mg/kg) + saline i.p. | 126.0 ± 2.36 | 277.7 ± 21.93 |
| Daily cort (2 mg/kg) + l-NAME (50 mg/kg) | 119.2±1.65 | 233.6±10.15 |
Data are presented as mean ± SD; Significance levels: Pairwise comparisons (Bonferroni): *P < 0.05, **P < 0.01 compared to control group.
Effect of BDNF infusions into the right lateral ventricle in intact rats with or without flattened diurnal corticosterone rhythms
One animal implanted with corticosterone and infused with BDNF died. Plasma corticosterone levels of the remainder are shown in Table 2. All values fell within the range of those expected at the start of the dark phase, and there were no significant differences between the four groups.
Table 2.
Plasma levels of corticosterone in rats implanted either with a cholesterol or a 30% corticosterone pellet s.c., and infused i.c.v. with either BDNF or solvent
| Treatment | n | Plasma corticosterone (ng/mL) |
|---|---|---|
| Saline (+RSA) i.c.v. + cholesterol s.c. | 5 | 187.2 ± 36.5 |
| Saline (+RSA) i.c.v. + corticosterone s.c. | 9 | 141.0 ± 45.4 |
| BDNF i.c.v. + cholesterol s.c. | 5 | 132.4 ± 72.6 |
| BDNF i.c.v. + corticosterone s.c. | 8 | 130.0 ± 23.6 |
Data are presented as mean ± SD; n, number of animals in each group.
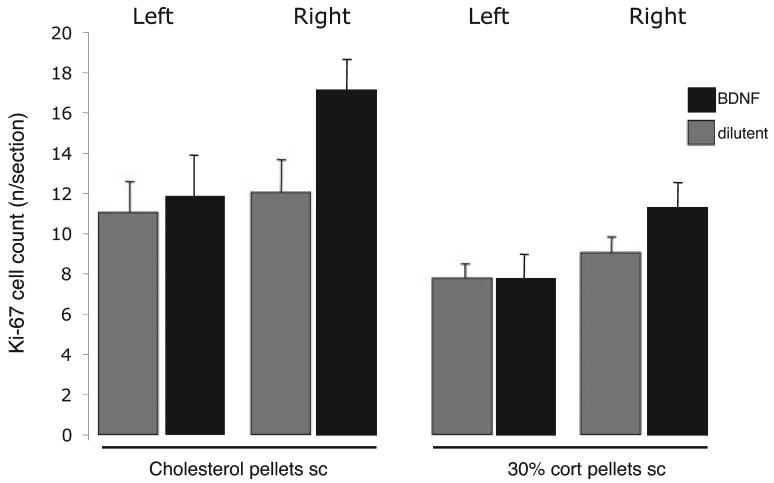
Overall, BDNF increased Ki-67 cell counts on the right (infused) side (F = 85.7, P = 0.022) but not on the other side (F = 0.05, P = n.s.; two-way anova). Corticosterone reduced Ki-67 cell counts in both the left and the right (BDNF-infused) dentate gyrus compared with control (right side: F = 8.13, P = 0.009; left: F = 4.99, P = 0.035; Fig. 3). There were no significant interactions between BDNF and corticosterone treatments. However, a pair-wise analysis showed that whereas BDNF significantly increased Ki-67 counts on the infused side in control animals (without a corticosterone pellet; F = 6.01, P = 0.04) this was not the case in the corticosterone-implanted animals (F = 1.37, P = n.s.). There were no significant changes on the other (non-infused) side in either group (one-way anova).
Fig. 3.
Effect of right-sided intra-cerebroventricular (i.c.v.) infusions of BDNF for 7 days on Ki-67 cell counts (mean ± SEM) in the left and right dentate gyrus in rats implanted subcutaneously with either cholesterol, or one 30% corticosterone pellet. Significance levels given in the text.
