Abstract
The tiger salamander lives in shallow water with bright light in the aquatic phase, and in dim tunnels or caves in the terrestrial phase. In the aquatic phase, there are five types of photoreceptors—two types of rods and three types of cones. Our previous studies showed that the green rods and blue-sensitive cones contain the same visual pigment and have the same absorbance spectra; however, the green rods have a larger photon-catch area and thus have higher light sensitivity than the blue-sensitive cones. Here we show that after metamorphosis, the terrestrial salamander looses the blue-sensitive cones, while the density of the green rods increases. Moreover, the size of the green rod outer segments is increased in the terrestrial phase, compared to that in the aquatic phase. This switch from the blue-sensitive cones to the green rods may represent an adaptation to the dim light environment of the terrestrial phase.
INTRODUCTION
The tiger salamander (Ambystoma tigrinum) is used extensively as an experimental model for electrophysiologic and patterning studies of rod and cone photoreceptors due to the large size of the photoreceptor cells and the duplex retina in which both rods and cones contain light-sensitive molecules to respond to light with different wavelengths (1–5). Based on the spectral sensitivity of single photoreceptors and the morphology of the outer segments, it has been concluded that aquatic salamanders contain five types of photoreceptors (5). There are two types of rods, the dominant red rods (λmax = 500 nm) and the low-density green rods (λmax = 432 nm), both having been named based on their color under the microscope when using white light (2–6). There are three types of cones, red- (λmax = 558 nm), blue- (λmax = 432 nm) and UV- (λmax = 356 nm) sensitive cones, named according to their spectral sensitivities (2,5,7–10).
We have previously shown that the aquatic phase salamander retina contains only four opsins, SWS1 (UV cone opsin), SWS2 (blue cone opsin), MLWS (red cone opsin) and Rh1 (rhodopsin) (11) (see Ebrey and Koutalos [12] for opsin classification). The green rods and blue-sensitive cones were shown to contain the same SWS2 opsin, but different transducins in the aquatic salamander (11,13). This finding that the same visual pigment is present in both the green rods and the blue-sensitive cones explains the earlier finding that these two types of photoreceptors have the same absorbance spectra (3,10). However, the green rod contains the rod transducin while the blue-sensitive cone uses the cone transducin (11,13). The functional significance of this single pigment expressing itself in both a rod and a cone in the salamander remains unknown.
Although the morphology, electrophysiology and patterning of photoreceptor cells in the aquatic salamander have been intensively investigated, the photoreceptor development in amphibians is poorly understood. The tiger salamander is a cold-blooded vertebrate urodele amphibian (14,15). Prior to metamorphosis gilled aquatic salamanders spend their lives in shallow water usually with bright light, while terrestrial adults which have undergone metamorphosis live in tunnels or caves with relative dim light (16,17). During metamorphosis, the skin color pattern of the salamander also undergoes a change from a light gray background with dark dots on the back in the aquatic phase, to a clearer color pattern with darker strips on a yellow background in the terrestrial adult, probably to adapt to changes in the living environment (16–18). Although it is known that the visual pigment chromophore changes from mainly A2 retinal (3,4-dehyroretinal) to A1 retinal as the animal enters the terrestrial phase (3), whether the photoreceptors themselves also undergo any changes during metamorphosis to accommodate environmental differences has not been studied.
In the present study, we have compared the densities and sizes of each type of photoreceptor in the salamander retina before and after metamorphosis using immunohistochemistry with antibodies specific for each opsin.
MATERIALS AND METHODS
Animals
All the animals in this study were treated in strict accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and the guidelines set forth in the Care and Use of Laboratory Animals by the University of Oklahoma Health Sciences Center. The aquatic tiger salamanders (A. tigrinum, Charles D, Sullivan, Inc., Nashville, TN) were maintained on a 12 h light/12 h dark cycle at 4–10°C to maintain them in the aquatic phase, or at room temperature to allow metamorphosis. After aquatic salamanders metamorphosed into terrestrial adults, they were transferred into highly humid hollow logs.
Antisera and antibodies
The mouse monoclonal antibody, 4D2, raised against the N-terminus of bovine rhodopsin, was a generous gift from Dr. Robert Molday (19) (Table 1). The rabbit polyclonal antibodies specific for salamander rod transducin α-subunit (Gtα1), TA1 and the cone transducin α-subunit (Gtα2), TA2, were raised and characterized as described in a previous study (13). CERN956, a rabbit polyclonal antibody against the red cone opsin (MLWS) was raised and characterized as described (20). UV-N (anti-SWS1 opsin) antibody and Blue-N (anti-SWS2 opsin) antibody were raised in rabbit as described previously (11). Secondary antibodies, goat antirabbit IgG, Texas Red-conjugated streptavidin, Cyanine (Cy)2 conjugated anti-FITC and Texas Red-conjugated antimouse IgG were purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA).
Table 1.
