Abstract
T-regulatory cell (T-reg) frequency is increased in HIV infection and with aging. We evaluated the effect of age on total, memory and naïve T-reg percentages in untreated HIV infection. Older HIV+ subjects had a total T-reg percent that is 2.8% (p = 0.02) higher than among younger HIV+, older HIV− and younger HIV− subjects. In HIV+ subjects, the total T-reg percentage is inversely correlated with the lymphocyte proliferative responses to tetanus (r = −0.45, p = 0.002) and Candida (r = −0.43, p =0.003) antigens. Similar correlations were seen between memory T-reg percentages and the lymphocyte proliferative response to tetanus and Candida in HIV+ subjects. T-reg percentages did not correlate consistently with markers of immune activation. T-reg percentages are increased in the older HIV+ population and may play a role in the accelerated disease progression seen in older HIV-infected persons.
Keywords: HIV, aging, T-regulatory cells
INTRODUCTION
T-regulatory cells (T-regs) are a critical T-cell population that profoundly inhibits T-cell activation, proliferation and effector function[1]. Deficiency in T-reg number or function is associated with autoimmune disease, while increased T-reg frequency is seen in certain cancers[2, 3] and chronic infections[1, 4, 5]. T-regs are characterized by the expression of the forkhead transcription factor (FoxP3), which is critical to their regulatory function. FoxP3 is a transcriptional repressor of nuclear factor of activated T-cells (NFAT) and nuclear factor-kappa B (NFκB), which leads to the suppression of interleukin (IL)-2 secretion[5, 6]. How best to identify human T-regs is controversial. Markers such as CD25 (the IL-2α-chain receptor), cytotoxic T-lymphocyte antigen-4 (CTLA-4), glucocorticosteroid-induced tumor necrosis factor receptor (GITR), and FoxP3 have been used to identify T-regs, but each of these markers is also upregulated in T-cells that have been activated by a variety of mechanisms. Recent studies show that T-regs can be identified reliably by the high-level expression of CD25 and the low-level expression of CD127, the IL-7 α-chain receptor[7–9]. The expression of CD127 inversely correlates with the expression of FoxP3, and over 80% of CD25+FoxP3+ cells are CD25+CD127lo [8]. Also, the isolation of CD25+CD127−CD4+ cells results in a highly purified population of T-regs[8].
Complicating the phenotypic identification of T-regs is the existence of many T-reg subsets that play divergent roles in various physiologic and disease settings. Abnormalities in these subsets play distinct roles in diseases ranging from multiple sclerosis to multiple myeloma. One way of categorizing T-regs is based on CD45RO isoform expression. Naïve T-regs are CD45RO−, are directly derived from the thymus, target self-antigens and are apoptosis-resistant[10, 11]. Memory T-regs, on the other hand, are CD45RO+, are generated in peripheral tissues from naïve T-cells after exposure to cognate peptide, and are activated and apoptosis-prone[11, 12].
Both a decrease in total T-reg numbers and an increase in T-reg proportions in peripheral blood are seen in HIV infection[9, 13, 14]. Memory T-reg proportions are increased in HIV infection, while naïve T-reg proportions are diminished[14]. The relative enrichment of the CD4+ T-cell pool with T-regs is associated with decreased immune responses to pathogens ex vivo[14], while the decrease in T-reg numbers is associated with the increased levels of immune activation [9, 14].
Aging, like HIV, is associated with impaired thymic function[15], a decreased ratio of naive to memory T-cells[16], impaired lymphocyte proliferative responses to antigens[17], and impaired delayed-type hypersensitivity (DTH) responses[18]. The damage to the immune system by HIV infection is more pronounced in association with older age. Naive and total CD4+ T-cell regeneration in response to highly active antiretroviral therapy (HAART) is less robust in older compared to younger HIV-infected (HIV+) patients[19], and older age at HAART initiation is associated with a higher risk of HIV-disease progression or death despite HAART[20–23]. Aging is also associated with an increase in T-reg activity and frequency[24–28]. In this study, we examine the effect of aging on the T-reg abnormalities seen in HIV infection.
