Abstract
Old age is associated with enhanced susceptibility to and poor recovery from brain injury. An exacerbated microglial and astrocyte response to brain injury might be involved in poor outcomes observed in the elderly. The present study was therefore designed to quantitate the expression of markers of microglia and astrocyte activation using real-time RT-PCR, immunoblot and immunohistochemical analysis in aging brain in response to brain injury. We examined the hippocampus, a region that undergoes secondary neuron death, in aged (21–24 month) and adult (5–6 month) mice following controlled cortical impact (CCI) injury to the sensorimotor cortex. Basal mRNA expression of CD11b and Iba1, markers of activated microglia, was higher in aged hippocampus as compared to the adult. The mRNA expression of microglial markers increased and reached maximum 3 days post injury in both adult and aged mice, but was higher in the aged mice at all time points studied, and in the aged mice the return to baseline levels was delayed. Basal mRNA expression of GFAP and S100B, markers of activated astrocytes, was higher in aged mice. Both markers increased and reached maximum 7 days post injury. The mRNA expression of astrocyte markers returned to near basal levels rapidly after injury in the adult mice, whereas again in the aged mice return to baseline was delayed. Immunochemical analysis using Iba1 and GFAP antibodies indicate accentuated glial responses in the aged hippocampus after injury. The pronounced and prolonged activation of microglia and astrocytes in hippocampus may contribute to worse cognitive outcomes in the elderly following TBI.
Keywords: Aging, Astrocyte, CD11b, GFAP, Iba1, Hippocampus, Inflammation, Microglia, Traumatic brain injury, S100B
Introduction
In the United States, an estimated 1.5 million people sustain traumatic brain injury (TBI) every year; about one every 21 seconds (Letarte, 2006). The cumulative burden of this epidemic is estimated to be 5.3 million Americans, which is nearly 2% of the population, living with disabilities resulting from TBI. The Centers for Disease Control and Prevention analyzed TBI related hospitalizations by age and found that TBI most commonly occurs in adolescents and young adults aged 15 to 24 years, and in the elderly (75 years and older). The incidence rates of TBI, starting at age 65, have been observed to double for every additional 10 years of age (Coronado, et al., 2005). Persons aged ≥ 75 years had the highest TBI-related hospitalization rate, at least twice the rate of any other age group (Coronado, et al., 2005). Older age is a variable known to negatively influence outcome after TBI (Hukkelhoven, et al., 2003). Studies indicate that outcome and mortality rates are generally worse for older people than for younger people with similar injuries (Ferrell and Tanev, 2002, Mohindra, et al., , Rapoport, et al., 2006). The aging of the United States population will further increase the incidence and associated disability of non-fatal TBI (Brown, et al., 2004).
Long-term cognitive and motor dysfunction have been recognized as the most debilitating consequences of human TBI (Klein, et al., 1996). TBI results in neurological dysfunction through primary and secondary (delayed) brain injury. Primary injury occurs at the time of the insult, whereas secondary injury occurs over a period of days. Secondary brain injury is incurred as a result of pathological processes initiated at the time of the primary injury and may be amenable to treatment, thereby improving prognosis. Secondary molecular, biochemical, and cellular events cause neuronal injury and loss in multiple brain areas, including the hippocampus (Bigler, et al., 2002). In both humans and rodents, age exacerbates the cognitive decline following TBI, suggesting synergistic action (Goldstein and Levin, 2001, Johnstone, et al., 1998, White-Gbadebo and Hamm, 1993). In addition, the hippocampus has been demonstrated to be a selectively vulnerable region after experimental TBI with neurons undergoing apoptotic death (Rink, et al., 1995).
We have recently investigated the effect of age on outcomes after controlled cortical impact (CCI) injury in the mouse using behavioral, magnetic resonance imaging and histological studies and found higher vulnerability of the aged brain to neurodegeneration along with diminished functional recovery (Onyszchuk, et al., 2008). Furthermore, a greater number of dying neurons was observed in hippocampus of aged mice following CCI. The mechanism of worse outcome in aging is unknown, but aging has been shown to exaggerate the inflammatory phase of wound healing, a finding that may be causally related to slower wound healing in older subjects (Ashcroft, et al., 2002).
Neuroinflammation contributes to the cascade of events that are responsible for development of secondary brain damage and adverse outcome following TBI (Schmidt, et al., 2004). In the context of inflammatory mechanisms, glial cells are recognized to play an active role in most degenerative CNS pathologies, and reactive gliosis is recognized as universal hallmark of both acute or chronic damage to the CNS (Marchetti and Abbracchio, 2005). Glial activation in response to cytokine exposure is enhanced in the aged brain (Deng, et al., 2006, Godbout, et al., 2005), and previous work from our laboratory has demonstrated higher expression of pro-inflammatory cytokines and chemokines during secondary neuron death in thalamus of aged mice following cortical aspiration injury (Sandhir, et al., 2004).
Despite extensive evidence implicating inflammatory mechanisms in CNS damage following traumatic brain injury, the influence of age on microglial and astrocytic responses has not been investigated. This study was therefore designed to quantitate glial responses in the hippocampus of aged (21–24 mo) and adult (5–6 mo) mice after CCI injury to the sensorimotor cortex by examining the expression of markers of microglia and actrocyte activation using real-time RT-PCR, western blot and immunochemical analysis.
Materials and methods
Animals
Studies were performed using adult (28–32 g, 5–6 months old) and aged (28–32 g, 21–24 months old) C57BL/6 male mice obtained from the National Institute of Aging colonies, which were barrier raised, monitored for genetic purity and screened for bacterial and viral pathogens strictly according to the NIA guidelines. The animals were housed in the AAALAC-accredited Laboratory Animal Resources (LAR) of the University of Kansas Medical Center and acclimatized for 2 weeks before commencing experiments. The LAR facility is supervised by a head veterinarian and is fully staffed with animal technicians. The mice were maintained on a 12-hour light–dark cycle and were provided standard rodent diet (8604, Harlan Teklad Laboratories, Madison, WI) and water without restriction. All the procedures followed protocols approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee (IACUC). A total of 106 (53 adult and 53 aged) mice were used for the study.
Controlled Cortical Impact Injury
Adult and aged mice were randomly assigned to undergo moderate impact injury utilizing a model that causes non-penetrating localized deformation of the cortex. The impactor consisted of a microprocessor controller linear motor device (Linmot, Zurich, Switzerland) mounted on an adjustable manipulator (Kopf, Tujunga, CA) that allowed precise positioning, and control of velocity and the level of cortical deformation as described previously (Onyszchuk, et al., 2007). A 3.0mm flat face tip with a strike velocity of 1.5 m/s, strike depth of 1.0 mm, and contact time of 85 ms was used for injury. Briefly, mice were anesthetized with isoflurane (induction, 2.5%; maintenance, 1.0%) and stabilized in a Cunningham stereotaxic frame (Stoelting, Wood Dale, IN), and body temperature maintained at 37 ± 1°C throughout the procedure. The skull was exposed after a midline incision, and a 3.5mm diameter circular craniotomy was performed using a burr drill, lateral to the mid-sagittal suture, with center coordinates: AP = 0, ML = +2.0 rostral to bregma. After the dural surface was exposed, the position of the impactor and tip was adjusted so that the tip contacted the dura. The tip contact area included motor (M1, M2) and sensory (S1FL, S1HL) cortical areas. The cortical impact was initiated through the device graphical user interface of the impactor control software. After the impact, the scalp was sutured closed, anesthesia was discontinued and animals allowed to recover in a temperature controlled environment. The injury to the sensorimotor cortex did not result in any direct damage to hippocampus. Sham control animals went through the same surgical procedures without the impact injury. We have previously shown that contusion volumes do not differ between the adult and aged mice measured at 3 days following CCI injury (Onyszchuk, et al., 2008).
Total RNA isolation
Tissues were harvested at survival times of 0, 1, 2, 3, 7, 14 and 28 days and stored in RNA Later (Ambion, Austin, TX) for 24 h at 4°C, sliced coronally at 1mm using a mouse brain matrix (Harvard Apparatus, Holliston, MA). The hippocampus was dissected from blocks taken from slices. Total RNA was extracted using TRIZOL Reagent (Invitrogen, Life Technologies, Carlsbad, CA) using with a Polytron homogenizer (Brinkmann Instruments, Westbury, NY) according to manufacturers instructions. Denatured total RNA (2µg/sample) was separated on 1.5%agarose/formaldehyde gels to check for RNA integrity.