Antibodies, secondaries, opsin and cell types.
| Name (unconjugated/conjugated) | Antigen | Host species | Species reactivity | Secondary antibodies |
|---|---|---|---|---|
| 4D2 | Rhodopsin | Mouse | Mammalian/salamander | Texas Red-conjugated antimouse |
| TA1 | Rods transducin α (Gtα1) | Rabbit | Salamander | FITC-conjugated antirabbit |
| TA2/fluorescein-conjugated | Cone transducin α (Gtα2) | Rabbit | Salamander | FITC-conjugated antirabbit/Cy2-conjugated antifluorescein |
| CERN956/biotinylated | Red cone opsin (MLWS) | Rabbit | Salamander | FITC-conjugated antirabbit/Texas Red-conjugated streptavidin |
| UV-N/fluorescein-conjugated | UV cone opsin (SWS1opsin) | Rabbit | Salamander | FITC-conjugated antirabbit/Cy2-conjugated antifluorescein |
| Blue-N/biotinylated | Blue cone and green rod opsin (SWS2 opsin) | Rabbit | Salamander | FITC-conjugated antirabbit/Texas Red-conjugated streptavidin |
Antibody conjugation
TA2 and Blue-N antibodies were purified as described previously (11). Blue-N was biotinylated (Immunoprobe Biotinylation kit; Sigma, St. Louis, MO), and TA2 conjugated with FITC (Fluro Tag Conjugation kit; Sigma), following protocols recommended by the manufacturer.
Tissue preparation
Aquatic salamanders (21–29 cm in length) and terrestrial salamanders (13–22 cm in length) were rapidly decapitated in ice, their eyes immediately enucleated and fixed in freshly prepared cold 4% paraformaldehyde in phosphate buffer, pH 7.4, overnight. For the retinal flat-mount, the cornea, choroid and sclera were removed, and the retina incubated in 1% NaBH4 in phosphate buffer for 5 min to eliminate the auto-fluorescence, and blocked with 3% sapine in 5% goat or donkey serum (depending on the secondary antibody) for 1 h to decrease any possible nonspecific signal (11,13).
Immunohistochemistry of flat-mounted retinas
Whole retinae were dissected and fixed in fresh 4% paraformaldehyde in phosphate-buffered saline (PBS) as described in the tissue preparation section. For retinal sections, the eyes were fixed and cryosectioned at 6 μm in thickness. The whole retina and retina sections were subjected to immunostaining with different procedures based on the antibody used. Triple immunostaining with TA2, UV-N and Blue-N was performed by incubation with rabbit UV-N antibody for 1 h, followed by staining with Cy5 conjugated antirabbit antibody for another 1 h. After five washes with PBS, the retina was blocked with normal rabbit serum. The FITC-TA2 antibody and biotinylated Blue-N antibody were then applied to the retina and incubated overnight at 4°C. A mixture of a Cy2-conjugated antifluorescein secondary antibody to visualize an amplified TA2 signal and a Texas Red-conjugated streptavidin to visualize the Blue-N signal were applied to the retina followed by three PBS washes. The retina was finally flat-mounted to the slide with a mounting medium (Vector Laboratories, Inc., Burlingame, CA).
To determine the density and distribution of photoreceptor subtypes, a mixture of a mouse monoclonal antibody 4D2 and a rabbit polyclonal antibody specific for each cone opsin (UV-N, Blue-N or CERN956) was applied to the retina for 1 h. The antirhodopsin antibody 4D2 was visualized using Texas Red-conjugated antimouse secondary antibody and the cone opsins were visualized using FITC-conjugated antirabbit secondary antibody.
Double labeling of two opsins on the whole-mounted retina
A mixture of fluorescein-conjugated UV-N and biotinylated Blue-N (or a mixture of fluorescein-conjugated TA2 and biotinylated CERN956 antibodies) was applied to the retina and incubated for 2 h, then Texas Red-conjugated streptavidin and Cy2-conjugated antifluorescein antibodies were incubated with the retina for 1 h followed by thorough washes.
All antibodies (primary and secondary) except 4D2 and CERN956 were diluted in 1% goat serum and 1% BSA at a dilution of 1:100 in the PBS. The dilution of 4D2 was 1:1000 and CERN956 was 1:500. For controls, whole retinae or sections were stained following the same procedure, but without primary antibody.
Image analysis
All slides were visualized with fluorescence microscopy. Low magnification images were captured with a digital camera (Axiocam; Carl Zeiss, Thornwood, NY) mounted on a Zeiss fluorescent microscope. Confocal images at high magnification were acquired with a Leica TCS-4D or a Zeiss 510 Meta series confocal microscope (Carl Zeiss laser scanning system; LSM 510) equipped with argon (excitation wavelength 488 nm), HeNe1 (568 nm) and HeNe2 (647 nm) lasers. All settings were adjusted to optimize the appearance of labeled outer photoreceptor segments in whole-mounted retinae or sections. Optical sections in stacks were collected at 1 μm increments using a 40× or 100× apochromate objective lens (N.A. = 1.3) with an aperture of 1.0 mm and working distance of 0.88 mm. Resolutions along the x-, y-, z-axis directions were 0.45, 0.45 and 1.0 m, respectively. Each series of optical sections was projected into a single in-focus montage image; and each image stack of around 25 sections spans the entire thickness of the outer segment. Image analysis was performed using Zeiss LSM Image Browser image analysis software (Zeiss). Quantitative analyses of the images were performed using AxioVision software (Zeiss). Length and diameter of the photoreceptor outer segments were measured and averaged (n = 60) in whole-mounted retinae and sections, at high magnification. Four independent measurements were made in different quadrants for each whole-mounted retina. Images were acquired with a 488 nm laser for FITC or Cy2, a 568 nm laser for Texas Red or Cy3, and a 647 nm laser for Cy5. The immunostaining signal of each antibody was then pseudo-colored as indicated in the figure legends.