MATERIALS AND METHODS
Blood was obtained from participants in AIDS Clinical Trials Group (ACTG) Protocol 5015 that enrolled treatment naïve HIV+ patients who were either 18 – 30 years old (younger HIV+) or ≥ 45 years (older HIV+). Age-matched, healthy HIV-uninfected (HIV−) subjects were enrolled to ACTG 5113 as controls Included among the baseline immune and viral indices that were measured in these studies were: plasma HIV-1 RNA (in HIV+ subjects only), CD4+ T-cells, T-cell subsets (naïve [CD45RA+CD62L+], memory [CD45RO+CD62L−] and activated [CD38+HLA-DR+] CD4+ and CD8+ T-cells), lymphocyte proliferative response to antigens (Candida albicans, tetanus toxoid, Mycobacterium avium intracellulare complex [MAC] and hepatitis A virus), serum levels of soluble tumor necrosis factor receptor (sTNFR)-II, TNF-α and IL-2R, and thymic volumes estimated by non-contrast chest CT. Detailed methods and results are reported elsewhere[19].
T-reg cell phenotyping
T-reg cell phenotypes were determined using cryopreserved PBMC obtained at baseline. Four-color flow-cytometry was performed using a FACSCalibur™ with CellQuest™ v2.1 software (Becton Dickinson, San Jose, CA). Mouse anti-human monoclonal antibodies to CD4, CD25, CD127, CD45RO, and isotype-control antibodies conjugated to fluoroscein isothiocyanate, phycoerythrin, peridinin chlorophyll-protein, PE-Cy5, or allophycocyanin (BD-Pharmingen, San Jose, CA) were used to determine the percentage of CD4+ T-cells that were CD25+CD127lo (all or total T-regs), CD25+CD127loCD45RO+(memory T-regs) and CD25+CD127loCD45RO− (naïve T-regs). The gating strategy we used for phenotyping of T-reg cells has been reported previously[14].
Statistical Methods
We categorized subjects into 4 groups: younger HIV−, older HIV−, younger HIV+ and older HIV+. Associations between a) % total, naïve or memory T-reg cells, and b) age group and HIV status were explored with a non-parametric method based on ranks that tested all pair-wise comparisons of the four groups defined by age group and HIV status with adjustment for multiple testing of a given outcome at the 0.05 level of significance[29] (denoted adjusted) and with a Wilcoxon Rank Sum test (denoted unadjusted), and also with multivariable linear regression using dummy variables. Spearman rank correlations were used for 486 univariate tests of association between T-reg proportions, age (in years) and immunologic variables. There was no formal adjustment for all of the multiple testing done using Spearman rank correlations, and p-values are presented for exploratory purposes only. S-Plus 3.4 (for SPARC, SunOS 5.3; 1996) was used for these analyses.
RESULTS
Forty-eight treatment-naïve HIV+ and 34 healthy HIV− subjects were included in this analysis. Plasma HIV-1 RNA levels were not significantly different between HIV+ younger and older subjects (Table 1). CD4+ T-cell number and percentage were significantly lower in the HIV+ groups, compared to the HIV− groups. Within each HIV serostatus category, CD4+ T-cell numbers and percentages were significantly lower only in older HIV+ versus younger HIV+ subjects.
Table 1.
Median (1st,3rd quartile) age, viral load, CD4+ T-cell count/percentage, and % CD4 that are CD25+CD127lo (total T-regs), CD25+CD127loCD45RO+(memory T-regs) and CD25+CD127loCD45RO− (naïve T-regs), for each age-HIV category.
| N | Age | Log10 plasma HIV-1 RNA (copies/ml) | CD4+ T-cells/μl | % CD4+ T- cells | % Total T-reg | % Memory T-reg | % Naïve T-reg | |
|---|---|---|---|---|---|---|---|---|
| Younger HIV− | 17 | 26 (25, 29) | NA | 787 (668, 880) | 46 (44, 49) | 5.16 (4.29, 6.54) | 2.39 (1.92, 2.80) | 2.67 (1.95, 4.13) |
| Older HIV− | 17 | 50 (46, 54) | NA | 949 (715, 1141) | 52 (47, 59) | 4.63 (3.97, 5.80) | 2.66 (2.11, 3.43) | 1.61 (0.87, 2.75) |
| Younger HIV+ | 25 | 26 (23, 28) | 4.31 (4.07,4.79) | 311 (234, 447) | 23 (17, 29) | 6.27 (4.72, 7.54) | 3.45 (2.87, 4.68) | 2.15 (1.57, 3.04) |
| Older HIV+ | 23 | 52 (48, 56) | 4.68 (3.92,5.39) | 199 (127, 392) | 15 (10, 20) | 7.19 (4.57, 10.15) | 4.32 (2.86, 7.47) | 2.11 (1.35, 3.53) |
We explored associations between HIV status and T-reg frequencies. We observed in the rank-based pair-wise comparison that among older subjects, HIV+ subjects had a significantly higher percent total T-reg cells compared to HIV− subjects (p < 0.05 adjusted; p = 0.001 unadjusted), but not among younger subjects. Both the linear regression, and adjusted and unadjusted rank-based pair-wise comparison analyses showed that HIV+ subjects, young (p = 0.0019, unadjusted) or old (p = 0.00196, unadjusted), have a significantly higher percent memory T-reg than the corresponding age group in HIV− subjects (p < 0.05 adjusted for the rank-based comparisons and p < 0.01 for the HIV+ main effect in the regression model). There were no significant differences in naïve T-reg percent between HIV+ and HIV− subjects among either younger or old individuals.