Real-time PCR analysis
Gene expression analysis was conducted using quantitative real-time PCR using SYBR Green dye. The following transcripts were analyzed: CD11b (Integrin αM), Iba1 (ionized calcium binding adapter molecule 1); GFAP (glial fibrillary acidic protein) and S100B (calcium-binding protein, beta) to quantitate microglial and astrocyte activation response in hippocampus of adult and aged mice following CCI injury. In brief, the RNA samples were treated with DNaseI to remove any contaminating DNA. Complementary DNA (cDNA) was synthesized by using 1 µg total RNA from each sample and random hexamers in a Taqman reverse transcription reaction (Applied Biosystems, Foster City, CA, USA). 10 ng cDNA and gene-specific primers were added to SYBR Green PCR Master Mix (SYBR Green I Dye, AmpliTaq-DNA polymerase, dNTPs mixture dUTP and optimal buffer components; Applied Biosystems, Foster City, CA), and subjected to PCR amplification (one cycle at 50°C for 2 min, one cycle at 95°C for 10 min, and 40 cycles at 95°C for 15 s and 60°C for 1 min) in a TaqMan 7500 sequence detection system (Applied Biosystems). PCR reactions were conducted in duplicate. Primer sequences used were designed using Primer Express software (Applied Biosystems) and based on GenBank accession numbers (Table 1). The resulting amplicon products were visualized on agarose gel to verify size and specificity of RT-PCR reaction (Fig. 1). The relative amounts of amplified transcripts were quantified with the comparative cycle threshold (CT) method using GAPDH as housekeeping gene using the delta-delta CT method (Pfaffl, et al., 2002).
Table 1.
Primer sequences used for quantitative real-time RT-PCR analysis.
| Gene | Genebank Accession Number | Primer Sequence (5’-3’) | Position | Amplicon Size (bp) |
|---|---|---|---|---|
| CD11b | NM019467 | F=CCTTGTTCTCTTTGATGCAG | 1127–1146 | 217 |
| R=GTGATGACAACTAGGATCTT | 1324–1343 | |||
| Iba1 | NM019467 | F=GTCCTTGAAGCGAATGCTGG | 518–537 | 157 |
| R=CATTCTCAAGATGGCAGATC | 655–674 | |||
| GFAP | NM010277 | F=TCCTGGAACAGCAAAACAAG | 271–290 | 224 |
| R=CAGCCTCAGGTTGGTTTCAT | 475–494 | |||
| S100B | NM009115 | F=TGCCCTCATTGATGTCTTCCA | 141–161 | 101 |
| R=GAGAGAGCTCGTTGTTGATAAGCT | 218–241 | |||
| GAPDH | XR003802 | F=ATGACATCAAGAAGGTGGTG | 769–788 | 177 |
| R=CATACCAGGAAATGAGSCTTG | 926–945 |
Fig. 1.
Electrophoresis of RT-PCR products corresponding to mRNA encoding mouse CD11b, Iba1, GFAP, S100B and GAPDH. Markers (100bp ladder as a size marker); CD11b (217 bp); Iba1 (157 bp); GFAP (224 bp); S100B (101 bp); GAPDH (177 bp)
Immunoblotting
Tissue specimens from the hippocampus were homogenized using cold lysis buffer (20 mM Hepes buffer, pH 7.4, 10 mM KCl, 1.5 mM MgCl 2 , 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.1 mM PMSF, 5 µg/ml pepstatin A, 10 µg/ml leupeptin and 10 µg/ml aprotanin) using a homogenizer. The brain homogenate was centrifuged at 12,000 g (10 min, 4°C). The clear supernatant was collected and the total protein concentration measured with a bicinchoninic acid assay kit using bovine serum albumin as a standard (Pierce Biotechnology Inc., Rockford, IL). Proteins were separated through 12 % SDS-PAGE, electrophoretically transferred to polyvinylidene difluoride membranes and probed with antibodies to Iba1 (019-19741, Wako, Richmond, VA), GFAP (AB-5804, Chemicon, Temecula, CA) and GAPDH (sc-20357, Santa Cruz Biotechnology, Santa Cruz, CA). Bands were visualized by the addition of IRDye 800 (Rockland Immunochemicals, Gilbertsville, PA) and Alexa 680 (Invitrogen, Eugene, OR)-conjugated secondary antibodies using Odyssey Infrared Imaging System (LI-COR, Lincoln, NE). Relative band intensity was determined using Odyssey software version 1.0 (LI-COR).
Immunohistochemical analysis
Microglia and astrocyte activation were detected by staining with anti-Iba1 and anti-GFAP. Briefly, sham and injured animals were deeply anesthetized with an overdose of Nembutal (sodium pentobarbitone). Trans-cardiac perfusion was carried out with 0.1 M phosphate buffered saline pH 7.4 (PBS) followed by 100 ml of 4% buffered formaldehyde. Brains were quickly removed and immersion fixed in 4% buffered formaldehyde for 12 hours. The brains were then cryoprotected and embedded in 5 × 5 arrays in gelatin blocks using the Multibrain™ process (Neuroscience Associates, Knoxville, TN). Tissue sheets of coronal sections (35µm) were collected from the each gelatin array. The sheets were used for free-floating immunohistochemistry. The sheets were rinsed in PBS, permeabilized with Triton X-100, quenched for peroxidase using H2O2 and incubated overnight with primary antibodies; GFAP (rabbit anti-mouse 1:2000, Chemicon AB-5804) or Iba1 (rabbit anti-mouse 1:2000; Wako 019-19741). The sheets were subsequently rinsed and incubated with biotinylated secondary antibody (1:500 goat anti-rabbit; Vector Labs BA-1000) for 2 hours at room temperature. Immunodetection was carried with Vectastain Elite ABC amplification kit (Vector Labs, Burlingame, CA) and developed using diaminobenzidine as chromogen. The sections were visualized under Nikon inverted-stage microscope (100 × magnification) and digital images were captured with a SPOT microscope camera (Diagnostic Instruments, Sterling Heights, MI).
Statistical analysis
Significant group differences were determined by a one-way analysis of variance (ANOVA), followed by a post-hoc analysis using the Student–Newman–Keuls test. In all cases, a p value of < 0.05 was considered significant. Results were expressed as the mean ± standard error.
Results
Microglial activation in hippocampus following CCI Injury
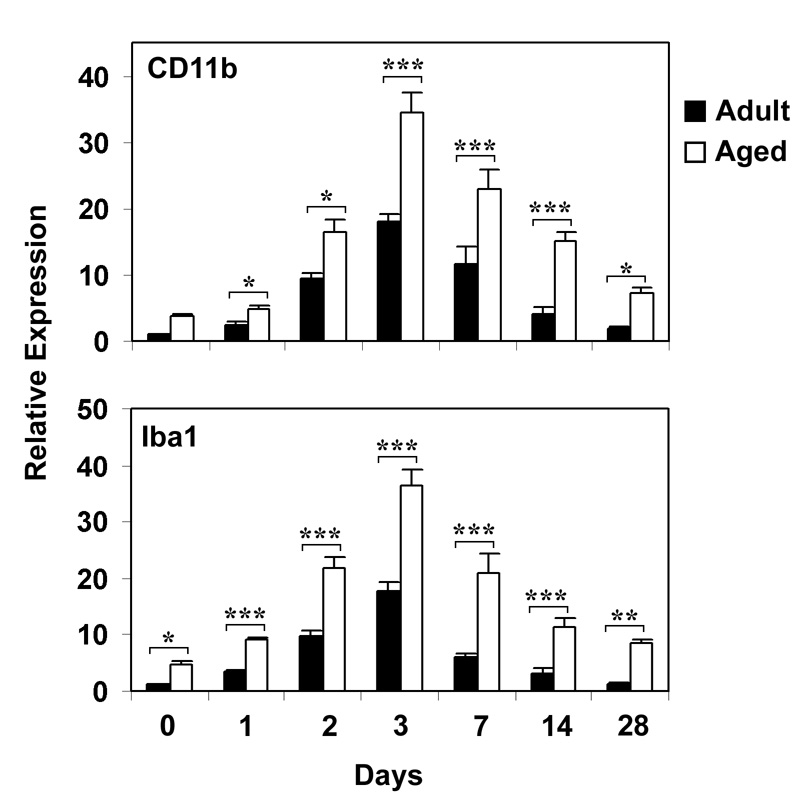
Hippocampal mRNA levels of microglial activation markers, CD11b and Iba1, were determined using real time RT-PCR 1, 2, 3, 7, 14 and 28 days following CCI injury in hippocampus of adult and aged mice (Fig. 2). Basal levels of both CD11b and Iba1 mRNA were higher in the aged hippocampus than in the adult tissue. Following injury, expression of CD11b and Iba1 increased after 24 hours and peaked at 3 days in both the adult and aged mice. Both microglial markers were significantly higher in the aged hippocampus after injury at all time points. Expression of CD11b mRNA was 1.9-fold higher in the aged hippocampus at 3 days and 2-fold higher 7 days post injury, and expression of Iba1 mRNA was 2-fold higher at 3 day time point and 3.5 fold higher at 7 day time point in the aged mice. Both microglial markers rapidly returned to baseline levels in the adult animals. At day 14 there was no significant difference in the expression of CD11b and Iba1 with respect to uninjured adult. However, in the aged mice the mRNA levels for both the markers remained significantly elevated at day 14 (p<0.001), suggesting that neuroinflammation was ongoing.
