Abstract
Bromelain (Br), an extract from pineapple stem with cysteine protease activity, exerts anti-inflammatory effects in a number of inflammatory models. We have previously shown that Br treatment decreased activated CD4+ T cells and has a therapeutic role in an ovalbumin-induced murine model of allergic airway disease. The current study was designed to determine the effect of Br on CD4+ T cell activation, specifically the expression of CD25 in vitro. CD25 is up regulated upon T cell activation, found as a soluble fraction (sCD25) and is a therapeutic target in inflammation, autoimmunity and allergy. Br treatment of anti-CD3 stimulated CD4+ T cells reduced CD25 expression in a dose and time dependent manner. This reduction of CD25 was dependent on the proteolytic action of Br as the addition of E64 (a cysteine protease inhibitor) abrogated this response. The concentration of sCD25 was increased in supernatants of Br treated activated CD4+ T cells as compared to control cells, suggesting that Br proteolytically cleaved cell-surface CD25. This novel mechanism of action identifies how Br may exert its therapeutic benefits in inflammatory conditions.
Keywords: CD4+ T cells, CD25, Cysteine protease, Natural product, Bromelain
1. Introduction
The hallmark of many immune mediated disorders is the dysregulated activation of T cells [1,2]. Primary events in the activation of T cells are the presentation of an antigenic stimulus via an antigen presenting cell (APC) to the T cell [3] and augmentation by co-stimulatory signals (CD80, CD86) on the APC [4]. Following this unique interaction the T cell up-regulates activation markers such as CD25, the high affinity alpha chain of the IL-2 receptor [5]. Once IL-2 binds to CD25 it triggers a signaling cascade, resulting in T cell proliferation and additional IL-2 production. CD25 is constitutively expressed on CD4+ CD25+ Foxp3+ T regulatory cells, up-regulated on CD4+ T cells in response to antigen and a fraction of CD25 can be released in soluble form(sCD25) [6]. Increased levels of sCD25 have been shown to correlate with disease severity in individuals with allergic asthma [7]. Serum levels of sCD25 rise in accordance with the IL-2 receptor and are currently being investigated as biomarkers in allergy and asthma. Therapeutic interventions that target T cell mediated diseases are often designed to either boost (cancer, infectious diseases) or lower (autoimmune, allergy) the threshold of T cell activation via alteration of cell surface receptors.
A number of natural products such as essential fatty acids [8], Ginseng extracts [9], Curcumin [10] and Bromelain (EC 3.4.22.32) an extract from the common pineapple [11], have been identified as therapeutic targets for inflammation due to their ability to modulate T cell activation and expansion. Bromelain (Br) is a combination of proteins which contain cysteine protease activity [12]. Br has been shown to enhance the expression of CD11c and CD86 [13,14] or suppress other surface markers such as CD44, B7-1, and CD80 [15,16] on distinct cell populations in a variety of immunological systems. Br can modulate T cell production of inflammatory cytokines IL-4, IFN-γ [15,16] and IL-13 [17] and down-regulate inflammatory responses in animal models of inflammatory bowel disease [18], experimental allergic encephalomyelitis [16] and allergic airway disease [19].
It has been previously demonstrated that Br inhibits downstream TCR signaling in CD4+ T helper cells [20] but the effect of Br on the expression of CD25 has been largely unexplored. The current study was designed to better understand the effect of Br on activated CD4+ T cells. In this manuscript we report that Br inhibits CD25 expression on activated CD4+ T cells, possibly via proteolytic cleavage.
2. Methods
2.1. Mice
Studies were performed on female C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME), 8–10 weeks of age and weighing 17 to 20 g. Mice were housed conventionally in the animal facility at the University of Connecticut Health Center in accordance with institutional and Office of Laboratory Animal Welfare guidelines. All experimental procedures were approved by the institution's Center for Laboratory Animal Care.
2.2. Reagents
To directly reflect the natural products utilized in the marketplace, we chose a commercially available, quality control tested product distributed to physicians, for use in our research. Br (EC 3.4.22.32) catalog VNBR, lot # 2272 was obtained from Vital Nutrients (Middle-town, CT) and stored at 4 °C in opaque containers. The identity of Br (Table 1) was confirmed by matching its Fourier transform infrared spectroscopy and HPLC profiles with the industry (Sigma) standards. Br was independently tested for activity/potency (2407 gdu/g) and endotoxin content (0.017 µg/ml), which was consistent with previously published levels [11]. Additional analytical testing completed for Solvent Residues, Microbiology, Aflatoxins, Heavy Metals, and Pesticides Residues were all within USP Limits or negative. The cysteine protease inhibitor E-64 [Trans-Epoxysuccinyl-L-Leucylamido-( 4-Guanidino) Butane] was obtained from Sigma (St. Louis, MO). For in vitro cell stimulation, anti-CD3 (145-2C11) was obtained from BD Pharmingen (San Jose, CA). Fluorescent labeled monoclonal antibodies used for flow analysis included anti-CD4-PE-Cy7 (L3T4) and anti-CD25-PE (PC61) BD Pharmingen (San Jose, CA).
Table 1.