We next explored the associations between age, HIV serostatus, and total, memory and naïve T-reg frequencies. Rank-based pair-wise adjusted comparisons of total T-reg percent between the four groups found only one significant difference: older HIV+ subjects had higher percent total T-reg than older HIV− subjects (p = 0.0011) (figure 1). Linear regression analyses showed that older HIV+ subjects have an expected T-reg percent that is 2.8% (95% C.I. = 0.41, 5.18) higher than all the other subgroups combined (p = 0.022). We found no significant differences between other groups. Spearman rank correlation also showed a positive correlation between age and total T-reg percent in HIV+ (r = 0.31, p = 0.031), but not in HIV− subjects. Overall, these findings indicate that total T-reg percent is higher in older HIV+ individuals compared to the other groups.
Figure 1.
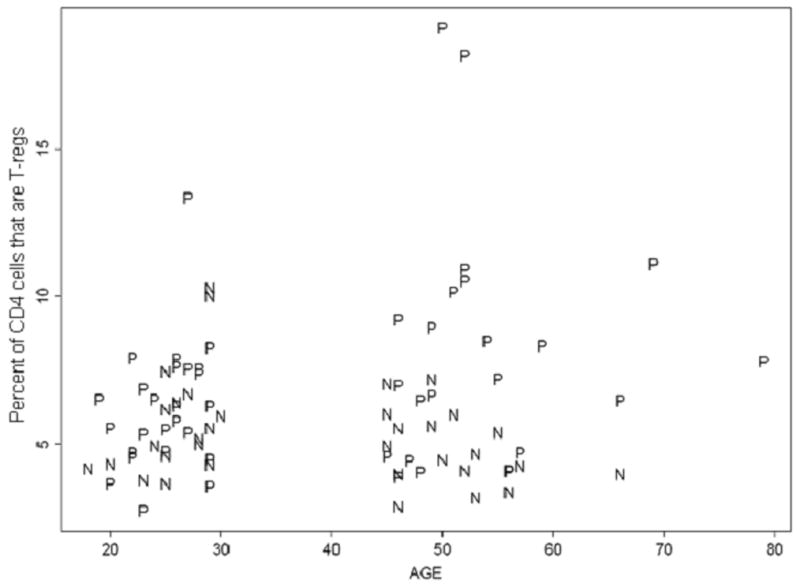
Plot of age versus the total T-reg percent. Total T-reg percentage is defined as the percentage of CD4+ T-cells that are CD25+CD127lo. P represents HIV+ subjects while N represents HIV− subjects.
We found no significant age group differences in memory T-reg percent in either HIV+ or HIV− subjects. However, in regard to naïve T-reg percent, we found that younger HIV− subjects were more likely to have a higher percentage of naïve T-regs than older HIV− subjects in the rank-based pair-wise analysis (p < 0.05 adjusted; p = 0.005 unadjusted), while Spearman rank correlation also showed that naïve T-reg percent is inversely correlated with age in HIV− subjects (r = −0.51, p = 0.004). No difference was found between younger and older HIV+ subjects.
Correlations between T-reg frequencies and other T-cell subsets and thymic volume (Table 2) were also evaluated. We found a significant inverse correlation between CD4+ T-cell percentage, and total (r = − 0.33, p = 0.025) and memory (r = − 0.31, p = 0.034) T-reg percent in HIV+, but not HIV−, subjects. We did not find a correlation between CD4+ T-cell numbers and any T-reg percent in any age or HIV serostatus category. We did not find a significant correlation between total, memory or naïve T-reg percent, and naïve or memory CD4+ or CD8+ T-cell number or percentage, or thymic score. We also did not find an association between the percentages of the various T-reg subsets and cellular (%CD38+HLA-DR+ CD4+ or CD8+ T-cells) or soluble (sTNFR-II, TNF-α and IL-2R) markers of activation in any of the HIV serostatus or age categories, or in the group as a whole, with the exception of an inverse correlation between soluble IL-2R and total (r = −0.50, p = 0.015) and memory (r = −0.46, p = 0.027) T-regs in younger HIV+ subjects.
