Abstract
Mitochondria play a key role in the energy metabolism in skeletal muscle. A new concept has emerged suggesting that impaired mitochondrial oxidative capacity in skeletal muscle may be the underlying defect that causes insulin resistance. According to current knowledge, the causes and the underlying molecular mechanisms at the origin of decreased mitochondrial oxidative capacity in skeletal muscle still remain to be elucidated. The present review focuses on recent data investigating these issues in the area of metabolic disorders and describes the potential causes, mechanisms and consequences of mitochondrial dysfunction in the skeletal muscle.
Keywords: Mitochondrial dysfunction, skeletal muscle, metabolic disorders, obesity, insulin resistance, type 2 diabetes
1. Introduction
Over the past decade, the list of publications suggesting an involvement of mitochondrial oxidative capacity in skeletal muscle in the aetiology of metabolic disorders such as obesity, insulin resistance or type 2 diabetes, has been growing steadily. By considering that lifestyle and physical activity, in addition to age, gender and genetic background, influence mitochondrial oxidative capacity in human muscle, it is clear that the understanding of the causes at the origin of oxidative phosphorylation (OXPHOS) activity impairment is far from being accomplished. In this context, the purpose of this review is to highlight recent knowledge regarding the potential causes, mechanisms and cellular consequences of muscle mitochondrial dysfunction.
2. Transcriptional regulation of muscle mitochondrial oxidative capacity
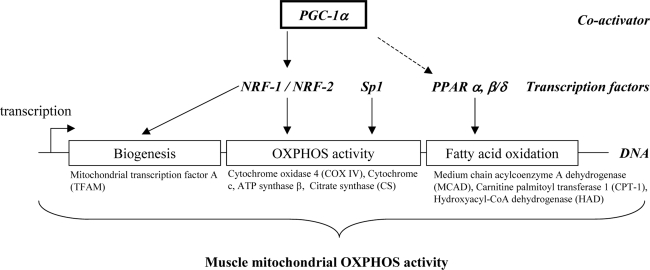
Muscle oxidative capacity is mainly determined by the mitochondrial density that depends on mitochondrial biogenesis (i.e. the cellular processes involved in the synthesis of the organelles), and the mitochondrial oxidative capacity, which relies on the oxidative enzyme content and activity. A number of transcriptional modulators have been implicated in the regulation of muscle mitochondrial biogenesis and OXPHOS activity. They include PPAR gamma coactivator 1 alpha (PGC-1α), in cooperation with several factors such as the peroxisome proliferator-activated receptors (PPAR), the nuclear respiratory factors 1 and 2 (NRF-1 and NRF-2) [1–9], or the specificity protein 1 (Sp1), an ubiquitous transcription factor known to regulate the constitutive expression of oxidative OXPHOS genes [10]. Of note, Sp1 can function as both a positive (e.g. cytochrome c1 and mitochondrial transcription factor A, TFAM) and a negative (e.g. adenine nucleotide translocator 2 and F1-ATPase beta subunit) regulator of transcription [11]. PGC-1α is a master modulator of gene expression in skeletal muscle [12]. It was found to drive the formation of oxidative type I fibres and to activate the expression of genes involved in mitochondrial oxidative capacity, through associated changes in the expression of NRF dependent genes [13]. These combined data have therefore suggested that decreased PGC-1α gene expression could be one of the primary contributors to decreased mitochondrial oxidative capacity. However, PPARs are also good candidates. When bound to their ligand (e.g. fatty acids for PPARα), PPARs form a heterodimeric complex with the retinoid X receptor (RXR) to regulate gene transcription involved in fatty acid metabolism. PGC-1α is also known to enhance the activity of the isoforms PPARα and PPARβ/δ in skeletal muscle, which may result in the enhanced expression of genes involved in mitochondrial fatty acid oxidation [14]. Major transcriptional modulators involved in the regulation of mitochondrial activity in skeletal muscle are illustrated in Figure 1 (see also [14–16] for reviews). All factors mentioned above co-regulate the transcriptional activity of a variety of genes involved in mitochondrial biogenesis, OXPHOS activity and fatty acid oxidation. For example, in muscle cells, overexpression of PGC-1α was shown to induce the gene expression of NRF-1, NRF-2, TFAM and mitochondrial-encoded cytochrome c oxidase (COX) subunits [15]. Likewise, muscle–specific overexpression of PPARβ/δ in mice was shown to increase oxidative enzyme activities such as citrate synthase or b-hydroxyacyl-CoA dehydrogenase, and to enhance expression of genes implicated in fatty acid catabolism [17].
Figure 1.
Major transcription factors involved in the regulation of muscle mitochondrial oxidative and phosphorylation (OXPHOS) activity. Non exhaustive key genes, whose expression is regulated by the transcription factors, are given for example.
3. Potential causes of impaired mitochondrial oxidative capacity
Emerging data support the hypothesis that in the particular case of young healthy insulin resistant offspring of parents with type 2 diabetes, alteration in mitochondrial activity would be partly inherited [18–21]. In this review, we will only focus on the potential causes of acquired impairment in muscle mitochondrial OXPHOS activity.
3.1. Aging
Most studies conducted in animals agree and evidence a decrease in mitochondrial oxidative capacity associated with aging [22, 23]. By contrast, data for humans are still controversial [24–29], although several studies have identified some major pathways altered with aging: 1) decreased maximal activity of key mitochondrial oxidative enzymes (e.g. cytochrome c oxidase or citrate synthase) [30, 31], 2) decreased mitochondrial ATP production rates [19,32], 3) decreased protein content in ATP synthase subunits β [33], and 4) decreased mitochondrial density [30, 34]. In addition, several studies have suggested the involvement of alterations in mitochondrial protein synthesis rate in the age-related decrease in mitochondrial oxidative capacity of skeletal muscle [35, 36]. Indeed, a selective decline in the fractional synthesis rate (FSR) of muscle mitochondrial proteins [37, 39] and a decreased stimulation of mitochondrial protein synthesis in response to insulin [39] have been described in elderly individuals compared to young adults. It remains, however, controversial whether the reduction in mitochondrial oxidative capacity is due to a consequence of aging per se or to environmental or lifestyle variables. Indeed, increased sedentary lifestyle and insulin resistance characterizes aging and both situations may contribute to age-related muscle mitochondrial dysfunction.
3.2. Insulin resistance
Several studies conducted in a miniature pig model [38], but also in humans [40, 41], have pointed out that insulin infusion acutely and specifically stimulates muscle whole mitochondrial protein synthesis. In addition, muscle mitochondrial ATP production, cytochrome c oxidase (COX) and citrate synthase enzyme activities in association with mRNA levels from both mitochondrial [NADH dehydrogenase subunit IV] and nuclear [cytochrome c oxidase (COX) subunit IV] genes encoding mitochondrial proteins were increased by insulin in skeletal muscle of healthy adults [41]. The study performed in insulin resistant patients with type 2 diabetes demonstrated a diminished stimulation of muscle mitochondrial ATP production by insulin [41]. Similarly, Petersen et al. reported decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents [20]. It was therefore suggested that impaired insulin action in skeletal muscle could contribute to the decline in mitochondrial OXPHOS activity [42]. However, in contrast to these results, Southgate et al. found using cultured human skeletal myotubes, that insulin acutely decreases the expression of genes involved in oxidative metabolism in healthy but not in insulin resistant muscle [43]. The blunted inhibitory effect of insulin was due to a decrease in the phosphorylation and nuclear exclusion of forkhead box class-O1 (FoxO1) that regulates PGC-1α transcription, secondary to reduced Akt activity. Hence, the hypothesis pointing insulin as a cause of alterations in mitochondrial OXPHOS activity deserves further investigation.