Bromelain identity and quality control data
| Item profile | Specification | Result | Method |
|---|---|---|---|
| Botanical –Pineapple | Ananas cosmosus | Ananas cosmosus | Visual |
| Plant parts used | Mature stem | Mature stem | Visual |
| Botanical extract | Bromelain | Bromelain | Methanol extraction |
| Identification | Standard Match(Sigma) | Conforms to Standard | FTIR*, HPLC‡ |
| Activity, potency | ≥2400 gdu**/g | 2407 gdu/g | gdu assay |
| Endotoxin content | 5.0–0.005 µg/ml | 0.017 µg/ml | Endosafe® |
| Solvent residues | USP Limits (ppm) | USP Limits (ppm) | |
| Methanol | 3000 | 2200 | Gas chromatography |
| Ethanol, 2-Propanol | 5000 | <1200 | Gas chromatography |
| Hexane | 290 | <2.0 | Gas chromatography |
| Xylenes (O, M, P, EB) | 2170 | <2.0 | Gas chromatography |
| Microbial Profile | (CFU/g) | (CFU/g) | |
| E. Coli | Negative | 40 | STVCA° |
| Salmonella | Negative | Negative | STVCA |
| Enterobacteriaceae | ≤10/g | Negative | STVCA |
| S. aureus | Negative | <10/g | USP 24 p. 1814 |
| P. aeruginosa | Negative | Negative | USP 24 p. 1814 |
| Aflatoxin Profile (B1, B2, G1, G2) | Aflatoxins ≤20 ppb | ≤2.0 ppb | HPLC |
| Heavy metal profile | |||
| Aluminum | ≤1000 ppm | 19±0.5 pp | ICP-MS! |
| Arsenic, cadmium | ≤3 ppm | <0.50±0.5 pp | ICP-MS! |
| Lead | ≤10 ppm | <0.06±0.05 ppm | ICP-MS! |
| Mercury | ≤2 ppm | <0.10±0.1 ppm | ICP-MS! |
| Pesticide residues | CDFA# | ||
| Organochlorines | ≤5ppm | Negative | Gas and liquid chromatography |
| Organophosphates | ≤7 ppm | Negative | coupled with mass spectrometry |
| Organonitrates | ≤5 ppm | Negative | |
| Carbamates | ≤5 ppm | Negative |
FTIR = Fourier transform infrared spectroscopy
HPLC = High-Performance Liquid Chromatography
GDU's = Gelatin Dissolving Units
STVCA = Soleris' Total Viable Count Assay
ICP-MS = Inductively Coupled Plasma–Mass Spectrometry
CDFA = California Department of Food and Agriculture.
2.3. CD4+ T cell isolation and activation
CD4+CD25− T cells were used in all in vitro experiments. To obtain CD4+CD25− T cells, spleens of naïve mice were mechanically disrupted and passed through a 70 µm nylon cell strainer (BD, Bedford, MA). Erythrocytes were lysed by rinsing with deionized H2O at room temperature for 15 s. Lysis was terminated by the addition of HANK's Balanced Salt Solution (Sigma). CD4+CD25− T cells were then isolated via negative selection from the leukocytes using a Treg isolation kit (Miltenyi Biotech, Auburn, CA). CD4+CD25− T cell isolations yielded 10–15×106 cells with a purity of >95%, as confirmed by flow cytometry. The CD4+CD25+ or Endogenous Regulatory T cell (Treg) fraction was used in selected experiments. Flat bottomed plates (24 well) were coated with anti-CD3 Ab (10 µg/ml) for 4 h in a CO2 incubator (10%) at 37 °C. The plate was subsequently washed three times with culture media to remove any unbound anti-CD3. CD4+CD25− cells (1×106) were added per well and plated for 18 h in RPMI 1640 culture media supplemented with 10% FCS and 50 µM 2-ME. L-Glutamine, antibiotics Penicillin–Streptomycin and Gentamycin were added to RPMI 1640 to maintain sterility and viability of cells. For in vitro assays, Br was dissolved in culture media, sterile filtered and administered following in vitro activation of CD4+ T cells. Br was co-cultured in a dose response manner (25–100 µg/ml) with activated CD4+ T cells for 0–18 h in culture media. E-64 (100 µM) was added to cultures containing Br to neutralize the cysteine protease activity. For re-stimulation assays: cells were washed three times after Br treatment, labeled with CFSE and placed in culture in anti-CD3 Ab (10 µg/ml) coated plate as above. CFSE labeling was performed by incubating CD4+CD25+ T cells (~1×106) with 2.5 µM CFSE at 37 °C for 15 min. CFSE-labeled cells were washed three times with 10% FCS. After 48 h of culture the cells were harvested from the plate, stained with CD4 and CD25 Abs, and observed for proliferation via CFSE dilution on flow cytometry.
2.4. Flow cytometry
CD4+ T cell samples were washed in FACS Buffer (PBS containing 0.2% BSA and 0.1% NaN3) and aliquots containing 106 cells were incubated with 100 µl of appropriately diluted antibodies (anti-CD4, CD25) for 30 min at 4 °C. After staining, the cells were washed with FACS Buffer, and relative fluorescence intensities were determined on a 4-decade log scale by flow cytometric analysis, using an LSRII (Becton Dickinson, Franklin Lakes, NJ). 500,000 cell events were collected and analysis was carried out with FACSDiva software (BD Biosciences, San Jose, CA).
