Abstract
The melanocortin pathway has emerged during this past decade as an important target area for the discovery and development of therapeutic agents related to obesity and type 2 diabetes. This peptide-G-protein coupled receptor (GPCR) pathway has evolved from peptide based ligands to small molecules possessing a variety of different molecular scaffolds. Herein, we summarize the originating hypothesis of the importance of the reverse β-turn secondary structure for agonist ligand potency at the melanocortin receptors and how that information was utilized for the discovery of small molecules based upon this type of turn structure.
Introduction
The melanocortin system is comprised of five G-Protein Coupled Receptor (GPCR) subtypes and a series of endogenous agonists and antagonists that interact and regulate the intracellular signaling of these receptors. The melanocortin receptors (MCRs) are found in a variety of tissues, and are associated with a myriad of vital functions. The melanocortin-1 receptor (MC1R) is best known for its role in skin pigmentation and hair coloration.1, 2 The melanocortin-2 receptor (MC2R) is unique among the melanocortin receptors in that it is only stimulated by the adrenocorticotropin hormone (ACTH) ligand, versus other endogenous agonist peptides (i.e. α-MSH, β-MSH, γ-MSH).1 The MC2R is expressed primarily in the adrenal glands and in adipocytes,1, 3, 4 and is involved in steroidogenesis and the body’s stress response mechanism in correlation with adrenocorticotropin hormone (ACTH) through the cortisol and other stress related pathways.3-5 The melanocortin-3 receptor (MC3R) is expressed throughout the body, notably in the central nervous system, the pancreas, the heart, and the gastrointestinal tract.6-8 The location of the MC3R in these tissues, as well as the phenotype of the knockout mouse, suggests a potential role in energy homeostasis, thermoregulation, and cardiovascular function. 9, 10 The melanocortin-4 receptor (MC4R) is primarily expressed in the central nervous system and the brain.11-14 The MC4R has been shown to be directly involved in feeding behavior, weight, and energy homeostasis by the changes in food intake observed after the administration of MC4R selective agonists and antagonists in mouse feeding studies as well as the phenotype of the knockout mouse. 15-18 Additionally, the MC4R plays a role in sexual function.19-21 The melanocortin-5 receptor (MC5R) is expressed in a plethora of tissues throughout the body.7, 22-24 Even though the MC5R remains largely uncharacterized, it has been tentatively linked to exocrine gland function and sebaceous gland lipid production.7, 22-24
The melanocortin receptors respond to endogenous agonists derived from the proopiomelanocortin (POMC) gene transcript as well as endogenous antagonists (Agouti and Agouti-related protein, AGRP).25, 26 α-Melanocyte stimulating hormone (α-MSH, Ac-Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-NH2) is a generated by enzymatic cleavage and modification of the POMC gene transcript.27, 28 α-MSH has potent nM agonist activity at all of the melanocortin receptor subtypes with the notable exception of the MC2R. ACTH is a 39 amino acid peptide that results from the first fragmentation of POMC by prohormone convertase 1 (PC1).29-34 ACTH is cleaved by PC2 to yield the precursor for α-MSH (which is further enzymatically modified at the N- and C- termini). Both α-MSH and ACTH, as well as the other endogenous melanocortin ligands, contain a core His-Phe-Arg-Trp sequence that has been postulated to be important for molecular recognition and receptor stimulation.35-39
Molecular recognition of peptides by their cognate receptors has been attributed to the ligand pharmacophore, secondary structure, and chemical functionality of the amino acid side chains. One of the most common and important secondary structures found in peptide hormones that stimulate GPCRs is a reverse turn, specifically a β-turn.40, 41 A β-turn is a turn of specific orientation in which the direction of the peptide chain is reversed within four residues with the distance between the first and fourth residue defined as less than 7 Å and an α-helix secondary structure is not formed. A hydrogen bond between the carbonyl group of residue i and the amide of the third peptide bond (i+3) stabilizes β-turns within the peptide chain.41-43 β-turns are classified based upon the φ and ψ angles observed between the side chains of residues i+1 and i+2.43 For a standard β-turn, the side chain of the i+1 residue is oriented equatorially, while the i+2 residue is oriented axially, either up or down, depending on the stereochemistry of the residue (Fig. 1).41, 43 This paper will focus on melanocortin peptides and small molecules designed to mimic type I and type II β-turns.
Figure 1.