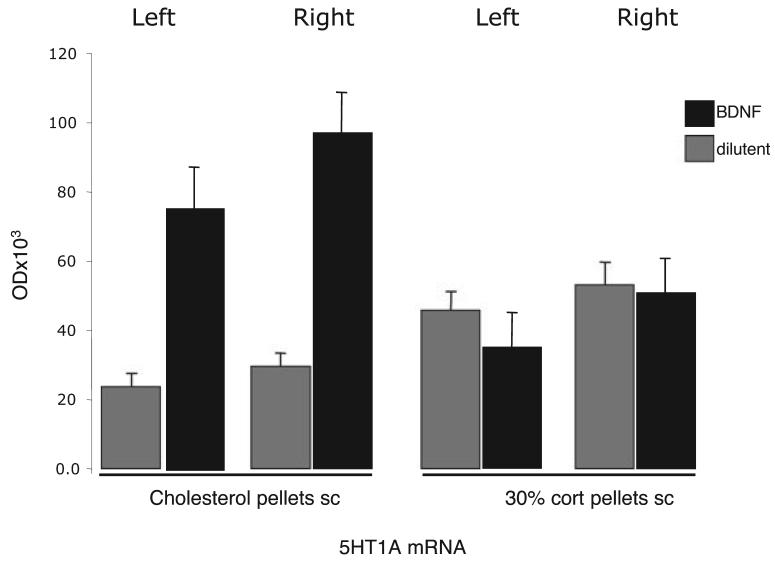
Overall, 5HT1A receptor mRNA reflected a highly significant interaction between BDNF and corticosterone on both the right (BDNF-infused) side (F = 19.85, P < 0.001) and the left (F = 16.75, P = 0.001) (two-way anova). BDNF increased 5HT1A mRNA on both sides (right: F = 16.5, P = 0.001; left: F = 7.2, P = 0.016), but corticosterone had no first-order effect (right, F = 2.1; left, F = 1.3; both P = n.s.). (Fig. 4). A pair-wise analysis confirmed that BDNF increased 5HT1A mRNA on both sides in animals receiving a cholesterol pellet (right: F = 35.2, P < 0.001; left: F = 20.7, P = 0.002). Repeated-measures analysis showed a greater response to BDNF on the infused side than on the other (F = 7.02, P = 0.029). However, BDNF had no effect on either side in animals treated with corticosterone (right, F = 0.08; left, F = 1.1; both P = n.s.).
Fig. 4.
Effect of right-sided intra-cerebroventricular infusions of BDNF for 7 days on the level of 5HT1A receptor mRNA in the dentate gyrus in intact rats implanted with either cholesterol pellets or one 30% corticosterone pellets. Values are mean optical density ± SEM. Significance levels are given in the text.
Discussion
Three significant findings were evident from the present study. l-NAME, a drug that is well established as a generalised inhibitor of NOS, has a powerful stimulating action on progenitor cell mitosis rates in the adult dentate gyrus (Packer et al., 2003; Park, 2003; Pinnock et al., 2007), which is prevented by administering an NO donor (Cheng et al., 2003). It also increases BDNF and trkB (its principal receptor) mRNA expression. A previous report, focused principally on the effects of exercise and with no measure of progenitor cell proliferation, and using a different dose of l-NAME, found a nonsignificant increase in BDNF alone (Chen et al., 2006). We show that this action, like that on the progenitor cells (Pinnock et al., 2007), is dependent on the presence of a diurnal rhythm in corticosterone, as it is not observed in ADX rats given a replacement corticosterone regime that replicates levels similar to that in intact rats, but lacking diurnal variation. Reinstating this rhythm by giving an additional, small, daily injection of corticosterone also reinstates the ability of l-NAME to increase BDNF mRNA. We measured the expression of both MR and GR to determine whether changes in these receptors might correlate with the effects of l-NAME or the efficacy of additional daily corticosterone. There was a main effect of l-NAME on MR; as this receptor type has been implicated in progenitor cell proliferation (Wong & Herbert, 2005), this might contribute to the actions of l-NAME on neurogenesis. However, the effects on GR were more complex, and the two treatments interacted. Although it might seem surprising that additional corticosterone increased GR expression in the dentate gyrus, we have found a similar result after 7 days of treatment of intact rats with higher daily doses of corticosterone (40 mg/kg; our unpublished data). It is evident that the two types of receptor react differentially to corticosterone (Conway-Campbell et al., 2007).