3.3. Sedentary behavior
Physical activity and sedentary behaviour play an important role in the regulation of muscle mitochondrial oxidative capacity. In this respect, it has been demonstrated that sedentary behaviour has deleterious consequences on muscle mitochondrial oxidative capacity [29, 44]. Furthermore, the inverse relationship between aging and mitochondrial respiration is no longer valid when the young and the older subjects are matched for a similar level of physical activity [24,29]. Indeed, Rimbert et al. used a cross-sectional protocol based on a Latin square design in association with a rigorous selection on subjects’ lifestyle to precisely determine the effects of age per se on muscle fat oxidative capacity [29]. The main conclusion arising from the experiment was that muscle mitochondrial OXPHOS activity and the resultant muscle fat oxidative capacity were not primarily impaired by age but by physical inactivity. The importance of physical activity in the prevention of muscle deconditioning and metabolic disorders in young and elderly people should be therefore taken into account.
3.4. Nutrition quality and diet-induced obesity
Nutritional state (e.g. obesity) and nutrients may contribute to skeletal muscle dysfunction. It is now recognized that nutrients have the ability to interact with transcription factors and contribute to biological processes. In this context, new disciplines have recently emerged in the field of nutrition i.e. nutrigenomics and nutrigenetics. Nutrigenomics examines the impact of dietary habits (nutrients provided by diet) on the genome. Nutrigenetics investigates the effect of genetic variation on the interaction between diet and disease. Humans considerably vary in their individual responses to diet, and such approaches can help to examine the impact of nutrition on skeletal muscle function.
Limited but significant data support the concept that nutrition, both quantitatively and qualitatively, may be directly responsible for changes in muscle mitochondrial function. Results have evidenced that in rats, high-fat or high-sucrose intake, and more importantly overfeeding (which effects have to be related to those observed with obesity), are major factors associated with decreased muscle mitochondrial OXPHOS activity, but in a muscle-specific fashion [45]. The mitochondrial OXPHOS activity within the oxidative muscle soleus, resistant to fatigue and dependent on mitochondrial activity for ATP production, were more affected than within the glycolytic muscle tibialis anterior. The main changes included a reduction in the respiratory chain activity with a concomitant decrease in mitochondrial ATP production [45]. Decreased mitochondrial respiration rates [46] and reduced expression of genes involved in mitochondrial oxidative capacity [47] have been reported in diet-induced obese rats. Judging by the available data, excess energy intake and obesity, can be directly associated with alterations in muscle mitochondrial activity. Yet, the role of obesity and in particular the type of obesity (i.e. android or gynoid) in the induction of muscle mitochondrial disturbances is an important question that needs to be addressed.
4. Potential mechanisms: intrinsic factors
As previously described, the transcriptional coactivator PGC-1α is a potent stimulator of mitochondrial biogenesis and OXPHOS activity (c.f. part 1). A decrease in the expression of mitochondrial genes associated with a concomitant reduction in NRF-1 and PGC-1α gene expression has been reported in the skeletal muscle of insulin resistant and diabetic patients [7, 48]. However the intrinsic factors responsible for depressed NRF-1 and PGC-1α gene expression remain to be fully elucidated. There are multiple processes by which environmental or physiologic factors might play a critical role in the control of mitochondrial biogenesis and function. Among these mechanisms, we selected lipotoxicity, inflammation and glucotoxicity.
First, lipotoxicity is the overall damage caused to tissues secondary to prolonged exposure to high levels of plasma non-esterified free fatty acids (NEFA). Excess NEFA and the accumulation of intramyocellular lipid metabolites (e.g., diacylglycerol, fatty acid acylcoA or ceramides) consecutive to increased NEFA availability, have been demonstrated to trigger insulin resistance [49, 50] but may also disturb mitochondrial activity. Recent findings on cultured skeletal muscle cells have brought evidence that fatty acids play a significant role in the regulation of muscle oxidative metabolism. First, a study on human myotubes reported that PGC-1α gene expression was increased two- to three-fold by unsaturated fatty acids but was unchanged with saturated fatty acids [51]. Mitochondrial activity was concomitantly enhanced by unsaturated fatty acids, but was impaired with the saturated fatty acid stearate [51]. Second, data obtained on C2C12 showed that the saturated fatty acid palmitate, by contrast to the monounsaturated fatty acid oleate, reduces PGC-1α gene expression through a mechanism involving mitogen-activated protein kinase (MAPK), extracellular signal-related kinase [52] and NF-κB activation [53]. Another study showed that saturated fatty acids decrease PGC-1α and mitochondrial gene expression and function via p38 MAPK-dependent transcriptional pathways [54]. Interestingly, Benton et al. [55] showed in two animal models in which muscle fatty acid accumulation was either increased (Zucker obese rats) or decreased (FAT/CD36 null mice) that PGC-1α protein expression was inversely correlated to the cellular ability to uptake and store lipids. Similarly, adult rats fed a high-fat diet for two weeks showed lower muscle mitochondrial respiration rates than low-fat fed rats [46].
An original study recently showed that resveratrol, a phytoalexin found in particular in the skin of red grapes, can reverse the deleterious effects of high-fat diet on muscle mitochondrial function in mice [56]. Resveratrol treatment greatly enhanced mitochondrial oxidative capacity, by induction of genes involved in oxidative phosphorylation and mitochondrial biogenesis. These adaptations were principally explained by a resveratrol-mediated activation of the protein deacetylase Sirt1, the result being a decrease in PGC-1α acetylation and an increase in PGC-1α activity [56]. Additional evidence came from research performed in humans. A study conducted in young men has demonstrated that 3-day high-fat diet decreases the expression of genes involved in mitochondrial oxidative capacity in skeletal muscle [8]. To strengthen the lipotoxic theory, infusion of intralipid for 48 hours in healthy humans decreases the muscle expression of both PGC-1α and several genes involved in oxidative phosphorylation [57]. Thus, consistent data obtained on various models, from cell to human, show that intramuscular lipid sensing may be involved in regulating the muscle PGC-1α expression and activity, and consequently muscle mitochondrial oxidative capacity, although further investigations are required to determine quality, dose and time-dependent effects of fatty acids on muscle mitochondrial OXPHOS activity. This single mechanism could be suitable to explain most of the muscle mitochondrial adaptations with metabolic disorders such as obesity and type 2 diabetes. However, additional complex interactions and pathways are likely to occur.