Fig. 2.
Relative mRNA Expression of Microglia activation markers in Hippocampus of adult and aged mice following CCI injury analyzed by real-time RT-PCR. CD11b (A) and Iba1 (B) mRNA expression was studied after 1, 2, 3, 7, 14 and 28 days after injury. The real time reactions were performed in duplicates for both the target gene and GAPDH used as a housekeeping control. The relative expression was calculated using delta delta CT method. Data are presented as mean + SEM (n=5/group). Asterisk denotes statistical significance after ANOVA and post-hoc testing where *p < 0.05, **p < 0.01, ***p < 0.001 vs. adult
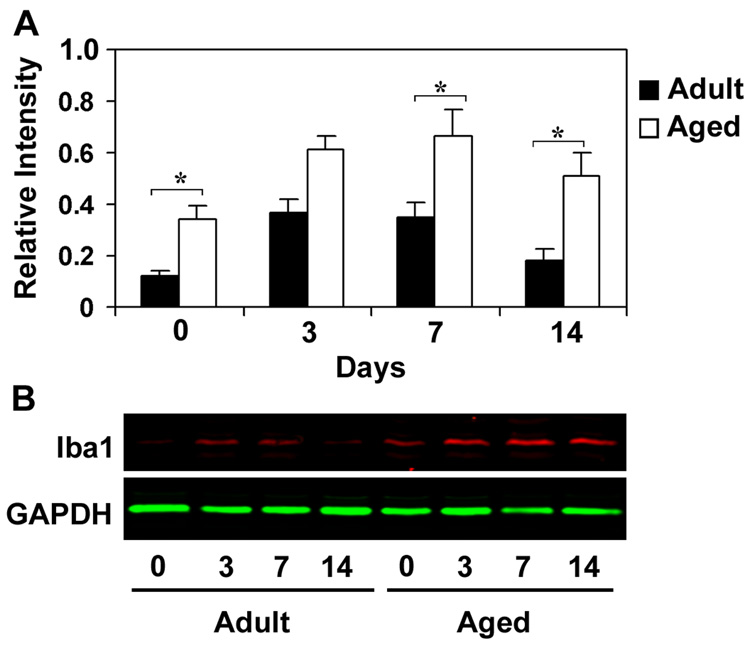
To determine if the increased mRNA expression of microglial markers corresponds with increased protein levels, immunoblot analysis of Iba1 was performed after 3, 7 and 14 days following CCI injury in adult and aged mice (Fig. 3). Iba1 was preferred for immunoblot analysis as it is specifically expressed in microglia and plays an important role in signaling events underlying microglial activation including phagocytosis (Ohsawa, et al., 2000). In addition, CD11b shows multiple isoforms on the western blot because of post-translational modifications (glycosylation) of the protein (Diamond, et al., 1991). A significantly higher amount of Iba1 protein was present in the hippocampus of aged animals at base line and at all time points post injury compared to the adult hippocampus. Relative expression of Iba1 protein was 1.6 fold higher at the 3 and 1.9 fold higher at 7 day time points, whereas it was 2.8 fold higher at the 14 day time point in the aged mice. This difference confirms enhanced and sustained increases in microglial markers in the aged hippocampus in response to injury.
Fig. 3.
Relative expression of Iba1 protein in hippocampus of adult and aged mice following CCI injury analyzed using immuoblotting. Protein extracts from hippocampus were analyzed by immunoblotting using anti-Iba1 antibodies after 3, 7 and 14 days after injury. Numbers represent relative levels of protein based on optical density normalized to GAPDH signal. The bottom panel shows the representative western blot for Iba1 along with GAPDH used as loading control. Data are presented as mean ± SEM (n=3/group). Asterisk denotes statistical significance after ANOVA and post-hoc testing where *p < 0.05 vs. adult
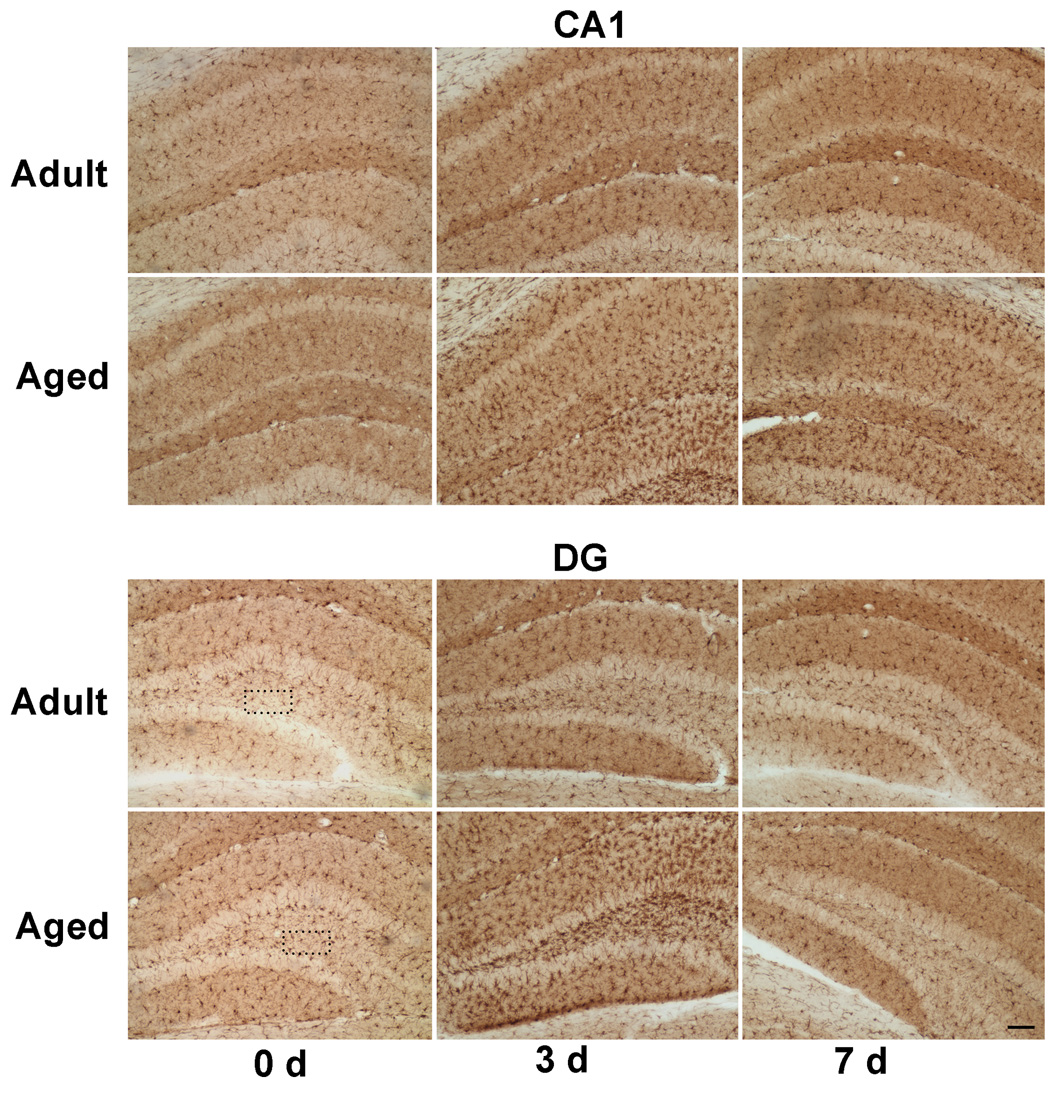
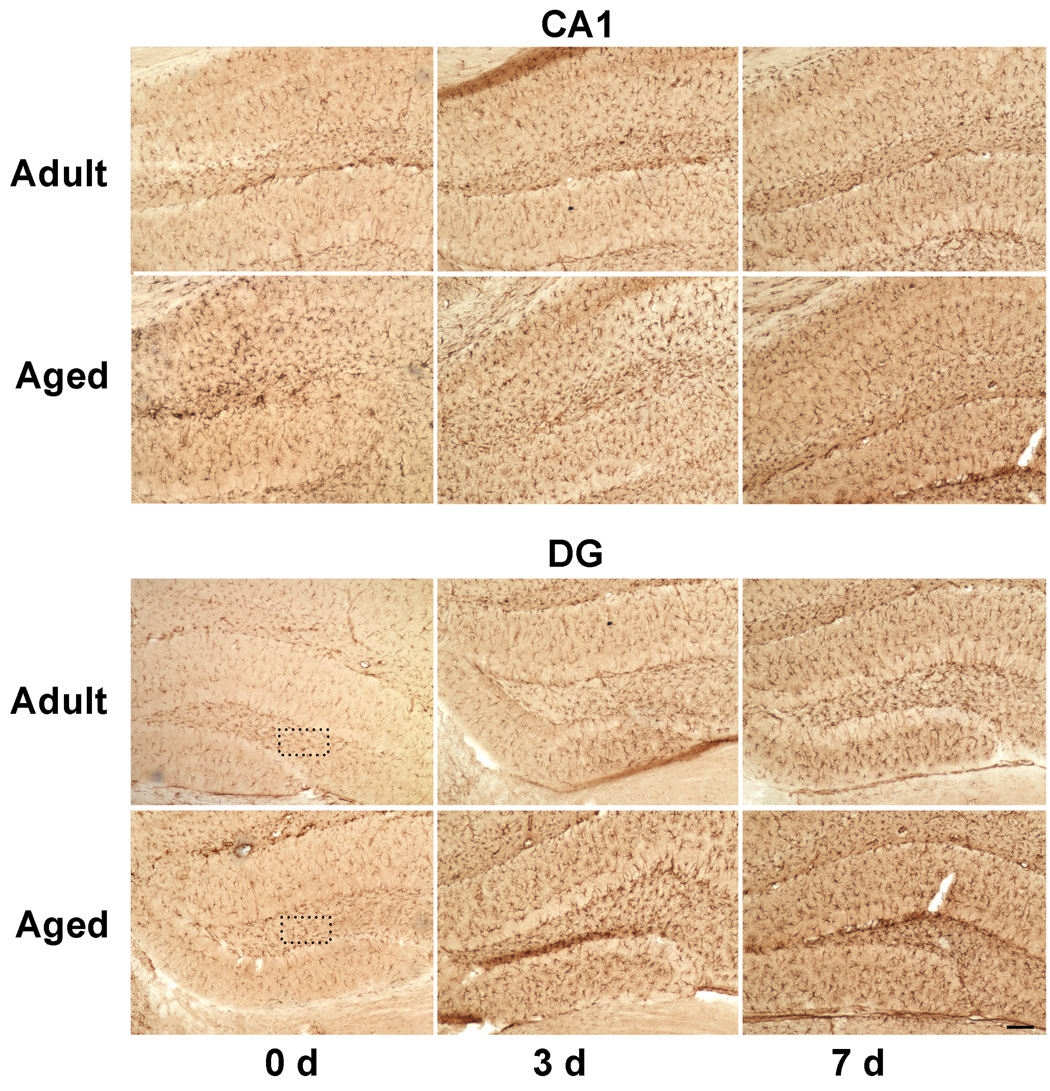
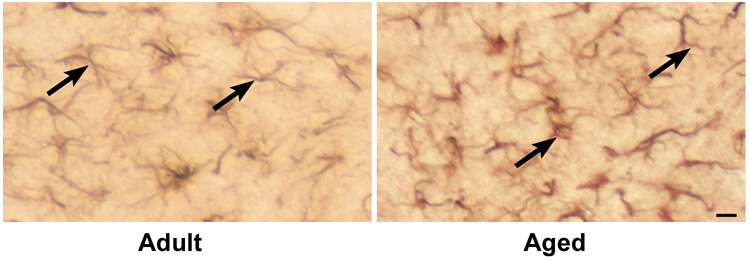
