Abstract
Background
Essential tremor (ET) is one of the most common neurological diseases. A basic understanding of its neuropathology is now emerging. Aside from Purkinje cell loss, a prominent finding is an abundance of torpedoes (rounded swellings of Purkinje cell axons). Such swellings often result from the mis-accumulation of cell constituents. Identifying the basic nature of these accumulations is an important step in understanding the underlying disease process. Torpedoes, only recently identified in ET, have not yet been characterized ultrastructurally.
Objectives
Light and electron microscopy were used to characterize the structural constituents of torpedoes in ET.
Methods
Formalin-fixed cerebellar cortical tissue from 4 prospectively-collected ET brains was sectioned and immunostained with a monoclonal phosphorylated neurofilament antibody (SMI-31, Covance, Emeryville, CA). Using additional sections from 3 ET brains, torpedoes were assessed using electron microscopy.
Results
Immunoreactivity for phosphorylated neurofilament protein revealed clear labeling of torpedoes in each case. Torpedoes were strongly immunoreactive; in many instances, two or more torpedoes were noted in close proximity to one another. On electron microscopy, torpedoes were packed with randomly arranged 10–12 nm neurofilaments. Mitochondria and smooth endoplasmic reticulum were abundant as well, particularly at the periphery of the torpedo.
Conclusions
We demonstrated that the torpedoes in ET represent the mis-accumulation of disorganized neurofilaments and other organelles. It is not known where in the pathogenic cascade these accumulations occur (i.e., whether these accumulations are the primary event or a secondary/downstream event) and this deserves further study.
Keywords: essential tremor, pathology, Purkinje cell, torpedo, neurofilament, electron microscopy
Introduction
Essential tremor (ET) is a progressive and highly-prevalent, age-associated neurological disease [3, 9]. Treatment options are limited, in part because the disease mechanisms have remained elusive [30]. Until recently, there had been few postmortems. With more intensive efforts to perform detailed postmortems; however, an understanding of the basic anatomic pathology is beginning to emerge. The majority of ET brains have pathological changes involving the cerebellum [1, 15, 16, 18, 28]. In addition to a modest yet significant loss of Purkinje cells, we have found in previous work that torpedoes are a prominent finding (six to seven times more abundant than in age-matched control brains) [15, 16, 18]. Based on these findings, the notion that this disorder might be degenerative has been proposed [16]. The torpedo is a rounded swelling of the proximal portion of the Purkinje cell axon; the swelling is thought to result from the mis-accumulation of normal or abnormal cell constituents in disease states. In primary cerebellar degenerative conditions, torpedoes consist primarily of neurofilament mis-accumulations [24]. We used both light and electron microscopy to further characterize the structural constituents of torpedoes in patients with ET, a disorder which similarly may be neurodegenerative.
Methods
Brain Collection
ET brains were prospectively-collected at the Essential Tremor Centralized Brain Repository (ETCBR) at Columbia University. As described previously [1, 15], brains were collected from members of the International Essential Tremor Foundation who during life had expressed an interest in brain donation. Each had been diagnosed during life with ET and additionally, as detailed previously [15], an ETCBR neurologist who specialized in movement disorders re-confirmed their diagnoses using ETCBR diagnostic criteria. All brains were well characterized, including complete neuropathological assessment and determination of any pathological findings [15]. As part of that assessment, a 3 × 20 × 25 mm parasagittal tissue block that came from the same region of the neocerebellum and including, in continuity, the cerebellar cortex, white matter and dentate nucleus was harvested from each brain and then immersion-fixed in 10% buffered formalin. Paraffin sections (7 μm) were stained with Luxol Fast Blue and Hematoxylin and Eosin (LH&E) for general tissue survey and quantification of torpedoes [15, 17, 18]. The numbers of torpedoes in one entire LH&E section and another entire Bielschowsky-stained section were counted, as described previously [15]. Purkinje cells were quantified as described previously [15]. We have shown that our counts on one section correlate robustly with counts on other sections from the same cases (r = 0.86, p <0.001), indicating that repeat sectioning and counting yields similar results. For the current analyses, we selected four ET cases with high torpedo counts.