In that respect, a number of adipocyte-derived factors may be responsible for the reduced mitochondrial oxidative capacity. Adipose tissue makes up ~ 15–25% of body mass in men and women with normal values of body mass index (BMI = 18–25 kg/m2) but can vary from 4–10% in athletes to 50% in obese patients (BMI > 30 kg/m2). Adipose tissue cells comprise adipocytes and non-adipose cells that constitute the stroma-vascular fraction, mainly endothelial cells, leucocytes, monocytes and macrophages. Recent studies support the hypothesis that obesity is associated with a state of chronic low-grade inflammation [56–61]. Tumor necrosis alpha (TNF-α) and interleukin 6 (IL-6), pro-inflammatory cytokines produced mainly by adipocytes and macrophages but also by muscle cells, are up-regulated in obesity [58–60]. Recently, it has been shown that TNF-α might positively autoregulate its own synthesis in adipose tissue [61], which might contribute to the maintenance of the elevated TNF-α observed in obesity [62, 63]. One should also keep in mind that the saturated fatty acid palmitate enhances TNF-α expression in skeletal muscle [64]. TNF-α signaling through TNF receptor has been implicated in the pathogenesis of insulin resistance [58–60, 62–63]. In vitro and in vivo data have shown that it suppresses AMPK activity via transcriptional upregulation of protein phosphatase 2C (PP2C). This in turn reduces the phosphorylation of the enzyme acetyl-CoA carboxylase, suppressing fatty-acid oxidation, increasing intramuscular diacylglycerol accumulation, and causing insulin resistance in skeletal muscle [65]. Importantly, TNF-α has been shown to increase the expression of the inducible isoform of the nitric oxide synthase (iNOS) [66] and to downregulate that of the endothelial isoform (eNOS) [67]. Decreased expression of the neuronal isoform (nNOS) has been also reported in skeletal muscle of streptozotocin-induced diabetic rats [68]. These enzymes catalyze the biosynthesis of NO (a short-lived highly diffusible hydrophobic free radical) from L-arginine and molecular oxygen utilizing NADPH as an electron donor and heme, FMN, FAD and tetrahydrobiopterin (H4B) as cofactors [69]. It is now demonstrated that NO generated by eNOS increases mitochondrial biogenesis, oxidative metabolism and ATP levels in several cell types, including muscle [70, 71]. Hickner et al. [72] have shown that in young women skeletal muscle eNOS protein content and activity are inversely related to body fat percentage. In addition recent evidences have demonstrated that eNOS expression and mitochondrial biogenesis are downregulated in adipose and muscle tissues of genetically and diet-induced obese mice and rats whereas iNOS is upregulated [67]. This process has been shown to be partly mediated by cGMP, resulting from NO-dependent activation of “soluble” guanylate cyclase, and involves the increased expression of PGC-1α, NRF-1 and TFAM [70]. Thus, in vitro and in vivo data support that TNFα may be involved in regulating muscle PGC-1a expression, and consequently muscle mitochondrial oxidative capacity.
Regarding IL-6, Al-Khalili et al. have recently established on culture cells from skeletal muscle that IL-6 regulates muscle substrate utilization, enhancing glycogen storage and lipid oxidation [72]. Yet based on the current data, it can be assumed that IL-6 exerts different effects according to the physiological situation, i.e. in response to exercise [73] or during low-grade inflammation in obesity [74, 75]. By contrast to the pro-inflammatory cytokines TNF-α and IL-6, IL-15 is a cytokine highly expressed in skeletal muscle which induces fatty acid oxidation [73] and facilitates glucose metabolism [74]. Furthermore, leptin and adiponectin are anti-inflammatory hormones exclusively produced by adipocytes [76, 77]. Leptin is an adipocytokine produced proportionally to adipose tissue size [78], initially described for its action in brain regions to reduce food intake. Adiponectin, which is present at a high concentration in the plasma, is downregulated with obesity [58]. Leptin and adiponectin have been shown to activate muscle fatty acid oxidation and this action appears to be mediated by AMP-activated protein kinase (AMPK) activation that triggers stimulation of mitochondrial function and biogenesis [79,80]. Indeed, activation of AMPK enhances PGC-1α gene expression [75] and stimulates PPARα [80, 82]. The balance between pro-inflammatory factors and adipocytokines may be therefore a connective link between adipose tissue mass and function, and metabolic disorders in skeletal muscle [81].
Finally, glucotoxicity is commonly defined by the overall damage caused to tissues, secondary to prolonged exposure to elevated plasma glucose concentration. The degree of mitochondrial failure has been correlated with the duration of diabetes. Complexes I, III and IV of the electron transport chain have been shown to be the main mitochondrial targets of hyperglycaemia-induced injury [76]. The presence of chronic hyperglycaemia can cause structural alterations of proteins through the Maillard reaction, and can lead to oxidative stress, a state of imbalance between the production of reactive oxygen species (ROS) and antioxidant defences, and consequently to cellular oxidative damage [77, 78]. In that respect, recent evidences have demonstrated that oxidative stress in skeletal muscle is probably one of the major determinants of the mitochondrial alterations in obesity and type 2 diabetes [79]. This is supported by in vivo and in vitro data showing that 1) an increase in muscle ROS production occurs specifically after hyperglycaemia and hyperlipidemia have appeared in high fat fed mice; 2) in this model, normalization of glycaemia by insulin or phlorizin and treatment with an antioxidant (N-acetylcysteine) decreases muscle ROS production and restores mitochondrial integrity; 3) incubation of cultured muscle cells with high glucose or lipid concentrations induces ROS production and alters mitochondrial density and functions; 4) these effects are blocked by an antioxidant treatment. Enhanced mitochondrial ROS production has been also shown to activate the redox-sensitive transcription factor NF-κB [80], which has been associated with PGC-1α downregulation in C2C12 skeletal muscle cells [53]. In addition, ROS overproduction is likely to enhance several metabolic pathways such as the hexosamine biosynthesis pathway (HBP) [81]. HBP is a nutrient-sensing pathway that has been implicated in the development of insulin resistance [81]. Obici et al. have reported that HBP activation in response to short-term overfeeding is accompanied by an inhibition of the expression of genes (e.g. malate dehydrogenase, acyl-CoA dehydrogenase, propionyl-CoA carboxylase, subunits of complexes I, III, IV and V, adenine nucleotide translocator 2, mitochondrial 2-oxoglutarate/malate carrier protein) involved in mitochondrial oxidative capacity within skeletal muscle [47]. The molecular mechanisms may in part relate to the control of Sp1 activity via O-linked N-acetylglucosamine (O-GlcNAc) modification [11].
5. Cellular consequences of impaired mitochondrial oxidative capacity
Skeletal muscle is highly dependent on mitochondrial oxidative phosphorylation for ATP production, the major energy source for prolonged muscle activity. It is obvious that muscle mitochondrial density and oxidative capacity adapt to muscle energy demand, and therefore decrease with physical inactivity. However, one can question why the muscle mitochondrial oxidative capacity should decrease in a situation of excess nutrient availability (excess intakes of energy, lipid or glucose, obesity). Can one consider this adaptation as a cell suicide? Do we reach the limits of the cellular adaptation? Or are they any beneficial consequences of such an adaptation? Interestingly, Obici et al. [47] suggested that, in line with the thrifty genotype hypothesis, decreased mitochondrial oxidative capacity in the presence of enhanced nutrient availability in skeletal muscle may have conferred a selective survival advantage by favouring the storage of excess nutrients as fat during periods of sporadic food availability. Far from willing to propose an answer, we will briefly review the potential cellular consequences of decreased muscle mitochondrial OXPHOS activity.