Because RT-PCR and western blotting showed quantitative increases in expression of Iba1, immunohistochemical analysis was carried out in brain sections with anti-Iba1 antibodies to confirm localization of Iba1 protein within microglia (Fig. 4a). A higher Iba1 staining was observed in DG of aged mice, whereas, in CA1 region there was reduced expression of Iba1 as compared to adult hippocampus. Immunoreactive cells demonstrating hypertrophied cytoplasm with thickening of proximal processes and decreased ramification of distal branches were observed in the dentate gyrus of aged mice (Fig. 4b). These morphological features are typical of activated microglia (Kreutzberg, 1996). In response to injury there was an increase in Iba1 staining throughout the hippocampus in both adult and aged hippocampus at 3 and 7 days after CCI injury. Maximal Iba1 staining was seen at 3 day post injury in both adult and aged hippocampus, in accordance with mRNA and protein expression data. These results demonstrate that the increase in activation markers occurs in microglia that exhibit the morphological features of activation.
Fig. 4.
a. Immunohistochemical labeling of microglia with anti-Iba1 antibodies in CA1 region and dentate gyrus from ipsilateral hippocampus of adult and aged mice following CCI injury. Coronal sections from hippocampus were stained with anti-Iba1 antibodies at day 3 and 7 post injury. Images taken at 100x magnification. Scale bar 0.1mm. Boxed area shows representative microglia from polymorphic layer of dentate gyrus from adult and aged hippocampus showing phenotypic changes in Fig 4b (scale bar 0.01).
Astrocyte activation in hippocampus following CCI Injury
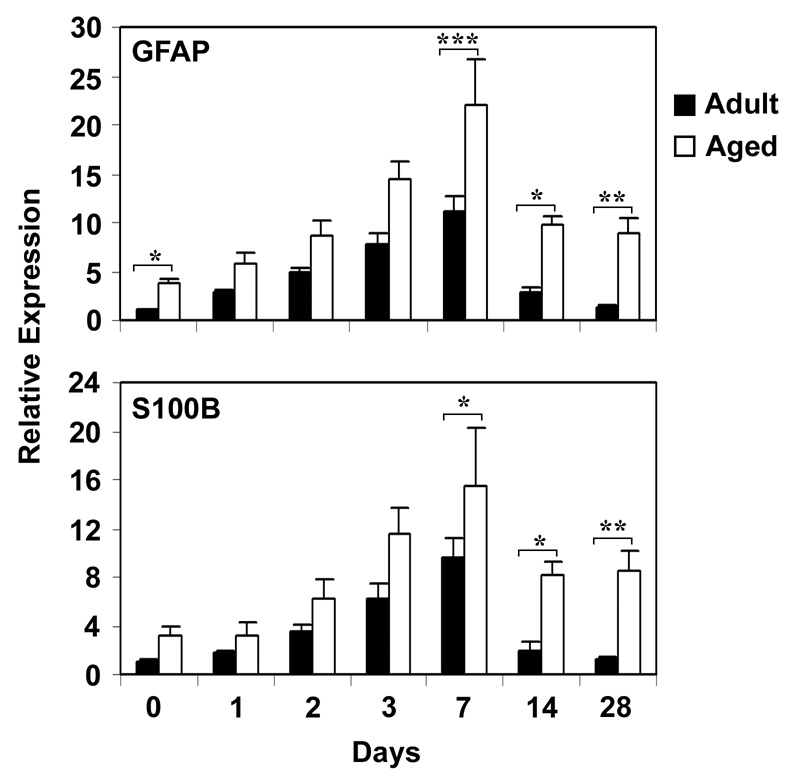
The time course of mRNA expression of astrocyte activation markers, GFAP and S100B, was studied in hippocampus using real time RT-PCR following CCI injury (Fig. 5). Higher basal levels of both GFAP and S100B were observed in the aged brain in comparison to the adult brain. Both mRNAs were upregulated beginning at day 1 following injury and peaking 7 days post-injury in both the adult and aged mice. Levels of GFAP and S100B mRNAs were 2-fold and 1.6-fold higher 7 days post injury in the aged animals compared to that in the adult mice (p<0.001 for GFAP and p<0.05 for S100B). Expression of astrocyte markers returned rapidly to the baseline in the adult mice; but, in the aged mice injury-induced increases in mRNAs for GFAP and S100B was prolonged.
