Abstract
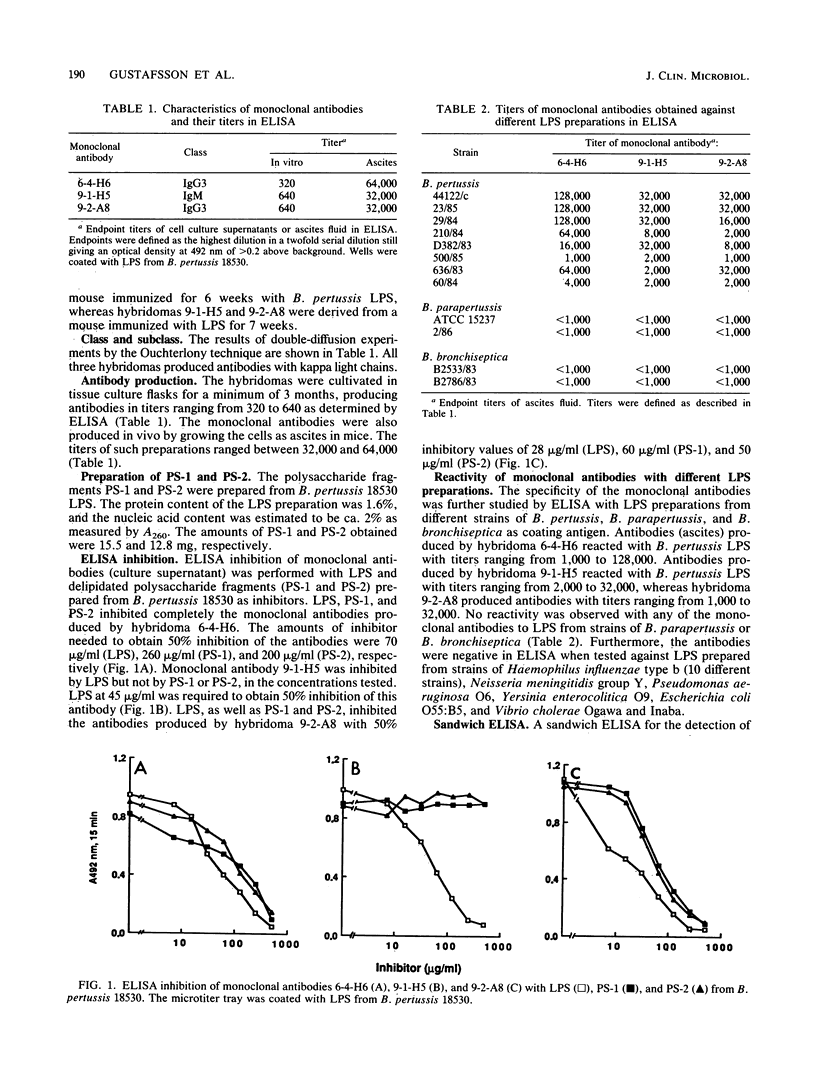
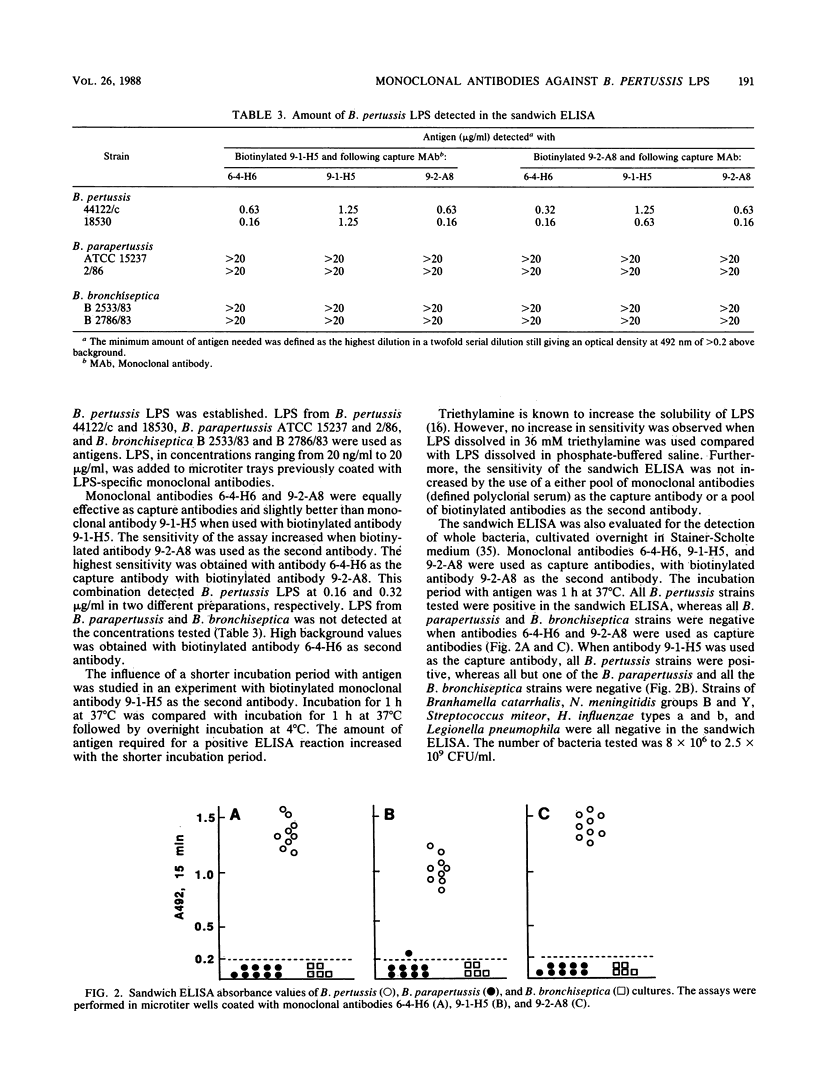
Hybrid cell lines producing monoclonal antibodies against Bordetella pertussis lipopolysaccharide (LPS) were established. The specificity of the antibodies was ascertained by enzyme-linked immunosorbent assay (ELISA) and ELISA-inhibition experiments with LPS and delipidated polysaccharide fragments (PS-1 and PS-2) prepared from B. pertussis LPS. Monoclonal antibody 9-1-H5 reacted with B. pertussis LPS only, whereas monoclonal antibodies 6-4-H6 and 9-2-A8 reacted with PS-1 and PS-2 as well as B. pertussis LPS. The antibodies did not react with LPS prepared from B. parapertussis and B. bronchiseptica in an LPS-specific ELISA. A monoclonal antibody-based sandwich ELISA was developed for detection of B. pertussis LPS. This assay had a detection limit of B. pertussis LPS in concentrations ranging from 0.16 to 0.32 microgram/ml. The assay was also shown to be specific for the detection of whole B. pertussis bacteria. No cross-reactions were observed with strains of Branhamella catarrhalis, Neisseria meningitidis, Streptococcus miteor, Haemophilus influenzae, or Legionella pneumophila. The monoclonal antibodies might be useful for the detection of soluble antigens and whole bacteria in clinical samples and for studies of the immunochemical structure of B. pertussis LPS.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackers J. P., Dolby J. M. The antigen of Bordetella pertussis that induces bactericidal antibody and its relationship to protection of mice. J Gen Microbiol. 1972 Apr;70(2):371–382. doi: 10.1099/00221287-70-2-371. [DOI] [PubMed] [Google Scholar]
- Aprile M. A., Wardlaw A. C. Immunochemical studies on the lipopolysaccharides of Bordetella pertussis. Can J Microbiol. 1973 Feb;19(2):231–239. doi: 10.1139/m73-035. [DOI] [PubMed] [Google Scholar]