Neurofilament Immunohistochemistry
Formalin-fixed tissue from the cerebellar cortex was sectioned (100 μm) using a TPI vibratome (TPI, St. Louis, MO). Sections were incubated in 0.1% proteinase-K (Sigma, St. Louis, MO), 10 mM TRIS (pH 8.0), and 0.1 mM EDTA at 37 ºC (10 minutes) and then at room temperature (20 minutes). Sections were washed in phosphate-buffered saline, pH 7.4 (PBS) before a 30 minute incubation in 3% H2O2 and then re-washed before a 2 hour antibody block (10% normal goat serum, 1% bovine serum albumin, 0.5 % Triton X in PBS) at room temperature. Sections were rinsed in PBS and then incubated overnight at 4 ºC in a monoclonal phosphorylated neurofilament antibody in 1% bovine serum albumin, 0.5 % Triton X in PBS (SMI-31, Covance, Emeryville, CA; 1:1000). Sections were washed again and then treated for 2 hours at room temperature with secondary antibody (peroxidase conjugated goat anti-mouse IgG)(Jackson Immunoresearch, West Grove, PA), and then visualized with chromogen 3,3-diaminobenzidine (DAB, Vector Labs, Burlingame, CA). For double-labeling immunofluorescence, 7 μm-thick paraffin-embedded sections were rehydrated, treated with Trilogy solution (Cell Marque, Rocklin, CA) in a conventional steamer for 40 minutes, and blocked with Image-iT FX Signal Enhancer (Invitrogen, Carlsbad, CA) for 30 minutes. Sections were incubated with rabbit polyclonal anti-calbindin-D28k (Sigma Chemical Co., St. Louis, MO) and mouse monoclonal SMI-31 (Covance, Princeton, NJ) in antibody diluent (Dako, Glostrup, Denmark) 1:1000 at 4 °C overnight. Sections were then incubated with anti-rabbit and anti-mouse goat secondary antibodies conjugated to Alexa Fluor 488 and Alexa Fluor 595, respectively (Invitrogen, Carlsbad, CA) in Dako antibody diluent 1:1000 at room temperature for one hour. All washes were done in 10 mM PBS-Triton X.
Electron Microscopy
Flat Eponate 12 (Ted Pella, Redding, CA) embedded vibratome sections of cerebellar cortical tissue were dissected into 3 × 4 mm sections and re-embedded in the same resin. Semi-thin sections were then stained with toluidine blue and examined by light microscopy for torpedoes. Once a torpedo was identified on semi-thin section, the block was further trimmed around the torpedo to the size required for ultrathin sectioning. Ultrathin sections were stained with uranyl acetate and lead citrate and then examined by transmission electron microscope (JEOL 1200EXII, Peabody, MA).