Fig. 5.
Relative mRNA Expression of astrocyte activation markers in Hippocampus of adult and aged mice following CCI injury analyzed by real-time RT-PCR. GFAP (A) and S100B (B) mRNA expression was studied after 1, 2, 3, 7, 14 and 28 days after injury. The real time reactions were performed in duplicates for both the target gene and GAPDH used as a housekeeping control. The relative expression was calculated using delta delta CT method. Data are presented as mean ± SEM (n=5/group). Asterisk denotes statistical significance after ANOVA and post-hoc testing where *p < 0.05, **p < 0.01, ***p < 0.001 vs. adult
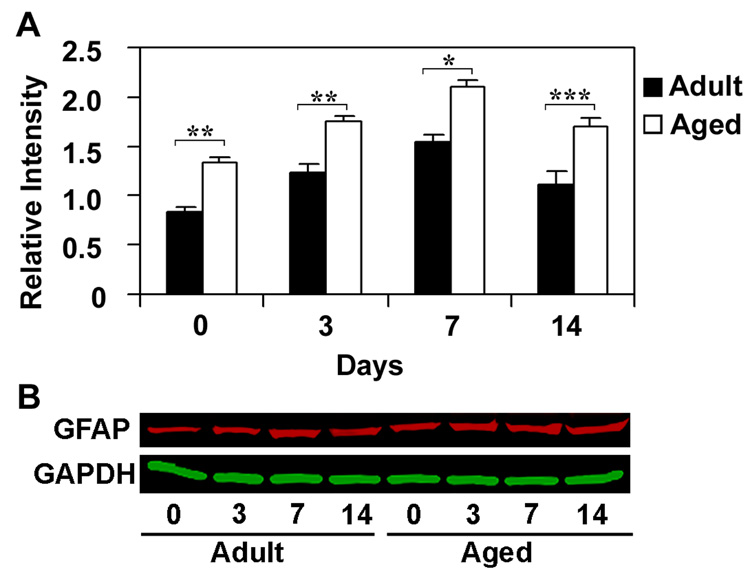
GFAP protein expression was analyzed by immunoblot to determine if increased transcription of astrocyte mRNAs resulted in higher protein levels. We chose GFAP for this analysis because it is better characterized than S100B. The immunoblot analysis for GFAP was carried out in hippocampus after 3, 7 and 14 days following CCI injury in adult and aged mice (Fig. 6). Relative expression of GFAP was significantly higher in the uninjured aged animals as compared to uninjured adult. Following CCI injury GFAP expression was significantly higher at all time points in the aged animals than in adult animals. The response to injury was also prolonged in the aged brain. In the aged mice GFAP expression was significantly higher 14 days post injury as compared to uninjured aged, whereas in the adult mice after 14 days of injury GFAP expression was comparable to that in uninjured adult.
Fig. 6.
Relative expression of GFAP protein in hippocampus of adult and aged mice following CCI injury analyzed using immuoblotting. Protein extracts from hippocampus were analyzed by immunoblotting using anti-GFAP antibodies after 3, 7 and 14 days after CCI injury. Numbers represent relative levels of protein based on optical density normalized to GAPDH signal. The bottom panel shows the representative western blot for GFAP along with GAPDH used as loading control. Data are presented as mean ± SEM (n=3/group). Asterisk denotes statistical significance after ANOVA and post-hoc testing where *p < 0.05, **p < 0.01, ***p < 0.001 vs. adult
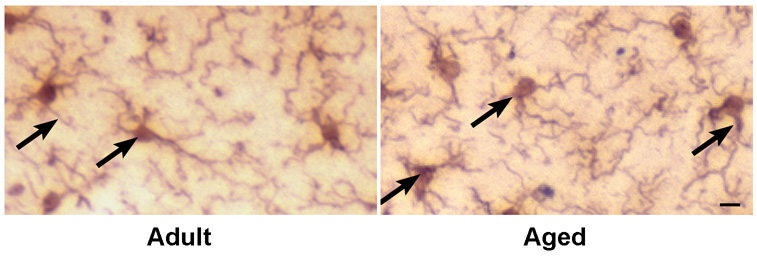
Immunohistochemical staining of CA1 and dentate gyrus regions of hippocampus with anti- GFAP antibodies in adult and aged mice after 3 and 7 days following CCI injury is shown in Fig. 7a. Astrocytes in the uninjured aged hippocampus had hypertrophic somata and thicker processes typical of reactive astrocytes that were not observed in uninjured adult hippocampus (Fig. 7b). In response to injury, there was a progressive increase in the GFAP staining reaching a maximum after 7 days of injury in both adult and aged mice. The results demonstrate that the increase in GFAP protein occurs in activated astrocytes following injury.
Fig. 7.
a. Immunohistochemical labeling of astrocytes with anti-GFAP antibodies in CA1 region and dentate gyrus from ipsilateral hippocampus of adult and aged mice following CCI injury. Coronal sections from hippocampus were stained with anti-Iba1 antibodies at day 3 and 7 post injury. Images taken at 100x magnification. Scale bar 0.1mm. Boxed area shows representative astrocytes from polymorphic layer of dentate gyrus from adult and aged hippocampus showing phenotypic changes in Fig. 7b (scale bar 0.01)
Discussion
Neuroinflammation following acute brain trauma plays a prominent role in both pathological and reconstructive response of the brain to injury (Lucas, et al., 2006). Activated microglia and astrocytes play a crucial role in the neuroinflammatory process. Activated glial cells contribute to the pathogenesis and progression of neurological disorders ranging from traumatic brain injury to Alzheimer's disease (Maeda, et al., 2007). While the glial response can provide trophic and metabolic support to the damaged neurons, deactivating toxic species and eliminating cell debris (Hauwel, et al., 2005), there is growing evidence that the response may also be detrimental to neurons, as activated microglia and astrocytes can produce a variety of potentially neurotoxic molecules that are implicated in neurodegeneration (Mrak and Griffin, 2005).
Microglial Response following Injury
Microglia are the primary intrinsic immune effector cells of the central nervous system and are involved in virtually all pathological processes of the brain involving inflammation (Berman, et al., 1999). Microglia exacerbate the neural damage that occurs in neurodegenerative diseases. However, activated microglia do not constitute a single, uniform cell population, but compromise a family of cells with diverse phenotypes-some that are beneficial and others that are destructive depending on whether they secrete neurotoxic or neurotrophic factors (Schwartz, et al., 2006).
Up-regulation of CD11b and Iba1 expression within the CNS is regarded as evidence of microglial activation (Scholz, et al., 2008). We observed higher expression of CD11b and Iba1 mRNA in hippocampus of uninjured aging mice as compared to adult mice, confirming previous studies demonstrating that the aged brain is in a pro-inflammatory state. Anti-Iba1 staining revealed higher immunoreactivity for activated microglia in the dentate gyrus of the aged brain, whereas, in the CA1 region there were fewer activated microglia. These results suggest that there might be regional differences in microglial activation in the aged brain. The increase in number of activated microglia with age has been reported in many human and animal studies (Conde and Streit, 2006, Felzien, et al., 2001, Sheffield and Berman, 1998), and chronic activation of microglia in the aging brain has been linked with increased neuronal loss (Stolzing, et al., 2005). In addition, microglia from the aging brain show an altered phenotype that is considered dysfunctional, associated with the loss of neuroprotective properties that likely contributes to age-related neurodegeneration (Streit, et al., 2004).