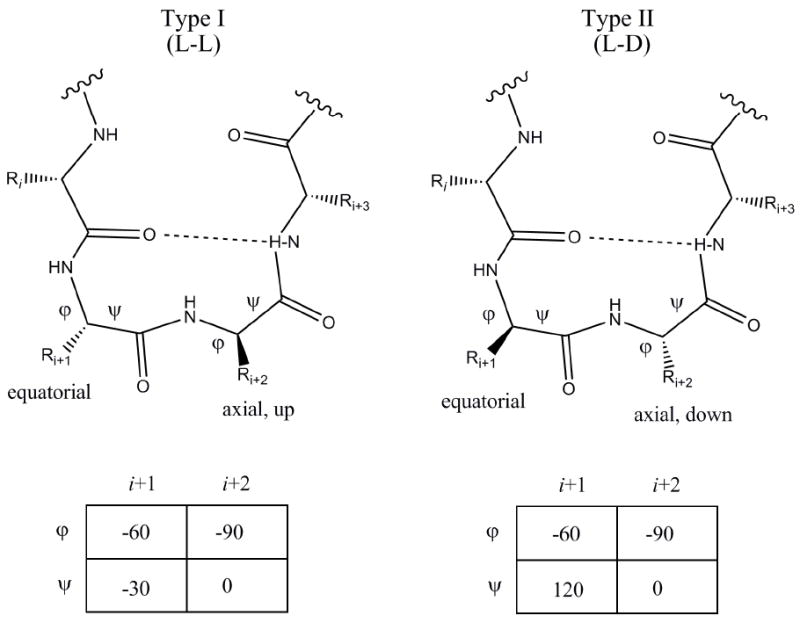
Type I and Type II ß-turn motif.L and D refers to the stereochemistry of the side chains
Design, Synthesis and Characterization of Melanocortin Peptides
α-MSH is a linear thirteen amino acid peptide that is highly flexible and can adopt a variety of conformational states depending upon local aqueous, protein, or membrane related environments. One of the elementary concepts in peptide related ligand design is that by reducing the degree of conformational freedom, ligands that are entropically favorable and mimic a “bioactive conformation” could be designed the use of conformational constraints ranging from side chain to cyclization strategies.44-47 Studies on melanocortin agonist ligands from the 1970’s-1980’s will start this perspective. Sawyer et al. conducted racemization experiments using quantitative gas chromatographic methods on α-MSH confirming that the Met4 and Phe7 (α-MSH numbering) residues were both racemized after heat-alkali treatment, that led to the design of diastereoisomeric analogues based on the observation that after heat-alkali treatments α-MSH experiences an increase in biological function in both in vitro and in vivo experiments.48 Subsequently, a linear 13 amino acid residue analogue of α-MSH was designed in which Met4 was replaced by Nle4 and lPhe7 was stereochemically substituted by dPhe7 [Nle4, dPhe7]-α-MSH, more commonly referred to as NDP-MSH.48 NDP-MSH exhibited strong biological activity in the classical frog skin pigmentation assay without having to undergo heat-alkali treatment, unlike the linear α-MSH peptide. NDP-MSH also showed increased potency as compared to both α-MSH and Nle4-α-MSH, and did not degrade when exposed to serum enzymes.49 NDP-MSH was more potent than α-MSH in activating mouse melanoma adenylate cyclase, and elicited a stronger response in the mouse melanoma cell tyrosinase assay.48 Interestingly, a DPhe residue is postulated to stabilize a reverse turn secondary structure around the α-MSH 5-9 residues. This hypothesis resulted in the design and synthesis of an analogue in which Cys residues replaced Met4 and Gly10 in α-MSH resulting in a covalently linked side chain to side chain disulfide bond. Cyclic [Cys4,Cys10]-α-MSH was shown to be more potent than α-MSH in the classical frog and lizard skin pigmentation assays and more potent in stimulating adenylate cyclase in the mouse melanoma adenylate cyclase assay.50, 51 It was during this time that mounting evidence suggested that the “biologically active” conformation of α-MSH may be due to the presence of a β-turn found within the His-Phe-Arg-Trp sequence and that cyclizing the peptide would produce a constrained conformation similar to a reverse turn that oriented the melanocortin pharmacophore moieties optimally for molecular recognition and receptor potency.50, 51
Knittel et al. then synthesized Ac-[Cys4,Cys10]-α-MSH(4-10)-NH2 and Ac-[Cys4,Cys10]-α-MSH(4-13)-NH2 to further the investigation of the biological role of the reverse turn within α-MSH.51 Since prolongation of skin darkening was not observed with Ac-[Cys4,Cys10]-α-MSH(4-10)-NH2, it supported the conclusion that a reverse turn is not the only structural feature that is important for melanocortin activity. Although the potencies of both synthesized cyclic analogues are greater than NDP-MSH, the prolongation of these peptides are less than that of NDP-MSH, suggesting that potency and prolongation are directed through different structural and conformational features. 39, 48, 51
The culmination of melanocortin peptide β-turns and cyclization studies was the discovery and characterization of the super potent melanocortin agonist Melanotan II (MTII) by Al-Obeidi et al. based upon structure-activity relationships, molecular dynamic calculations, and examining other agonist peptides such as NDP-MSH and Ac-[Cys4,Cys10]-α-MSH-NH2.52, 53 The structural requirements for melanotropic potency at positions five and ten of α-MSH were examined.53, 54 A series of NDP-MSH based peptide analogues were synthesized using the 1-13, 4-10, and 4-13 fragment templates as core sequences. Substitutions were made in which Glu5 was replaced by Asp and Gly10 was replaced with amino acids containing basic side chains. It was reported that one analogue displayed enhanced potency when compared to α-MSH and Ac-[Nle4,DPhe7]α-MSH(4-10)-NH2 in the frog skin and the lizard skin assays.53, 54