Results
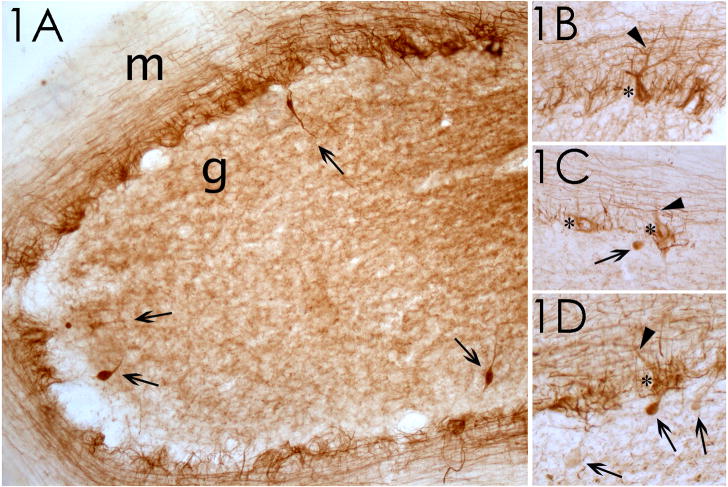
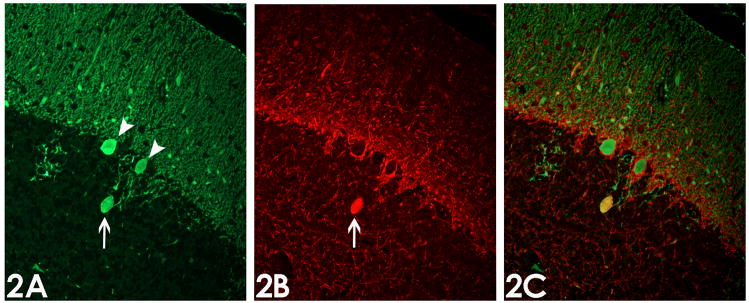
The four ET cases (median age at death = 89.5 years, all women, median duration of tremor = 42.5 years) had high torpedo counts (17.8 ± 3.5 per LH&E-stained section and 22.0 ± 9.7 per Bielschowsky-stained section) and low Purkinje cell counts (5.7 ± 2.9 per 100x field). On LH&E stained sections, as demonstrated previously (Figure 2 in [16] and Figure 2 in [17]), the torpedoes appeared in the granular layer, near the origin of the Purkinje cell axons, as rounded or ovoid structures, having a homogeneous, glassy, eosinophilic appearance. Immunoreactivity for phosphorylated neurofilament protein revealed a discrete pattern of labeling in each case (Figure 1). Immunoreactivity was concentrated in basket cell processes in the Purkinje cell layer and axonal processes in the lower molecular layer, which likely represent parallel fibers. Torpedoes were strongly immunoreactive, providing a distinct contrast between more weakly stained axonal profiles in the granule cell layer; in many instances, two or more torpedoes were observed in close proximity to one another (Figure 1A, D). The torpedoes had a characteristic ovoid shape and, in most instances, the thin, flanking segments of the axon in continuity to the torpedo were also strongly immunoreactive. Occasional Purkinje cell somata and dendrites were also immunoreactive (Figure 1B, C, D); in some instances the axonal process associated with these labeled Purkinje cells contained a torpedo. Thirty-four (94.4%) of 36 torpedoes that were immunoreactive for calbindin were immunoreactive for phosphorylated neurofilament protein as well (Figure 2).
Figure 1.
Phosphorylated neurofilament staining of cerebellum in ET cases. (1A) Case 1; the granular cell layer (g) and molecular layer (m) are identified. 100x magnification. Four torpedoes, in the granular cell layer, are marked by arrows. (1B–1D) Cases 2 and 3; pathologic accumulation of phosphorylated neurofilaments in Purkinje cell body (asterisks) and proximal dendrites (arrowheads). In some instances, the axon of these Purkinje cells contains a torpedo (arrows in 1C, 1D). 200x magnification.
Figure 2.
Immunofluorescence analysis of torpedoes. (2A) Purkinje cell bodies (arrow heads) and torpedo (arrow) are immunoreactive for calbindin. (2B) Torpedo (arrow) but not Purkinje cell bodies are immunoreactive for phosphorylated neurofilament protein. (2C) Overlay of calbindin and phosphorylated neurofilament protein immunostains shows co-localization of these proteins (yellow color). Magnification 200x.