5.1. Aerobic capacity
Aerobic capacity is quantified through VO2 max, the maximal oxygen uptake capacity. Limiting factors for VO2 max involved principally the cardiorespiratory system. But endurance performance is also strongly related to mitochondrial oxidative capacity [82]. The latter, especially in sedentary individuals, has been proposed to play a critical role in limiting the oxidative metabolism of skeletal muscle, to a larger extent than oxygen supply [83]. Intolerance to prolonged exercise and early fatigability are common features associated with defects in muscle oxidative capacity [84, 85]. Abnormal response to exercise has notably been associated with a reduced oxidative enzyme activity of complexes III [86] and IV [87] of the mitochondrial respiratory chain.
5.2. Oxidative stress
Mitochondria are critical organelles involved in the generation of ROS. The normal functioning of the mitochondrial respiratory chain continually produces ROS, principally superoxide anion and nitric oxide. ROS have a very short half-life but can rapidly react with DNA, proteins and lipids causing damages to all cell components, including an increased mutation rate for DNA or an increased formation of oxidized proteins and lipids. The mitochondrial membranes and DNA are particularly vulnerable to oxidative stress but all cellular structures are concerned. Oxidation of proteins may alter their structure and function either by loss of catalytic enzyme activity and structural integrity or by interruption of regulatory pathways. Fatty acids are also particularly prone to oxidative damage, resulting in the formation of lipid peroxides. Russel et al. found that skeletal muscle of obese insulin-resistant subjects contained a higher amount of intramyocellular lipids, and a higher degree of lipid peroxidation [88]. Alterations of membrane components also lead to cell dysfunction and even to cell death. For instance, the oxidation of mitochondrial cardiolipins is a key factor in the initiation of cell apoptosis [89]. Although consequences of increased oxidative stress have been clearly identified [90–93], the involvement of mitochondrial dysfunction in increased ROS production associated with metabolic disorders is still under debate. In that respect, Chanseaume et al. have shown that adaptations in mitochondrial OXPHOS activity in rats receiving high-energy diets were associated with a reduction in muscle mitochondrial superoxide anion production [45]. Consistent with comments from Obici et al. [47], decreasing respiratory chain activity in skeletal muscle may not only be considered detrimental to ATP synthesis but may also be responsible for reduced ROS production. This could be related to decreased levels of mitochondrial oxidative protein damage observed in the muscle of diabetic Sprague-Dawley rats [94].
5.3. Metabolic flexibility
Metabolic flexibility describes the ability of muscle to switch between glucose and fatty acids as oxidative energy source depending on metabolic conditions and energy demand. In the healthy state, muscle may switch from fatty acid oxidation under fasting conditions to increased glucose oxidation in the postprandial state. Such capacity to switch between fuels is strongly reduced in obese and diabetic individuals as a consequence of impaired oxidative capacity or/and insulin resistance [95]. This observation supports the hypothesis that alterations in skeletal muscle mitochondrial activity might lead to abnormalities in fuel selection and partitioning, and participate to metabolic inflexibility [96].
5.4. Intracellular lipid content and insulin sensitivity
Although controversial [97, 98], a recent concept has proposed that any impairment of mitochondrial function might predispose to intramyocellular lipid (IMCL) accumulation (fatty acids and/or lipid metabolites). Some evidence has been brought by Benton et al. using animal models characterized by high or low muscle ability to uptake and store fatty acids [56]. PGC-1α protein expression was inversely correlated to the cellular IMCL synthesis rate in the presence of fatty acids. Furthermore, increased triglyceride storage has been observed in parallel to decreased fatty acid oxidative capacity in skeletal muscle of obese and diabetic subjects [99–101].
It is now well established that mitochondrial oxidative capacity is linearly correlated to insulin sensitivity within skeletal muscle [29]. In addition, a decrease in mitochondrial content and function has been described in the skeletal muscle of obese, insulin resistant and type 2 diabetic patients compared to healthy individuals [7, 19–20, 30–31, 52, 101–104]. Hence in the past few years, muscle mitochondrial dysfunction has been suggested as the leading cause for impaired insulin sensitivity [21]. The close association between mitochondrial oxidative capacity and insulin sensitivity possibly involves alterations in intracellular trafficking of fat metabolites [103, 105]. Recent studies using in vivo nuclear magnetic resonance (NMR) spectroscopy have evidenced an inverse relationship between insulin sensitivity and ICML content [106–108]. The molecular mechanism underlying defective insulin-stimulated glucose transport activity may be attributed to an accumulation of intramyocellular lipid metabolites such as ceramides, fatty acyl CoAs and diacylglycerol which could potentially disrupt the insulin signalling pathway through Ser/Thr phosphorylation of insulin receptor substrate [48]. A convincing demonstration has been brought by Petersen et al. [27] who compared IMCL content and mitochondrial function in healthy young and elderly individuals using NMR spectroscopy. Data demonstrated that elderly individuals showed significantly higher ICML, but reduced muscle mitochondrial ATP synthesis and higher plasma insulin concentration during oral glucose tolerance test compared to young adults [27]. But while several authors support this hypothesis using transversal studies, results from recent work have questioned this concept. In a chronological model of diet-induced obesity in rats, it has been demonstrated that insulin resistance can not be attributed to a decrease in mitochondrial oxidative capacity, which appears later, after changes in lipid metabolism and insulin sensitivity have occurred [109]. The main result of the latter experiment was that before being a “victim”, muscle mitochondrial oxidative capacity first positively adjusts to excess energy and contributes to limit the diet-induced metabolic disorders i.e. alteration of lipid metabolism, IMCL accumulation and insulin resistance [109]. Therefore new possibility has emerged that mitochondrial dysfunction is not necessarily the primary cause of IMCL accumulation and insulin resistance within skeletal muscle but that stimulating mitochondrial OXPHOS activity may be of importance in the prevention of these metabolic disorders [2, 117].
6. Conclusions
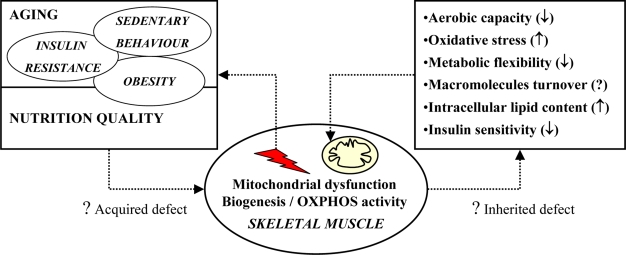
According to current knowledge, the underlying molecular mechanisms of muscle mitochondrial dysfunction in metabolic disorders are far from being elucidated. One or a combination of mechanisms involving genetics, disturbances in glucose and lipid homeostasis, oxidative stress and also a state of chronic low-grade inflammation, might be involved in the process leading to these defects. Figure 2 illustrates potential causes and cellular consequences of impaired mitochondrial oxidative capacity in skeletal muscle. Nowadays, understanding the molecular and biochemical defects responsible for muscle mitochondrial dysfunction is of importance to specify the role of mitochondrial dysfunction in the aetiology of metabolic disorders and to define preventive and therapeutic targets for the treatment of these pathologies.
Figure 2.
Summary of potential causes and cellular consequences of impaired mitochondrial oxidative and phosphorylation (OXPHOS) activity in skeletal muscle.
Physiological factors associated to aging, hormonal changes, lifestyle behavior and diet, may impair muscle mitochondrial biogenesis and OXPHOS activity during lifespan. These alterations, named “acquired defects”, in association with “inherited defects” due to genetic or epigenetic processes, may favor the apparition of metabolic disorders which then hasten a vicious circle that can ultimately lead to pathological states.