Our results show up-regulation of microglial activation markers, CD11b and Iba1, in hippocampus at the transcriptional level and Iba1 at the translational level following CCI injury. Maximum activation was observed after 3 days of injury in both adult and aged mice. It is clear from the data that the microglial response to injury was exaggerated and prolonged in the old mice compared to that in the adult mice. This observation is consistent with reports of elevated microglial responses in the aged nervous system following diverse types of injury e.g. cerebral ischemia (Popa-Wagner, et al., 2007), retinal ischemia (Kim, et al., 2004), MPTP neurotoxicity (Sugama, et al., 2003), peripheral nerve injury (Conde and Streit, 2006). It has been proposed that after injury microglia in the aged brain are primed to produce faster and more pronounced inflammatory responses (Godbout, et al., 2005) and to proliferate more vigorously than in the younger brain (Conde and Streit, 2006). These activated dysfunctional microglia might contribute to worse outcome in the elderly following TBI. In accord with that hypothesis, it has been shown that LPS activated microglia in aged mice promote DA neuron death whereas in neonatal mice microglia protect DA neurons against the PD-producing neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, MPTP (Sawada, et al., 2008).
The prolonged microglial response observed in the aging hippocampus after injury may be an important contributor to the progressive damage observed following TBI. Several studies have provided evidence for chronic nature of inflammatory response in TBI and SCI patients for up to 1 year after trauma (Bramlett and Dietrich, 2007). Our results are certainly consistent with this clinical evidence.
Astrocyte Response following Injury
Astrocytes, the predominant cell type of the nervous system, have important neuroregulatory functions that include regulation of neuron communication, neurosecretion, metabolism and synaptic plasticity (Mahesh, et al., 2006). In addition, astrocytes respond to pathological insults such as TBI with a graded cellular activation in relation to severity of injury referred to as reactive gliosis. They make essential contributions to many homeostatic functions that could directly influence neuronal survival, tissue integrity and functional outcome after TBI (Floyd and Lyeth, 2007). The wide range of astrocyte functions contributes to uncertainty over whether these cells exert beneficial or detrimental effects after CNS injuries. For example, potential protective effects include maintenance of extracellular ion and fluid balance, glutamate uptake and neurotrophin release, while potential detrimental effects include the release of inflammatory cytokines and cytotoxic radicals (Myer, et al., 2006).
In this study, higher basal expression of GFAP and S100B in hippocampus was observed in the aged mice as compared to adult. A progressive age-related increase in basal expression of GFAP mRNA and protein levels has been observed in rodents and humans (Yoshida, et al., 1996). The increase in GFAP expression has been shown to roughly parallel an increase in the number of reactive astrocytes with age (Goss, et al., 1991). Age related increases in GFAP mRNA or protein in extracts of the whole hippocampus have been attributed to more GFAP molecules per cell (Major, et al., 1997), likely due to morphological changes associated with activation. In accord with that observation, the aged hippocampus shows an altered astrocyte phenotype with hypertrophic soma and thickening of the processes. Astrocyte hyperplasia and hypertrophy has been observed in several areas of the aging brain accompanied by an elevated content of GFAP and S100B by many investigators (Francesco Amenta, 1998), which is associated with a reduction in neuroprotective capacity (Pertusa, et al., 2007).
We observed upregulation of GFAP and S100B mRNA expression in response to injury in both adult and aged hippocampus, but the aged animals had significantly higher expression of GFAP and S100B mRNA in response to injury. Maximum mRNA and protein expression was observed at day 7 post-injury in both aged and adult hippocampus. A large number of studies indicates that GFAP, part of the astroglial skeleton, and S100B, an astroglial protein, are upregulated after TBI (Pelinka, et al., 2004). Increased astrocyte responses have been observed in the aged brain after hippocampal stab (Zhu, et al., 2003) and cortical stab injuries (Kyrkanides, et al., 2001). Higher trauma induced proliferation of astrocytes in aged rats has also been observed (Topp, et al., 1989). Interestingly, astrocytes respond to TBI by pronounced changes in gene expression, cellular hypertrophy and cell proliferation, all of which occur in a gradated fashion in relation to the severity of the injury. Thus the present results suggest that in the aged brain, astrocytes react as if the injury were more severe than it actually is. The hypertrophy of processes of reactive astrocytes appears to be of fundamental importance in inhibiting CNS regeneration. In a recent study increased regeneration after CNS trauma in the GFAP−/− Vim−/− mice was observed (Wilhelmsson, et al., 2004). In this study increased regeneration was attributed to reduced hypertrophy of cellular processes of individual reactive astrocytes. Clinical data also support the contention that increased astrocytic proteins in the aged brain may be detrimental, as a correlation has been observed between increase in serum GFAP and S100B levels and poor outcome in humans after TBI (Pelinka, et al., 2004).
It is clear from the results that microglial activation precedes astrocyte activation following CCI injury, suggesting that the inflammatory response in brain is spearheaded by microglia. These results confirm other studies demonstrating that microglia respond faster than astrocytes in response to CNS injury (Liu, et al., 2003). For example, LPS-induced production of NO occurred within the first 6–12 hr in microglia, compared with a much delayed time point (24 hr) in astrocytes (Liu, et al., 2002). It appears that astrocytes rely on secondary responses to produce certain proinflammatory factors. These observations strongly imply that microglia, as the first line of response to immunologic challenges in the brain, play the primary role in the brain inflammatory process following TBI.
This is the first study involving quantitation of the time course of the microglial and astrocyte response in aging following TBI. Most of the studies reporting enhanced glial response in aging have utilized only immunohistochemical staining to demonstrate glial activation. Furthermore, higher expression of glial markers in aged brain might involve more glial activation marker molecules per cell, which might be overlooked by conventional histological techniques.
Enhanced glial activation and poor outcome in aging
The results demonstrate increased transcription of genes involved in microglia and astrocyte activation in hippocampus of aged animals following injury. Gene expression profiling in aging brain of rodents has also shown increased transcription of markers indicative of primed microglia and astocytic proteins (Lee, et al., 2000). The increased transcription of genes involved in activation of microglia and astrocytes in aging might be responsible for altered phenotype of microglia and astrocytes with loss of their neuroprotective properties consquently limiting their ability to rescue damaged and dying neurons following TBI. In addition, accentuated activation of microglia and astrocyte might also be responsible for enhanced production of proinflammatory cytokines and chemokines in the aged brain following CCI. We have observed higher expression of proinflammatory cytokines and chemokines during secondary neuron death in thalamus of aged mice following cortical ablation injury (Sandhir, et al., 2004). Higher glial activation may explain the enhanced neurodegeneration observed in various brain regions including hippocampus of aged mice following CCI (Onyszchuk, et al., 2008). Studies with a stroke model found larger infarct area characterized by higher rate of cellular degeneration, and a large number of apoptotic cells in aged brains accompanied by acceleration in formation of a glial scar (Petcu, et al., 2007). In addition, the response of plasticity associated proteins such as MAP1B was delayed, whereas, the amount of neurotoxic c-terminal fragment of β-amyloid precursor protein was increased in the aged animals. Furthermore, higher microglial response in the aged brain in response to injury might have an adverse affect on the ability of the aged brain to repair.
The mechanism involved in enhanced microglial and astrocyte activation in the aged brain following injury is not understood. However, evidence from many recent studies suggests poorly controlled inflammatory process in aging and age-related diseases. For example, reduced activity of peroxisome proliferators-activated receptors (PPAR-α and γ) has been linked to increased inflammatory mediators during aging process (Chung, et al., 2008), and increased activation of the transcription factor NF-kappaβ has been linked to accentuated inflammatory response in aging (Wu, et al., 2007).
In conclusion, the findings demonstrate that aging is associated with increased glial responses in the hippocampus following CCI injury that may ultimately lead to augmented neurodegeneration and/ or reduced neuronal plasticity required for optimal recovery.