NMR studies conducted by Sugg et al. provided experimental evidence that the DPhe7 melanocortin ligand residue appeared to stabilize the β-turn within the core tetrapeptide sequence, resulting in the conclusion relating the structure and bioactivity of NDP-MSH and its analogues.55 It was hypothesized based upon these NMR studies, that the hydrophobic side chains of the His, DPhe and Trp residues are on the same side of the peptide in close proximity to one another, while the hydrophilic Arg side chain is oriented away from the aromatic moieties.55 This NMR data and structure-activity relationship data of the linear α-MSH fragment analogues, NDP-MSH, and Ac-[Cys4,Cys10]-α-MSH resulted in the design of cyclic α-MSH analogues using the 4-10 and 4-13 fragments.48, 50, 51, 53, 54 Al-Obeidi et al. synthesized a series of cyclic peptides with the following modifications: Ac-Nle4-c[Xxx5, DPhe7, Yyy10]-Gly11-α-MSH(4-13)-NH2 and Ac-Nle4-c[ Xxx5, DPhe7, Yyy10]-α-MSH(4-10)-NH2, in which Xxx was substituted by an acidic amino acid residue and the Yyy position was substituted for by a basic residue. A lactam bridge was formed between the side chains of the Xxx5 and Yyy10 residues to further constrain the molecule.52, 53 The cyclic peptide, Ac-Nle4-c[Asp5-His6-DPhe7-Arg8-Trp9-Lys10]-NH2, was shown to exhibit a potency equivalent to α-MSH in the frog skin assay, however, it was 90-100 times more potent in the lizard skin assay and demonstrated prolonged biological activity in comparison to α-MSH.52, 53 This cyclic, super potent compound later came to be known as Melanotan II (MTII).
Although MTII is a potent peptide, it is not a selective agonist for any of the frog, lizard, mouse, or human melanocortin receptors.52, 53, 56, 57 Additional NMR studies have shown that MTII exhibits a type II β-turn within the His-DPhe-Arg-Trp region.45 Molecular modeling has further supported the separation of side chains with the His, DPhe and Trp on one side and Arg on the opposite side.58, 59 Ying et al. synthesized a series of peptide analogues based on the NMR structure of MTII incorporating disulfide or lactam bridge macrocycles as means of further constraining the conformation of the compounds.59 Based on ROESY NMR data for MTII, the hydrogens bonded to the Asp5 and Arg8 were identified to be in close proximity leading the authors to hypothesize that replaced of these amino acids by sulfur that could be used to form sulfide bonds and introduce a conformational constraint.59 Further modifications were made by altering the Nα-alkylation residue (intended to mimic the Arg8 pharmacophore), inverting the chirality of the C atom of the residue being Nα-alkylated, or substitution with bulky aromatic residues.60
The compounds were tested for their binding affinity and adenylate cyclase activity at the hMC1,3-5R. Three peptides were reported to be antagonists that exhibited high selectivity for the hMC4R. It was hypothesized that the selectivity was due to the increased conformational constraint as compared to the predicted values for MTII.59 The compounds containing a disulfide bond instead of a lactam bridge had higher binding efficiencies, leading to the hypothesis that the disulfide linked peptides were better ligands because of the increased flexibility of a disulfide bond compared to that of a lactam bridge. The data that the peptides exhibited only 50% binding efficiency was interpreted to suggest the presence of an allosteric binding site at the hMC4R to which some of the synthesized peptides bound in lieu of the site used by MTII.60
Melanocortin Small Molecule Agonists
The ease with which peptides are degraded in the body and their general lack of selectivity has led to the desire for and the generation of a new class of compounds, termed peptidomimetics, designed to copy the general shape and chemistry of the endogenous peptide ligands with the goal of increased resistance to degradation, resulting in a longer half-life in the body and greater selectivity between receptor subtypes. The advent of small molecules targeting the melanocortin receptors has also meant the development of ligands that are capable of being selective for only one receptor subtype. Since the melanocortin receptor subtypes have such diverse roles that are tissue dependent, it is important to continue to seek more potent and selective ligands for use as effective drug therapy treatments with minimal side effects.