Table 2.
Spearman rank correlation (r) between % CD4 that are CD25+CD127lo (total T-regs) and various immune parameters in each age-HIV serostatus category.
| Immune Parameter | HIV+ | Younger HIV+ | Older HIV+ | HIV− | Younger HIV− | Older HIV− |
| % CD4+ T-cell | −0.33* | −0.31 | −0.36† | −0.16 | 0.18 | −0.25 |
| % naïve CD4+ T-cell | −0.24 | −0.16 | −0.17 | −0.06 | 0.05 | −0.23 |
| % memory CD4+ T-cell | 0.25† | 0.26 | 0.12 | 0.11 | −0.08 | 0.31 |
| % CD4+CD38+HLA-DR+ | 0.21 | 0.17 | 0.22 | 0.15 | 0.44† | −0.15 |
| % CD8+CD38+HLA-DR+ | 0.04 | 0.17 | −0.10 | 0.01 | −0.12 | −0.05 |
| Soluble TNFr2 | 0.10 | −0.11 | 0.16 | 0.04 | −0.27 | 0.22 |
| TNF-α | 0.09 | −0.03 | 0.18 | −0.11 | −0.49† | 0.19 |
| Soluble IL-2r | −0.05 | −0.50* | 0.23 | 0.15 | 0.15 | 0.12 |
| Thymic score | −0.10 | −0.01 | 0 | 0.25 | −0.17 | 0.34 |
P < 0.05
P = 0.05 – 0.10
We also asked whether there were significant associations between T-reg percent and pathogen-specific immune responses. Among HIV+ subjects, the total T-reg percentage inversely correlated with the lymphocyte proliferative response (log10 SI) to tetanus (r = −0.45, p = 0.002) and to Candida antigens(r = −0.43, p = 0.003) (figure 2), but not with the lymphocyte proliferative response to MAC or hepatitis A antigens. A similar correlation was seen between memory T-reg percent and lymphocyte proliferative response to tetanus (r = −0.37, p = 0.01) and to Candida (r = −0.33, p = 0.02) in HIV+ subjects. Among HIV− subjects, the various measures of pathogen-specific immunity did not correlate with T-reg frequencies.
Figure 2.
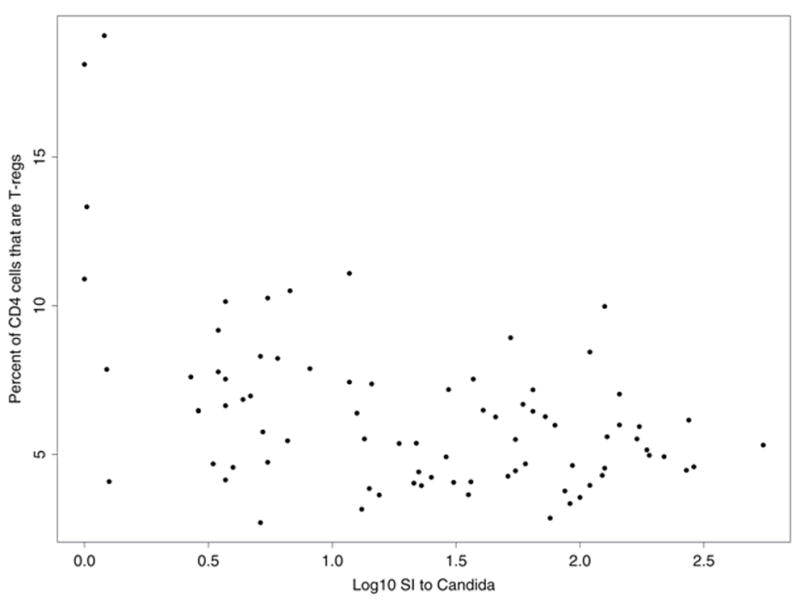
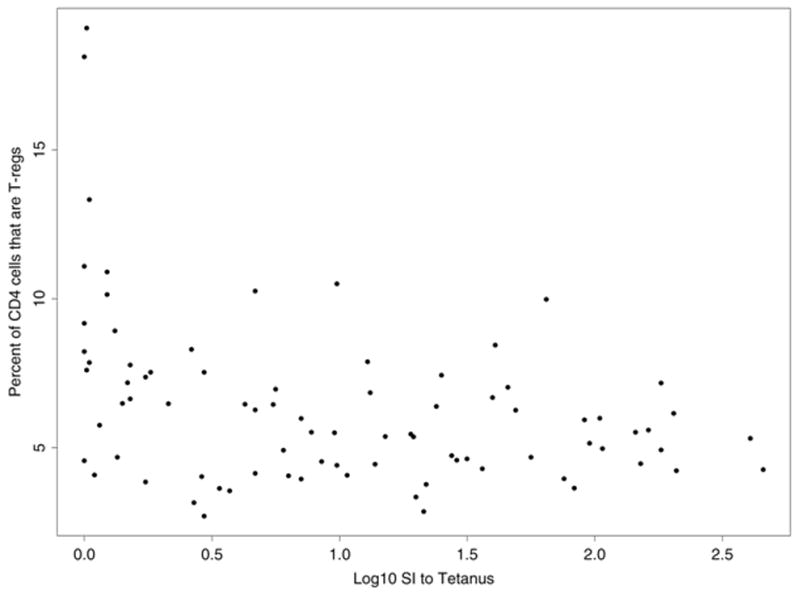
Plot of percentage of CD4+ T-cells that are CD25+CD127lo (total T-reg %) and the lymphocyte proliferative response to tetanus (A) and Candida albicans (B) in HIV+ subjects. Lymphoproliferation was assayed by measurement of [3H] thymidine incorporation in response to Candida albicans antigen (20 mg/mL; Greer Laboratories) and tetanus toxoid (2 limit-of-flocculation units [lfu]/mL; Aventis Pasteur). Results are expressed as the log10 of the stimulation index, defined as the ratio of the median counts per minute of quadruplicate cultures with antigen to the median counts per minute in culture medium alone without antigen.
DISCUSSION
In this cross-sectional exploratory analysis, we showed an increase in total T-reg percent in older HIV+ individuals. This is consistent with an effect of aging on the relationship between HIV and T-reg percent, or vice versa, an effect of HIV on age-related changes in T-reg percent. We can not exclude the possibility that a longer duration of HIV infection in older HIV+ individuals contributed to the increased T-reg frequencies in this group. Nevertheless, aging is associated with an increase in total T-reg activity and frequency[24–28], an increase that is seen whether FoxP3, CD25 or the absence of CD127 expression on CD4+ T-cells are used to measure T-reg frequency[28]. We may have even underestimated the frequency of T-regs in our older subjects since the proportion of FoxP3+CD4+ T-cells that express CD25 declines as humans age[28].