Acknowledgements
The authors acknowledge the assistance of Eugene Gregory in carrying out the work and Eileen Roach with processing of the images. Special thanks to Dr. Y.Y. He for his help with controlled cortical impact injury. The study was supported in part by the Steve Palermo Endowment and grant from the National Institute of Aging (AG026482).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ashcroft GS, Mills SJ, Ashworth JJ. Ageing and wound healing. Biogerontology. 2002;3:337–345. doi: 10.1023/a:1021399228395. [DOI] [PubMed] [Google Scholar]
- 2.Berman NE, Marcario JK, Yong C, Raghavan R, Raymond LA, Joag SV, Narayan O, Cheney PD. Microglial activation and neurological symptoms in the SIV model of NeuroAIDS: association of MHC-II and MMP-9 expression with behavioral deficits and evoked potential changes. Neurobiol Dis. 1999;6:486–498. doi: 10.1006/nbdi.1999.0261. [DOI] [PubMed] [Google Scholar]
- 3.Bigler ED, Anderson CV, Blatter DD. Temporal lobe morphology in normal aging and traumatic brain injury. AJNR Am J Neuroradiol. 2002;23:255–266. [PMC free article] [PubMed] [Google Scholar]
- 4.Bramlett HM, Dietrich WD. Progressive damage after brain and spinal cord injury: pathomechanisms and treatment strategies. Prog Brain Res. 2007;161:125–141. doi: 10.1016/S0079-6123(06)61009-1. [DOI] [PubMed] [Google Scholar]
- 5.Brown AW, Leibson CL, Malec JF, Perkins PK, Diehl NN, Larson DR. Long-term survival after traumatic brain injury: a population-based analysis. NeuroRehabilitation. 2004;19:37–43. [PubMed] [Google Scholar]
- 6.Chung JH, Seo AY, Chung SW, Kim MK, Leeuwenburgh C, Yu BP, Chung HY. Molecular mechanism of PPAR in the regulation of age-related inflammation. Ageing Res Rev. 2008;7:126–136. doi: 10.1016/j.arr.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Conde JR, Streit WJ. Effect of aging on the microglial response to peripheral nerve injury. Neurobiol Aging. 2006;27:1451–1461. doi: 10.1016/j.neurobiolaging.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Conde JR, Streit WJ. Microglia in the aging brain. J Neuropathol Exp Neurol. 2006;65:199–203. doi: 10.1097/01.jnen.0000202887.22082.63. [DOI] [PubMed] [Google Scholar]
- 9.Coronado VG, Thomas KE, Sattin RW, Johnson RL. The CDC traumatic brain injury surveillance system: characteristics of persons aged 65 years and older hospitalized with a TBI. J Head Trauma Rehabil. 2005;20:215–228. doi: 10.1097/00001199-200505000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Deng XH, Bertini G, Xu YZ, Yan Z, Bentivoglio M. Cytokine-induced activation of glial cells in the mouse brain is enhanced at an advanced age. Neuroscience. 2006;141:645–661. doi: 10.1016/j.neuroscience.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 11.Diamond MS, Staunton DE, Marlin SD, Springer TA. Binding of the integrin Mac-1 (CD11b/CD18) to the third immunoglobulin-like domain of ICAM-1 (CD54) and its regulation by glycosylation. Cell. 1991;65:961–971. doi: 10.1016/0092-8674(91)90548-d. [DOI] [PubMed] [Google Scholar]
- 12.Felzien LK, McDonald JT, Gleason SM, Berman NE, Klein RM. Increased chemokine gene expression during aging in the murine brain. Brain Res. 2001;890:137–146. doi: 10.1016/s0006-8993(00)03090-0. [DOI] [PubMed] [Google Scholar]
- 13.Ferrell R, Tanev K. Traumatic brain injury in older adults. Current Psychiatry Reports. 2002;4:354–362. doi: 10.1007/s11920-002-0083-9. [DOI] [PubMed] [Google Scholar]
- 14.Floyd CL, Lyeth BG. Astroglia: important mediators of traumatic brain injury. Prog Brain Res. 2007;161:61–79. doi: 10.1016/S0079-6123(06)61005-4. [DOI] [PubMed] [Google Scholar]
- 15.Francesco Amenta EBMSJAV. Astrocyte changes in aging cerebral cortex and hippocampus: A quantitative immunohistochemical study. Microsc. Res. Tech. 1998:29–33. doi: 10.1002/(SICI)1097-0029(19981001)43:1<29::AID-JEMT5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 16.Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein FC, Levin HS. Cognitive outcome after mild and moderate traumatic brain injury in older adults. J Clin Exp Neuropsychol. 2001;23:739–753. doi: 10.1076/jcen.23.6.739.1028. [DOI] [PubMed] [Google Scholar]
- 18.Goss JR, Finch CE, Morgan DG. Age-related changes in glial fibrillary acidic protein mRNA in the mouse brain. Neurobiol Aging. 1991;12:165–170. doi: 10.1016/0197-4580(91)90056-p. [DOI] [PubMed] [Google Scholar]
- 19.Hauwel M, Furon E, Canova C, Griffiths M, Neal J, Gasque P. Innate (inherent) control of brain infection, brain inflammation and brain repair: the role of microglia, astrocytes, "protective" glial stem cells and stromal ependymal cells. Brain Res Brain Res Rev. 2005;48:220–233. doi: 10.1016/j.brainresrev.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Hukkelhoven CW, Steyerberg EW, Rampen AJ, Farace E, Habbema JD, Marshall LF, Murray GD, Maas AI. Patient age and outcome following severe traumatic brain injury: an analysis of 5600 patients. J Neurosurg. 2003;99:666–673. doi: 10.3171/jns.2003.99.4.0666. [DOI] [PubMed] [Google Scholar]
- 21.Johnstone B, Childers MK, Hoerner J. The effects of normal ageing on neuropsychological functioning following traumatic brain injury. Brain Inj. 1998;12:569–576. doi: 10.1080/026990598122331. [DOI] [PubMed] [Google Scholar]
- 22.Kim KY, Ju WK, Neufeld AH. Neuronal susceptibility to damage: comparison of the retinas of young, old and old/caloric restricted rats before and after transient ischemia. Neurobiol Aging. 2004;25:491–500. doi: 10.1016/j.neurobiolaging.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Klein M, Houx PJ, Jolles J. Long-term persisting cognitive sequelae of traumatic brain injury and the effect of age. J Nerv Ment Dis. 1996;184:459–467. doi: 10.1097/00005053-199608000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 25.Kyrkanides S, O'Banion MK, Whiteley PE, Daeschner JC, Olschowka JA. Enhanced glial activation and expression of specific CNS inflammation-related molecules in aged versus young rats following cortical stab injury. J Neuroimmunol. 2001;119:269–277. doi: 10.1016/s0165-5728(01)00404-0. [DOI] [PubMed] [Google Scholar]
- 26.Lee CK, Weindruch R, Prolla TA. Gene-expression profile of the ageing brain in mice. Nat Genet. 2000;25:294–297. doi: 10.1038/77046. [DOI] [PubMed] [Google Scholar]
- 27.Letarte P. In: The Brain. Feliciano D, Nattox KL, Moore EE, editors. New York: Trauma. McGraw Hill; 2006. pp. 397–418. [Google Scholar]
- 28.Liu B, Gao HM, Hong JS. Parkinson's disease and exposure to infectious agents and pesticides and the occurrence of brain injuries: role of neuroinflammation. Environ Health Perspect. 2003;111:1065–1073. doi: 10.1289/ehp.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu B, Gao HM, Wang JY, Jeohn GH, Cooper CL, Hong JS. Role of nitric oxide in inflammation-mediated neurodegeneration. Ann N Y Acad Sci. 2002;962:318–331. doi: 10.1111/j.1749-6632.2002.tb04077.x. [DOI] [PubMed] [Google Scholar]
- 30.Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS njury and disease. Br J Pharmacol. 2006;147 Suppl 1:S232–S240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maeda J, Higuchi M, Inaji M, Ji B, Haneda E, Okauchi T, Zhang M-R, Suzuki K, Suhara T. Phase-dependent roles of reactive microglia and astrocytes in nervous system injury as delineated by imaging of peripheral benzodiazepine receptor. Brain Research. 2007;1157:100–111. doi: 10.1016/j.brainres.2007.04.054. [DOI] [PubMed] [Google Scholar]