RESULTS
Multiple opsins in UV-sensitive cones in both aquatic and terrestrial phases
Makino and Dodd (9) have demonstrated by electrophysiology that, unlike other photoreceptors, the salamander UV-sensitive cone has three absorbance peaks which match those of the SWS1, SWS2 and MLWS pigments, thus, suggesting that the UV-sensitive salamander cone contains all the three cone pigments. In our study, we set out to confirm this finding at the protein level using immunohistochemistry with antibodies specific for these pigments. As shown by double-labeling of the SWS1 and the SWS2 opsins, a subset of cones in both the aquatic and terrestrial phases express both the SWS1 and SWS2 opsins, suggesting that UV-sensitive cones express more than one opsin (Fig. 1), and consistent with the previous physiologic evidence. However, the MLWS opsin was not detected in the UV-sensitive cones by immunostaining (data not shown), possibly due to the fact that the expression level of the MLWS opsin is the lowest among the three pigments, and thus its detection is beyond the sensitivity of the antibody detection in the UV-sensitive cone (9).
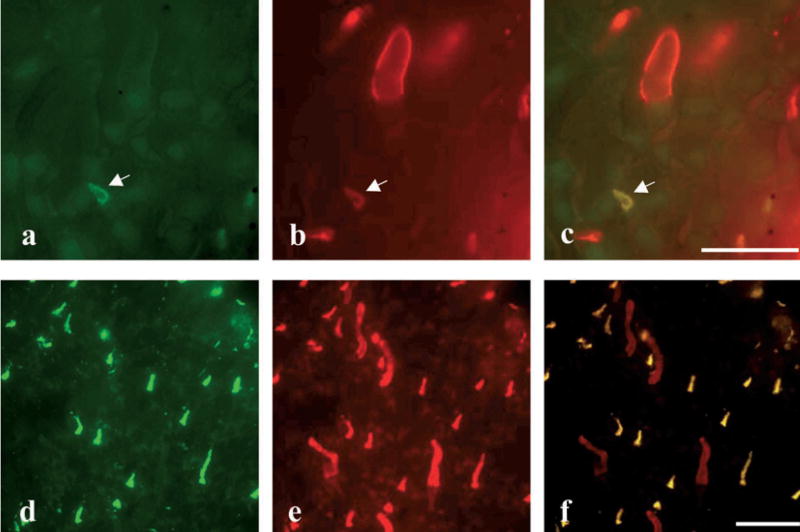
Figure 1.
The UV-sensitive cones contain both the SWS1 and SWS2 opsins. The retinas from aquatic phase (a–c) and terrestrial phase (d–f) salamanders were double stained with UV-N (green) and Blue-N (red) antibodies specific for the SWS1 opsin and SWS2 cone opsin, respectively. (a, d) UV-N antibody labeled only the UV-sensitive cones; (b, e) Blue-N antibody-labeled green rods, blue-sensitive cones and UV-sensitive cones. Note that the white arrow indicates a UV-sensitive cone in (a, b). (c, f) Superimposed images of cells labeled by both antibodies (bar = 16 μm for a, b and c; bar = 8 μm for d–f).
Existence of blue-sensitive cones which express only the SWS2 opsin in salamanders of the aquatic phase
As shown by the triple staining of the SWS1 opsin, MLWS opsin and the cone transducin α-subunit (Gtα2) which is expressed in all types of cones, there are clearly three types of cones in the retina of aquatic salamanders. The first type is the red-sensitive cone expressing MLWS opsin only. The second type expresses the SWS1 cone opsin and SWS2 opsin, indicating that they are the UV-sensitive cones. The third type does not express either SWS1 or MLWS opsin but expresses Gtα2, indicating that they are indeed cones, but not the red- or the UV-sensitive cones (Fig. 2a–d).
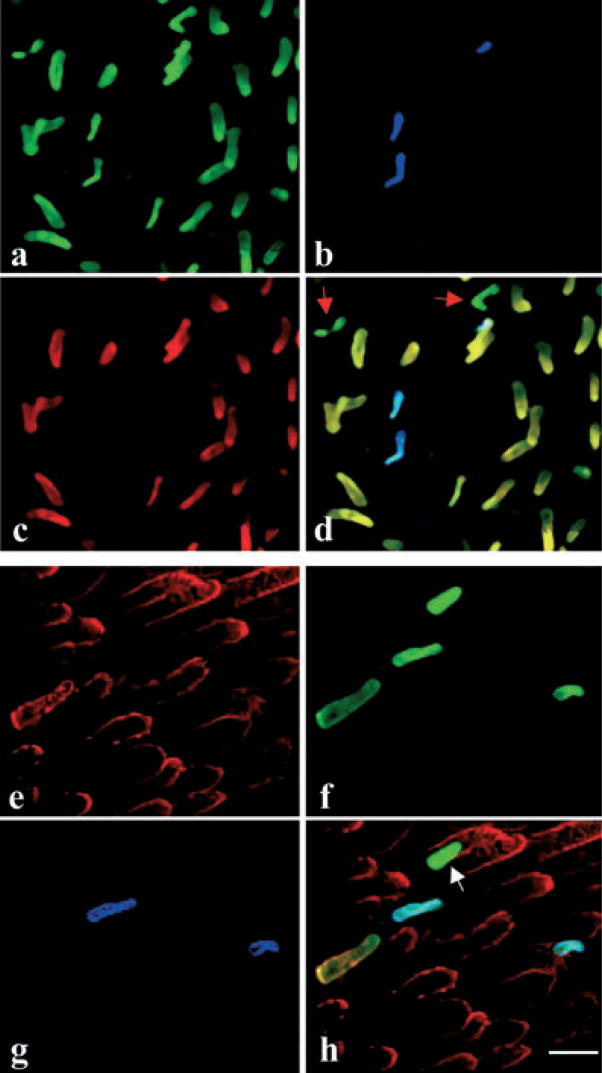
Figure 2.