2.5. Immunoblotting and ELISA
To quantify the amount of sCD25, Western blot analysis was performed on cell culture supernatants obtained from activated T cells treated with media alone (control), Br (50 µg/ml) or E-64 Inactivated Br. The cell culture supernatants were run on 12% (SDS-PAGE) gels. The separated proteins were then electrophoretically transferred to Immobilon-P membranes (Millipore Corp., Bedford, MA) using a semidry transfer system (Bio-Rad, Hercules, CA). The blots were blocked in Tris-buffered saline/Tween-20 (TBS-T containing 20 mM Tris base, pH 7.6,137mM NaCl, 0.1% Tween-20) supplemented with 5% (wt/vol) non-fat dry milk for 60 min; blots were incubated overnight at 4 °C with the primary antibody (CD25) R&D Systems Inc. (Minneapolis, MN). Membranes were washed three times in TBS-T before incubation for 60 min with horseradish peroxidase-conjugated secondary antibody Stressgen (Ann Arbor, MI) diluted 1:2000 in TBS-Tand 5% (wt/vol) nonfat dry milk. Membranes were washed three times with TBS-T for 10 min each, blots were treated with Enhanced Chemi-Luminescence (ECL from Amersham) reagents and the required proteins were detected by autoradiography for variable lengths of time with Kodak X-Omat film. In addition to Western blot the amount of sCD25 in cell culture fluid was determined by ELISA catalogue #DY2438 (R&D Systems Inc. Minneapolis, MN) via manufacturer's instructions.
2.6. Statistical analysis
Statistical comparisons between groups were made with analysis of variance and unpaired t-tests using JMP® Software (SAS Institute Inc., Cary, NC). All data were expressed as means±standard error of the mean, and differences were considered significant at p≤0.05.
3. Results
3.1. Bromelain treatment reduces CD25 expression on activated CD4+ T cells
Previous work in our laboratory suggests that Br may reduce murine allergic asthma by preferentially modulating CD4+CD25+ T cells [17]. This experiment was designed to evaluate the effect of Br on CD25+ expression on activated CD4+ T cells, independent of TCR stimulus and therefore Br treatment was initiated on CD4+CD25+ T cells after the TCR stimulus was removed. As anticipated, CD25 expression was greatly increased on anti-CD3 stimulated naïve CD4++ T cells with 78% of the cells positive for CD25 as compared to only 0.5% at baseline (Fig. 1A). Br treatment reduced the expression of CD25 in a dose response manner, from 78% to 52% (Br 25 µg/ml), 40% (Br 50 µg/ml), and 18% (Br 100 µg/ml). Treatment with the protease inhibitor E64 abrogated the ability of Br treatment to reduce the percentage of CD25+ cells (72%) as compared to controls 78%. Br treatment did not negatively affect cell viability (as determined by trypan blue exclusion) as the viability was >95% for all doses used in cell culture experiments.
Fig. 1.
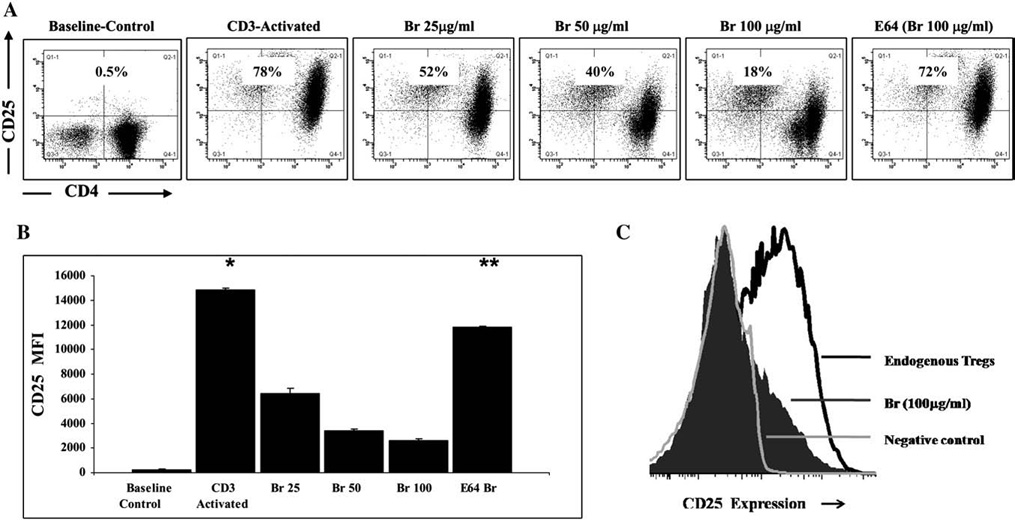
Br treatment reduces CD25 expression on CD4+ T cells. A) CD4+CD25− T cells (1×106) were stimulated in vitro with anti-CD3 (10 µg/ml) for 48 h. Activated T cells were removed from stimulation, washed thrice, and re-plated with Br (25, 50, 100 µg/ml) for 8 h. Post culture, cells were analyzed for cell surface expression of CD25. As compared to baseline (0.5%), activated control CD4+ T cells up regulated CD25 surface expression (78%). Br treatment reduced the expression of CD25 to 52% (Br 25 µg/ml), 40% (Br 50 µg/ml) and 18% (Br 100 µg/ml). Inactivation of the cysteine protease activity of Br (100 µg/ml) with E64 prevents the loss of CD25 surface expression (72%). Experiments were carried out in triplicate. CD4 T cells (x-Axis) are compared to CD25 expression (y-Axis). B) The mean fluorescent intensity (MFI) of CD25 was compared between groups from A) above. C) CD4+CD25+ Endogenous Tregs (1×105) were treated with Br (100 µg/ml) or media for 8 h and CD25 expression was compared between groups and to CD4+CD25− (Negative) controls. The * represents a significant difference between the control anti-CD3 stimulated cells and all other groups, while the ** denotes significant differences between the E64 Inactivated Br and all other treatment groups. The ‡ represents p=0.05 when comparing the Br 50 µg/ml and Br 100 µg /ml treatment groups. Experiments were carried out in triplicate.