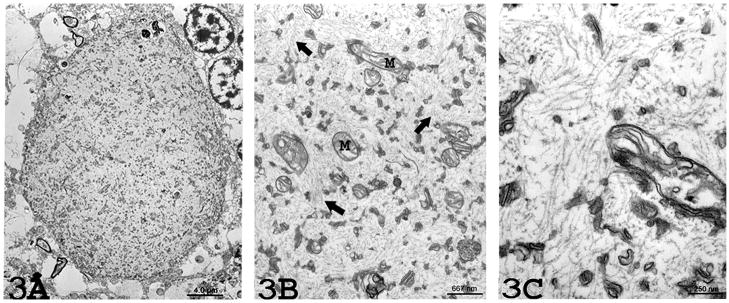
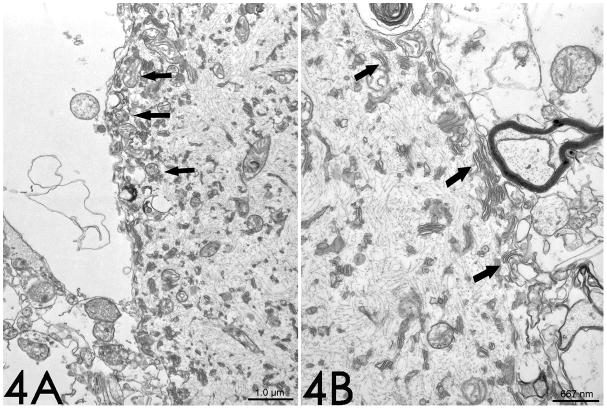
Electron microscopy revealed an absence of myelin around the torpedo in each of the three cases. The torpedo was filled with numerous randomly-arranged neurofilaments, measuring 10 – 12nm in diameter (Figures 3 and 4); there was no consistent orientation of these neurofilaments, which were cut longitudinally, diagonally, or transversely. Scattered mitochondria and stacks of smooth endoplasmic reticulum were also noted among the neurofilaments. These organelles also occurred particularly at the periphery of the torpedo, where their concentration was the greatest (Figure 4). The mitochondria were often intimately associated with the smooth endoplasmic reticulum. A few autophagic vacuoles were also observed (Figure 3A).
Figure 3.
(3A) Electron microscopy (2,500x magnification) from Case 1 reveals an absence of myelin around the torpedo. The torpedo is comprised of an aggregate of randomly-arranged neurofilaments with scattered organelles. (3B) Higher (15,000x) magnification revealing mitochondria (M) and occasional membrane stacks in a matrix of numerous, disorganized neurofilaments (arrows). (3C) Higher (40,000x) magnification reveals numerous randomly arranged neurofilaments measuring 10 – 12nm nm in diameter.
Figure 4.
Case 1. On electron microscopy, (4A) mitochondria (arrows) (10,000x magnification) and (4B) smooth endoplasmic reticulum (arrows) (15,000x magnification) are present predominantly at the periphery of the torpedo, and are often intimately associated.
Discussion
In light microscopic studies [1, 15], we have found that torpedoes are a prominent finding in ET. In primary cerebellar degenerative conditions, torpedoes consist primarily of neurofilament mis-accumulations [24]. The notion that ET is a degenerative disease is relatively new and the ultrastructural features of these lesions had not previously characterized in ET.
Torpedoes occur when cellular material over-accumulates in the Purkinje cell axon, resulting in a swelling. Depending on the underlying disease mechanism, the accumulated material can differ. In this study, we demonstrated that torpedoes in ET consist of mis-accumulation of disordered neurofilaments. Other structures (mitochondria and endoplasmic reticulum) were also abundant, especially at the periphery of the torpedo, where they may have been displaced by the disorganized accumulation of neurofilaments.
