Abstract
Dysregulation of localized iron homeostasis is implicated in several degenerative diseases, including Parkinson’s, Alzheimer’s, and age-related macular degeneration, wherein iron-mediated oxidative stress is hypothesized to contribute to cell death. Inhibiting toxic iron without altering normal metal-dependent processes presents significant challenges for standard small molecule chelating agents. We previously introduced BSIH (isonicotinic acid [2-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-benzylidene]-hydrazide) prochelators that are converted by hydrogen peroxide into SIH (salicylaldehyde isonicotinoyl hydrazone) chelating agents that inhibit iron-catalyzed hydroxyl radical generation. Here, we show that BSIH protects a cultured cell model for retinal pigment epithelium against cell death induced by hydrogen peroxide. BSIH is more stable than SIH in cell culture medium and is more protective during long-term experiments. Repetitive exposure of cells to BSIH is nontoxic, whereas SIH and desferrioxamine induce cell death after repeated exposure. Combined, our results indicate that cell protection by BSIH involves iron sequestration that occurs only when the cells are stressed by hydrogen peroxide. These findings suggest that prochelators discriminate toxic iron from healthy iron and are promising candidates for neuro- and retinal protection.
Keywords: Iron, Oxidative stress, Reactive oxygen species, Fenton chemistry, Chelation therapy
1. Introduction
Iron is the most plentiful transition metal in the human body and is a required cofactor for essential life processes such as oxygen transport, DNA synthesis, and cellular respiration [1]. Because of its notorious role in the Fenton reaction, in which ferrous iron reacts with H2O2 to produce highly reactive hydroxyl radicals, nearly all of the total iron in healthy individuals is bound by proteins that utilize, transport, or store iron. The importance of maintaining iron homeostasis is highlighted by the fact that imbalance in either direction causes disease: deficiency leads to anemia, and overload leads to tissue damage and organ failure as a result of iron-promoted oxidative stress. A complex communication network therefore exists among different cell and tissue types to regulate systemic iron balance in order to meet metabolic needs without accumulating pools of toxic iron [2]. The designation of iron as toxic or detrimental is used here to describe iron in any form that participates in undesirable reactions that promote oxidative stress. It is defined therefore by its reactivity, not by an identifiable set of coordinating ligands or by a specific oxidation state. Such reactivity implies that toxic iron is also redox-active iron that shuttles between Fe(II) and Fe(III) oxidation states, has open coordination sites that enable reactivity, and is likely to be labile, as opposed to tightly bound by transport or storage proteins.
Hereditary diseases of iron overload are caused by mutations in genes of both iron regulation and usage [3, 4]. Examples include hemochromatosis, where defects in genes associated with intestinal iron absorption lead to liver cirrhosis and heart failure; aceruloplasminemia, where defects in the ferroxidase protein ceruloplasmin result in iron deposition in the brain, retina, and pancreas; and thalassemia, where faulty hemoglobin production requires chronic blood transfusions that instigate secondary iron overload.
In addition to diseases of systemic iron overload, evidence is also pointing to localized iron dysregulation as a factor in many aging-related degenerative diseases like Parkinson’s, Alzheimer’s, and age-related macular degeneration (AMD) [5–8]. These diseases have characteristic signs of oxidative stress, diminished antioxidant defenses, and higher than normal localized iron loads. For example, elevated iron accumulation has been documented in brain regions affected by Parkinson’s and Alzheimer’s diseases [9–13]. In AMD, increased amounts of lipid peroxidation products and decreased levels of antioxidant enzymes are found [14, 15]. Concurrently, the iron level in AMD-affected maculas is higher than in healthy maculas [16]. In support of the deleterious effect of such retinal iron accumulation, patients with aceruloplasminemia have retinal iron overload that results in a maculopathy with features of AMD [17].
Strategies to reduce iron levels in systemic iron overload diseases include serial phlebotomy, dietary iron restriction, and in some cases iron chelation therapy. Desferrioxamine B (DFO) has been used clinically since the 1970s to treat transfusion-induced iron overload. DFO is a hydrophilic, hexadentate chelator that is administered in large doses via subcutaneous transfusion. Recently, deferiprone (L1) and deferasirox (Exjade) have been introduced as chelating agents with improved pharmacological properties [18]. The targets of these drugs are non-transferrin bound iron in the plasma, followed by intracellular iron stores in the liver, heart and endocrine tissues.
Reducing the intracellular iron load by using small molecule chelators holds potential for reducing oxidative stress in AMD and other degenerative conditions. However, there are significant challenges associated with selectively sequestering detrimental iron in the brain or retina. Crossing the blood-brain or blood-retinal borders will be necessary in these cases, whereas avoiding chelator access to the brain is critical for transfusional iron overload. Furthermore, the risk of chelator competition with iron-requiring enzymes or other metal ions is more significant than in cases of systemic iron overload where the target iron is plentiful and more readily available for sequestration. For diseases associated with localized iron stress, an optimal chelator needs to be cell membrane permeable, sequester only that fraction of iron that is causing damage, avoid inhibiting metalloenzymes, and prevent redistribution of iron or other metals within or between cells.