In addition to evaluating Br's effect on the percentage of CD4+CD25+ cells, the change in mean fluorescent intensity (MFI) of CD25 was also compared between groups (Fig. 1B). The MFI of CD25 was significantly increased in anti-CD3 stimulated CD4+ T cells 14,821±144 as compared to naïve control baseline CD4+ T cells 201±76 (p≤0.001). Br treatment reduced the MFI of CD25 6416±409 (Br 25 µg/ml), 3404± 143 (Br 50 µg/ml) and 2630±118 (Br 100 µg/ml) as compared to anti- CD3 stimulated CD4++ T cells, p≤0.001. As anticipated E-64 inactivation of Br inhibited the ability of Br (100 µg/ml) to reduce the MFI of CD25, 11,839±13.5, (p≤0.001).
The effect of Br on CD25 expression was also evaluated onCD4+CD25+ Endogenous T Regulatory cells (Tregs), which are known to constitutively express high levels of CD25 (Fig. 1C). As compared to un-stimulated CD4+ CD25− negative controls, Tregs were indeed 25hi. Br (100 µg/ml) treatment greatly diminished CD25 expression on Tregs. The reduction of CD25 on Tregs was also inhibited with E-64 inactivation of Br (not shown).
3.2. Inhibition of CD25 expression on activated CD4+ T cells by Br is time dependent
The reduction in CD25 expression by Br was dependent on proteolytic activity but the time in which the effect occurred was unknown. To address the time effect of Br on activated CD4+CD25+ T cells, splenic CD4+CD25− T cells were isolated, and stimulated in vitro for 48 h and then treated with Br or E-64 inactivated Br as in Fig. 1. Br (50 µg/ml) was chosen for these experiments as the dose that consistently reduced CD25 ~50% from activated CD4+ T cells. Representative dot plots are shown (Fig. 2) for CD3 activated control and Br (50 µg/ml) treated cells cultured for 3, 6 and 18 h. After 3 h in culture there was no difference in the percentage of CD25+ cells between CD3 activated control (68%) and Br treated cells (72%). At 6 h in culture the percentage of CD25+ activated control cells was slightly reduced to 54% but there was marked reduction with Br 50 µg/ml treatment from 72% to 11%. The reduction in CD25 expression continued to slowly decline on Control cells 43% after 18 h as compared to 5% with Br (50 µg/ml) treatment. In duplicate experiments 50 µg/ml of Br exhibited the greatest reduction in CD25 expression at 6 h. Similar to the data in Fig. 1, this ability of Br to reduce the percentage of cells expressing CD25 was inhibited by E64 at 3 h (59%), 6 h (45%) and 18 h (38%).
Fig. 2.
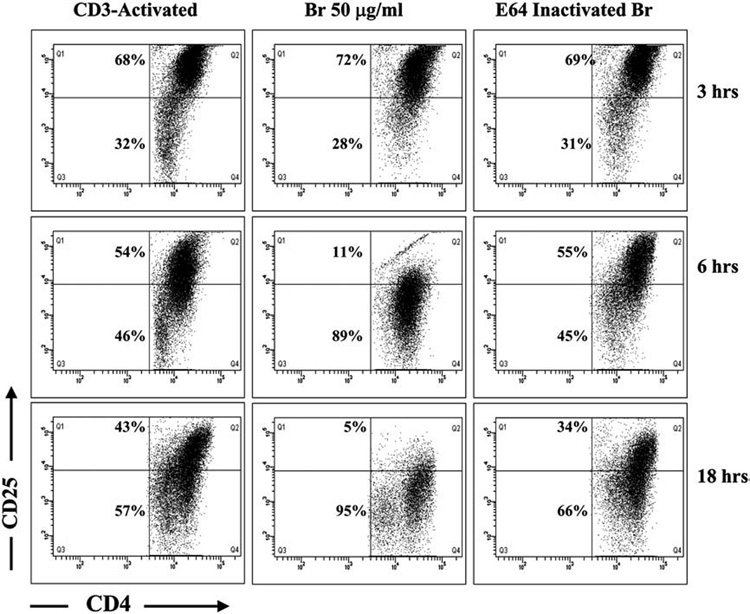
Inhibition of CD25 expression on activated CD4+ T cells by Br is time dependent. CD4+CD25− T cells (1×106) were stimulated in vitro with anti-CD3 (10 µg/ml) for 48 h. Activated T cells were removed from stimulation, washed thrice, and re-plated with or without Br 50 µg/ml or E64-inactivated Br, for up to 18 h. Representative dot plots of activated T cells at 3, 6, and 18 h of incubation are shown. There was no difference in surface expression of CD25 on control (68%) and Br treated (72%) CD4+ T cells at 3 h. A considerable reduction in CD25 expression was observed at 6 h (54% vs 11%) and 18 h (43% vs 5%) between activated controls and Br treated cells, which was largely abrogated with E64. Experiments were carried out in duplicate. CD4+ T cells (x-Axis) are compared to CD25 expression (y-Axis).
3.3. Br proteolyticly cleaves CD25 from activated CD4+ T cells
In order to determine whether Br was inhibiting the expression or cleaving off the CD25 receptor, a Western Blot for CD25 was carried out on cell culture supernatant from duplicate experiments on anti- CD3 stimulated (Activated Control) cells and Br (25 and 50 µg/ml) treated cells. The molecular weight is depicted in Lane 1 (31–66.2 Kd) and corresponds to the molecular mass of IL-2Rα which ranges from 40 to 50 Kd depending on the state of glycosylation. The CD25 protein is present at low levels in the supernatant of control cells (Fig. 3A, lane 2) and is increased with Br 25 µg/ml (lanes 3 and 4) and Br 50 µg/ml (lanes 5 and 6). The presence of CD25 in culture supernatant was inhibited with E64 treatment (lane 7). The pattern of CD25 protein deposition by Western blot is replicated in an ELISA for sCD25 (Fig. 3B). As compared to control cells (1226±163 pg/ml) the amount of sCD25 was significantly elevated in the culture supernatant of Br 50 µg/ml treated cells (2253±143 pg/ml) p≤0.01. In contrast, when E64 is used to inactivate Br's proteotytic activity, the presence of sCD25 is greatly diminished (306±25 pg/ml).