In 1999, Haskell-Luevano et al. screened a series of small molecules based on a heterocyclic, nine membered ring structure. The focus of the experiment was to identify small molecules whose structure incorporates a β-turn within the Phe-Arg-Trp region that exhibits agonist activity at the mMC1R.35-37, 61 Two nonpeptide, heterocyclic compounds were identified as low micromolar agonists at the mMC1R by the screening of this library and were the first non-peptidic β-turn mimetic ligands reported in the literature able to stimulate the melanocortin receptors.57 While the compounds (Fig. 3, 1) did not contain a basic side chain at the Arg8 position, they still possessed low micromolar agonist activity at the mMC1R. This data refuted the belief that the basic side chain of Arg8 was required for activity.35, 37, 57, 61 The results of this study further supported the hypothesis that a β-turn secondary structure present in the Phe-Arg-Trp sequence of agonist peptides may be key to biological activity at the melanocortin receptors and showed that biological activity could be reproduced by incorporating the secondary structure into small molecule ligands.35 The identification of small molecules with agonist activity opened the gate for future researchers to design nonpeptide agonists for the melanocortin receptors.
Figure 3.
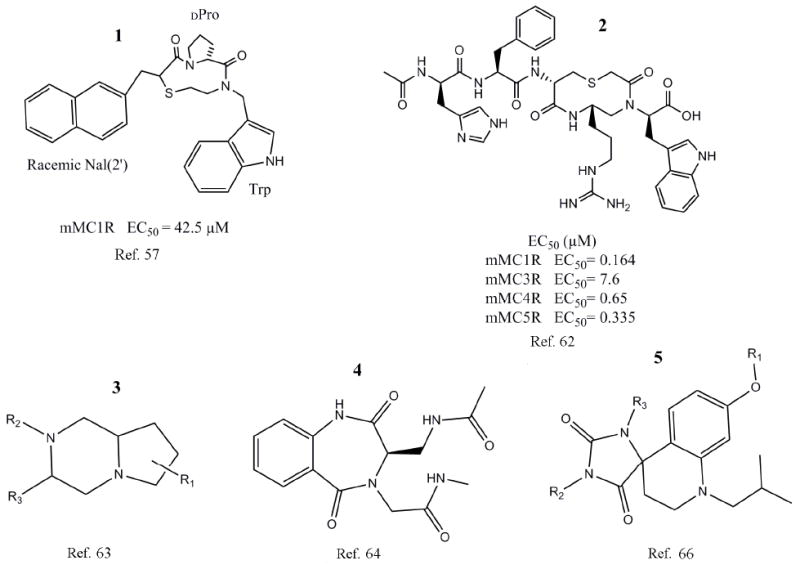
Structures and scaffolds of melanocortin targeting molecules incorporate a β-turn motif.