This study also showed that HIV infection is associated with increases in memory T-reg percent, a finding consistent with previous studies[9, 14]. We also showed that naïve T-reg percent is increased in younger versus older seronegative individuals, but not in HIV+ individuals. It is surprising that we did not find associations between HIV infection, age and memory and naïve T-reg percent. However, due to the study’s small sample size, we may underestimate any significant relationships between age, HIV serostatus and memory or naïve T-reg frequencies. Naïve T-regs undergo a preferential differentiation into effector memory T-regs after exposure to their cognate peptides and this leads to the accumulation of memory T-regs as persons age[12]. In older persons, T-reg expansion may also be driven by reactivation of endogenous pathogens as a result of immune senescence [15, 30]. Similarly, in HIV disease, the accumulation of total and memory T-reg cells may be driven by chronic exposure to HIV and other antigens such as those expressed by endogenous microbes[31]. HIV disease is characterized by frequent reactivation of endogenous pathogens[32, 33] and elevated levels of bacterial products in plasma[31] as a result of immunodeficiency. These convergent pathways may explain how aging can enhance the increases in total T-reg frequency seen with HIV infection.
We show a positive correlation between total and memory T-reg percent and soluble IL-2R in younger HIV+ subjects. However, unlike other studies[9, 34, 35], we did not observe a consistent relationship between T-reg percent and other cellular and soluble markers of immune activation. The study’s small sample size and relatively narrow range of CD4+ T-cells may have prevented us from detecting significant associations between activation and T-regulatory cells. Also, unlike others[34, 35], we used the high-level expression of CD25 and low-level expression of CD127 as markers of T-regulatory cells. The use of different markers for T-reg identification prevents inter-study comparisons as each study may be measuring different T-reg subsets. For instance, FoxP3+CD127loCD4+ T-reg cells, but not FoxP3+CD25+CD127loCD4+ T-reg cells, are closely associated with immune activation in primary HIV infection[35]. Each T-reg subset may be affected by or may contribute to immune activation in a variable manner, and may account for the apparent contradictory observations in the literature.