Currently available chelating agents have significant drawbacks with respect to these challenges. DFO is a poor candidate as it does not readily cross biological membranes. While increasing lipophilicity may improve chelator uptake, it does not prevent unwanted inhibition of essential metalloenzymes. Deferiprone, for example, is taken up in the brain but inhibits catechol-O-methyltransferase as well as tyrosine and tryptophan hydroxylases by binding to iron at the enzyme active site [19]. A finding that emphasizes the importance of avoiding indiscriminant iron binding is that DFO treatment of patients with thalassemia and sickle-cell disease has been associated with retinal toxicity hypothesized to result from iron deficiency in the retinal pigment epithelium [20]. Interactions of chelators with other metal ions, particularly zinc and copper, is also a concern. If a chelator encounters these cations prior to iron complexation, even a thermodynamic preference for trivalent over divalent metal ions will not avoid disruption of healthy metal homeostasis. Deferasirox, for example, readily forms insoluble polymers with Zn(II) and Cu(II) that could lead to precipitation of these complexes in vivo [18].
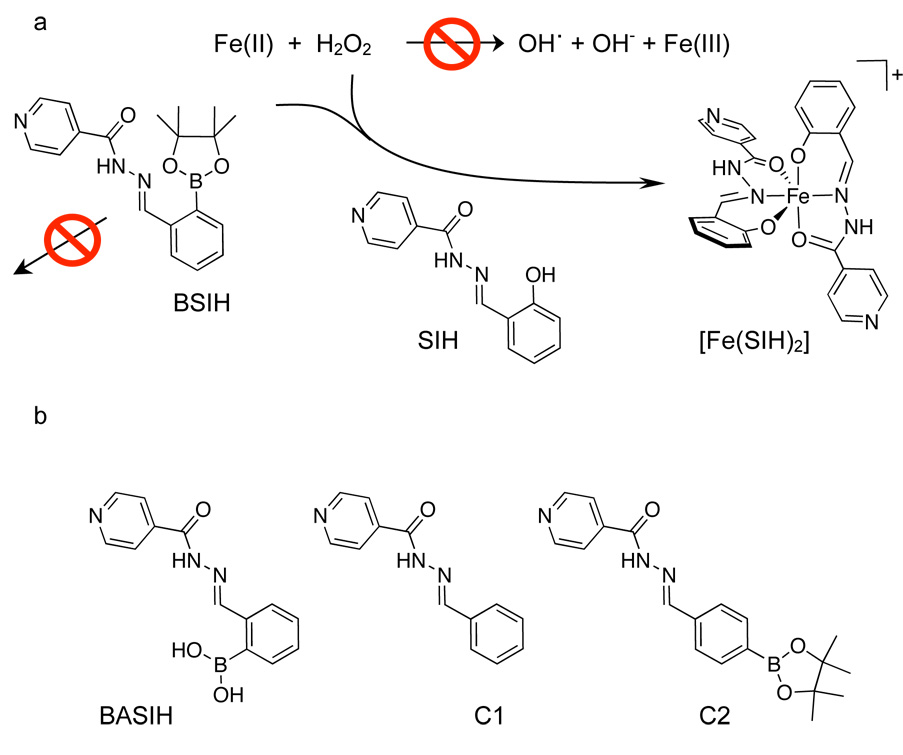
In order to overcome these challenges, we recently introduced prochelators as a novel approach to decrease oxidative damage by activating a chelating agent only under conditions of oxidative stress [21, 22]. The prochelators do not bind metal ions until a protective mask is removed by H2O2 to reveal an effective chelating agent that sequesters iron that is the source of hydroxyl radical generation (Fig. 1a). Unlike typical chelators that interfere with healthy metal ion status or inhibit metalloenzymes by indiscriminant binding, these prochelators are designed to activate only where they are needed. Our first-generation prochelator, BSIH (isonicotinic acid [2-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-benzylidene]-hydrazide) [21] (see structure in Fig. 1), contains a boronic ester in place of a phenolic oxygen that is a key donor atom of SIH (salicylaldehyde isonicotinoyl hydrazone). SIH is a well-studied, membrane permeable chelating agent that scavenges and incapacitates redox-active iron in cell culture [23–26]. Herein we report the first cell culture data that validate the prochelator strategy for discriminating toxic versus healthy iron and protecting cells from oxidative stress.
Figure 1.
a) Prochelator strategy to release an iron chelator at the site of oxidative stress. Boronate-masked aroyl hydrazone prochelator BSIH does not bind metal ions, but is converted by H2O2 to SIH, which binds Fe(III) to form the bis-ligated [Fe(SIH)2]+ complex that prevents iron from promoting hydroxyl radical formation via Fenton chemistry. b) Additional compounds used in the cell culture studies.
2. Experimental
2.1. General
Chemicals were obtained from Fisher Scientific or Acros Organics unless otherwise noted and used without further purification; (4,4,5,5-tetramethyl-1,2,3-dioxaborolan-2-yl)benzaldehyde was purchased from Oakwood Products. All solvents were reagent grade and all aqueous solutions were prepared with nanopure water. UV-visible spectra were recorded on a Photonics Model 420 Fiber Optic CCD Array spectrophotometer. 1H and 13C NMR spectra were recorded on a Varian Mercury 300 or Inova 400 spectrometer; δ values are in ppm and J values are in Hz. IR Spectra were recorded on a Nicolet 360 FT-IR. High-resolution mass spectra (HRMS) were recorded on a Jeol JMS-SX102 mass spectrometer.