Bondebjerg et al. synthesized a series of peptidomimetics based on the β-turn configuration of the endogenous peptide ligands. A novel thioether cyclized peptidomimetic scaffold capable of being modified at up to four different positions was used as the basis for the compounds synthesized in the described study.62 The resulting small molecules exhibited low micromolar activity at the mMC1,3-5Rs with some compounds demonstrating moderate selectivity for the mMC1R. Additionally, the study was able to synthesize and identify one peptidomimetic (Fig. 3, 2) with higher than average activity at all four assayed receptors compared to the other synthesized compounds.62
In 2006, Cain et al. synthesized a series of small molecules based on a pyrrolopiperazine template (Fig. 3, 3) intended to mimic the type II β-turn configuration. Based on the observation that the majority of small molecules with activity at the melanocortin receptors contain two aromatic hydrophobic groups and a group with a basic nitrogen, the group synthesized a series of small molecules to investigate the effects of substitution of the hydrophobic residues, the orientation of the hydrophobic groups, and the significance of the basic Arg residue based on a template synthesized from lPro and d,l-Phe.63 Many of the resulting small molecules were capable of binding to one or more of the hMCRs at nanomolar concentrations, some exhibiting selectivity for the hMC5R. Though many of the compounds were unable to stimulate a cAMP response, a new small molecule template capable of binding to the melanocortin receptors was identified.63
In 2007, Verdie et al. developed a method for the incorporation of benzodiazepinone templates into sequences of α-MSH and other known agonists. The synthetic route used was to insert the benzodiazepinone like an amino acid residue during solid phase peptide synthesis.64 Based upon the NMR studies performed by Ying et al., core sequences were selected to be used as templates for the benzodiazepinone structures incorporated into the peptides.59 Further molecular modeling predicted that the substitution of these amino acid residues with the benzodiazepinone template would not disrupt the natural folding conformation.64 Two consecutive amino acids, either His6-DPhe7 or DPhe7-Arg8, were then replaced by a modified 1,4-benzondiazepine-2,5-dione building block (Fig. 3, 4).64 Originally, five α-MSH analogues were modified with this benzodiazepinone template, with continued studies leading to the design of many additional analogues based on the primary results. The study found that when the His6-dPhe7 dipeptide sequence was replaced by the benzodiazepinone functional activity was lost, however, weak binding still occurred at the MC1R. Furthermore, compounds in which dPhe7-Arg8 were substituted with the small molecule template resulted in no binding at the receptor.64 While the study did not result in potent agonist molecules, it did present the important discovery of the chemistry needed to substitute small molecules into a peptide chain using on-resin synthetic techniques.64 The development of this approach which allows for the direct substitution of small molecules into a peptide will advance the field of peptide chemistry because of the ability to introduce privileged structures into known peptide ligands.
Chianelli et al. previously generated a series of ligands possessing activity for the somatostatin receptors using a novel method for the design of templates suitable for synthetic modification yielding libraries of small molecules possessing drug like properties as well as exhibiting peptide pharmacophores.65 This study was done to develop melanocortin nonpeptide agonists to demonstrate that this approach is applicable to ligands of other receptors. Scaffolds were designed and synthesized with the goal of achieving small molecules of comparable size and shape to the β-turn sequence backbone. Three libraries were generated during this study, each based upon the results of the previous library.66 From the first library, one compound was reported to show weak agonist activity at the MC4R. The second synthesized series produced one compound with low micromolar activity at the MC4R. In the third round generation of MC4R targeted ligands, 11 compounds demonstrated MC4R agonist activity greater than the activity shown by the compound selected from the second library as the model for modification for the third library.66 The generation of several compounds capable of agonist activity at the MC4R demonstrates that the approach employed in the development of the compound libraries is applicable to more than just one class of GPCRs that recognize a β-turn secondary structure.66 (Fig. 3, 5)
Conclusion
This perspective has reviewed the evolution of melanocortin peptide agonists that were hypothesized to contain a reverse β-turn secondary structure postulated to be important for the “bioactive” conformation of melanocortin agonist ligands. These hypotheses were supported by biophysical studies (NMR and computational molecular modeling) and further validated by the development of small molecules that were proposed to mimic the β-turn secondary structure. It is well recognized that an extremely large number of different molecular scaffolds have been used and identified as ligands that are able to potentially stimulate the melanocortin receptors that are not remotely related to the β-turn secondary structure, but that is outside the focus of this perspective and has been reviewed elsewhere. Since this special issue is a tribute to the groundbreaking contributions by Professor Jonathon Ellman, and the first β-turn small molecule melanocortin receptor agonist was a result of a collaborative effort between Haskell-Luevano, Cone, and Ellman, we thought it a fitting topic for this special tribute.
Figure 2.
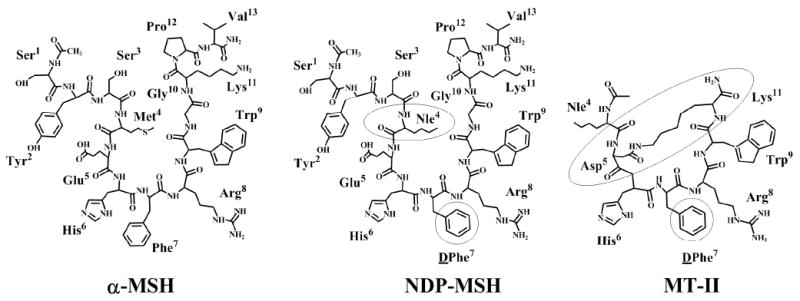
Structures of melanocortin ligands with boxed regions indicating important structural changes.