We also found an inverse correlation in HIV+, but not HIV−, subjects between T-reg percent and the lymphocyte proliferative responses to both Candida and tetanus. While the study’s small sample size may magnify the effect of outliers in our dataset, the association between T-reg frequency and pathogen-specific response has been shown previously[14] and is consistent with the known immune suppressive effects of T-regs[36, 37]. The current study suggests that the relationship is strongest in the context of HIV infection. HIV infection is characterized by lower CD4+ T-cell numbers and the enrichment of the CD4+ T-cell pool with T-regs, a combination that may magnify the immune suppressive effect of T-regs. Unexpectedly, memory T-regs and pathogen-specific response had a lower rank coefficient than total T-regs and pathogen-specific response, which suggests that CD45RO− T-reg subsets may also influence pathogen-specific immune response. The effect of T-reg frequency on pathogen-specific immunity appears variable as we did not see associations with MAC- or hepatitis A-specific responses. Inconsistent exposure to either MAC or hepatitis A may account for this lack of association. We could not explore this possibility as the study did not collect information regarding previous exposure to these antigens (or to tetanus toxoid). Also, typically, the proliferative responses to hepatitis A antigens are very low, which may have led us to underestimate this response. It is also possible that HIV, just like aging[28], may have variable effects on T-reg function.
The significance of our observations is limited by the study’s small sample size. Nevertheless, the primary study’s design controlled for variables such as plasma HIV-1 RNA within each HIV serostatus category, which strengthens the associations that we have observed. We have shown an enhancing effect of age on the increase in total T-reg percent in HIV infection, as well as an enrichment of memory T-reg cells in HIV infection. As T-reg cells have profound suppressive effects on immune response[36, 37], the increase in T-reg percent may contribute to the accelerated disease progression seen in HIV infection in the elderly.
Acknowledgments
This work was funded by the following grants from the National Institutes of Health: U01-AI069471 (A.R.T.), U01-AI068634 (J.S.), U01-AI069501 (R.C.K.), AI38858 (M.M.L.), AI068636 (A.L.L.). The clinical trials registry numbers for these studies are: NCT00006144 & NCT00014053
This study was supported by the AIDS Clinical Trials Group, funded by the National Institute of Allergy and Infectious Diseases. We gratefully acknowledge the study volunteers and the A5015 and A5113 study sites and site staff: Helen Fitch (Beth Israel Deaconess Medical Center, Boston, MA); Jane Baum, Kim Whitely, and Ronald Johnson (Case Western Reserve University, Cleveland, OH); Vicki Stocker, Jennifer Janik and Nancy Webb (Frontier Science and Technology Research Foundation, Amherst, NY); Minya Pu, Ronald Bosch, Miriam Chernoff (Harvard School of Public Health, Boston, MA); James Richardson, Greta Clement, Michael P. Dube (Indiana University, Indianapolis); Charles Flexner, Albert Wu (Johns Hopkins University, Baltimore, MD); Paul Fidel (Louisiana State University, Baton Rouge); Amy Sbrolla (Massachusetts General Hospital, Boston); Mary Sarah Dolan (Mount Sinai Medical Center, New York, NY); Susan Cu-Uvin, Joan Gormley, Karen T. Tashima (Miriam Hospital, Providence, RI); Robert L. Murphy (Northwestern University, Chicago, IL); Susan L. Koletar, Laura Laughlan (Ohio State