2.2. Synthesis
SIH, BSIH, BASIH and BIH (benzaldehyde isonicotinoyl hydrazone; denoted hereafter as C1) were prepared as described previously [21, 22]. C2 ([4-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-benzylidene]-hydrazide) was synthesized in a similar manner by reacting 0.5 mmol of 4-(4,4,5,5-tetramethyl-1,2,3-dioxaborolan-2-yl)benzaldehyde with 0.5 mmol of isonicotinic acid hydrazide in 4 mL of MeOH. After stirring for 5 min at 100 °C, the reaction mixture was cooled over ice and the white precipitate was collected via vacuum filtration, washed with water, and dried in vacuo to yield a clean white powder (136 mg, 76%). 1H NMR (DMSO 400 MHz, s, singlet; d, doublet; m, multiplet): δ 1.26 (12H, s), 7.71 (4H, m), 7.78 (2H, d, J = 5.8), 8.41 (1H, s), 8.74 (2H, d, J = 5.7), 12.09 (1H, s). 13C NMR (DMSO 100 MHz): δ 25.34, 84.52, 122.20, 127.27, 135.51, 137.35, 141.05, 149.17, 151.01. HRMS m/z 351.1868 [M+H]+, calcd for C19H23BN3O3, 351.1869 (10B). IR (neat, νmax/cm−1): 651, 686, 1090, 11430, 1297, 1361, 1555, 1663, 2359. λmax(MeOH) 307 nm (29,200 M−1cm−1).
2.3. UV-Vis
50 µM solutions of BSIH or SIH were prepared in 20 mM NaHPO4 buffer (pH 7.4) or MEM cell culture medium. UV-vis spectra were recorded after the solutions were incubated at 37 °C for 10 min, 2 h, 7 h and 24 h. To determine the half-life of SIH, the absorbance at 325 nm was recorded every 30 minutes for 8 h for a solution of 100 µM SIH in MEM at pH 7.4 incubated at 37 °C.
2.4. Cell Culture
All cell culture agents, including minimal essential medium (MEM), Dulbecco’s modified eagle medium (DMEM), F12 Ham’s nutrient mix (F12), fetal bovine serum (FBS), pencillin-streptomycin (pen-strep), L-glutamine, and trypsin-EDTA were purchased from Gibco. LDH Release Assay Kit was obtained from Roche Diagnostics and CellTiter-Blue Cell Viability Assay from Promega. Cell viability assays were performed on a Perkin Elmer Victor3 1420 plate reader.
The spontaneously immortalized human retinal pigment epithelial cell line ARPE-19 was purchased from American Type Culture Collection. These cells, commonly used to study the RPE, retain some features of in vivo RPE cells, although non-necessarily polarization in our short-term cultures [27]. The cells were grown in 1:1 DMEM and F12 medium with FBS (10%), pen-strep (1%), and glutamine (1%). Cells were cultured until confluent in 24-well Falcon plates. Fresh DMEM/F12 medium was added one day prior to treatment. An initial kill curve experiment was performed in which cells were treated with 0 – 5 mM H2O2 in MEM. Cell death increased with increasing peroxide concentration; 500 µM H2O2 was the minimal dose causing significant cell death. Chelator/prochelator solutions at or below 200 µM were freshly prepared by dissolving the compounds directly in MEM; for higher concentrations, stock solutions were prepared in 50 mM NaOH and stored at −20 °C. Just prior to use, frozen stocks were thawed and diluted in MEM to the desired working concentration, then adjusted to pH 7.6 with HCl and filter sterilized. UV-vis and NMR analyses were performed to verify that all chelators/prochelators were unchanged following this solution preparation.
2.4.1. Assays for cell viability
To evaluate the ability of prochelators/chelators to protect ARPE-19 cells from H2O2 toxicity, confluent cells were washed three times with MEM prior to treatment with prochelator or chelator solutions ranging in concentration from 0 – 200 µM. After a specified pretreatment period (30 min or 8 h), the cells were washed with fresh MEM to remove residual prochelator/chelator solution and MEM with 500 µM H2O2 was added. After 15 hours, cell viability was assayed by one of two methods, the CellTiter-Blue assay or the lactate dehydrogenase (LDH) release assay. Both assays, run on separate days, returned similar results. Results are reported as the mean and standard deviation from experiments done at least in triplicate; an MEM-only control was run in triplicate on each 24-well plate. Statistical significance was calculated using the Student’s t-test. Cell culture experiments were repeated by using MEM that had been treated with Chelex 100 to remove residual metal ions. No considerable differences were observed for experiments done with Chelex-treated vs non-treated medium.
CellTiter-Blue is a fluorometric method based on the ability of live cells to reduce nonfluorescent resazurin into highly fluorescent resorufin. The CellTiter-Blue reagent (200 µL) was added directly into each well and the plates were incubated for 4 hours at 37 °C with 5 % CO2 prior to recording the fluorescence (560Ex/590Em) using a plate reader. Percent protection was calculated as shown in Eq. 1, where Emtreatment, EmH2O2, and EmMEM are the emission signals for a treated sample, the H2O2-only control that gives maximal cell death for 500 µM H2O2, and the MEM-only control, which indicates maximum viability, respectively:
| Eq. 1 |
Potential interactions between the chelators/prochelators and the CellTiter-Blue reagent that might interfere with the assay were found to be negligible. The maximal fluorescence obtained from control experiments in which resazurin was incubated with the chelators/prochelators in the absence or presence of 500 µM H2O2 at 37 °C for 4 hours was less than 4% of the MEM-only control in the cell viability experiments, which is within the experimental error of the measurement.