Fig. 3.
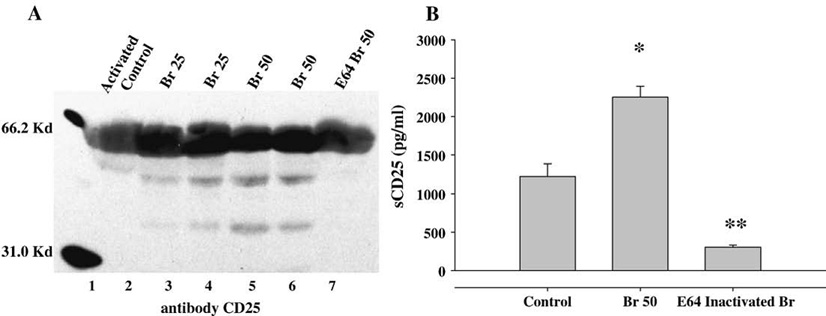
Br proteolyticly cleaves CD25 from activated CD4+ T cells. CD4+CD25− T cells (1×106) were stimulated in vitro with anti-CD3 (10 µg/ml) for 48 h. Activated T cells (1×106) cells were removed from stimulation, washed thrice, and re-plated with or without Br (25 and 50 µg/ml) for 8 h. Post Treatment cell culture supernatant (CS) was evaluated for the presence of CD25 protein via A)Western Blot and B) ELISA. A) Lane 2 = CS from control-media treated cells; lanes 3 and 4 = CS from Br 25 µg/ml; lanes 5 and 6 = CS from Br 50 µg/ml; and lane 7 = CS from E64 Inactivated Br cell cultures. B) The presence of CD25 (IL-2Rα) was detected at low levels in control-media treated cells and was significantly elevated in the CS of Br (50 µg/ml) treated cells. E64 Inactivated Br cell cultures had decreased detectable levels of CD25. Data presented are means of duplicate experiments. * represents p≤0.01 as compared to control, and ** represents p≤0.01 as compared to Br 50.
3.4. CD4+ T cells retain ability to divide after Br treatment
With the reduction of the CD25 receptor on activated T cells after Br treatment it was necessary to determine if these cells remain functional. Cells were isolated, activated and treated with Br (50 µg/ml or 100 µg/ml), culture media, or with E64 -inactivate Br as described in Section 3.1. Cells were then washed, labeled with CFSE and cultured with media or re stimulated with anti-CD3 alone or anti-CD3 and IL-2 for 48 h. Cells were analyzed via Flow Cytometry for their ability to dilute CFSE (Fig. 4). In groups which did not received stimulation (no αCD3) very few cells divided (5.4–7.6%). With the addition of αCD3, an average of 33% of cells divided, with no difference observed between treatment groups. The addition of exogenous IL-2 did not significantly alter division with ~37% cells dividing.
Fig. 4.
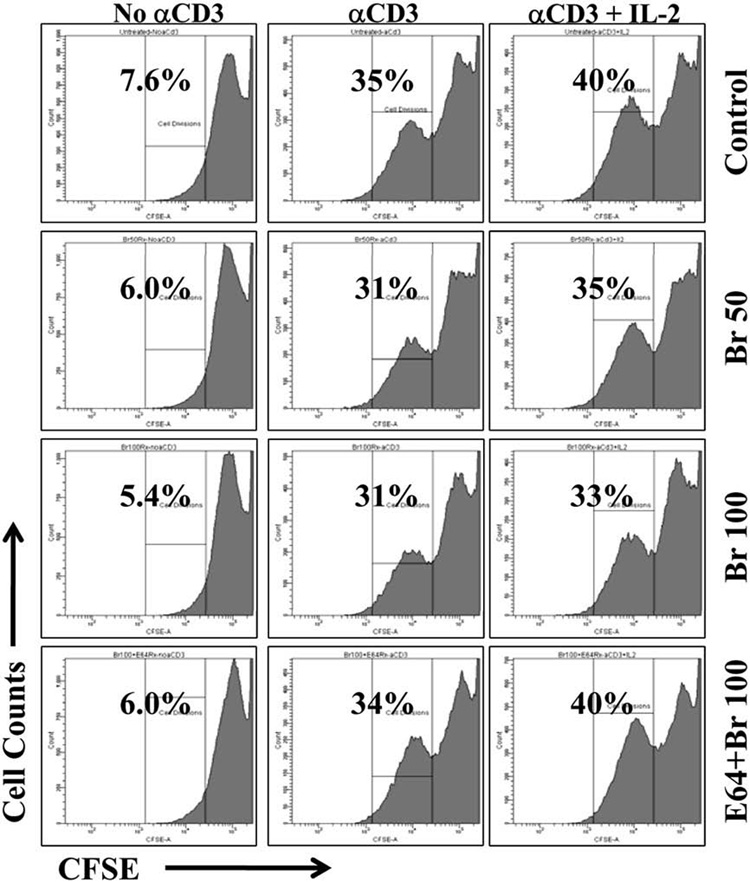
CD4+ T cells retain ability to divide after Br Treatment. CD4+CD25− T cells (1×106) were stimulated in vitro with CD3 (10 µg/ml) for 48 h. Activated T cells (1×106) cells were removed from stimulation, washed thrice, and cultured with Br (50 and 100 µg/ml), media alone (Control) or with E64-Inactivated Br for 8 h. Cells were then CFSE labeled and re-stimulated with anti-CD3, exogenous IL-2, or media alone. Cells without re-stimulation failed to dilute CFSE. The percentage of cell division was similar in those stimulated with anti-CD3 (Mean 33%±2) and the addition of exogenous IL-2 did not significantly alter cell division.