University, Columbus); Ruth Davis, Elke Narkiewicz (Rush University Medical Center, Chicago, IL); David Shugarts (University of Colorado, Denver); Nancy Mantz (University of Pittsburgh, PA); Susan Fiscus, Alexandria Nesbit, Jackie Kaufman (University of North Carolina, Chapel Hill); Jose G. Castro, Roberto A. Monroig (University of Miami, Coral Gables, FL); Ian Frank, Wayne Wagner (University of Pennsylvania, Philadelphia); Connie A. Funk, Sona Avedian (University of Southern California, Los Angeles); Philip Keiser, Tianna Petersen (University of Texas, Southwest, Galveston); Joanne Stekler, Shelia Dunaway (University of Washington, Seattle); and Pablo Tebas, GeYoul Kim, Eric Lawrence (Washington University, St. Louis, MO).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Xu D, Fu J, Jin L, Zhang H, Zhou C, Zou Z, Zhao JM, Zhang B, Shi M, Ding X, Tang Z, Fu YX, Wang FS. J Immunol. 2006;177:739–747. doi: 10.4049/jimmunol.177.1.739. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T, Nakajima A, Hirohashi S. Clin Cancer Res. 2007;13:902–911. doi: 10.1158/1078-0432.CCR-06-2363. [DOI] [PubMed] [Google Scholar]
- 3.Enarsson K, Lundgren A, Kindlund B, Hermansson M, Roncador G, Banham AH, Lundin BS, Quiding-Jarbrink M. Clin Immunol. 2006;121:358–368. doi: 10.1016/j.clim.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Yang G, Liu A, Xie Q, Guo TB, Wan B, Zhou B, Zhang JZ. Int Immunol. 2007;19:133–140. doi: 10.1093/intimm/dxl130. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Zhou B, Li M, Deng Q, Wu X, Le X, Wu C, Larmonier N, Zhang W, Zhang H, Wang H, Katsanis E. Clin Immunol. 2007;123:50–59. doi: 10.1016/j.clim.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Lopes JE, Torgerson TR, Schubert LA, Anover SD, Ocheltree EL, Ochs HD, Ziegler SF. J Immunol. 2006;177:3133–3142. doi: 10.4049/jimmunol.177.5.3133. [DOI] [PubMed] [Google Scholar]
- 7.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, Fazekas de St Groth B. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim A, Tan D, Price P, Kamarulzaman A, Tan HY, James I, French MA. Aids. 2007;21:1525–1534. doi: 10.1097/QAD.0b013e32825eab8b. [DOI] [PubMed] [Google Scholar]
- 10.Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 11.Fritzsching B, Oberle N, Pauly E, Geffers R, Buer J, Poschl J, Krammer P, Linderkamp O, Suri-Payer E. Blood. 2006;108:3371–3378. doi: 10.1182/blood-2006-02-005660. [DOI] [PubMed] [Google Scholar]
- 12.Santner-Nanan B, Seddiki N, Zhu E, Quent V, Kelleher A, de St Groth BF, Nanan R. Int Immunol. 2008;20:375–383. doi: 10.1093/intimm/dxm151. [DOI] [PubMed] [Google Scholar]
- 13.Tsunemi S, Iwasaki T, Imado T, Higasa S, Kakishita E, Shirasaka T, Sano H. Aids. 2005;19:879–886. doi: 10.1097/01.aids.0000171401.23243.56. [DOI] [PubMed] [Google Scholar]
- 14.Tenorio AR, Martinson J, Pollard D, Baum L, Landay A. J Acquir Immune Defic Syndr. 2008;48:577–580. doi: 10.1097/QAI.0b013e31817bbea5. [DOI] [PubMed] [Google Scholar]
- 15.Vescovini R, Biasini C, Fagnoni FF, Telera AR, Zanlari L, Pedrazzoni M, Bucci L, Monti D, Medici MC, Chezzi C, Franceschi C, Sansoni P. J Immunol. 2007;179:4283–4291. doi: 10.4049/jimmunol.179.6.4283. [DOI] [PubMed] [Google Scholar]
- 16.Utsuyama M, Hirokawa K, Kurashima C, Fukayama M, Inamatsu T, Suzuki K, Hashimoto W, Sato K. Mech Ageing Dev. 1992;63:57–68. doi: 10.1016/0047-6374(92)90016-7. [DOI] [PubMed] [Google Scholar]
- 17.Hessen MT, Kaye D, Murasko DM. Mech Ageing Dev. 1991;58:61–73. doi: 10.1016/0047-6374(91)90120-o. [DOI] [PubMed] [Google Scholar]