- 32.Mahesh VB, Dhandapani KM, Brann DW. Role of astrocytes in reproduction and neuroprotection. Mol Cell Endocrinol. 2006;246:1–9. doi: 10.1016/j.mce.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 33.Major DE, Kesslak JP, Cotman CW, Finch CE, Day JR. Life-long dietary restriction attenuates age-related increases in hippocampal glial fibrillary acidic protein mRNA. Neurobiol Aging. 1997;18:523–526. doi: 10.1016/s0197-4580(97)00102-4. [DOI] [PubMed] [Google Scholar]
- 34.Marchetti B, Abbracchio MP. To be or not to be (inflamed)--is that the question in anti-inflammatory drug therapy of neurodegenerative disorders? Trends Pharmacol Sci. 2005;26:517–525. doi: 10.1016/j.tips.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Mohindra S, Mukherjee KK, Gupta R, Chhabra R. Continuation of poor surgical outcome after elderly brain injury. Surgical Neurology In Press. doi: 10.1016/j.surneu.2007.02.031. Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 36.Mrak RE, Griffin WS. Glia and their cytokines in progression of neurodegeneration. Neurobiol Aging. 2005;26:349–354. doi: 10.1016/j.neurobiolaging.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 37.Myer DJ, Gurkoff GG, Lee SM, Hovda DA, Sofroniew MV. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain. 2006;129:2761–2772. doi: 10.1093/brain/awl165. [DOI] [PubMed] [Google Scholar]
- 38.Ohsawa K, Imai Y, Kanazawa H, Sasaki Y, Kohsaka S. Involvement of Iba1 in membrane ruffling and phagocytosis of macrophages/microglia. J Cell Sci. 2000;113(Pt 17):3073–3084. doi: 10.1242/jcs.113.17.3073. [DOI] [PubMed] [Google Scholar]
- 39.Onyszchuk G, Al-Hafez B, He YY, Bilgen M, Berman NE, Brooks WM. A mouse model of sensorimotor controlled cortical impact: characterization using longitudinal magnetic resonance imaging, behavioral assessments and histology. J Neurosci Methods. 2007;160:187–196. doi: 10.1016/j.jneumeth.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Onyszchuk G, He YY, Berman NE, Brooks WM. Detrimental Effects of Aging on Outcome from Traumatic Brain Injury: A Behavioral, Magnetic Resonance Imaging, and Histological Study in Mice. J Neurotrauma. 2008;25:153–171. doi: 10.1089/neu.2007.0430. [DOI] [PubMed] [Google Scholar]
- 41.Pelinka LE, Kroepfl A, Leixnering M, Buchinger W, Raabe A, Redl H. GFAP versus S100B in serum after traumatic brain injury: relationship to brain damage and outcome. J Neurotrauma. 2004;21:1553–1561. doi: 10.1089/neu.2004.21.1553. [DOI] [PubMed] [Google Scholar]
- 42.Pertusa M, Garcia-Matas S, Rodriguez-Farre E, Sanfeliu C, Cristofol R. Astrocytes aged in vitro show a decreased neuroprotective capacity. J. Neurochem. 2007:794–805. doi: 10.1111/j.1471-4159.2006.04369.x. [DOI] [PubMed] [Google Scholar]
- 43.Petcu EB, Sfredel V, Platt D, Herndon JG, Kessler C, Popa-Wagner A. Cellular and Molecular Events Underlying the Dysregulated Response of the Aged Brain to Stroke. Gerontology. 2007 doi: 10.1159/000112845. [DOI] [PubMed] [Google Scholar]
- 44.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Popa-Wagner A, Carmichael ST, Kokaia Z, Kessler C, Walker LC. The response aged brain to stroke: too much, too soon? Curr Neurovasc Res. 2007;4:216–227. doi: 10.2174/156720207781387213. [DOI] [PubMed] [Google Scholar]
- 46.Rapoport MJ, Herrmann N, Shammi P, Kiss A, Phillips A, Feinstein A. Outcome after traumatic brain injury sustained in older adulthood: a one-year longitudinal study. Am J Geriatr Psychiatry. 2006;14:456–465. doi: 10.1097/01.JGP.0000199339.79689.8a. [DOI] [PubMed] [Google Scholar]
- 47.Rink A, Fung KM, Trojanowski JQ, Lee VM, Neugebauer E, McIntosh TK. Evidence of apoptotic cell death after experimental traumatic brain injury in the rat. Am J Pathol. 1995;147:1575–1583. [PMC free article] [PubMed] [Google Scholar]
- 48.Sandhir R, Puri V, Klein RM, Berman NE. Differential expression of cytokines and chemokines during secondary neuron death following brain injury in old and young mice. Neurosci Lett. 2004;369:28–32. doi: 10.1016/j.neulet.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 49.Sawada M, Sawada H, Nagatsu T. Effects of aging on neuroprotective and neurotoxic properties of microglia in neurodegenerative diseases. Neurodegener Dis. 2008;5:254–256. doi: 10.1159/000113717. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt O, Infanger M, Heyde C, Ertel W, Stahel P. The Role of Neuroinflammation in Traumatic Brain Injury. European Journal of Trauma. 2004;30:135–149. [Google Scholar]
- 51.Scholz J, Abele A, Marian C, Haussler A, Herbert TA, Woolf CJ, Tegeder I. Low-dose methotrexate reduces peripheral nerve injury-evoked spinal microglial activation and neuropathic pain behavior in rats. Pain. 2008 doi: 10.1016/j.pain.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwartz M, Butovsky O, Bruck W, Hanisch UK. Microglial phenotype: is the commitment reversible? Trends Neurosci. 2006;29:68–74. doi: 10.1016/j.tins.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 53.Sheffield LG, Berman NE. Microglial expression of MHC class II increases in normal aging of nonhuman primates. Neurobiol Aging. 1998;19:47–55. doi: 10.1016/s0197-4580(97)00168-1. [DOI] [PubMed] [Google Scholar]
- 54.Stolzing A, Sethe S, Grune T. Chronically active: activation of microglial proteolysis in ageing and neurodegeneration. Redox Rep. 2005;10:207–213. doi: 10.1179/135100005X70198. [DOI] [PubMed] [Google Scholar]
- 55.Streit WJ, Sammons NW, Kuhns AJ, Sparks DL. Dystrophic microglia in the aging human brain. Glia. 2004;45:208–212. doi: 10.1002/glia.10319. [DOI] [PubMed] [Google Scholar]
- 56.Sugama S, Yang L, Cho BP, DeGiorgio LA, Lorenzl S, Albers DS, Beal MF, Volpe BT, Joh TH. Age-related microglial activation in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurodegeneration in C57BL/6 mice. Brain Res. 2003;964:288–294. doi: 10.1016/s0006-8993(02)04085-4. [DOI] [PubMed] [Google Scholar]
- 57.Topp KS, Faddis BT, Vijayan VK. Trauma-induced proliferation of strocytes in the brains of young and aged rats. Glia. 1989;2:201–211. doi: 10.1002/glia.440020309. [DOI] [PubMed] [Google Scholar]
- 58.White-Gbadebo D, Hamm RJ. Chronic corticosterone treatment potentiates deficits following traumatic brain injury in rats: implications for aging. J Neurotrauma. 1993;10:297–306. doi: 10.1089/neu.1993.10.297. [DOI] [PubMed] [Google Scholar]
- 59.Wilhelmsson U, Li L, Pekna M, Berthold CH, Blom S, Eliasson C, Renner O, Bushong E, Ellisman M, Morgan TE, Pekny M. Absence of glial fibrillary acidic protein and vimentin prevents hypertrophy of astrocytic processes and improves post-traumatic regeneration. J Neurosci. 2004;24:5016–5021. doi: 10.1523/JNEUROSCI.0820-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu D, Ren Z, Pae M, Guo W, Cui X, Merrill AH, Meydani SN. Aging up-regulates expression of inflammatory mediators in mouse adipose tissue. J Immunol. 2007;179:4829–4839. doi: 10.4049/jimmunol.179.7.4829. [DOI] [PubMed] [Google Scholar]
- 61.Yoshida T, Goldsmith SK, Morgan TE, Stone DJ, Finch CE. Transcription supports age-related increases of GFAP gene expression in the male rat brain. Neurosci Lett. 1996;215:107–110. [PubMed] [Google Scholar]
- 62.Zhu W, Umegaki H, Shinkai T, Kurotani S, Suzuki Y, Endo H, Iguchi A. Different glial reactions to hippocampal stab wounds in young adult and aged rats. J Gerontol Biol Sci Med Sci. 2003;58:117–122. doi: 10.1093/gerona/58.2.b117. [DOI] [PubMed] [Google Scholar]