Acknowledgments
The authors thank all members of the Human Nutrition Laboratory of Clermont-Ferrand.
References
- 1.Fredenrich A, Grimaldi PA. Roles of peroxisome proliferator-activated receptor delta in skeletal muscle function and adaptation. Curr. Opin. Clin. Nutr. Metab. Care. 2004;7:377–381. doi: 10.1097/01.mco.0000134370.93686.0a. [DOI] [PubMed] [Google Scholar]
- 2.Grimaldi PA. Regulatory role of peroxisome proliferator-activated receptor delta (PPAR delta) in muscle metabolism. A new target for metabolic syndrome treatment? Biochimie. 2005a;87:5–8. doi: 10.1016/j.biochi.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Grimaldi PA. Roles of PPARdelta in skeletal muscle physiology. Med. Sci. (Paris) 2005b;21:239–240. doi: 10.1051/medsci/2005213239. [DOI] [PubMed] [Google Scholar]
- 4.Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard JM, Lehmann JM. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc. Natl. Acad. Sci. USA. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lapsys NM, Kriketos AD, Lim-Fraser M, Poynten AM, Lowy A, Furler SM, Chisholm DJ, Cooney GJ. Expression of genes involved in lipid metabolism correlate with peroxisome proliferator-activated receptor gamma expression in human skeletal muscle. J. Clin. Endocrinol. Metab. 2000;85:4293–4297. doi: 10.1210/jcem.85.11.6973. [DOI] [PubMed] [Google Scholar]
- 6.Lazennec G, Canaple L, Saugy D, Wahli W. Activation of peroxisome proliferator-activated receptors (PPARs) by their ligands and protein kinase A activators. Mol. Endocrinol. 2000;14:1962–1975. doi: 10.1210/mend.14.12.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc. Natl. Acad. Sci. USA. 2003;100:8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sparks LM, Xie H, Koza RA, Mynatt R, Hulver MW, Bray GA, Smith SR. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes. 2005;54:1926–1933. doi: 10.2337/diabetes.54.7.1926. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka T, Yamamoto J, Iwasaki S, Asaba H, Hamura H, Ikeda Y, Watanabe M, Magoori K, Ioka RX, Tachibana K, Watanabe Y, Uchiyama Y, Sumi K, Iguchi H, Ito S, Doi T, Hamakubo T, Naito M, Auwerx J, Yanagisawa M, Kodama T, Sakai J. Activation of peroxisome proliferator-activated receptor delta induces fatty acid beta-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc. Natl. Acad. Sci. USA. 2003;100:15924–15929. doi: 10.1073/pnas.0306981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaid A, Li R, Luciakova K, Barath P, Nery S, Nelson BD. On the role of the general transcription factor Sp1 in the activation and repression of diverse mammalian oxidative phosphorylation genes. J. Bioenerg. Biomembr. 1999;31:129–135. doi: 10.1023/a:1005499727732. [DOI] [PubMed] [Google Scholar]
- 11.Yang X, Su K, Roos MD, Chang Q, Paterson AJ, Kudlow JE. O-linkage of N-acetylglucosamine to Sp1 activation domain inhibits its transcriptional capability. Proc. Natl. Acad. Sci. USA. 2001;98:6611–661. doi: 10.1073/pnas.111099998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moyes CD. Controlling muscle mitochondrial content. J. Exp. Biol. 2003;206:4385–4391. doi: 10.1242/jeb.00699. [DOI] [PubMed] [Google Scholar]
- 13.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002a;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 14.Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): Transcriptional coactivator and metabolic regulator. Endocr. Rev. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- 15.Chabi B, Adhihetty PJ, Ljubicic V, Hood DA. How is mitochondrial biogenesis affected in mitochondrial disease? Med. Sci. Sports Exerc. 2005;37:2102–2110. doi: 10.1249/01.mss.0000177426.68149.83. [DOI] [PubMed] [Google Scholar]
- 16.Hood DA, Irrcher I, Ljubicic V, Joseph AM. Coordination of metabolic plasticity in skeletal muscle. J. Exp. Biol. 2006;209:2265–2275. doi: 10.1242/jeb.02182. [DOI] [PubMed] [Google Scholar]
- 17.Luquet S, Lopez-Soriano J, Holst D, Fredenrich A, Melki J, Rassoulzadegan M, Grimaldi PA. Peroxisome proliferator-activated receptor delta controls muscle development and oxidative capability. FASEB J. 2003;17:2299–22301. doi: 10.1096/fj.03-0269fje. [DOI] [PubMed] [Google Scholar]
- 18.Befroy DE, Falk Petersen K, Dufour S, Mason GF, de Graaf RA, Rothman DL, Shulman GI. Impaired Mitochondrial Substrate Oxidation in Muscle of Insulin-Resistant Offspring of Type 2 Diabetic Patients. Diabetes. 2007;57:1376–1381. doi: 10.2337/db06-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N. Engl. J. Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen KF, Dufour S, Shulman GI. Decreased Insulin-Stimulated ATP Synthesis and Phosphate Transport in Muscle of Insulin-Resistant Offspring of Type 2 Diabetic Parents. PLoS Med. 2005;2:e233. doi: 10.1371/journal.pmed.0020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petersen KF, Shulman GI. Etiology of insulin resistance. Am. J. Med. 2006;119:S10–S16. doi: 10.1016/j.amjmed.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drew B, Phaneuf S, Dirks A, Selman C, Gredilla R, Lezza A, Barja G, Leeuwenburgh C. Effects of aging and caloric restriction on mitochondrial energy production in gastrocnemius muscle and heart. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R474–R480. doi: 10.1152/ajpregu.00455.2002. [DOI] [PubMed] [Google Scholar]
- 23.Kwong LK, Sohal RS. Age-related changes in activities of mitochondrial electron transport complexes in various tissues of the mouse. Arch. Biochem. Biophys. 2000;373:16–22. doi: 10.1006/abbi.1999.1495. [DOI] [PubMed] [Google Scholar]
- 24.Barrientos A, Casademont J, Rotig A, Miro O, Urbano-Marquez A, Rustin P, Cardellach F. Absence of relationship between the level of electron transport chain activities and aging in human skeletal muscle. Biochem. Biophys. Res. Commun. 1996;229:536–539. doi: 10.1006/bbrc.1996.1839. [DOI] [PubMed] [Google Scholar]
- 25.Brierley EJ, Johnson MA, James OF, Turnbull DM. Effects of physical activity and age on mitochondrial function. QJM. 1996;89:251–258. doi: 10.1093/qjmed/89.4.251. [DOI] [PubMed] [Google Scholar]
- 26.Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and ageing in human muscle. J. Physiol. 2000;526:203–210. doi: 10.1111/j.1469-7793.2000.t01-1-00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: Possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasmussen UF, Krustrup P, Kjaer M, Rasmussen HN. Human skeletal muscle mitochondrial metabolism in youth and senescence: no signs of functional changes in ATP formation and mitochondrial oxidative capacity. Pflugers Arch. 2003;446:270–278. doi: 10.1007/s00424-003-1022-2. [DOI] [PubMed] [Google Scholar]