Acknowledgments
We would like to acknowledge financial support from NIH Grants RO1DK57080, RO1DK64250, RO1DK063974, and an American Diabetes Research Award. We would like to thank Mic E. Mouse for his inspirational thoughts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. Science. 1992;257:1248. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- 2.Lu D, Väge DI, Cone RD. Mol Endo. 1998;12:592. doi: 10.1210/mend.12.4.0091. [DOI] [PubMed] [Google Scholar]
- 3.Grunfeld C, Hagman J, Sabin EA, Buckley DI, Jones DS, Ramachandran J. Endocrinology. 1985;116:113. doi: 10.1210/endo-116-1-113. [DOI] [PubMed] [Google Scholar]
- 4.Boston BA, Cone RD. Endocrinology. 1996;137:2043. doi: 10.1210/endo.137.5.8612546. [DOI] [PubMed] [Google Scholar]
- 5.Halkerston ID. Advances in Cyclic Nucleotide Research. 1975;6:99. [PubMed] [Google Scholar]
- 6.Gantz I, Konda Y, Tashiro T, Shimoto Y, Miwa H, Munzert G, Watson SJ, DelValle J, Yamada T. J Biol Chem. 1993;268:8246. [PubMed] [Google Scholar]
- 7.Chhajlani V. Biochem Mol Biol Int. 1996;38:73. [PubMed] [Google Scholar]
- 8.Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB. Proc Natl Acad Sci U S A. 1993;90:8856. doi: 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Baetscher M, Cone RD. Endocrinology. 2000;141:3518. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- 10.Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao L, Metzger JM, Strack AM, Camacho RE, Mellin TN, Nunes CN, Min W, Fisher J, Gopal-Truter S, MacIntyre DE, Chen HY, Van Der Ploeg LH. Nat Genet. 2000;26:97. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 11.Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson SJ, Delvalle J, Yamada T. Journal of Biological Chemistry. 1993;268:15174. [PubMed] [Google Scholar]
- 12.Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Mol Endocrinol. 1994;8:1298. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- 13.Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, Friedman JM, Elmquist JK. J Neurosci. 2003;23:7143. doi: 10.1523/JNEUROSCI.23-18-07143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. J Comp Neurol. 2003;457:213. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- 15.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Nature. 1997;385:165. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 16.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Smith FJ, Kesterson RA, Boston BA, Fang Q, Berkemeir LR, Gu W, Cone RD, Campfield LA, Lee F. Cell. 1997;88:131. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 17.Schioth HB, Muceniece R, Mutulis F, Bouifrouri AA, Mutule I, Wikberg JES. Neuropeptides. 1999;33:191. doi: 10.1054/npep.1999.0760. [DOI] [PubMed] [Google Scholar]
- 18.Benoit SC, Schwartz MW, Lachey JL, Hagan MM, Rushing PA, Blake KA, Yagaloff KA, Kurylko G, Franco L, Danhoo W, Seeley RJ. J Neurosci. 2000;20:3442. doi: 10.1523/JNEUROSCI.20-09-03442.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Der Ploeg LH, Martin WJ, Howard AD, Nargund RP, Austin CP, Guan X, Drisko J, Cashen D, Sebhat I, Patchett AA, Figueroa DJ, DiLella AG, Connolly BM, Weinberg DH, Tan CP, Palyha OC, Pong SS, MacNeil T, Rosenblum C, Vongs A, Tang R, Yu H, Sailer AW, Fong TM, Huang C, Tota MR, Chang RS, Stearns R, Tamvakopoulos C, Christ G, Drazen DL, Spar BD, Nelson RJ, MacIntyre DE. Proc Natl Acad Sci U S A. 2002;99:11381. doi: 10.1073/pnas.172378699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irani BG, Xiang Z, Moore MC, Mandel RJ, Haskell-Luevano C. Biochem Biophys Res Commun. 2005;326:638. doi: 10.1016/j.bbrc.2004.11.084. [DOI] [PubMed] [Google Scholar]