4. Discussion
We and others have previously demonstrated that Br exerts immuno-modulatory effects in CD4+ T cell mediated inflammation [16,18,21]. Our previous observations utilizing a murine model of ovalbumin-induced allergic asthma demonstrated that in vivo Br administration significantly reduced activated CD4+ T cells in particular CD4+CD25+ cells. There was no effect on the Foxp3 expressing T regulatory cells which constitutively express CD25. This observation led us to the current experiments which were designed to determine if Br modulates CD25 expression. By utilizing an in vitro model of T cell activation, we have shown that Br reduced CD25 expression on CD4+ T cells in a dose and time dependent manner. Additionally, Br treatment appears to cleave off CD25 from the cell surface since increased sCD25 can be found in cell culture supernatants. The process by which Br reduced expression of CD25 required the cysteine protease activity of Br, since it was abrogated with E64.
The immuno-modulatory capacity of Br has been a topic of investigation for several decades and its exact mechanism of action remains undetermined [22–25]. The diversity of results obtained from Br research may be due in part to the inconsistencies of natural product material in the marketplace, in addition to diverse experimental designs [26,27]. For these experiments we chose a commercially available, quality control tested product distributed to physicians through Vital Nutrients (Middletown, CT). The identity of Br was confirmed by matching its FITR and HPLC profiles with the industry standard (Table 1). Br was analytically tested by independent laboratories for purity, quality and enzymatic stability and degree of degradation. A subset of the results is provided for Solvent Residues, Microbials, Alfatoxins, Heavy Metals and Pesticide Residues, all of which are within acceptable USP limits or undetectable (Table 1).
Research into the mechanism of action of Br has demonstrated effects on a myriad of immune cell populations depending on the model of study. Br modulates leukocyte trafficking [28], inhibits MAP kinase and T cell receptor signaling [20], and modulates expression of cell surface molecules such as CD44 and CD45RA [15], CD28 [29] and CD86 [14]. As discussed by Lehmann et al. [30], Br may act as a physiological regulator of the inflammatory response primarily by shifting the T cell activation threshold. In addition to the key molecules involved with T cell activation listed above, CD25, the high affinity IL-2 receptor α subunit is also critical. IL-2 is the primary T cell growth factor which binds to its cognate receptor CD25 on T cells to initiate T cell proliferation. CD25 has been identified as a therapeutic target in conditions such as cancer [31,32] autoimmunity [33] and asthma susceptibility [34] and levels or soluble CD25 are being evaluated for their diagnostic potential [35,36].
Different models of in vitro T cell activation exist and a commonly used protocol is to provide CD4+ T cells with CD3 and CD28 stimulation. Levels of activation may fluctuate depending on the concentration and method of anti-CD3 delivery (such as plate bound or soluble) and the dose and timing of drug delivery. Similarly, the effect of Br on T cells has been evaluated prior to T cell activation, concurrently with T activation, or after the T cells have become activated (or express CD25). A previous study examined the effect of Br on T cells prior to stimulation and found no change in CD25 expression [29]. Others [37] investigating the effect of compounds such as Der p 1 with cyteine protease activity found significant reduction in CD25 expression with treatment after anti-CD3 stimulation. In our studies we have utilized both concurrent and post T cell activation administration of Br where CD25 expression was significantly reduced as compared to controls (Fig. 1). This effect was consistent and even when CD4+CD25− T cells were treated concurrently with anti-CD3 stimulation where the percentage of CD25+ cell decreased from 35% (controls) to 2% with Br (100 µg/ml) treatment. The reduction in CD25 expression was dependent on the cysteine protease activity of Br as E-64 reversed the loss of CD25. Br also reduced CD25 expression on T regulatory cells (Fig. 1C) when treated in vitro. This is an important control due to the fact that endogenous Tregs do not require stimulation to increase CD25, confirming the affinity of Br for CD25. The expression of CD25 was also shown to be the reduction of CD25 by Br began after ~6–8 h in culture (Fig. 2) which was consistent with similar timing observed by when assessing CD25 cleavage and T cell replication with a cysteine proteases [37].
Other compounds with cysteine protease activity have been shown to cleave CD25 such as house dust mite Der p1 [37] and Aspergillus fungal proteases [38]. In agreement with these findings and as evidence for cleavage, Br treatment of CD4+CD25+ cells (Fig. 3A and B) resulted in an increase in antibody detectable sCD25 protein isolated from the cell culture supernatant as compared to baseline levels which have been shown to increase in anti-CD3 stimulated controls. CD25 cleavage by Br was inhibited by E64 similar to the inhibition of CD25 cleavage by antipain inactivated Der p 1 [37].