Neurofilaments represent the predominant filamentous structures in the axon and they are essential for axonal maintenance [14]. Neurofilament-filled axonal swellings can occur in neurons other than Purkinje cells. In spinal motor neurons, these neurofilament-filled axonal swellings, referred to as “spheroids”, have long been known to be a pathological hallmark of fundamental importance in motor neuron diseases, including amyotrophic lateral sclerosis and infantile spinal muscular atrophy [2, 4, 7, 8, 14, 29]. Neurofilament mis-accumulations and resulting axonal swellings are thought to inhibit both anterograde and retrograde axonal transports, ultimately leading to cell “strangulation” [2, 6, 14, 25]. This strangulation then leads to the selective degeneration, and eventually death, of these neurons [19, 21, 22, 26, 27]. Thus, defects in transport of neurofilaments clearly lead to abnormal accumulations of axonal intermediate filaments and neuronal degenerative changes [14, 29]. The pathological accumulation of phosphorylated neurofilament epitopes in occasional Purkinje cell bodies and dendrites in ET cases further suggests that impaired axoplasmic transport may play a role in this disorder [11, 13].
There have been very few electron microscopic studies of torpedoes and none of torpedoes in ET. Prior studies include a report of biopsy tissue from a five-year old boy with a cerebellar astrocytoma [20], an autopsy case with “circulatory disturbances” [23], and two cases of olivopontocerebellar atrophy [24]; in each of these electron microscopic studies, torpedoes were also comprised of a mis-accumulation of disorganized neurofilaments and cellular organelles. In all three ET cases in this study, the torpedoes examined ultrastructurally were unmyelinated, suggesting their origin within the initial segment of the Purkinje cell axon per se or a nearby demyelinated proximal segment [20]. The spheroids in amyotrophic lateral sclerosis also occur in the proximal portion of the motor neuron axon, although these are also observed in myelinated segments [10]. While mouse models employing abnormal expression of the various neurofilament proteins often result in spheroids in proximal spinal motor neuron axons [14], overexpression of the α-internexin neuronal intermediate filament protein led to numerous cerebellar torpedoes in myelinated segments of Purkinje cell axons [5]. Ultrastructural identification of the connection of a torpedo to its parent axon is needed to more closely define the point of axonal dysfunction in ET.
This was a focused study of the structural characteristics of torpedoes in ET; future study of additional structural changes in ET as well as other forms of cerebellar degeneration would be of interest.
We demonstrated that the swellings in the ET axon represent the mis-accumulation of disorganized neurofilaments and organelles. It is not known where in the pathogenic cascade these accumulations occur. One possibility is that these filament accumulations are a primary or initiating event. For example, in motor neuron disease, glycation products, perhaps through the generation of neurofilament aggregates, are thought to play an early role in the pathomechanism [12]. A second possibility is that these filament accumulations represent a secondary or downstream occurrence in the molecular pathological cascade. A third possibility is that they may represent an attempt at axonal and cellular reorganization and regeneration. Further study of these structures within the context of the pathological anatomy of this common yet poorly-understood disease is warranted.
Acknowledgments
We would also like to acknowledge the guidance of William T. Dauer MD, Columbia University.
Funding Source
R01 NS42859 from the National Institutes of Health (Bethesda, MD); the Parkinson’s Disease Foundation (New York, NY); the Arlene Bronstein Essential Tremor Research Fund (Columbia University); and the Claire O’Neil Essential Tremor Research Fund (Columbia University).