Given that it seems clear that both l-NAME and fluoxetine, a drug acting on serotonin, require the presence of an intact corticosterone rhythm to be effective, and both increase BDNF expression (Sairanen et al., 2005; present study), a major question was whether the action of BDNF itself also depended on the same rhythm. Our results show clearly that it does, as i.c.v. infusions of BDNF increased the number of Ki-67-labelled cells - an established index of mitotic activity, and one that correlates closely with BrdU labelling (Kee et al., 2002; Pinnock et al., 2007) - in the dentate gyrus after 7 days treatment in intact rats, but not in those whose daily rhythm had been flattened by a subcutaneous implant of corticosterone. Increased 5HT1A mRNA expression was also prevented by flattening the corticoid rhythm, a procedure that diminishes 5HT release in response to SSRIs (Gartside et al., 2003a).
It is very important to distinguish two actions of glucocorticoids on neurogenesis in the adult dentate gyrus, although it is not always so easy to separate them. The original observation that neurogenesis is markedly suppressed by increased levels of glucocorticoids (Cameron & Gould, 1994) has been amply confirmed, and extended to show that survival of newly generated neurons is also impaired (Wong & Herbert, 2004). Flattening the diurnal rhythm does not, in general, alter progenitor cell activity, although it should be noted that maintaining corticosterone levels near, say, normal peak values may actually increase overall exposure of the brain to glucocorticoids, as the usual daily nadir will be eliminated. However, an intact diurnal rhythm seems essential for alterations either in serotonin (e.g. induced by treatment with an SSRI such as fluoxetine) or in NO (following l-NAME treatment) to activate progenitor cells in the adult dentate gyrus (Huang & Herbert, 2006; Pinnock et al., 2007). These major regulatory factors thus depend on an intact diurnal glucocorticoid rhythm for their action. In contrast, the activity of the neurogenetic niche in the dentate gyrus is highly sensitive to absolute levels of glucocorticoids.
There is considerable evidence that BDNF may be implicated in the control of neurogenesis in the adult hippocampus. This area is the site of active axonal growth and synaptogenesis, as well as the formation of new neurons, and BDNF is known to play a central role in these processes (Monteggia et al., 2004) as well as in plastic alterations in the function of the hippocampus (e.g. long-term potentiation; Lu, 2003; Bramham & Messaoudi, 2005; Thomas & Davies, 2005). More specifically, treatments that increase neurogenesis (e.g. anti-depressant drugs such as fluoxetine, exercise) also increase BDNF (Dias et al., 2003; Adlard et al., 2004; Altieri et al., 2004; De Foubert et al., 2004; Sairanen et al., 2005). BDNF infusions stimulate progenitor cell mitosis (Scharfman et al., 2005; present study). Exposure to stress or to excess glucocorticoids, which depress neurogenesis, have been generally found to decrease BDNF (Schaaf et al., 1998; Hellsten et al., 2002; Rasmusson et al., 2002; Dwivedi et al., 2006; Hansson et al., 2006; Prickaerts et al., 2006), although there have been exceptions (Chao et al., 1998). These findings give rise to two further questions: is the regulation of BDNF by other regulatory factors dependent on the diurnal corticoid rhythm? And is its action on neurogenesis also moderated by the diurnal glucocorticoid rhythm? These are the questions addressed herein.
We show that whereas l-NAME stimulates both progenitor cells and BDNF in control rats, these are prevented by flattening the glucocorticoid rhythm, further reinforcing the putative link between stimulation of proliferation and increased BDNF expression. The essential role of the diurnal corticoid rhythm was confirmed in ADX corticosterone-implanted rats (which, of course, have no corticoid rhythm), and in which a daily additional injection of corticosterone (which restores this rhythm) also reinstated the effect l-NAME had on BDNF mRNA expression. It also, as we show elsewhere, enables l-NAME to activate the progenitor cells (Pinnock et al., 2007). If, however, the role of the diurnal corticoid rhythm was simply to enable alterations in NO or serotonin to increase BDNF (and hence stimulate the progenitor cells), then i.c.v. infusions of BDNF itself would bypass this control gate and still be effective even when the corticoid rhythm had been flattened. But this is not what we found. BDNF was unable to increase Ki-67 counts in rats in which corticosterone had been implanted subcutaneously, a procedure previously shown to flatten the daily rhythm (Gartside et al., 2003a,b; Huang & Herbert, 2006). In our experiments, the mean levels of corticosterone were not altered by this procedure, which argues against the actions of BDNF being inhibited by excess corticoid, although we did find that flattened rhythms in this experiment reduced overall levels of Ki-67-labelled cells. The caveat mentioned above regarding overall exposure of the brain to glucocorticoid under these conditions should be borne in mind. We conclude that the action of BDNF on progenitor cell mitosis is also gated by the diurnal rhythm in corticoids.