The LDH Release Kit is a colorimetric assay for the quantification of cell death based on the measurement of lactate dehydrogenase activity released from the cytosol of dead cells into the medium. In brief, the medium from each well was removed, fractionated by centrifugation (240 × g for 10 min), and 25 µL of the supernatant solution were placed in a 96-well plate. Phosphate buffered saline (PBS, 75 µL) was added to each well, followed by freshly prepared LDH reaction mixture (100 µL). After 30 minutes of incubation at room temperature in the dark, the absorbance of each solution at 490 nm was measured using a plate reader.
2.4.2. Repetitive treatment
For the repetitive treatment experiment, confluent ARPE-19 cells were washed three times with MEM, and then treated with 100 µM SIH, 100 µM BSIH or 1 mM DFO (all prepared in MEM as described above); a control received MEM only with no treatment added. The larger dose of DFO was used since it does not enter cells by passive diffusion but rather relies on endocytosis for cellular access. Every 2 hours, the medium was replaced with freshly prepared chelator/prochelator solutions (or MEM only for the control), and viability was assessed using the CellTiter-Blue assay after 16 h (7 treatments) and 26 h (12 treatments). Viability for the treated samples was compared to viability of the untreated control at the same time point, which was normalized to 100%.
3. Results and Discussion
We chose to test the biological efficacy of BSIH in retinal pigment epithelial (RPE) cells because of their implication in AMD. RPE cells support the photoreceptors of the retina and are known to experience oxidative insult from several sources, including O2−• and H2O2 produced during mitochondrial respiration, by peroxide produced during phagocytosis of photoreceptor outer segments, and by photo-oxidation [28]. Furthermore, SIH protects a human adult RPE cell line against cell death induced by exogenous hydrogen peroxide and other toxicants (N. Lukinova, T. Dentchev, and J.L. Dunaief, manuscript in preparation).
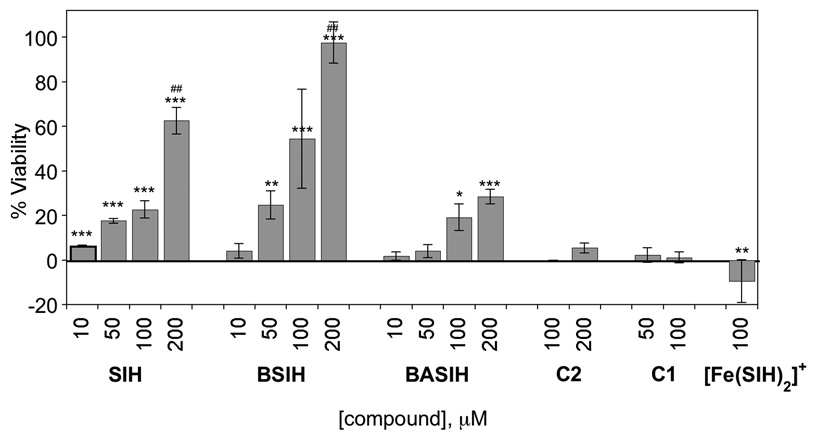
The compounds shown in Fig. 1 were tested for their ability to protect cultured ARPE-19 cells from cell death induced by an otherwise toxic dose of H2O2. BSIH and its boronic acid version, BASIH, are prochelators that we have previously shown convert in vitro to SIH upon reaction with H2O2 [21, 22]. Compounds C1 and C2 are both non-chelating controls. Cells were first incubated with increasing concentrations of these compounds for the specified time frame, then washed with fresh medium to remove residual test compound just prior to addition of H2O2. Cells were assayed after overnight incubation using two separate assays of cell viability (Fig. 2 and Supplementary Material Fig. S1).
Figure 2.
Cell viability of ARPE-19 cells pretreated with increasing concentrations of chelator SIH, prochelator BSIH or BASIH, [Fe(SIH)2]NO3, or control compound C1 or C2, for 8 h prior to addition of 500 µM H2O2. Error bars represent the standard deviation from triplicate runs (n=3), except for 100 and 200 µM SIH and BSIH where n=6. Statistical significance is indicated relative to samples exposed to H2O2 but given no chelator/prochelator treatment by * p<0.05, ** p<0.01, *** p<0.001, or as a comparison of SIH and BSIH at the same concentrations by ##p<0.01.
3.1. BSIH and SIH protect cells against H2O2-induced cell death
Preincubating the cells for 8 h with either SIH or BSIH provides protection against H2O2-induced cell death that increases with concentration (Fig. 2). Significantly, a 200 µM dose of BSIH provides complete protection against cell death, whereas SIH provides only 70% protection under this protocol (Fig. 2). Unlike BSIH, BASIH provides only modest protection, reaching only 30% viability. The difference in the protective effect of BSIH vs BASIH is not yet understood, although altered bioavailability may be a contributor. BASIH reacts with H2O2 in vitro at the same rate as BSIH to convert to SIH; however, it is less lipophilic than either BSIH or SIH which may result in less cellular uptake and diminished biological efficacy [22].