Footnotes
Disclosure: The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Axelrad JE, Louis ED, Honig LS, Flores I, Ross GW, Pahwa R, Lyons KE, Faust PL, Vonsattel JP. Reduced Purkinje cell number in essential tremor: a postmortem study. Archives of Neurology. 2008;65:101–107. doi: 10.1001/archneurol.2007.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaulieu JM, Nguyen MD, Julien JP. Late onset of motor neurons in mice overexpressing wild-type peripherin. J Cell Biol. 1999;147:531–544. doi: 10.1083/jcb.147.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benito-Leon J, Louis ED. Essential tremor: emerging views of a common disorder. Nature clinical practice. 2006;2:666–678. doi: 10.1038/ncpneuro0347. [DOI] [PubMed] [Google Scholar]
- 4.Brady ST. Motor neurons and neurofilaments in sickness and in health. Cell. 1993;73:1–3. doi: 10.1016/0092-8674(93)90151-f. [DOI] [PubMed] [Google Scholar]
- 5.Ching GY, Chien CL, Flores R, Liem RK. Overexpression of alpha-internexin causes abnormal neurofilamentous accumulations and motor coordination deficits in transgenic mice. J Neurosci. 1999;19:2974–2986. doi: 10.1523/JNEUROSCI.19-08-02974.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cleveland DW, Rothstein JD. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci. 2001;2:806–819. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- 7.Corbo M, Hays AP. Peripherin and neurofilament protein coexist in spinal spheroids of motor neuron disease. Journal of Neuropathology and Experimental Neurology. 1992;51:531–537. doi: 10.1097/00005072-199209000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Cote F, Collard JF, Julien JP. Progressive neuronopathy in transgenic mice expressing the human neurofilament heavy gene: a mouse model of amyotrophic lateral sclerosis. Cell. 1993;73:35–46. doi: 10.1016/0092-8674(93)90158-m. [DOI] [PubMed] [Google Scholar]
- 9.Dogu O, Sevim S, Camdeviren H, Sasmaz T, Bugdayci R, Aral M, Kaleagasi H, Un S, Louis ED. Prevalence of essential tremor: door-to-door neurologic exams in Mersin Province, Turkey. Neurology. 2003;61:1804–1806. doi: 10.1212/01.wnl.0000099075.19951.8c. [DOI] [PubMed] [Google Scholar]
- 10.Hirano A, Donnenfeld H, Sasaki S, Nakano I. Fine structural observations of neurofilamentous changes in amyotrophic lateral sclerosis. Journal of Neuropathology and Experimental Neurology. 1984;43:461–470. doi: 10.1097/00005072-198409000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Kato S, Hayashi H, Mikoshiba K, Hirano A, Yen SH, Ohama E. Purkinje cells in olivopontocerebellar atrophy and granule cell-type cerebellar degeneration: an immunohistochemical study. Acta Neuropathol. 1998;96:67–74. doi: 10.1007/s004010050861. [DOI] [PubMed] [Google Scholar]
- 12.Kikuchi S, Ogata A, Shinpo K, Moriwaka F, Fujii J, Taniguchi N, Tashiro K. Detection of an Amadori product, 1-hexitol-lysine, in the anterior horn of the amyotrophic lateral sclerosis and spinobulbar muscular atrophy spinal cord: evidence for early involvement of glycation in motoneuron diseases. Acta Neuropathol. 2000;99:63–66. doi: 10.1007/pl00007407. [DOI] [PubMed] [Google Scholar]
- 13.Koeppen AH. The Purkinje cell and its afferents in human hereditary ataxia. Journal of Neuropathology and Experimental Neurology. 1991;50:505–514. doi: 10.1097/00005072-199107000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Liem RK, Leung CL. Neuronal intermediate filament overexpression and neurodegeneration in transgenic mice. Exp Neurol. 2003;184:3–8. doi: 10.1016/s0014-4886(03)00291-7. [DOI] [PubMed] [Google Scholar]