The ability of cysteine protease containing compounds to cleave CD25 may result in contradictory effects. The consequence of protease exposure either as allergens or fungal pathogens may lead to enhanced innate immune recognition and then skewing towards a Th2 adaptive immune response [39]. In contrast, therapeutic treatment with natural products which contain cysteine proteases such as Br, may result in a reduced Th2 response and an anti-inflammatory effect. Constant treatment or contact with Br is required for down-regulation of CD25 as cells which are removed from treatment retain their ability to up regulate CD25. Cells also maintain their functionality (Fig. 4) and do not become anergic. This may be an optimal therapy where the desired effect occurs without the loss of immune functionality. The data described here suggest an additional mechanism through which Br may alter T cell activation and positively modulate the immune response.
Footnotes
Grant support: This research was supported by NIH/AI R01 HL-43573, NIH/NCCAM FG32-AT001569, NIH/NCCAM K08 and Competitive Technologies Inc.
References
- 1.Akkoc T, de Koning PJ, Ruckert B, Barlan I, Akdis M, Akdis CA. Increased activation-induced cell death of high IFN-gamma-producing T(H)1 cells as a mechanism of T(H)2 predominance in atopic diseases. J Allergy Clin Immunol. 2008;121(3):652–658. doi: 10.1016/j.jaci.2007.12.1171. [e1] [DOI] [PubMed] [Google Scholar]
- 2.Cope AP, Schulze-Koops H, Aringer M. The central role of T cells in rheumatoid arthritis. Clin Exp Rheumatol. 2007;25 5 Suppl 46:S4–S11. [PubMed] [Google Scholar]
- 3.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 4.Corthay A. A three-cell model for activation of naive T helper cells. Scand J Immunol. 2006;64(2):93–96. doi: 10.1111/j.1365-3083.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- 5.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4(9):665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 6.Ostroukhova M, Ray A. CD25+ T cells and regulation of allergen-induced responses. Curr Allergy Asthma Rep. 2005;5(1):35–41. doi: 10.1007/s11882-005-0052-6. [DOI] [PubMed] [Google Scholar]
- 7.Hoeger PH, Niggemann B, Ganschow R, Dammann C, Haeuser G. Serum levels of sCD23 and sCD25 in children with asthma and in healthy controls. Allergy. 1994;49(4):217–221. doi: 10.1111/j.1398-9995.1994.tb02652.x. [DOI] [PubMed] [Google Scholar]
- 8.Brassard P, Larbi A, Grenier A, Frisch F, Fortin C, Carpentier AC, et al. Modulation of T-cell signalling by non-esterified fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2007;77(5–6):337–343. doi: 10.1016/j.plefa.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 9.Biondo PD, Goruk S, Ruth MR, O'Connell E, Field CJ. Effect of CVT-E002 (COLD-fX) versus a ginsenoside extract on systemic and gut-associated immune function. Int Immunopharmacol. 2008;8(8):1134–1142. doi: 10.1016/j.intimp.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Varalakshmi C, Ali M, Pardhasaradhi BV, Srivastava RM, Singh S, Khar A. Immunomodulatory effects of curcumin: in-vivo. Int Immunopharmacol. 2008;8(5):688–700. doi: 10.1016/j.intimp.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Hale LP, Fitzhugh DJ, Staats HF. Oral immunogenicity of the plant proteinase bromelain. Int Immunopharmacol. 2006;6(13–14):2038–2046. doi: 10.1016/j.intimp.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Harrach T, Eckert K, Maurer HR, Machleidt I, Machleidt W, Nuck R. Isolation and characterization of two forms of an acidic bromelain stem proteinase. J Protein Chem. 1998;17(4):351–361. doi: 10.1023/a:1022507316434. [DOI] [PubMed] [Google Scholar]
- 13.Roep BO, van den Engel NK, van Halteren AG, Duinkerken G, Martin S. Modulation of autoimmunity to beta-cell antigens by proteases. Diabetologia. 2002;45(5):686–692. doi: 10.1007/s00125-002-0797-6. [DOI] [PubMed] [Google Scholar]
- 14.Kleef R, Delohery TM, Bovbjerg DH. Selective modulation of cell adhesion molecules on lymphocytes by bromelain protease 5. Pathobiology. 1996;64(6):339–346. doi: 10.1159/000164070. [DOI] [PubMed] [Google Scholar]
- 15.Hale LP, Haynes BF. Bromelain treatment of human T cells removes CD44, CD45RA, E2/MIC2, CD6, CD7, CD8, and Leu 8/LAM1 surface molecules and markedly enhances CD2-mediated T cell activation. J Immunol. 1992;149(12):3809–3816. [PubMed] [Google Scholar]
- 16.Targoni OS, Tary-Lehmann M, Lehmann PV. Prevention of murine EAE by oral hydrolytic enzyme treatment. J Autoimmun. 1999;12(3):191–198. doi: 10.1006/jaut.1999.0271. [DOI] [PubMed] [Google Scholar]
- 17.Secor ER, Jr, Carson WF, IV, Cloutier MM, Guernsey LA, Schramm CM, Wu CA, et al. Bromelain exerts anti-inflammatory effects in an ovalbumin-induced murine model of allergic airway disease. Cell Immunol. 2005;237(1):68–75. doi: 10.1016/j.cellimm.2005.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hale LP, Greer PK, Trinh CT, Gottfried MR. Treatment with oral bromelain decreases colonic inflammation in the IL-10-deficient murine model of inflammatory bowel disease. Clin Immunol. 2005;116(2):135–142. doi: 10.1016/j.clim.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Secor ER, Carson WF, IV, Singh A, Pensa M, Guernsey LA, Schramm CM, et al. Oral bromelain attenuates inflammation in an ovalbumin-induced murine model of asthma. Evid Based Complement Alternat Med. 2008;5(1):61–69. doi: 10.1093/ecam/nel110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mynott TL, Ladhams A, Scarmato P, Engwerda CR. Bromelain, from pineapple stems, proteolytically blocks activation of extracellular regulated kinase-2 in T cells. J Immunol. 1999;163(5):2568–2575. [PubMed] [Google Scholar]