In view of the role of serotonin in the regulation of neurogenesis, our finding that BDNF increased the expression of 5HT1A mRNA is interesting. Agonists of this receptor increase progenitor cell proliferation (Huang & Herbert, 2005), although whether the response to SSRIs such as fluoxetine is preserved in mice with deleted 5HT1A receptors seems uncertain (Santarelli et al., 2003; Holick et al., 2008). Whereas there is considerable literature on BDNF in the hippocampus being increased by drugs acting on serotonin (see above), the converse is not true (Deltheil et al., 2007), although BDNF-deficient mice may have decreased 5HT1A activity (Daws et al., 2007) and interactions between serotonin and BDNF have been found to predict depression (Kaufman et al., 2006). In the present study, unilateral BDNF infusions increased 5HT1A mRNA expression in the dentate gyrus on both sides of the brain. But there was no associated increase in Ki-67 labelling on the contralateral side, suggesting that increased receptor expression by itself (without increased agonist activity) is not sufficient to stimulate progenitor cell division. However, just as a flattened corticosterone rhythm prevented the activating action of BDNF on progenitor cell division, so it did on the ability of BDNF to stimulate levels of 5HT1A mRNA. Is seems likely therefore that actions of BDNF on the brain in addition to neurogenesis may be dependent on the prevailing rhythm or levels of corticoids. What we do not yet know is whether these receptors are required for BDNF to increase neurogenesis.
Our results, together with those of earlier studies, allow us to generate a partial model of the control of the rate of proliferation of progenitor cells in the dentate gyrus. The regulating actions of at least two major control systems (serotonin and NO) are themselves subject to regulation by the presence of an intact diurnal corticoid rhythm, and this may limit access to activation of BDNF. The latter may represent at least part of the final common path of the control exerted by these two factors, but is itself subject to the corticoid rhythm. The mechanism responsible for this gating action is not yet known, but might involve rhythm-sensitive target genes such as the per clock genes, which are known to be expressed in the hippocampus, and are sensitive to corticoids (Hastings et al., 2003).
The results here do not add directly to the evidence linking neurogenesis in the dentate gyrus with the clinical phenomenon of depression, although they do suggest additional approaches for further research. Altered neurogenesis has been suggested to contribute to depression (Jacobs et al., 2000), and to its recovery following treatment with anti-depressants (Santarelli et al., 2003). BDNF is also thought to play a role in the occurrence of, or recovery from, depression (Kalueff et al., 2006; Hilt et al., 2007; Martinowich et al., 2007). Whether response to anti-depressants depends both on BDNF and, in turn, on current patterns (rhythms) of daily cortisol remains to be investigated. It is striking that higher levels of cortisol predispose to depression (Goodyer et al., 2000; Harris et al., 2000), that diurnal rhythms are often disturbed during the illness (Bridges & Jones, 1966), and that genetic polymorphisms in both serotonin and BDNF have been implicated in the risk for this condition (Caspi et al., 2003; Kaufman et al., 2006; Castren et al., 2007).
Acknowledgements
This research was supported by a grant from the Wellcome Trust. We thank Helen Shiers and Sarah Cleary for the corticosterone assays, and Wolfram Tetzlaff for help and guidance with the experiments.
Abbreviations
- ADX
adrenalectomized
- BDNF
brain-derived neurotropic factor
- i.c.v.