- 21.Wessells H, Fuciarelli K, Hansen J, Hadley ME, Hruby VJ, Dorr R, Levine N. J Urol. 1998;160:389. [PubMed] [Google Scholar]
- 22.Griffon N, Mignon V, Facchinetti P, Diaz J, Schwartz J, Sokoloff P. Biochem Biophys Res Commun. 1994;200:1007. doi: 10.1006/bbrc.1994.1550. [DOI] [PubMed] [Google Scholar]
- 23.Chen W, Kelly MA, Opitz-Araya X, Thomas RE, Low MJ, Cone RD. Cell. 1997;91:789. doi: 10.1016/s0092-8674(00)80467-5. [DOI] [PubMed] [Google Scholar]
- 24.Van Der Kraan M, Adan RAH, Entwistle ML, Gispen WH, Burbach JPH, Tatro JB. Endocrinology. 1998;139:2348. doi: 10.1210/endo.139.5.6008. [DOI] [PubMed] [Google Scholar]
- 25.Ollmann MM, Wilson BD, Yang Y-K, Kerns JA, Chen Y, Gantz I, Barsh GS. Science. 1997;278:135. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 26.Lerner AB, McGuire JS. Nature. 1961;189:176. doi: 10.1038/189176a0. [DOI] [PubMed] [Google Scholar]
- 27.Eberle AN. The Melanotropins: Chemistry, Physiology and Mechanism of Action. Karger; Basel: 1988. [Google Scholar]
- 28.Smith AI, Funder JW. Endocr Rev. 1988;9:159. doi: 10.1210/edrv-9-1-159. [DOI] [PubMed] [Google Scholar]
- 29.Riniker B, Sieber P, Rittel W, Zuber H. Nat New Biol. 1972;235:114. doi: 10.1038/newbio235114b0. [DOI] [PubMed] [Google Scholar]
- 30.Notake M, Tobimatsu T, Watanabe Y, Takahashi H, Mishina M, Numa S. FEBS Lett. 1983;156:67. doi: 10.1016/0014-5793(83)80250-6. [DOI] [PubMed] [Google Scholar]
- 31.Drouin J, Chamberland M, Charron J, Jeannotte L, Nemer M. FEBS Lett. 1985;193:54. doi: 10.1016/0014-5793(85)80078-8. [DOI] [PubMed] [Google Scholar]
- 32.Boileau G, Barbeau C, Jeannotte L, Chretien M, Drouin J. Nucleic Acids Res. 1983;11:8063. doi: 10.1093/nar/11.22.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang AC, Cochet M, Cohen SN. Proc Natl Acad Sci U S A. 1980;77:4890. doi: 10.1073/pnas.77.8.4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel PD, Sherman TG, Watson SJ. DNA. 1988;7:627. doi: 10.1089/dna.1988.7.627. [DOI] [PubMed] [Google Scholar]
- 35.Hruby VJ, Wilkes BC, Hadley ME, Al-Obeidi F, Sawyer TK, Staples DJ, de Vaux AE, Dym O, Castrucci AM, Hintz MF, et al. J Med Chem. 1987;30:2126. doi: 10.1021/jm00394a033. [DOI] [PubMed] [Google Scholar]
- 36.Castrucci AM, Hadley ME, Sawyer TK, Wilkes BC, al-Obeidi F, Staples DJ, de Vaux AE, Dym O, Hintz MF, Riehm JP, et al. Gen Comp Endocrinol. 1989;73:157. doi: 10.1016/0016-6480(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 37.Haskell-Luevano C, Sawyer TK, Hendrata S, North C, Panahinia L, Stum M, Staples DJ, Castrucci AM, Hadley MF, Hruby VJ. Peptides. 1996;17:995. doi: 10.1016/0196-9781(96)00141-6. [DOI] [PubMed] [Google Scholar]
- 38.Haskell-Luevano C, Holder JR, Monck EK, Bauzo RM. J Med Chem. 2001;44:2247. doi: 10.1021/jm010061n. [DOI] [PubMed] [Google Scholar]
- 39.Hruby VJ, Wilkes BC, Cody WL, Sawyer TK, Hadley ME. Peptide Protein Rev. 1984;3:1. [Google Scholar]
- 40.Smith JA, Pease LG. Reverse Turns in Peptides and Proteins. CRC Pres; Boca Raton: 1980. [DOI] [PubMed] [Google Scholar]
- 41.Rose GD, Gierasch LM, Smith JA. Adv Protein Chem. 1985;37:1. doi: 10.1016/s0065-3233(08)60063-7. [DOI] [PubMed] [Google Scholar]
- 42.Souers A, Ellman JA. Tetrahedron. 2001;57:7431. [Google Scholar]
- 43.Eguchi M, Kahn M. Mini Rev Med Chem. 2002;2:447. doi: 10.2174/1389557023405783. [DOI] [PubMed] [Google Scholar]