In order to show that the protective effect of BSIH is not due to the reactivity of its aryl boronic ester functional group with H2O2, the control compound C2 was tested. C2 also contains a boronic ester that reacts with H2O2, but in this case the phenol of the resulting compound is ill-positioned for iron chelation. C2 provides no protection against H2O2-induced cell death, providing strong evidence that the protective effect of BSIH is due to its iron binding capacity following activation.
Further support that iron chelation is the mechanism of cell protection is the absence of any protective effect for either C1, which lacks the iron-binding phenol of SIH, or for the pre-formed [Fe(SIH)2]+ complex, which actually slightly sensitizes cells to toxicity (Fig. 2). In addition, cells that are over-loaded with iron by treatment with ferric ammonium citrate are more sensitive to H2O2 (Supplementary Material Fig. S2), supporting the hypothesis that cell death induced by H2O2 is linked to its reactivity with iron.
3.2. Conversion of BSIH to SIH
If the protective effect of BSIH is due to its conversion to SIH, then the rate of unmasking of BSIH by H2O2 should be a critical factor in its efficacy. We previously reported a value of k = 0.05 M−1 s−1 for the rate expression in Eq. 2. This value was obtained from reactions carried out in a 50/50 mixture of methanol/phosphate buffer. These experiments have been repeated here in 100% phosphate buffer and in MEM cell culture medium (Supplementary Material Fig. S3) and the same value was obtained, indicating that the methanol co-solvent does not influence the reaction rate. Given this value, the maximum rate of SIH production would be 0.3 µM/min for 200 and 500 µM doses of BSIH and H2O2, respectively. Within the first 30 min following H2O2 exposure, no more than 9 µM of SIH would therefore be available for iron chelation. The concentration of labile iron is expected to be low enough that this amount should be adequate to suppress iron-mediated damage. The data in Fig. 2, however, indicate that 9 µM SIH is not sufficient to provide significant protection, at least when administered in the pre-incubation protocol described above. As described below in section 3.4, an alternative protocol for administering SIH indicates that low µM concentrations of SIH are indeed effective for cellular protection.
| Eq. 2 |
3.3. Improved stability of BSIH compared to SIH
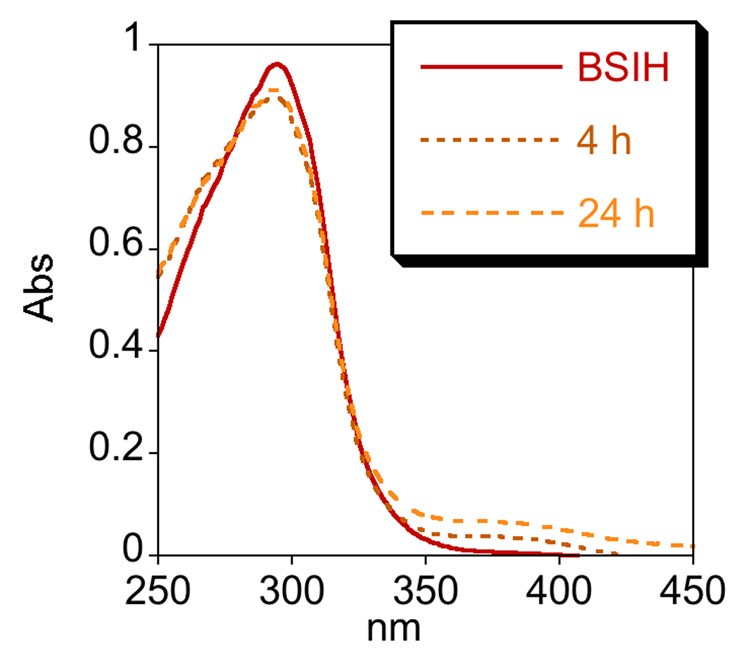
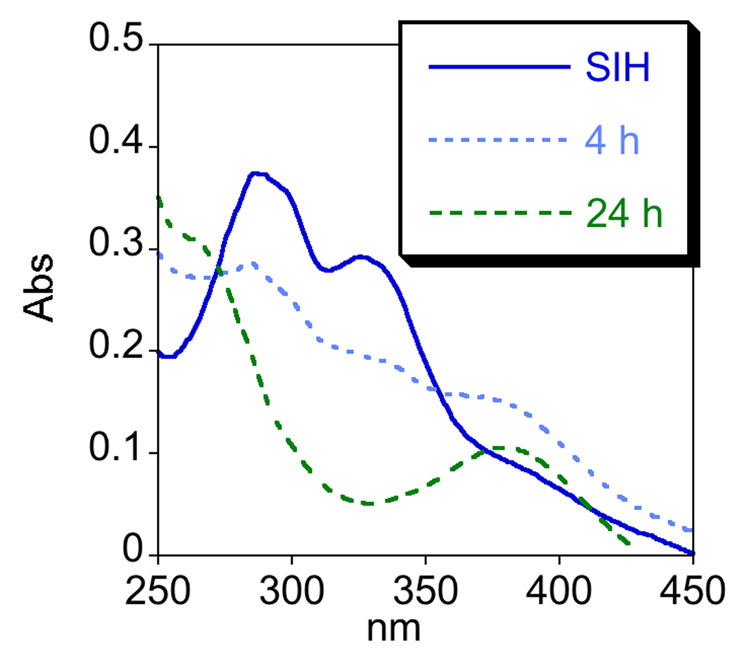
One factor that may contribute to the modest protection (70% in Fig. 2) observed for SIH following an 8 h incubation time is the stability of SIH. The degradation of aroylhydrazones has been recognized for SIH and its derivatives, which are stable in aqueous buffer at neutral pH but hydrolyze into their hydrazine and aldehyde components in cell culture media, where amino acids promote hydrolysis [29, 30]. Hydrolysis rates vary depending on the derivative and the conditions, but the iron complexes remain stable. In order to test the stability of BSIH and SIH, we monitored their UV-vis spectra in 20 mM NaHPO4 buffer at pH 7.4 and in minimal essential cell culture medium (MEM) at 37 °C over 24 h. Both compounds are stable in buffer (data not shown). Whereas BSIH is stable in MEM over 24 h (Fig. 3a), SIH hydrolyzes with a half-life of 7 h (Fig. 3b and Supplementary Material Fig. S4). These results verify that SIH and BSIH remain intact during in vitro experiments done in aqueous buffer, but they emphasize that SIH partially decomposes in cell culture conditions.