intra-cerebroventricular
- NO
nitric oxide
- NOS
nitric oxide synthase
- OD
optical density
- RSA
rat serum albumin
- SSRI
selective serotonin-reuptake inhibitor
References
- Adlard PA, Perreau VM, Engesser-Cesar C, Cotman CW. The timecourse of induction of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus following voluntary exercise. Neurosci. Lett. 2004;363:43–48. doi: 10.1016/j.neulet.2004.03.058. [DOI] [PubMed] [Google Scholar]
- Al-Majed AA, Brushart TM, Gordon T. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur. J. Neurosci. 2000;12:4381–4390. [PubMed] [Google Scholar]
- Altieri M, Marini F, Arban R, Vitulli G, Jansson BO. Expression analysis of brain-derived neurotrophic factor (BDNF) mRNA isoforms after chronic and acute antidepressant treatment. Brain Res. 2004;1000:148–155. doi: 10.1016/j.brainres.2003.12.028. [DOI] [PubMed] [Google Scholar]
- Barbacid M. The Trk family of neurotrophin receptors. J. Neurobiol. 1994;25:1386–1403. doi: 10.1002/neu.480251107. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Oliff HS, Isackson P, Cotman CW. Hippocampal BDNF mRNA shows a diurnal regulation, primarily in the exon III transcript. Brain Res. Mol. Brain Res. 1999;71:11–22. doi: 10.1016/s0169-328x(99)00137-0. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog. Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Bridges PK, Jones MT. The diurnal rhythm of plasma cortisol concentration in depression. Br. J. Psychiatry. 1966;112:1257–1261. doi: 10.1192/bjp.112.493.1257. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Castren E, Voikar V, Rantamaki T. Role of neurotrophic factors in depression. Curr. Opin. Pharmacol. 2007;7:18–21. doi: 10.1016/j.coph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Chao HM, Sakai RR, Ma LY, McEwen BS. Adrenal steroid regulation of neurotrophic factor expression in the rat hippocampus. Endocrinology. 1998;139:3112–3118. doi: 10.1210/endo.139.7.6114. [DOI] [PubMed] [Google Scholar]
- Chen X, Herbert J. Regional changes in c-fos expression in the basal forebrain and brainstem during adaptation to repeated stress: correlations with cardiovascular, hypothermic and endocrine responses. Neuroscience. 1995;64:675–685. doi: 10.1016/0306-4522(94)00532-a. [DOI] [PubMed] [Google Scholar]
- Chen MJ, Ivy AS, Russo-Neustadt AA. Nitric oxide synthesis is required for exercise-induced increases in hippocampal BDNF and phosphatidylinositol 3′ kinase expression. Brain Res. Bull. 2006;68:257–268. doi: 10.1016/j.brainresbull.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Cheng A, Wang S, Cai J, Rao MS, Mattson MP. Nitric oxide acts in a positive feedback loop with BDNF to regulate neural progenitor cell proliferation and differentiation in the mammalian brain. Dev. Biol. 2003;258:319–333. doi: 10.1016/s0012-1606(03)00120-9. [DOI] [PubMed] [Google Scholar]
- Conway-Campbell BL, McKenna MA, Wiles CC, Atkinson HC, de Kloet ER, Lightman SL. Proteasome-dependent downregulation of activated nuclear hippocampal glucocorticoid receptors determines dynamic responses to corticosterone. Endocrinology. 2007;148:5470–5477. doi: 10.1210/en.2007-0585. [DOI] [PubMed] [Google Scholar]
- Daws LC, Munn JL, Valdez MF, Frosto-Burke T, Hensler JG. Serotonin transporter function, but not expression, is dependent on brain-derived neurotrophic factor (BDNF): in vivo studies in BDNF-deficient mice. J. Neurochem. 2007;101:641–651. doi: 10.1111/j.1471-4159.2006.04392.x. [DOI] [PubMed] [Google Scholar]
- De Foubert G, Carney SL, Robinson CS, Destexhe EJ, Tomlinson R, Hicks CA, Murray TK, Gaillard JP, Deville C, Xhenseval V, Thomas CE, O’Neill MJ, Zetterstrom TS. Fluoxetine-induced change in rat brain expression of brain-derived neurotrophic factor varies depending on length of treatment. Neuroscience. 2004;128:597–604. doi: 10.1016/j.neuroscience.2004.06.054. [DOI] [PubMed] [Google Scholar]
- Deltheil T, Guiard BP, Guilloux JP, Nicolas L, Delomenie C, Reperant C, Maitre EL, Leroux-Nicollet I, Benmansour S, Coudore F, David DJ, Gardier AM. Consequences of changes in BDNF levels on serotonin neurotransmission, 5-HT transporter expression and function: studies in adult mice hippocampus. Pharmacol. Biochem. Behav. 2007 doi: 10.1016/j.pbb.2007.09.018. in press. [DOI] [PubMed] [Google Scholar]