- 44.Rizo J, Gierasch LM. Annu Rev Biochem. 1992;61:387. doi: 10.1146/annurev.bi.61.070192.002131. [DOI] [PubMed] [Google Scholar]
- 45.Hruby VJ, Li G, Haskell-Luevano C, Shenderovich M. Biopolymers, Peptide Science. 1997;43:219. doi: 10.1002/(SICI)1097-0282(1997)43:3<219::AID-BIP3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 46.Kessler H. Angew Chem Int Ed Engl. 1982;21:512. [Google Scholar]
- 47.Hruby VJ. Life Sci. 1982;31:189. doi: 10.1016/0024-3205(82)90578-1. [DOI] [PubMed] [Google Scholar]
- 48.Sawyer TK, Sanfilippo PJ, Hruby VJ, Engel MH, Heward CB, Burnett JB, Hadley ME. Proc Natl Acad Sci U S A. 1980;77:5754. doi: 10.1073/pnas.77.10.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castrucci AML, Hadley ME, Sawyer TK, Hruby VJ. Comp Biochem Physiol. 1984;78B:519. doi: 10.1016/0305-0491(84)90090-7. [DOI] [PubMed] [Google Scholar]
- 50.Sawyer TK, Hruby VJ, Darman PS, Hadley ME. Proc Natl Acad Sci U S A. 1982;79:1751. doi: 10.1073/pnas.79.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Knittel JJ, Sawyer TK, Hruby VJ, Hadley ME. J Med Chem. 1983;26:125. doi: 10.1021/jm00356a002. [DOI] [PubMed] [Google Scholar]
- 52.Al-Obeidi F, Hadley ME, Pettitt BM, Hruby VJ. J Am Chem Soc. 1989;111:3413. [Google Scholar]
- 53.Al-Obeidi F, Castrucci AM, Hadley ME, Hruby VJ. J Med Chem. 1989;32:2555. doi: 10.1021/jm00132a010. [DOI] [PubMed] [Google Scholar]
- 54.Al-Obeidi F, Hruby VJ, Castrucci AM, Hadley ME. J Med Chem. 1989;32:174. doi: 10.1021/jm00121a032. [DOI] [PubMed] [Google Scholar]
- 55.Sugg EE, Castrucci AM, Hadley ME, van Binst G, Hruby VJ. Biochemistry. 1988;27:8181. doi: 10.1021/bi00421a029. [DOI] [PubMed] [Google Scholar]
- 56.Haskell-Luevano C, Nikiforovich G, Sharma SD, Yang YK, Dickinson C, Hruby VJ, Gantz I. J Med Chem. 1997;40:1738. doi: 10.1021/jm960845e. [DOI] [PubMed] [Google Scholar]
- 57.Haskell-Luevano C, Rosenquist A, Souers A, Khong KC, Ellman JA, Cone RD. J Med Chem. 1999;42:4380. doi: 10.1021/jm990190s. [DOI] [PubMed] [Google Scholar]
- 58.Al-Obeidi F, O’Connor SD, Job C, Hruby VJ, Pettitt BM. J Pept Res. 1998;51:420. doi: 10.1111/j.1399-3011.1998.tb00640.x. [DOI] [PubMed] [Google Scholar]
- 59.Ying J, Kover KE, Gu X, Han G, Trivedi DB, Kavarana MJ, Hruby VJ. Biopolymers. 2003;71:696. doi: 10.1002/bip.10596. [DOI] [PubMed] [Google Scholar]
- 60.Ying J, Gu X, Cai M, Dedek M, Vagner J, Trivedi DB, Hruby VJ. J Med Chem. 2006;49:6888. doi: 10.1021/jm060768f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haskell-Luevano C, Hendrata S, North C, Sawyer TK, Hadley ME, Hruby VJ, Dickinson C, Gantz I. J Med Chem. 1997;40:2133. doi: 10.1021/jm960840h. [DOI] [PubMed] [Google Scholar]
- 62.Bondebjerg J, Xiang Z, Bauzo RM, Haskell-Luevano C, Meldal M. J Am Chem Soc. 2002;124:11046. doi: 10.1021/ja0123913. [DOI] [PubMed] [Google Scholar]
- 63.Cain JP, Mayorov AV, Cai M, Wang H, Tan B, Chandler K, Lee Y, Petrov RR, Trivedi D, Hruby VJ. Bioorg Med Chem Lett. 2006;16:5462. doi: 10.1016/j.bmcl.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verdie P, Subra G, Feliu L, Sanchez P, Berge G, Garcin G, Martinez J. J Comb Chem. 2007;9:254. doi: 10.1021/cc060054q. [DOI] [PubMed] [Google Scholar]
- 65.Chianelli D, Kim YC, Lvovskiy D, Webb TR. Bioorg Med Chem. 2003;11:5059. doi: 10.1016/j.bmc.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 66.Webb TR, Jiang L, Sviridov S, Venegas RE, Vlaskina AV, McGrath D, Tucker J, Wang J, Deschenes A, Li R. J Comb Chem. 2007;9:704. doi: 10.1021/cc0601581. [DOI] [PubMed] [Google Scholar]