Figure 3.
BSIH is stable in cell culture medium whereas SIH decomposes within hours. a) UV-vis spectra of BSIH dissolved in MEM and incubated at 37 °C change negligibly even after 24 h. b) The spectral changes of SIH that appear within 4 h of incubation under the same conditions are consistent with decomposition of SIH into its components salicylaldehyde and isonicotinoyl hydrazine. Both SIH and BSIH are stable in neutral aqueous buffer for 24 h (not shown).
Consistent with the diminished stability of SIH is the observation that SIH is more effective at protecting cells against H2O2 when the incubation time is reduced from 8 h to 30 min, presumably because more intact chelator is available at the time of the oxidative insult (Supplementary Material Fig. S1). The difference in efficacy of SIH between these incubation times and the disconnect between the projected amount of SIH released from BSIH vs the amount of SIH needed for complete protection is not as straightforward as expected simply from its hydrolysis half-life, however. Additional factors that influence the efficacy of SIH for cell protection appear to be important, but remain to be identified.
3.4. Dosing strategy influences efficacy
In contrast to the SIH results shown in Fig. 2, previous studies have found that low µM concentrations of SIH can completely protect cells stressed with a deadly concentration of H2O2 [25], (N. Lukinova, T. Dentchev, and J.L. Dunaief, manuscript in preparation). In those protocols, a dose of SIH was pre-incubated with cells followed by an additional dose of chelator added simultaneously with H2O2. To test this protocol in our system, we incubated ARPE-19 cells with 1 – 10 µM SIH for 30 min, then added an additional dose of the same concentration along with 500 µM H2O2. This “double-dosing” strategy greatly improves the efficacy of SIH, as two treatments of 5 µM SIH provide complete protection against H2O2 toxicity (Table 1 and Supplementary Material Fig. S5). In comparison, a single pre-incubation dose of 10 µM SIH provides less than 10% protection (Fig. 2) while a single 5 µM dose added along with peroxide provides only 15% protection (Table 1 and Supplementary Fig. S5). Taken together, these data confirm that low µM concentrations of SIH are indeed sufficient to protect cells from H2O2-induced toxicity, but that appropriate dosing is necessary.
Table 1.
Comparison of the effect that the application protocol has on the ability of SIH to keep cells viable when stressed with a toxic dose of hydrogen peroxide. A pre-incubation dose followed by a second dose added at the same time as H2O2 is more effective than a single, higher-concentration dose of SIH. Details for each experiment are noted in the associated figure caption and in the Experimental section.
| Compound | pre-incubation dose (µM) | additional dose added with H2O2 (µM) | predicted [SIH] (µM) after 30 min of H2O2 exposure | viability | data |
|---|---|---|---|---|---|
| BSIH | 200 | – | 9a | 90–100 % | Fig. 2 |
| SIH | 200 | – | 90b | 60–70 % | Fig. 2 |
| SIH | 5 | 5 | 9b | 90–100 % | Fig. S5 |
| SIH | – | 5 | 4.76b | <15 % | Fig. S5 |
| SIH | 10 | – | 9b | <10% | Fig. 2 |
based on the initial rate of conversion of BSIH to SIH by H2O2.
based on the hydrolysis rate for decomposition of SIH in cell culture.
The observations that the timing and application protocol are critical for optimal protection by SIH suggest that suboptimal protection may be a consequence of processes that compromise its capacity to sequester iron that is released upon oxidative insult. Such processes likely include hydrolysis, non-specific metal loading, and perhaps others yet to be identified. The actual concentration of SIH that provides protection appears to be low µM, which is consistent with the projected amount of SIH that would be released from a 200 µM dose of BSIH, as described above and compared in Table 1. These data therefore reinforce the conclusion that the mechanism of cell protection by BSIH is due to iron sequestration following its conversion to SIH.