- Dias BG, Banerjee SB, Duman RS, Vaidya VA. Differential regulation of brain derived neurotrophic factor transcripts by antidepressant treatments in the adult rat brain. Neuropharmacology. 2003;45:553–563. doi: 10.1016/s0028-3908(03)00198-9. [DOI] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch. Gen. Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Pandey GN. Antidepressants reverse corticosterone-mediated decrease in brain-derived neurotrophic factor expression: differential regulation of specific exons by antidepressants and corticosterone. Neuroscience. 2006;139:1017–1029. doi: 10.1016/j.neuroscience.2005.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild G, Leitch MM, Ingram CD. Acute and chronic effects of corticosterone on 5-HT1A receptor-mediated autoinhibition in the rat dorsal raphe nucleus. Neuropharmacology. 2003;45:925–934. doi: 10.1016/s0028-3908(03)00269-7. [DOI] [PubMed] [Google Scholar]
- Gartside SE, Leitch MM, McQuade R, Swarbrick DJ. Flattening the glucocorticoid rhythm causes changes in hippocampal expression of messenger RNAs coding structural and functional proteins: implications for aging and depression. Neuropsychopharmacology. 2003a;28:821–829. doi: 10.1038/sj.npp.1300104. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Herbert J, Tamplin A, Altham PM. Recent life events, cortisol, dehydroepiandrosterone and the onset of major depression in high-risk adolescents. Br. J. Psychiatry. 2000;177:499–504. doi: 10.1192/bjp.177.6.499. [DOI] [PubMed] [Google Scholar]
- Gould E, Cameron HA, Daniels DC, Woolley CS, McEwen BS. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J. Neurosci. 1992;12:3642–3650. doi: 10.1523/JNEUROSCI.12-09-03642.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Sommer WH, Metsis M, Stromberg I, Agnati LF, Fuxe K. Corticosterone actions on the hippocampal brain-derived neurotrophic factor expression are mediated by exon IV promoter. J. Neuroendocrinol. 2006;18:104–114. doi: 10.1111/j.1365-2826.2005.01390.x. [DOI] [PubMed] [Google Scholar]
- Harris TO, Borsanyi S, Messari S, Stanford K, Cleary SE, Shiers HM, Brown GW, Herbert J. Morning cortisol as a risk factor for subsequent major depressive disorder in adult women. Br. J. Psychiatry. 2000;177:505–510. doi: 10.1192/bjp.177.6.505. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat. Rev. Neurosci. 2003;4:649–661. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- Hellsten J, Wennstrom M, Mohapel P, Ekdahl CT, Bengzon J, Tingstrom A. Electroconvulsive seizures increase hippocampal neurogenesis after chronic corticosterone treatment. Eur. J. Neurosci. 2002;16:283–290. doi: 10.1046/j.1460-9568.2002.02093.x. [DOI] [PubMed] [Google Scholar]
- Hilt LM, Sander LC, Nolen-Hoeksema S, Simen AA. The BDNF Val66Met polymorphism predicts rumination and depression differently in young adolescent girls and their mothers. Neurosci. Lett. 2007;429:12–16. doi: 10.1016/j.neulet.2007.09.053. [DOI] [PubMed] [Google Scholar]
- Holick KA, Lee DC, Hen R, Dulawa SC. Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology. 2008;33:406–417. doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- Huang GJ, Herbert J. The role of 5-HT1A receptors in the proliferation and survival of progenitor cells in the dentate gyrus of the adult hippocampus and their regulation by corticoids. Neuroscience. 2005;135:803–813. doi: 10.1016/j.neuroscience.2005.05.056. [DOI] [PubMed] [Google Scholar]
- Huang GJ, Herbert J. Stimulation of neurogenesis in the hippocampus of the adult rat by fluoxetine requires rhythmic change in corticosterone. Biol. Psychiatry. 2006;59:619–624. doi: 10.1016/j.biopsych.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Praag H, Gage FH. Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol. Psychiatry. 2000;5:262–269. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Avgustinovich DF, Kudryavtseva NN, Murphy DL. BDNF in anxiety and depression. Science. 2006;312:1598–1599. doi: 10.1126/science.312.5780.1598. Author reply 1598-1599. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Grasso D, Lipschitz D, Houshyar S, Krystal JH, Gelernter J. Brain-derived neurotrophic factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biol. Psychiatry. 2006;59:673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Kee N, Sivalingam S, Boosntra R, Wojtowicz JM. The utility of KI-67 and BrdU as proliferative markers of adult neurogenesis. J. Neurosci. Methods. 2002;115:97–105. doi: 10.1016/s0165-0270(02)00007-9. [DOI] [PubMed] [Google Scholar]