Existence of blue-sensitive cones expressing only the SWS2 opsin in the retina of aquatic salamanders. (a–d) The aquatic salamander retina was triple-labeled with the TA2 antibody specific for cone transducin Gtα2 (green color), which labels all types of cones (a); UV-N (blue color), which labels the UV-sensitive cones (b); and antibody for MLWS opsin (red color), which labels the red-sensitive cones (c). (d) Superimposed image of panels (a)–(c) showing a type of cone (indicated by red arrows) that expresses Gtα2, but not the MLWS or the SWS1 opsin. (e–h) The retina from an aquatic phase salamander was triple-labeled with an antibody for TA1 (red color), which labels both the red and green rods (e); Blue-N (green color), which labels green rods and blue-sensitive cones (f); and UV-N (blue color), which labels the UV-sensitive cones (g). (h) Superimposed image of panels (e)–(g). The white arrow indicates a blue-sensitive cone expressing the SWS2 opsin, but not the SWS1 opsin (bar = 16 μm).
Further analyses using triple immunolabeling of the SWS1 opsin, SWS2 opsin and the rod transducin α-subunit (Gtα1), which is expressed in both the red and green rods, showed that there are two types of cells with different sizes and morphology expressing the SWS2 (blue cone opsin), but not the SWS1, opsin. One type of these cells also expresses Gtα1, indicating that they are the green rods (Fig. 2) (11); while the other type of stained cells does not express Gtα1 (Fig. 2e–h), suggesting that they are the blue-sensitive cones, which is consistent with our previous report that both the green rod and blue-sensitive cone express the SWS2 opsin in the aquatic phase (11).
Lack of blue-sensitive cones in salamanders of the terrestrial phase
We have also examined the retinae of salamanders in the terrestrial phase with the Blue-N (specific for SWS2 opsin) and UV-N (specific for SWS1 opsin) antibodies, and compared the stained cells with those in the aquatic phase salamander retinae. In contrast to that in the aquatic phase retina, all the cones expressing the SWS2 opsin also express the SWS1 cone opsin in the terrestrial phase (Fig. 3d–f), suggesting that they are UV-sensitive cones. Unlike the aquatic phase, there are no blue-sensitive cones (which express only the SWS2 opsin) in the entire retina of all the terrestrial-phase salamanders analyzed, while the green rods were detected in each of the same retinae, suggesting that the terrestrial salamanders lack the blue-sensitive cones.
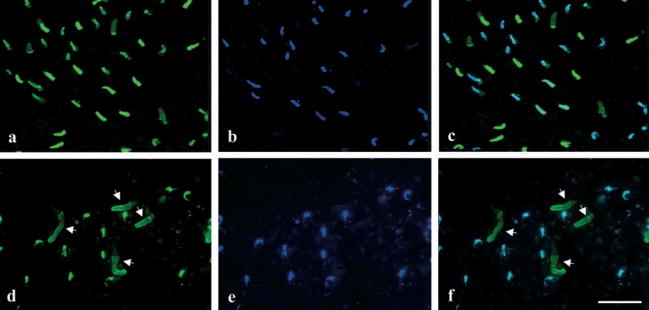
Figure 3.
Lack of the blue-sensitive cones in the terrestrial salamander. Aquatic (a–c) and terrestrial (d–f) salamander retinae were double-labeled with antibodies specific for Blue-N (green color), which labels the green rods, blue-sensitive cones and UV-sensitive cones (a, d); and UV-N (blue color), which labels the UV-sensitive cones (b, e). (c, f) Superimposed images of (a) and (b), or (d) and (e), respectively. (a–c) The retina from an aquatic salamander shows that there are blue-sensitive cones expressing only the SWS2 opsin, but not the SWS1 opsin in the retina. (d–f) In the retina from the terrestrial phase salamander, every cone expressing the SWS2 opsin also expresses the SWS1 opsin, indicating that they are UV-sensitive cones. The white arrows indicate green rods (bar = 16 μm).