- 15.Louis ED, Faust PL, Vonsattel JP, Honig LS, Rajput A, Robinson CA, Pahwa R, Lyons KE, Ross GW, Borden S, Moskowitz CB, Lawton A, Hernandez N. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130:3297–3307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 16.Louis ED, Vonsattel JP. The emerging neuropathology of essential tremor. Mov Disord. 2007;23:174–182. doi: 10.1002/mds.21731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louis ED, Vonsattel JP, Honig LS, Lawton A, Moskowitz C, Ford B, Frucht S. Essential tremor associated with pathologic changes in the cerebellum. Archives of Neurology. 2006;63:1189–1193. doi: 10.1001/archneur.63.8.1189. [DOI] [PubMed] [Google Scholar]
- 18.Louis ED, Vonsattel JP, Honig LS, Ross GW, Lyons KE, Pahwa R. Neuropathologic findings in essential tremor. Neurology. 2006;66:1756–1759. doi: 10.1212/01.wnl.0000218162.80315.b9. [DOI] [PubMed] [Google Scholar]
- 19.Ma WY, Vacca-Galloway LL. Reduced branching and length of dendrites detected in cervical spinal cord motoneurons of Wobbler mouse, a model for inherited motoneuron disease. The Journal of Comparative Neurology. 1991;311:210–222. doi: 10.1002/cne.903110204. [DOI] [PubMed] [Google Scholar]
- 20.Mann DM, Stamp JE, Yates PO, Bannister CM. The fine structure of the axonal torpedo in Purkinje cells of the human cerebellum. Neurol Res. 1980;1:369–378. doi: 10.1080/01616412.1980.11739567. [DOI] [PubMed] [Google Scholar]
- 21.March PA, Thrall MA, Brown DE, Mitchell TW, Lowenthal AC, Walkley SU. GABAergic neuroaxonal dystrophy and other cytopathological alterations in feline Niemann-Pick disease type C. Acta Neuropathol (Berl) 1997;94:164–172. doi: 10.1007/s004010050689. [DOI] [PubMed] [Google Scholar]
- 22.Mentis GZ, Diaz E, Moran LB, Navarrete R. Early alterations in the electrophysiological properties of rat spinal motoneurones following neonatal axotomy. J Physiol. 2007;582:1141–1161. doi: 10.1113/jphysiol.2007.133488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizushima OSS. An ultrastructural observation of torpedoes in the human degenerative cerebellum. J Clinical Electron Microscopy. 1976;9:672–673. [Google Scholar]
- 24.Petito CK, Hart MN, Porro RS, Earle KM. Ultrastructural studies of olivopontocerebellar atrophy. Journal of Neuropathology and Experimental Neurology. 1973;32:503–522. doi: 10.1097/00005072-197310000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Robertson J, Kriz J, Nguyen MD, Julien JP. Pathways to motor neuron degeneration in transgenic mouse models. Biochimie. 2002;84:1151–1160. doi: 10.1016/s0300-9084(02)00025-1. [DOI] [PubMed] [Google Scholar]
- 26.Rossi F, Borsello T, Strata P. Exposure to kainic acid mimics the effects of axotomy in cerebellar Purkinje cells of the adult rat. Eur J Neurosci. 1994;6:392–402. doi: 10.1111/j.1460-9568.1994.tb00282.x. [DOI] [PubMed] [Google Scholar]
- 27.Sasaki S, Iwata M. Dendritic synapses of anterior horn neurons in amyotrophic lateral sclerosis: an ultrastructural study. Acta Neuropathol. 1996;91:278–283. doi: 10.1007/s004010050426. [DOI] [PubMed] [Google Scholar]
- 28.Shill HA, Adler CH, Sabbagh MN, Connor DJ, Caviness JN, Hentz JG, Beach TG. Pathologic findings in prospectively ascertained essential tremor subjects. Neurology. 2008;70:1452–1455. doi: 10.1212/01.wnl.0000310425.76205.02. [DOI] [PubMed] [Google Scholar]
- 29.Xu Z, Cork LC, Griffin JW, Cleveland DW. Increased expression of neurofilament subunit NF-L produces morphological alterations that resemble the pathology of human motor neuron disease. Cell. 1993;73:23–33. doi: 10.1016/0092-8674(93)90157-l. [DOI] [PubMed] [Google Scholar]
- 30.Zesiewicz TA, Elble R, Louis ED, Hauser RA, Sullivan KL, Dewey RB, Jr, Ondo WG, Gronseth GS, Weiner WJ. Practice parameter: therapies for essential tremor: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2005;64:2008–2020. doi: 10.1212/01.WNL.0000163769.28552.CD. [DOI] [PubMed] [Google Scholar]