- Lu B. BDNF and activity-dependent synaptic modulation. Learn. Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat. Neurosci. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- McQuade RLM, Gartside SE, Young AH. Effect of chronic lithium treatment on glucocorticoid and 5-HT1A receptor messenger RNA in hippocampal and dorsal raphe nucleus regions of the rat brain. J. Psychopharmacol. 2004;18:496–501. doi: 10.1177/026988110401800406. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T, Meuth S, Nagy A, Greene RW, Nestler EJ. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc. Natl Acad. Sci. USA. 2004;101:10827–10832. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Kim JE, Lee R, Chen J, Fujioka T, Malberg J, Tsuji S, Duman RS. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J. Neurosci. 2002;22:3673–3682. doi: 10.1523/JNEUROSCI.22-09-03673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J. Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer MA, Stasiv Y, Benraiss A, Chmielnicki E, Grinberg A, Westphal H, Goldman SA, Enikolopov G. Nitric oxide negatively regulates mammalian adult neurogenesis. Proc. Natl Acad. Sci. USA. 2003;100:9566–9571. doi: 10.1073/pnas.1633579100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C. The chronic inhibition of nitric oxide synthase enhances cell proliferation in the adult rat hippocampus. Neurosci. Lett. 2003;339:9–12. doi: 10.1016/s0304-3940(02)01422-2. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson CR. The Rat Brain in Sterotaxic Coordinates. Academic Press; New York: 1998. [Google Scholar]
- Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J. Neurosci. 2001;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnock SB, Balendra R, Chan M, Hunt LT, Turner-Stokes T, Herbert J. Interactions between nitric oxide and corticosterone in the regulation of progenitor cell proliferation in the dentate gyrus of the adult rat. Neuropsychopharmacology. 2007;32:493–504. doi: 10.1038/sj.npp.1301245. [DOI] [PubMed] [Google Scholar]
- Prickaerts J, van den Hove DL, Fierens FL, Kia HK, Lenaerts I, Steckler T. Chronic corticosterone manipulations in mice affect brain cell proliferation rates, but only partly affect BDNF protein levels. Neurosci. Lett. 2006;396:12–16. doi: 10.1016/j.neulet.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Shi L, Duman R. Downregulation of BDNF mRNA in the hippocampal dentate gyrus after re-exposure to cues previously associated with footshock. Neuropsychopharmacology. 2002;27:133–142. doi: 10.1016/S0893-133X(02)00286-5. [DOI] [PubMed] [Google Scholar]
- van Riel EMO, Steenbergen PJ, Joels M. Chronic unpredictable stress causes attenuation of serotonin responses in cornu ammonis 1 pyramidal neurons. Neuroscience. 2003;120:649–658. doi: 10.1016/s0306-4522(03)00355-5. [DOI] [PubMed] [Google Scholar]
- Sairanen M, Lucas G, Ernfors P, Castren M, Castren E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J. Neurosci. 2005;25:1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Schaaf MJ, de Jong J, de Kloet ER, Vreugdenhil E. Downregulation of BDNF mRNA and protein in the rat hippocampus by corticosterone. Brain Res. 1998;813:112–120. doi: 10.1016/s0006-8993(98)01010-5. [DOI] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp. Neurol. 2005;192:348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J. Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K, Davies A. Neurotrophins: a ticket to ride for BDNF. Curr. Biol. 2005;15:R262–R264. doi: 10.1016/j.cub.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Wong EY, Herbert J. The corticoid environment: a determining factor for neural progenitors’ survival in the adult hippocampus. Eur. J. Neurosci. 2004;20:2491–2498. doi: 10.1111/j.1460-9568.2004.03717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EY, Herbert J. Roles of mineralocorticoid and glucocorticoid receptors in the regulation of progenitor proliferation in the adult hippocampus. Eur. J. Neurosci. 2005;22:785–792. doi: 10.1111/j.1460-9568.2005.04277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]