Possible degeneration of the blue-sensitive cone during metamorphosis from the aquatic phase to the terrestrial phase
There are two possible causes for the lack of blue-sensitive cones in the terrestrial phase: (1) the blue-sensitive cones may start to express the SWS1 pigment after metamorphosis and become the UV-sensitive cones, and (2) the blue-sensitive cones may selectively degenerate during metamorphosis. To address this question, we have labeled the blue-sensitive cones and the green rods in the retinae from salamanders during metamorphosis. Double-labeling of the retina of the salamander at metamorphosis using the Blue-N (SWS2) antibody and the UV-N (SWS1) antibody showed that the outer segments of blue-sensitive cones become apparently thinner, compared to that in the aquatic phase and to other cones in the same retina (Fig. 4c,f), suggesting that these cells may be in the process of degeneration. In contrast, the green rods showed increased size of the outer segments (Fig. 4a,d). As a reference, the size of the UV-sensitive cone is not changed in the same retina during metamorphosis (Fig. 4b,e). These results suggest a possible selective elimination of the blue-sensitive cones during metamorphosis.
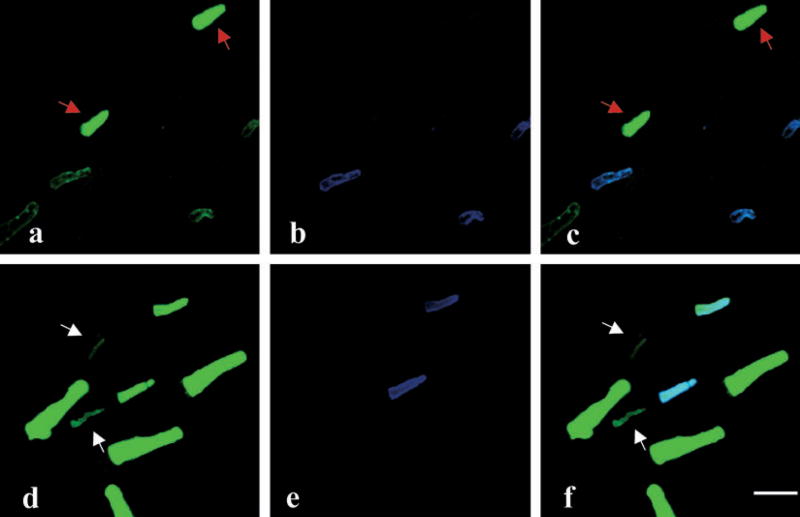
Figure 4.
Possible degeneration of blue-sensitive cones during metamorphosis. Flat-mounted retinae from salamanders of the aquatic phase (a–c) and those in the process of metamorphosis (d–f), which had developed lungs but had not lost their gills, were double-labeled with Blue-N (green color), which labels the green rods and blue-sensitive cones (a, d); and UV-N (blue color), which labels the UV-sensitive cones (b, e). (c, f) Superimposed images of (a) and (b), or (d) and (e), respectively. Note that the blue-sensitive cones have substantially thinner outer segments (indicated by white arrows in [d] and [f]) in the metamorphosing salamanders compared to those in the aquatic phase (indicated by red arrows in [a] and [c]) (bar = 16 μm).
Increased density and size of the green rods in the terrestrial phase
To compare the size of each type of photoreceptor in the aquatic and terrestrial salamanders, we have measured the dimensions of the outer segments of 60 randomly selected photoreceptors of each type in whole-mounted retinae from three salamanders in the aquatic phase, and 40 cells from those in the terrestrial phase, using the average size of the red rods as the reference for size and density. As shown in Table 2, the average length of the green rod outer segment is increased from 15.0 μm in the aquatic phase to 18.6 μm in the terrestrial phase. The diameter of the green rod outer segment is also increased slightly (Fig. 5). However, in the same retinas, the other cell types, including the red rods and the red- and UV-sensitive cones, showed similar sizes in terrestrial and aquatic phases (Table 2), suggesting that the enlarged size of the green rod outer segment is not a general change in all types of photoreceptors.
Table 2.
The outer segment size (diameter × length, recorded in μm) of rods and cones in aquatic and terrestrial salamanders.
| Aquatic (n = 3†) | Terrestrial (n = 3†) | P* | |
|---|---|---|---|
| Red cone | 6.0 ± 0.4 × 8.2 ± 0.2 | 6.0 ± 0.3 × 8.0 ± 0.2 | >0.05 |
| Red rod | 12.4 ± 0.5 × 20.0 ± 1.3 | 11.0 ± 0.7 × 20.6 ± 0.9 | >0.05 |
| Green rod | 9.8 ± 0.3 × 15.0 ± 0.7 | 10.2 ± 0.4 × 18.6 ± 0.3 | <0.01 |
| UV cone | 3.1 ± 0.1 × 5.1 ± 0.2 | 2.9 ± 0.2 × 4.9 ± 0.3 | <0.05 |
Values are mean ± standard deviation of 60 cells from each quadrant of three retinae in aquatic salamanders or 40 cells from each quadrant of three retinae in terrestrial salamanders.
Number of animals examined per category.
Statistical significance when comparing aquatic and terrestrial measurements per rod or cone type; significant values are in bold.