- 21.Manhart N, Akomeah R, Bergmeister H, Spittler A, Ploner M, Roth E. Administration of proteolytic enzymes bromelain and trypsin diminish the number of CD4+ cells and the interferon-gamma response in Peyer's patches and spleen in endotoxemic balb/c mice. Cell Immunol. 2002;215(2):113–119. doi: 10.1016/s0008-8749(02)00019-9. [DOI] [PubMed] [Google Scholar]
- 22.Murachi T, Neurath H. Fractionation and specificity studies on stem bromelain. J Biol Chem. 1960;235:99–107. [PubMed] [Google Scholar]
- 23.Knill-Jones RP, Pearce H, Batten J, Williams R. Comparative trial of Nutrizym in chronic pancreatic insufficiency. Br Med J. 1970;4(5726):21–24. doi: 10.1136/bmj.4.5726.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Felton GE. Fibrinolytic and antithrombotic action of bromelain may eliminate thrombosis in heart patients. Med Hypotheses. 1980;6(11):1123–1133. doi: 10.1016/0306-9877(80)90134-6. [DOI] [PubMed] [Google Scholar]
- 25.Bouwstra JB, Spoelstra EC, De Waard P, Leeflang BR, Kamerling JP, Vliegenthart JF. Conformational studies on the N-linked carbohydrate chain of bromelain. Eur J Biochem. 1990;190(1):113–122. doi: 10.1111/j.1432-1033.1990.tb15553.x. [DOI] [PubMed] [Google Scholar]
- 26.Barnes J. Quality, efficacy and safety of complementary medicines: fashions, facts and the future. Part II: Efficacy and safety. Br J Clin Pharmacol. 2003;55(4):331–340. doi: 10.1046/j.1365-2125.2003.01811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan IA. Issues related to botanicals. Life Sci. 2006;78(18):2033–2038. doi: 10.1016/j.lfs.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 28.Fitzhugh DJ, Shan S, Dewhirst MW, Hale LP. Bromelain treatment decreases neutrophil migration to sites of inflammation. Clin Immunol. 2008;128(1):66–74. doi: 10.1016/j.clim.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engwerda CR, Andrew D, Ladhams A, Mynott TL. Bromelain modulates T cell and B cell immune responses in vitro and in vivo. Cell Immunol. 2001;210(1):66–75. doi: 10.1006/cimm.2001.1807. [DOI] [PubMed] [Google Scholar]
- 30.Lehmann PV. Immunomodulation by proteolytic enzymes. Nephrol Dial Transplant. 1996;11(6):952–955. doi: 10.1093/oxfordjournals.ndt.a027510. [DOI] [PubMed] [Google Scholar]
- 31.El Houda Agueznay N, Badoual C, Hans S, Gey A, Vingert B, Peyrard S, et al. Soluble interleukin-2 receptor and metalloproteinase-9 expression in head and neck cancer: prognostic value and analysis of their relationships. Clin Exp Immunol. 2007;150(1):114–123. doi: 10.1111/j.1365-2249.2007.03464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murakami S. Soluble interleukin-2 receptor in cancer. Front Biosci. 2004;9:3085–3090. doi: 10.2741/1461. [DOI] [PubMed] [Google Scholar]
- 33.Glisic-Milosavljevic S, Wang T, Koppen M, Kramer J, Ehlenbach S, Waukau J, et al. Dynamic changes in CD4+ CD25+(high) T cell apoptosis after the diagnosis of type 1 diabetes. Clin Exp Immunol. 2007;150(1):75–82. doi: 10.1111/j.1365-2249.2007.03475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hubeau C, Apostolou I, Kobzik L. Targeting of CD25 and glucocorticoid-induced TNF receptor family-related gene-expressing T cells differentially modulates asthma risk in offspring of asthmatic and normal mother mice. J Immunol. 2007;178(3):1477–1487. doi: 10.4049/jimmunol.178.3.1477. [DOI] [PubMed] [Google Scholar]
- 35.Maeta M, Saito H, Katano K, Kondo A, Tsujitani S, Makino M, et al. A progressive postoperative increase in the serum level of soluble receptors for interleukin-2 is an indicator of a poor prognosis in patients with gastric cancer. Int J Mol Med. 1998;1(1):113–116. doi: 10.3892/ijmm.1.1.113. [DOI] [PubMed] [Google Scholar]
- 36.Kaito K, Otsubo H, Ogasawara Y, Kimura H, Kurihara E, Koike M, et al. Serum soluble interleukin-2 receptor in eosinophilia. Acta Haematol. 2003;109(1):23–28. doi: 10.1159/000067274. [DOI] [PubMed] [Google Scholar]
- 37.Schulz O, Sewell HF, Shakib F. Proteolytic cleavage of CD25, the alpha subunit of the human T cell interleukin 2 receptor, by Der p 1, a major mite allergen with cysteine protease activity. J Exp Med. 1998;187(2):271–275. doi: 10.1084/jem.187.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamhamedi-Cherradi SE, Martin RE, Ito T, Kheradmand F, Corry DB, Liu YJ, et al. Fungal proteases induce Th2 polarization through limited dendritic cell maturation and reduced production of IL-12. J Immunol. 2008;180(9):6000–6009. doi: 10.4049/jimmunol.180.9.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2007;21:21. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]