- 29.Rimbert V, Boirie Y, Bedu M, Hocquette JF, Ritz P, Morio B. Muscle fat oxidative capacity is not impaired by age but by physical inactivity: association with insulin sensitivity. FASEB J. 2004;18:737–739. doi: 10.1096/fj.03-1104fje. [DOI] [PubMed] [Google Scholar]
- 30.Kelley D, He J, Menshikova E, Ritov V. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 31.Simoneau JA, Kelley DE. Altered glycolytic and oxidative capacities of skeletal muscle contribute to insulin resistance in NIDDM. J. Appl. Physiol. 1997;83:166–171. doi: 10.1152/jappl.1997.83.1.166. [DOI] [PubMed] [Google Scholar]
- 32.Petersen KF, Dufour S, Shulman GI. Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of Type 2 Diabetic Parents. PLoS Med. 2005a;2:e233. doi: 10.1371/journal.pmed.0020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hojlund K, Wrzesinski K, Larsen PM, Fey SJ, Roepstorff P, Handberg A, Dela F, Vinten J, McCormack JG, Reynet C, Beck-Nielsen H. Proteome analysis reveals phosphorylation of ATP synthase beta -subunit in human skeletal muscle and proteins with potential roles in type 2 diabetes. J. Biol. Chem. 2003;278:10436–10442. doi: 10.1074/jbc.M212881200. [DOI] [PubMed] [Google Scholar]
- 34.Liang P, Hughes V, Fukagawa NK. Increased prevalence of mitochondrial DNA deletions in skeletal muscle of older individuals with impaired glucose tolerance: possible marker of glycemic stress. Diabetes. 1997;46:920–923. doi: 10.2337/diab.46.5.920. [DOI] [PubMed] [Google Scholar]
- 35.Guillet C, Prod’homme M, Balage M, Gachon P, Giraudet C, Morin L, Grizard J, Boirie Y. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. Faseb J. 2004;18:1586–7. doi: 10.1096/fj.03-1341fje. [DOI] [PubMed] [Google Scholar]
- 36.Rooyackers OE, Adey DB, Ades PA, Nair KS. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci USA. 1996a;93:15364–15369. doi: 10.1073/pnas.93.26.15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balagopal P, Rooyackers OE, Adey DB, Ades PA, Nair KS. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. Am. J. Physiol. 1997;273:E790–E800. doi: 10.1152/ajpendo.1997.273.4.E790. [DOI] [PubMed] [Google Scholar]
- 38.Boirie Y, Short KR, Ahlman B, Charlton M, Nair KS. Tissue-specific regulation of mitochondrial and cytoplasmic protein synthesis rates by insulin. Diabetes. 2001;50:2652–2658. doi: 10.2337/diabetes.50.12.2652. [DOI] [PubMed] [Google Scholar]
- 39.Rooyackers OE, Adey DB, Ades PA, Nair KS. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc. Natl. Acad. Sci. USA. 1996;93:15364–15369. doi: 10.1073/pnas.93.26.15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halvatsiotis P, Short KR, Bigelow M, Nair KS. Synthesis rate of muscle proteins, muscle functions, and amino acid kinetics in type 2 diabetes. Diabetes. 2002;51:2395–2404. doi: 10.2337/diabetes.51.8.2395. [DOI] [PubMed] [Google Scholar]
- 41.Stump CS, Short KR, Bigelow ML, Schimke JM, Nair KS. Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc. Natl. Acad. Sci. USA. 2003;100:7996–8001. doi: 10.1073/pnas.1332551100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boirie Y. Insulin regulation of mitochondrial proteins and oxidative phosphorylation in human muscle. Trends Endocrinol. Metab. 2003;14:393–394. doi: 10.1016/j.tem.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Southgate RJ, Bruce CR, Carey AL, Steinberg GR, Walder K, Monks R, Watt MJ, Hawley JA, Birnbaum MJ, Febbraio MA. PGC-1alpha gene expression is down-regulated by Akt- mediated phosphorylation and nuclear exclusion of FoxO1 in insulin-stimulated skeletal muscle. Faseb J. 2005;19:2072–2074. doi: 10.1096/fj.05-3993fje. [DOI] [PubMed] [Google Scholar]
- 44.Short KR, Vittone JL, Bigelow ML, Proctor DN, Rizza RA, Coenen-Schimke JM, Nair KS. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes. 2003;52:1888–1896. doi: 10.2337/diabetes.52.8.1888. [DOI] [PubMed] [Google Scholar]
- 45.Chanseaume E, Malpuech-Brugere C, Patrac V, Bielicki G, Rousset P, Couturier K, Salles J, Renou JP, Boirie Y, Morio B. Diets high in sugar, fat, and energy induce muscle type-specific adaptations in mitochondrial functions in rats. J. Nutr. 2006;136:2194–2200. doi: 10.1093/jn/136.8.2194. [DOI] [PubMed] [Google Scholar]
- 46.Iossa S, Lionetti L, Mollica MP, Crescenzo R, Botta M, Barletta A, Liverini G. Effect of high-fat feeding on metabolic efficiency and mitochondrial oxidative capacity in adult rats. Br. J. Nutr. 2003;90:953–960. doi: 10.1079/bjn2003000968. [DOI] [PubMed] [Google Scholar]
- 47.Obici S, Wang J, Chowdury R, Feng Z, Siddhanta U, Morgan K, Rossetti L. Identification of a biochemical link between energy intake and energy expenditure. J. Clin. Invest. 2002;109:1599–1605. doi: 10.1172/JCI15258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 49.Chavez JA, Holland WL, Bar J, Sandhoff K, Summers SA. Acid ceramidase overexpression prevents the inhibitory effects of saturated fatty acids on insulin signaling. J. Biol. Chem. 2005;280:20148–20153. doi: 10.1074/jbc.M412769200. [DOI] [PubMed] [Google Scholar]
- 50.Shulman GI. Cellular mechanisms of insulin resistance. J. Clin. Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Staiger H, Staiger K, Haas C, Weisser M, Machicao F, Haring HU. Fatty acid-induced differential regulation of the genes encoding peroxisome proliferator-activated receptor-gamma coactivator-1alpha and -1beta in human skeletal muscle cells that have been differentiated in vitro. Diabetologia. 2005;48:2115–2118. doi: 10.1007/s00125-005-1895-z. [DOI] [PubMed] [Google Scholar]
- 52.Simoneau JA, Veerkamp JH, Turcotte LP, Kelley DE. Markers of capacity to utilize fatty acids in human skeletal muscle: relation to insulin resistance and obesity and effects of weight loss. Faseb J. 1999;13:2051–2060. doi: 10.1096/fasebj.13.14.2051. [DOI] [PubMed] [Google Scholar]
- 53.Coll T, Jove M, Rodriguez-Calvo R, Eyre E, Palomer X, Sanchez RM, Merlos M, Laguna JC, Vazquez-Carrera M. Palmitate-mediated downregulation of peroxisome proliferator-activated receptor-gamma coactivator 1alpha in skeletal muscle cells involves MEK1/2 and nuclear factor-kappaB activation. Diabetes. 2006;55:2779–2787. doi: 10.2337/db05-1494. [DOI] [PubMed] [Google Scholar]