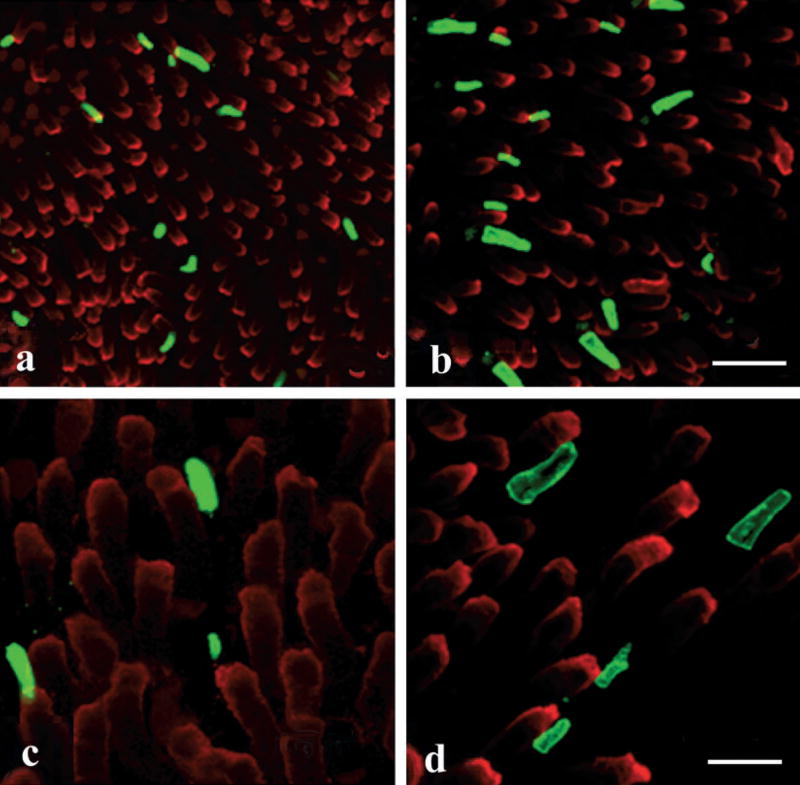
Figure 5.
Comparison of green rod outer segment sizes in the aquatic and terrestrial phases. The retinae of aquatic (a, c) and terrestrial (b, d) salamanders were double-stained with 4D2 (red), which labels the red rods; and with Blue-N (green), which labels the green rods and blue-sensitive cones ([a, b] low magnification, bar = 40 μm; [c, d] high magnification, bar = 16 μm). Note that the green rod outer segments are larger in size in terrestrial salamanders, than in aquatic salamanders.
We have also quantified the relative densities of each type of cone and rod in whole-mounted retinae after specific opsin labeling, using the average density of red rods as the reference. The number of each cell type was counted in 10 random fields of each flat-mounted retina after labeling with antibodies specific for each opsin, averaged and numerically expressed for each cell type per 100 red rods in the field. As shown in Table 3, the average density of green rods was 3.03 per 100 red rods in the aquatic phase, while the number was increased to 6.14 in the terrestrial phase, which is almost double that from the aquatic phase. The blue-sensitive cones were 1.5 per 100 red rods in the aquatic phase, and none in the terrestrial phase. The rest of the photoreceptors, including the red- and UV-sensitive cones, showed a similar density in both phases (Table 3). The finding that the relative number of UV-sensitive cones remains unchanged further supports the interpretation that the blue-sensitive cones did not evolve to UV-sensitive cones during metamorphosis.
Table 3.
Densities of rods and cones in aquatic and terrestrial salamanders.
| Red rod | Green rod | Red-sensitive cone | Blue-sensitive cone | UV-sensitive cone | ||
|---|---|---|---|---|---|---|
| Aquatic (n = 3 eyes) | Mean ± SD | 1033 ± 250 | 31 ± 6.8 | 805 ± 31.7 | 16 ± 1.9 | 59.5 ± 5.8 |
| Ratio (%)† | 100 | 3.0 | 79.0 | 1.5 | 5.8 | |
| Terrestrial (n = 3 eyes) | Mean ± SD | 1205 ± 119.5 | 74 ± 2.8 | 921 ± 26.8 | 0 | 64 ± 8.5 |
| Ratio (%)† | 100 | 6.1 | 75.3 | 0 | 5.3 | |
| P* | >0.05 | <0.01 | >0.05 | >0.05 |
Values are the mean ± standard deviation of photoreceptors in 10 random retinal fields per rod or cone type.
Ratio represents the cell number of each type of photoreceptor per 100 red rods.
Statistical significance when comparing aquatic and terrestrial ratios; significant value in bold.
DISCUSSION
In the present study, we have shown that salamanders lose the blue-sensitive cone while increasing the density and size of the green rod after metamorphosis from the aquatic to the terrestrial phase. As both the green rods and blue-sensitive cones have the same spectral sensitivity, but the green rods have higher light sensitivity than the blue-sensitive cones, substitution of the blue-sensitive cones with more sensitive green rods may represent an adaptation to changes in their living environment from the shallow water aquatic phase to the dim environment of their terrestrial phase.