3.5. Repetitive administration of chelators and prochelator
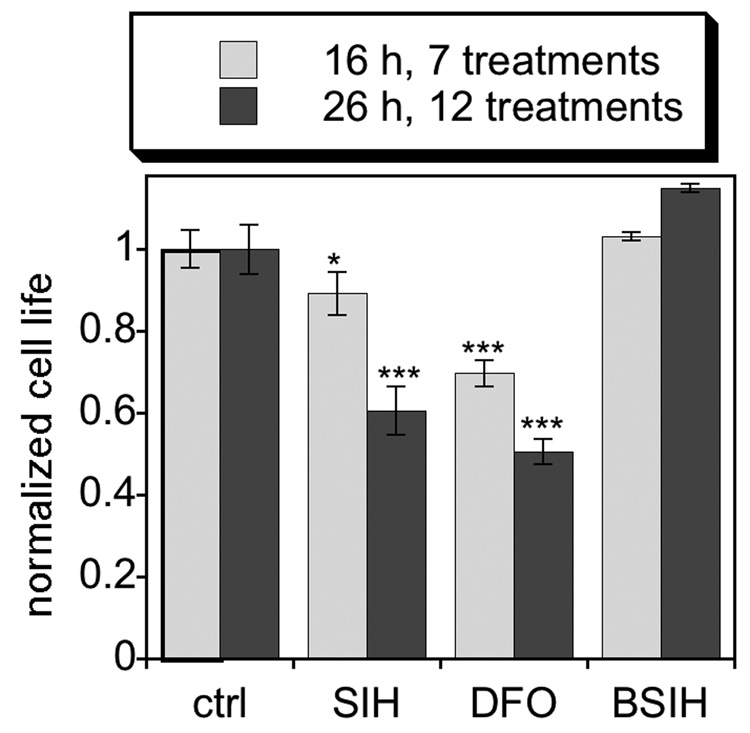
Initial toxicity studies of SIH and BSIH indicated that RPE cells are not adversely affected by either compound up to 2 mM doses, the solubility limit of BSIH, when viability is monitored 24 h after single-dose treatment (data not shown). The fact that SIH at such high concentrations is not toxic was surprising given the essential role of iron in numerous cellular processes. Reasoning that SIH toxicity may not manifest itself after only a single dose, especially since a portion would degrade before establishing iron deficiency, we tested the toxicity of repetitive treatments of SIH, BSIH, and DFO. Fresh compound was added to cells every 2 h for 26 h. After seven treatments, the DFO-treated cells began to deteriorate, and after 12 treatments the viability of SIH and DFO-treated cells was decreased by nearly half (Fig. 4), likely as a result of diminished iron availability. Significantly, cells treated with BSIH under this chronic protocol remained as viable as the untreated control cells (Fig. 4), suggesting that the unactivated prochelator does not interfere with normal metal homeostasis.
Figure 4.
Assessment of chelator toxicity: Repeated treatments of ARPE-19 cells with iron chelators SIH and DFO induce cell death, while prochelator BSIH is non-toxic. Cells were treated every 2 h with 1 mM DFO or 100 µM SIH or BSIH and cell viability was compared to the untreated control (“ctrl”, normalized to 100% viability) at the same timepoint. Relative to the untreated control, * p<0.05, *** p<0.001.
4. Conclusions
The results presented above provide the first cellular validation of the prochelator strategy for inhibiting oxidative stress. Important questions about iron’s role in neurodegeneration, for example whether it is a cause or consequence of disease, remain to be elucidated. Small molecules like the prochelators presented here that differentiate deleterious iron from healthy iron will be valuable reagents for probing the chemical biology of oxidative stress. Furthermore, the improved stability and activation by peroxide make prochelators better candidates than chelators for iron overload diseases that affect the brain and eye. This hypothesis will be tested in animal models of iron overload, including ceruloplasmin/hephaestin knockout mice that have an age-dependent retinal degeneration following retinal iron overload [31].
Supplementary Material
Acknowledgements
We thank Dr. Leon Charkoudian for initiating the collaboration between the Franz and Dunaief labs. We thank Duke University’s Arts and Sciences Faculty Committee on Research and NIH EY018922 for funding this study.
Abbreviations
- AMD
age-related macular degeneration
- BSIH
boronic acid pinacol ester version of SIH (isonicotinic acid [2-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-benzylidene]-hydrazide)
- BASIH
boronic acid version of BSIH
- DFO
desferrioxamine
- LDH
lactate dehydrogenase
- MEM
minimal essential medium
- PBS
Phosphate buffered saline
- RPE
retinal pigment epithelial
- SIH
salicylaldehyde isonicotinoyl hydrazone
Appendix A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crichton R. Inorganic Biochemistry of Iron Metabolism. 2nd edn. New York: John Wiley & Sons; 2001. [Google Scholar]
- 2.Andrews NC, Schmidt PJ. Ann. Rev. Physiol. 2007;69:69–85. doi: 10.1146/annurev.physiol.69.031905.164337. [DOI] [PubMed] [Google Scholar]
- 3.Hentze MW, Muckenthaler MU, Andrews NC. Cell. 2004;117:285–297. doi: 10.1016/s0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- 4.Madsen E, Gitlin JD. Annu. Rev. Neurosci. 2007;30:317–337. doi: 10.1146/annurev.neuro.30.051606.094232. [DOI] [PubMed] [Google Scholar]
- 5.Halliwell B. J. Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 6.Zecca L, Youdim MBH, Riederer P, Conner JR, Crichton RR. Nat. Rev. Neurosci. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- 7.Barnham KJ, Masters CL, Bush AI. Nat. Rev. Drug Disc. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 8.Dunaief JL. Invest. Ophthalmol. Vis. Sci. 2006;47:4660–4664. doi: 10.1167/iovs.06-0568. [DOI] [PubMed] [Google Scholar]