- 54.Crunkhorn S, Dearie F, Mantzoros C, Gami H, da Silva WS, Espinoza D, Faucette R, Barry K, Bianco AC, Patti ME. Peroxisome proliferator activator receptor gamma coactivator-1 expression is reduced in obesity: Potential pathogenic role of saturated fatty acids and p38 mitogen-activated protein kinase activation. J. Biol. Chem. 2007;282:15439–15450. doi: 10.1074/jbc.M611214200. [DOI] [PubMed] [Google Scholar]
- 55.Benton CR, Han XX, Febbraio M, Graham TE, Bonen A. Inverse relationship between PGC-1alpha protein expression and triacylglycerol accumulation in rodent skeletal muscle. J. Appl. Physiol. 2006;100:377–383. doi: 10.1152/japplphysiol.00781.2005. [DOI] [PubMed] [Google Scholar]
- 56.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 57.Richardson DK, Kashyap S, Bajaj M, Cusi K, Mandarino SJ, Finlayson J, DeFronzo RA, Jenkinson CP, Mandarino LJ. Lipid infusion decreases the expression of nuclear encoded mitochondrial genes and increases the expression of extracellular matrix genes in human skeletal muscle. J. Biol. Chem. 2005;280:10290–10297. doi: 10.1074/jbc.M408985200. [DOI] [PubMed] [Google Scholar]
- 58.Brindley DN, Wang CN, Mei J, Xu J, Hanna AN. Tumor necrosis factor-alpha and ceramides in insulin resistance. Lipids. 1999;(34 Suppl):S85–S88. doi: 10.1007/BF02562240. [DOI] [PubMed] [Google Scholar]
- 59.Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52:1779–1785. doi: 10.2337/diabetes.52.7.1779. [DOI] [PubMed] [Google Scholar]
- 60.Saghizadeh M, Ong JM, Garvey WT, Henry RR, Kern PA. The expression of TNF alpha by human muscle. Relationship to insulin resistance. J. Clin. Invest. 1996;97:1111–1116. doi: 10.1172/JCI118504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neels JG, Pandey M, Hotamisligil GS, Samad F. Autoamplification of tumor necrosis factor-alpha: A potential mechanism for the maintenance of elevated tumor necrosis factor-alpha in male but not female obese mice. Am. J. Pathol. 2006;168:435–444. doi: 10.2353/ajpath.2006.050699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Halse R, Pearson SL, McCormack JG, Yeaman SJ, Taylor R. Effects of tumor necrosis factor-alpha on insulin action in cultured human muscle cells. Diabetes. 2001;50:1102–1109. doi: 10.2337/diabetes.50.5.1102. [DOI] [PubMed] [Google Scholar]
- 63.Rieusset J, Bouzakri K, Chevillotte E, Ricard N, Jacquet D, Bastard JP, Laville M, Vidal H. Suppressor of cytokine signaling 3 expression and insulin resistance in skeletal muscle of obese and type 2 diabetic patients. Diabetes. 2004;53:2232–2241. doi: 10.2337/diabetes.53.9.2232. [DOI] [PubMed] [Google Scholar]
- 64.Jove M, Planavila A, Sanchez RM, Merlos M, Laguna JC, Vazquez-Carrera M. Palmitate induces tumor necrosis factor-alpha expression in C2C12 skeletal muscle cells by a mechanism involving protein kinase C and nuclear factor-kappaB activation. Endocrinology. 2006;147:552–561. doi: 10.1210/en.2005-0440. [DOI] [PubMed] [Google Scholar]
- 65.Steinberg GR, Michell BJ, van Denderen BJ, Watt MJ, Carey AL, Fam BC, Andrikopoulos S, Proietto J, Gorgun CZ, Carling D, Hotamisligil GS, Febbraio MA, Kay TW, Kemp BE. Tumor necrosis factor alpha-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab. 2006;4:465–474. doi: 10.1016/j.cmet.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 66.Munoz-Fernandez MA, Fresno M. The role of tumour necrosis factor, interleukin 6, interferon-gamma and inducible nitric oxide synthase in the development and pathology of the nervous system. Prog. Neurobiol. 1998;56:307–340. doi: 10.1016/s0301-0082(98)00045-8. [DOI] [PubMed] [Google Scholar]
- 67.Valerio A, Cardile A, Cozzi V, Bracale R, Tedesco L, Pisconti A, Palomba L, Cantoni O, Clementi E, Moncada S, Carruba MO, Nisoli E. TNF-alpha downregulates eNOS expression and mitochondrial biogenesis in fat and muscle of obese rodents. J. Clin. Invest. 2006;116:2791–2798. doi: 10.1172/JCI28570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perreault M, Dombrowski L, Marette A. Mechanism of impaired nitric oxide synthase activity in skeletal muscle of streptozotocin-induced diabetic rats. Diabetologia. 2000;43:427–437. doi: 10.1007/s001250051325. [DOI] [PubMed] [Google Scholar]
- 69.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem. J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 71.Nisoli E, Falcone S, Tonello C, Cozzi V, Palomba L, Fiorani M, Pisconti A, Brunelli S, Cardile A, Francolini M, Cantoni O, Carruba MO, Moncada S, Clementi E. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc. Natl. Acad. Sci. USA. 2004;101:16507–16512. doi: 10.1073/pnas.0405432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hickner RC, Kemeny G, Stallings HW, Manning SM, McIver KL. Relationship between body composition and skeletal muscle eNOS. Int. J. Obes. (Lond) 2006;30:308–312. doi: 10.1038/sj.ijo.0803134. [DOI] [PubMed] [Google Scholar]
- 73.Almendro V, Busquets S, Ametller E, Carbo N, Figueras M, Fuster G, Argiles JM, Lopez-Soriano FJ. Effects of interleukin-15 on lipid oxidation: disposal of an oral [(14)C]-triolein load. Biochim. Biophys. Acta. 2006;1761:37–42. doi: 10.1016/j.bbalip.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 74.Busquets S, Figueras M, Almendro V, Lopez-Soriano FJ, Argiles JM. Interleukin-15 increases glucose uptake in skeletal muscle. An antidiabetogenic effect of the cytokine. Biochim. Biophys. Acta. 2006;1760:1613–1617. doi: 10.1016/j.bbagen.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 75.Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc. Natl. Acad. Sci. USA. 2002;99:15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Turko IV, Li L, Aulak KS, Stuehr DJ, Chang JY, Murad F. Protein tyrosine nitration in the mitochondria from diabetic mouse heart. Implications to dysfunctional mitochondria in diabetes. J. Biol. Chem. 2003;278:33972–33977. doi: 10.1074/jbc.M303734200. [DOI] [PubMed] [Google Scholar]
- 77.Brownlee M. A radical explanation for glucose-induced beta cell dysfunction. J. Clin. Invest. 2003;112:1788–1790. doi: 10.1172/JCI20501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 79.Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, Morio B, Vidal H, Rieusset J. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced nsulin-resistant mice. J. Clin. Invest. 2008;118:789–800. doi: 10.1172/JCI32601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 81.Zachara NE, Hart GW. O-GlcNAc a sensor of cellular state: the role of nucleocytoplasmic glycosylation in modulating cellular function in response to nutrition and stress. Biochim. Biophys. Acta. 2004;1673:13–28. doi: 10.1016/j.bbagen.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 82.Bassett DR, Jr, Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med. Sci. Sports Exerc. 2000;32:70–84. doi: 10.1097/00005768-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 83.Haseler LJ, Lin AP, Richardson RS. Skeletal muscle oxidative metabolism in sedentary humans: 31P-MRS assessment of O2 supply and demand limitations. J. Appl. Physiol. 2004;97:1077–1081. doi: 10.1152/japplphysiol.01321.2003. [DOI] [PubMed] [Google Scholar]