- 18.Roberts-Thomson IC, Whittingham S, Youngchaiyud U, Mackay IR. Lancet. 1974;2:368–370. doi: 10.1016/s0140-6736(74)91755-3. [DOI] [PubMed] [Google Scholar]
- 19.Kalayjian RC, Landay A, Pollard RB, Taub DD, Gross BH, Francis IR, Sevin A, Pu M, Spritzler J, Chernoff M, Namkung A, Fox L, Martinez A, Waterman K, Fiscus SA, Sha B, Johnson D, Slater S, Rousseau F, Lederman MM. J Infect Dis. 2003;187:1924–1933. doi: 10.1086/375372. [DOI] [PubMed] [Google Scholar]
- 20.Egger M, May M, Chene G, Phillips AN, Ledergerber B, Dabis F, Costagliola D, D’Arminio Monforte A, de Wolf F, Reiss P, Lundgren JD, Justice AC, Staszewski S, Leport C, Hogg RS, Sabin CA, Gill MJ, Salzberger B, Sterne JA. Lancet. 2002;360:119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 21.Grabar S, Kousignian I, Sobel A, Le Bras P, Gasnault J, Enel P, Jung C, Mahamat A, Lang JM, Costagliola D. Aids. 2004;18:2029–2038. doi: 10.1097/00002030-200410210-00007. [DOI] [PubMed] [Google Scholar]
- 22.Stringer JS, Zulu I, Levy J, Stringer EM, Mwango A, Chi BH, Mtonga V, Reid S, Cantrell RA, Bulterys M, Saag MS, Marlink RG, Mwinga A, Ellerbrock TV, Sinkala M. Jama. 2006;296:782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 23.Viard JP, Mocroft A, Chiesi A, Kirk O, Roge B, Panos G, Vetter N, Bruun JN, Johnson M, Lundgren JD. J Infect Dis. 2001;183:1290–1294. doi: 10.1086/319678. [DOI] [PubMed] [Google Scholar]
- 24.Bryl E, Witkowski JM. Exp Gerontol. 2004;39:587–595. doi: 10.1016/j.exger.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 25.Trzonkowski P, Szmit E, Mysliwska J, Mysliwski A. Clin Immunol. 2006;119:307–316. doi: 10.1016/j.clim.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Rosenkranz D, Weyer S, Tolosa E, Gaenslen A, Berg D, Leyhe T, Gasser T, Stoltze L. J Neuroimmunol. 2007;188:117–127. doi: 10.1016/j.jneuroim.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 27.Gregg R, Smith CM, Clark FJ, Dunnion D, Khan N, Chakraverty R, Nayak L, Moss PA. Clin Exp Immunol. 2005;140:540–546. doi: 10.1111/j.1365-2249.2005.02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lages CS, Suffia I, Velilla PA, Huang B, Warshaw G, Hildeman DA, Belkaid Y, Chougnet C. J Immunol. 2008;181:1835–1848. doi: 10.4049/jimmunol.181.3.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zar J. Prentice-Hall, Inc.; Englewood Cliffs, N. J: 1984. p. 200. [Google Scholar]
- 30.Stowe RP, Kozlova EV, Yetman DL, Walling DM, Goodwin JS, Glaser R. Exp Gerontol. 2007;42:563–570. doi: 10.1016/j.exger.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 32.Bronke C, Palmer NM, Jansen CA, Westerlaken GH, Polstra AM, Reiss P, Bakker M, Miedema F, Tesselaar K, van Baarle D. J Infect Dis. 2005;191:873–880. doi: 10.1086/427828. [DOI] [PubMed] [Google Scholar]
- 33.Kim HN, Meier A, Huang ML, Kuntz S, Selke S, Celum C, Corey L, Wald A. J Infect Dis. 2006;194:420–427. doi: 10.1086/505879. [DOI] [PubMed] [Google Scholar]
- 34.Eggena MP, Barugahare B, Jones N, Okello M, Mutalya S, Kityo C, Mugyenyi P, Cao H. J Immunol. 2005;174:4407–4414. doi: 10.4049/jimmunol.174.7.4407. [DOI] [PubMed] [Google Scholar]
- 35.Ndhlovu LC, Loo CP, Spotts G, Nixon DF, Hecht FM. J Leukoc Biol. 2008;83:254–262. doi: 10.1189/jlb.0507281. [DOI] [PubMed] [Google Scholar]
- 36.Aandahl EM, Michaelsson J, Moretto WJ, Hecht FM, Nixon DF. J Virol. 2004;78:2454–2459. doi: 10.1128/JVI.78.5.2454-2459.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kinter AL, Horak R, Sion M, Riggin L, McNally J, Lin Y, Jackson R, O’Shea A, Roby G, Kovacs C, Connors M, Migueles SA, Fauci AS. AIDS Res Hum Retroviruses. 2007;23:438–450. doi: 10.1089/aid.2006.0162. [DOI] [PubMed] [Google Scholar]