In most types of vertebrates, each type of photoreceptor contains only one visual pigment and has only one absorbance peak. However, it has been shown from electrophysiologic studies on single cells that in the aquatic salamander the UV-sensitive cones contain all three of the cone pigments; that is, high levels of the SWS1 pigment, relatively low abundance of the SWS2 pigment and very low levels of the MLWS pigment (9). However, the existence of these opsins in the UV-sensitive cones has not previously been demonstrated at the protein level. In the present study, we show that the UV-sensitive cones in both the aquatic and terrestrial phases indeed contain both the SWS1 and SWS2 opsins, consistent with the physiologic evidence. However, double-antibody labeling did not detect the MLWS opsin in the UV-sensitive cones. A possible explanation is that the abundance of the MLWS pigment in the UV-sensitive cones is approximately 10 000-fold lower than that of the SWS1 opsin, as shown by the spectral sensitivity measurement (9). At this low level, the MLWS opsin may not be detectable by immunostaining due to the physical limitation of sensitivity of fluorescent immunohistochemistry.
Our previous studies have shown that the green rod and the blue-sensitive cone both contain the same visual pigment (SWS2 opsin) (11). However, the green rod is more sensitive to light than the blue-sensitive cone, due to the larger size of the green rod outer segment which provides a larger photon-catch area compared to the outer segment of the blue-sensitive cone. Therefore, the substitution of the less sensitive blue-sensitive cones by more sensitive green rods during salamander metamorphosis may increase the sensitivity of the salamander to light in the wavelength range of the SWS2 pigment. Further, the increased size of the outer segment of green rods in the terrestrial salamander supports the notion that the terrestrial salamander has an enlarged area for photon catch, which increases the sensitivity to light in the blue range. In contrast, all other types of photoreceptors are relatively unchanged in their densities and sizes, again suggesting that the photoreceptor cell changes during metamorphosis are specific to the blue-sensitive cones and the green rods. The switch of photoreceptors might represent an adaptation of the salamander from bright light in shallow water in the aquatic phase to dim light in caves or tunnels in the terrestrial phase.
There are two possible mechanisms responsible for the loss of the blue-sensitive cones in terrestrial salamanders. One is that the blue-sensitive cones might alter their gene expression to express the SWS1 opsin and become the UV-sensitive cones. However, cell counts after specific immunostaining show that the density of UV-sensitive cones is not increased in the terrestrial salamander. This finding does not support the possibility that the blue-sensitive cones have phylogenetically evolved to UV-sensitive cones during metamorphosis. The other explanation is that the blue-sensitive cone is selectively eliminated while the green rod is increased in number and size. Our immunostaining of salamander cones during the process of metamorphosis showed significantly decreased diameters of the outer segments in the blue-sensitive cones, while the other cones have sizes of outer segments similar to those in the aquatic phase. The shrinking outer segment in the blue-sensitive cones might represent progression toward degeneration of this type of cell during metamorphosis. These results seem to support the selective elimination of blue-sensitive cones via regression in the terrestrial phase. It is reasonable to speculate that the terrestrial salamanders produce more green rods to enlarge the photon-catch area in order to meet the requirement of dim light in the living environment of the terrestrial phase. It remains to be investigated how the switch of photoreceptor type is regulated in salamanders. It is not clear whether thyroid hormones play a role in the selective elimination of the blue-sensitive cones during metamorphosis of salamanders.
Visual system reorganization during metamorphosis has been documented in the deep-sea hydrothermal vent crab and the winter flounder (21,22). The vent crab looses its image-forming optics and develops a highly sensitive naked retina after metamorphosis. Moreover, the spectral absorbance of the visual pigment shifts toward longer wavelengths, from larva to postlarva to adult crab, possibly to adapt to the environmental change (21). In the winter flounder, there is only one opsin (the MLWS opsin) in the premetamorphic retina (22). In contrast, in the postmetamorphic flounder retina, there is one type of rod opsin and three types of cone opsins. The altered expression of visual pigments may impact color vision and light sensitivity (22). Selective elimination of a specific type of cone, and substitution with an increase in rods with the same absorbance spectra, but higher light sensitivity, such as that found in this study, represents a novel mechanism for metamorphic amphibians to adapt to environmental changes in light.
Acknowledgments
This study was supported by NIH grants EY12231 and EY15650 (J.-x.M.), a Vision Centers of Biomedical Biomedical Research Excellence (J.-x.M.) and the American Diabetes Association (J.-x.M.) and Juvenile Diabetes Research Foundation (J.-x.M.), EY04939 (R.K.C.) and EY14793 (vision core Medical University of South Carolina), and grants from The Foundation Fighting Blindness (RKC) and an unrestricted grant to the Department of Ophthalmology at Medical University of South Carolina from Research to Prevent Blindness. R.K.C. is a Research to Prevent Blindness Senior Scientific Investigator. We thank Zsolt Ablonczy for help with the figures and Luanna Bartholomew for editorial assistance.
Footnotes
This invited paper is part of the Symposium-in-Print: Photoreceptors and Signal Transduction.