- 84.Arenas J, Martin MA. Metabolic intolerance to exercise. Neurologia. 2003;18:291–302. [PubMed] [Google Scholar]
- 85.Coggan AR, Abduljalil AM, Swanson SC, Earle MS, Farris JW, Mendenhall LA, Robitaille PM. Muscle metabolism during exercise in young and older untrained and endurance-trained men. J. Appl. Physiol. 1993;75:2125–2133. doi: 10.1152/jappl.1993.75.5.2125. [DOI] [PubMed] [Google Scholar]
- 86.Mousson B, Collombet JM, Dumoulin R, Carrier H, Flocard F, Bouzidi M, Godinot C, Maire I, Mathieu M, Quard S. An abnormal exercise test response revealing a respiratory chain complex III deficiency. Acta Neurol. Scand. 1995;91:488–493. doi: 10.1111/j.1600-0404.1995.tb00451.x. [DOI] [PubMed] [Google Scholar]
- 87.Haller RG, Lewis SF, Estabrook RW, DiMauro S, Servidei S, Foster DW. Exercise intolerance, lactic acidosis, and abnormal cardiopulmonary regulation in exercise associated with adult skeletal muscle cytochrome c oxidase deficiency. J. Clin. Invest. 1989;84:155–161. doi: 10.1172/JCI114135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Russell AP, Gastaldi G, Bobbioni-Harsch E, Arboit P, Gobelet C, Deriaz O, Golay A, Witztum JL, Giacobino JP. Lipid peroxidation in skeletal muscle of obese as compared to endurance-trained humans: a case of good vs. bad lipids? FEBS Lett. 2003;551:104–106. doi: 10.1016/s0014-5793(03)00875-5. [DOI] [PubMed] [Google Scholar]
- 89.Nakagawa Y. Initiation of apoptotic signal by the peroxidation of cardiolipin of mitochondria. Ann. NY Acad. Sci. 2004;1011:177–184. doi: 10.1007/978-3-662-41088-2_18. [DOI] [PubMed] [Google Scholar]
- 90.Bulteau AL, Szweda LI, Friguet B. Mitochondrial protein oxidation and degradation in response to oxidative stress and aging. Exp. Gerontol. 2006;41:653–657. doi: 10.1016/j.exger.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 91.Fridlyand LE, Philipson LH. Reactive species and early manifestation of insulin resistance in type 2 diabetes. Diabetes Obes. Metab. 2006;8:136–145. doi: 10.1111/j.1463-1326.2005.00496.x. [DOI] [PubMed] [Google Scholar]
- 92.Jezek P, Hlavata L. Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism. Int. J. Biochem. Cell Biol. 2005;37:2478–2503. doi: 10.1016/j.biocel.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 93.Lenaz G, D’Aurelio M, Merlo Pich M, Genova ML, Ventura B, Bovina C, Formiggini G, Parenti Castelli G. Mitochondrial bioenergetics in aging. Biochim. Biophys. Acta. 2000;1459:397–404. doi: 10.1016/s0005-2728(00)00177-8. [DOI] [PubMed] [Google Scholar]
- 94.Kayali R, Cakatay U, Telci A, Akcay T, Sivas A, Altug T. Decrease in mitochondrial oxidative protein damage parameters in the streptozotocin-diabetic rat. Diabetes Metab. Res. Rev. 2004;20:315–321. doi: 10.1002/dmrr.456. [DOI] [PubMed] [Google Scholar]
- 95.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: A reexamination. Diabetes. 2000;49:677–683. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- 96.Storlien L, Oakes ND, Kelley DE. Metabolic flexibility. Proc. Nutr. Soc. 2004;63:363–368. doi: 10.1079/PNS2004349. [DOI] [PubMed] [Google Scholar]
- 97.Kraegen EW, Cooney GJ, Turner N. Muscle insulin resistance: A case of fat overconsumption, not mitochondrial dysfunction. Proc. Natl Acad. Sci. USA. 2008;105:7627–7628. doi: 10.1073/pnas.0803901105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Holloszy JO. Skeletal muscle “mitochondrial deficiency” does not mediate insulin resistance. Am. J. Clin. Nutr. 2008 Dec 3; doi: 10.3945/ajcn.2008.26717C. [DOI] [PubMed] [Google Scholar]
- 99.Bonen A, Parolin ML, Steinberg GR, Calles-Escandon J, Tandon NN, Glatz JF, Luiken JJ, Heigenhauser GJ, Dyck DJ. Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. Faseb J. 2004;18:1144–1146. doi: 10.1096/fj.03-1065fje. [DOI] [PubMed] [Google Scholar]
- 100.He J, Watkins S, Kelley D. Skeletal muscle lipid content and oxidative enzyme activity in relation to muscle fiber type in type 2 diabetes and obesity. Diabetes. 2001;50:817–823. doi: 10.2337/diabetes.50.4.817. [DOI] [PubMed] [Google Scholar]
- 101.Schrauwen P, Hesselink MK. Oxidative capacity, lipotoxicity, and mitochondrial damage in type 2 diabetes. Diabetes. 2004;53:1412–1417. doi: 10.2337/diabetes.53.6.1412. [DOI] [PubMed] [Google Scholar]
- 102.Bruce CR, Anderson MJ, Carey AL, Newman DG, Bonen A, Kriketos AD, Cooney GJ, Hawley JA. Muscle oxidative capacity is a better predictor of insulin sensitivity than lipid status. J. Clin. Endocrinol. Metab. 2003;88:5444–5451. doi: 10.1210/jc.2003-030791. [DOI] [PubMed] [Google Scholar]
- 103.Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, Pypaert M, Shulman GI. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J. Clin. Invest. 2005;115:3587–3593. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes. 2005;54:8–14. doi: 10.2337/diabetes.54.1.8. [DOI] [PubMed] [Google Scholar]
- 105.Petersen KF, Shulman GI. Cellular mechanism of insulin resistance in skeletal muscle. J. R. Soc. Med. 2002;95(Suppl 42):8–13. [PMC free article] [PubMed] [Google Scholar]
- 106.Forouhi NG, Jenkinson G, Thomas EL, Mullick S, Mierisova S, Bhonsle U, McKeigue PM, Bell JD. Relation of triglyceride stores in skeletal muscle cells to central obesity and insulin sensitivity in European and South Asian men. Diabetologia. 1999;42:932–935. doi: 10.1007/s001250051250. [DOI] [PubMed] [Google Scholar]
- 107.Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: A 1H NMR spectroscopy study. Diabetologia. 1999;42:113–116. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 108.Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, Vanzulli A, Testolin G, Pozza G, Del Maschio A, Luzi L. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: A 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes. 1999;48:1600–1606. doi: 10.2337/diabetes.48.8.1600. [DOI] [PubMed] [Google Scholar]
- 109.Chanseaume E, Tardy AL, Salles J, Giraudet C, Rousset P, Tissandier A, Boirie Y, Morio B. Chronological approach of diet-induced alterations in muscle mitochondrial functions in rats. Obesity (Silver Spring) 2007;15:50–59. doi: 10.1038/oby.2007.511. [DOI] [PubMed] [Google Scholar]