- 9.Dexter DT, Wells FR, Lees AJ, Agid F, Agid Y, Jenner P, Marsden CD. J. Neurochem. 1989;52:1830–1836. doi: 10.1111/j.1471-4159.1989.tb07264.x. [DOI] [PubMed] [Google Scholar]
- 10.Sofic E, Paulus W, Jellinger K, Riederer P, Youdim MBH. J. Neurochem. 1991;56:978–982. doi: 10.1111/j.1471-4159.1991.tb02017.x. [DOI] [PubMed] [Google Scholar]
- 11.Gotz ME, Double K, Gerlach M, Youdim MBH, Riederer P. Ann. N.Y. Acad. Sci. 2004;1012:193–208. doi: 10.1196/annals.1306.017. [DOI] [PubMed] [Google Scholar]
- 12.Chwiej J, Adamek D, Szczerbowska-Boruchowska M, Krygowska-Wajs A, Wojcik S, Falkenberg G, Manka A, Lankosz M. J. Biol. Inorg. Chem. 2007;12:204–211. doi: 10.1007/s00775-006-0179-5. [DOI] [PubMed] [Google Scholar]
- 13.Smith MA, Harris PLR, Sayre LM, Perry G. Proc. Natl Acad. Sci. USA. 1997;94:9866–9868. doi: 10.1073/pnas.94.18.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nowak M, Swietochowska E, Wielkoszynski T, Marek B, Karpe J, Gorski J, Glogowska-Szelag J, Kos-Kudla B, Ostrowska Z. Eur J Ophthalmol. 2003;13:281–286. doi: 10.1177/112067210301300307. [DOI] [PubMed] [Google Scholar]
- 15.Evereklioglu C, Er H, Doganay S, Cekmen M, Turkoz Y, Otlu B, Ozerol E. Doc. Ophthalmol. 2003;106:129–136. doi: 10.1023/a:1022512402811. [DOI] [PubMed] [Google Scholar]
- 16.Hahn P, Milam AH, Dunaief JL. Arch. Ophthalmol. 2003;121:1099–1105. doi: 10.1001/archopht.121.8.1099. [DOI] [PubMed] [Google Scholar]
- 17.Dunaief JL, Richa C, Franks EP, Schultze RL, Aleman TS, Schenck JF, Zimmerman EA, Brooks DG. Ophthalmology. 2005;112:1062–1065. doi: 10.1016/j.ophtha.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 18.Hider RC, Zhou TAO. Ann NY Acad Sci. 2005;1054:141–154. doi: 10.1196/annals.1345.017. [DOI] [PubMed] [Google Scholar]
- 19.Waldmeier PC, Buchle AM, Steulet AF. Biochem. Pharmacol. 1993;45:2417–2424. doi: 10.1016/0006-2952(93)90222-i. [DOI] [PubMed] [Google Scholar]
- 20.Lakhanpal V, Schocket SS, Jiji R. Ophthalmology. 1984;91:443–451. doi: 10.1016/s0161-6420(84)34267-1. [DOI] [PubMed] [Google Scholar]
- 21.Charkoudian LK, Pham DM, Franz KJ. J. Am. Chem. Soc. 2006;128:12424–12425. doi: 10.1021/ja064806w. [DOI] [PubMed] [Google Scholar]
- 22.Charkoudian LK, Pham DM, Kwan A, Vangeloff A, Franz KJ. Dalton Trans. 2007;43:4873–5092. doi: 10.1039/b705199a. [DOI] [PubMed] [Google Scholar]
- 23.Richardson DR, Ponka P. Am J Hematol. 1998;58:299–305. doi: 10.1002/(sici)1096-8652(199808)58:4<299::aid-ajh9>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 24.Horackova M, Ponka P, Byczko Z. Cardiovasc. Res. 2000;47:529–536. doi: 10.1016/s0008-6363(00)00088-2. [DOI] [PubMed] [Google Scholar]
- 25.Simunek T, Boer C, Bouwman RA, Vlasblom R, Versteilen AMG, Sterba M, Gersl V, Hrdina R, Ponka P, de Lange JJ, Paulus WJ, Musters RJP. J. Mol. Cell. Cardiol. 2005;39:345–354. doi: 10.1016/j.yjmcc.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Kurz T, Gustafsson B, Brunk UT. Febs Journal. 2006;273:3106–3117. doi: 10.1111/j.1742-4658.2006.05321.x. [DOI] [PubMed] [Google Scholar]
- 27.Dunn KC, AotakiKeen AE, Putkey FR, Hjelmeland LM. Exp. Eye Res. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- 28.Sarks JP, Sarks SH, Killingsworth MC. Eye. 1988;2:552–577. doi: 10.1038/eye.1988.106. [DOI] [PubMed] [Google Scholar]
- 29.Buss JL, Ponka P. Biochim. Biophys. Acta. 2003;1619:177–186. doi: 10.1016/s0304-4165(02)00478-6. [DOI] [PubMed] [Google Scholar]
- 30.Kovarikova P, Klimes J, Sterba M, Popelova O, Mokry M, Gersl V, Ponka P. J. Sep. Sci. 2005;28:1300–1306. doi: 10.1002/jssc.200500077. [DOI] [PubMed] [Google Scholar]
- 31.Hahn P, Qian Y, Dentchev T, Chen L, Beard J, Harris ZL, Dunaief JL. Proc. Natl Acad. Sci. USA. 2004;101:13850–13855. doi: 10.1073/pnas.0405146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.