References
- 1.Braekevelt CR. Fine structure of the retinal photoreceptors of the tiger salamander (Ambystoma tigrinum) Histol Histopathol. 1993;8:265–272. [PubMed] [Google Scholar]
- 2.Cornwall MC, MacNichol EF, Jr, Fein A. Absorptance and spectral sensitivity measurements of rod photoreceptors of the tiger salamander, Ambystoma tigrinum. Vis Res. 1984;24:1651–1659. doi: 10.1016/0042-6989(84)90323-7. [DOI] [PubMed] [Google Scholar]
- 3.Harosi FI. Absorption spectra and linear dichroism of some amphibian photoreceptors. J Gen Physiol. 1975;66:357–382. doi: 10.1085/jgp.66.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makino CL, Taylor WR, Baylor DA. Rapid charge movements and photosensitivity of visual pigments in salamander rods and cones [published erratum appears in J. Physiol. (Lond.) 1992; 448:781] J Physiol. 1991;442:761–780. doi: 10.1113/jphysiol.1991.sp018818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mariani AP. Photoreceptors of the larval tiger salamander retina. Proc R Soc Lond, B, Biol Sci. 1986;227:483–492. doi: 10.1098/rspb.1986.0035. [DOI] [PubMed] [Google Scholar]
- 6.Boll F. Zur anatomie und physiologie der retina [original 1877, Archieve fur Physiologie 4–36, Translation by R. Hubbard] Vis Res. 1977;17:1249–1265. [Google Scholar]
- 7.Attwell D, Werblin FS, Wilson M. The properties of single cones isolated from the tiger salamander retina. J Physiol. 1982;328:259–283. doi: 10.1113/jphysiol.1982.sp014263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das J, Crouch RK, Ma JX, Oprian DD, Kono M. Role of the 9-methyl group of retinal in cone visual pigments. Biochemistry. 2004;43:5532–5538. doi: 10.1021/bi036097u. [DOI] [PubMed] [Google Scholar]
- 9.Makino CL, Dodd RL. Multiple visual pigments in a photoreceptor of the salamander retina. J Gen Physiol. 1996;108:27–34. doi: 10.1085/jgp.108.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perry RJ, McNaughton PA. Response properties of cones from the retina of the tiger salamander [published erratum appears in J. Physiol. (Lond.) 1991; 436:771] J Physiol. 1991;433:561– 587. doi: 10.1113/jphysiol.1991.sp018444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma J, Znoiko S, Othersen KL, Ryan JC, Das J, Isayama T, Kono M, Oprian DD, Corson DW, Cornwall MC, Cameron DA, Harosi FI, Makino CL, Crouch RK. A visual pigment expressed in both rod and cone photoreceptors. Neuron. 2001;32:451–461. doi: 10.1016/s0896-6273(01)00482-2. [DOI] [PubMed] [Google Scholar]
- 12.Ebrey T, Koutalos Y. Vertebrate photoreceptors. Prog Retin Eye Res. 2001;20:49–94. doi: 10.1016/s1350-9462(00)00014-8. [DOI] [PubMed] [Google Scholar]
- 13.Ryan JC, Znoiko S, Xu L, Crouch RK, Ma JX. Salamander rods and cones contain distinct transducin alpha subunits. Vis Neurosci. 2000;17:847–854. doi: 10.1017/s0952523800176047. [DOI] [PubMed] [Google Scholar]
- 14.Shaffer HB, McKnight ML. The polytypic species revisited: Genetic differentiation and molecular phylogenetics of the tiger salamander (Ambystoma tigrinum) (Amphibia: Caudata) complex. Evolution. 1996;50:417–433. doi: 10.1111/j.1558-5646.1996.tb04503.x. [DOI] [PubMed] [Google Scholar]
- 15.Wilbur HM, Collins JP. Ecological aspects of amphibian metamorphosis: Nonnormal distributions of competitive ability re.ect selection for facultative metamorphosis. Science. 1973;182:1305–1314. doi: 10.1126/science.182.4119.1305. [DOI] [PubMed] [Google Scholar]
- 16.Collins JP. Distribution, habitats and life history variation in the tiger salamander, Ambystoma tigrinum, in east-central and southeast Arizona. Copeia. 1981;1981:666–675. [Google Scholar]
- 17.Rose FL, Armentrout D. Adaptive strategies of Ambystoma tigrinum Green inhabiting the Llano Estacado of West Texas. J Anim Ecol. 1975;45:713–739. [Google Scholar]
- 18.Voss SR, Shaffer HB. Adaptive evolution via a major gene effect: Paedomorphosis in the Mexican axolotl. Proc Natl Acad Sci USA. 1997;94:14185–14189. doi: 10.1073/pnas.94.25.14185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laird DW, Molday RS. Evidence against the role of rhodopsin in rod outer segment binding to RPE cells. Invest Ophthalmol Vis Sci. 1988;29:419–428. [PubMed] [Google Scholar]
- 20.Vissers PM, DeGrip WJ. Functional expression of human cone pigments using recombinant baculovirus: Compatibility with histidine tagging and evidence for N-glycosylation. FEBS Lett. 1996;396:26–30. doi: 10.1016/0014-5793(96)01064-2. [DOI] [PubMed] [Google Scholar]
- 21.Jinks RN, Markley TL, Taylor EE, Perovich G, Dittel AI, Epifanio CE, Cronin TW. Adaptive visual metamorphosis in a deep-sea hydrothermal vent crab. Nature. 2002;420:68–70. doi: 10.1038/nature01144. [DOI] [PubMed] [Google Scholar]
- 22.Mader MM, Cameron DA. Photoreceptor differentiation during retinal development, growth, and regeneration in a metamorphic vertebrate. J Neurosci. 2004;24:11463–11472. doi: 10.1523/JNEUROSCI.3343-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]