- Ayme G., Caroff M., Chaby R., Haeffner-Cavaillon N., Le Dur A., Moreau M., Muset M., Mynard M. C., Roumiantzeff M., Schulz D. Biological activities of fragments derived from Bordetella pertussis endotoxin: isolation of a nontoxic, Shwartzman-negative lipid A possessing high adjuvant properties. Infect Immun. 1980 Mar;27(3):739–745. doi: 10.1128/iai.27.3.739-745.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundle D. R., Gidney M. A., Perry M. B., Duncan J. R., Cherwonogrodzky J. W. Serological confirmation of Brucella abortus and Yersinia enterocolitica O:9 O-antigens by monoclonal antibodies. Infect Immun. 1984 Nov;46(2):389–393. doi: 10.1128/iai.46.2.389-393.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroff M., Bundle D. R., Perry M. B., Cherwonogrodzky J. W., Duncan J. R. Antigenic S-type lipopolysaccharide of Brucella abortus 1119-3. Infect Immun. 1984 Nov;46(2):384–388. doi: 10.1128/iai.46.2.384-388.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroff M., Bundle D. R., Perry M. B. Structure of the O-chain of the phenol-phase soluble cellular lipopolysaccharide of Yersinia enterocolitica serotype O:9. Eur J Biochem. 1984 Feb 15;139(1):195–200. doi: 10.1111/j.1432-1033.1984.tb07994.x. [DOI] [PubMed] [Google Scholar]
- Caroff M., Szabó L. Identification of 2-amino-6-O-(2-amino-2-deoxy-beta-D-glucopyranosyl)-2-deoxy-D-glucose as a major constituent of the hydrophobic region of the Bordetella pertussis endotoxin. Carbohydr Res. 1983 Mar 16;114(1):95–102. doi: 10.1016/0008-6215(83)88176-2. [DOI] [PubMed] [Google Scholar]
- Chaby R., Moreau M., Szabó L. 2-O-(beta-D-glucuronyl)-7-O-(2-amino-2-deoxy-alpha-D-glucopyranosyl)-L-glycero-D-manno-heptose: a constituent of the Bordetella pertussis endotoxin. Eur J Biochem. 1977 Jun 15;76(2):453–460. doi: 10.1111/j.1432-1033.1977.tb11615.x. [DOI] [PubMed] [Google Scholar]
- Chaby R., Szabó L. 3-Deoxy-2-octulosonic acid 5-phosphate: a component of the endotoxin of Bordetella pertussis. Eur J Biochem. 1975 Nov 1;59(1):277–280. doi: 10.1111/j.1432-1033.1975.tb02452.x. [DOI] [PubMed] [Google Scholar]
- Chaby R., Szabó L. 7-O-(2-Amino-2-deoxy-alpha-D-glucopyranosyl)-L-glycero-D-manno-heptose. A constituent of the endotoxin of Bordetella pertussis. Eur J Biochem. 1976 Nov 1;70(1):115–122. doi: 10.1111/j.1432-1033.1976.tb10962.x. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Field L. H., Parker C. D. Pertussis outbreak in Austin and Travis County, Texas, 1975. J Clin Microbiol. 1977 Aug;6(2):154–160. doi: 10.1128/jcm.6.2.154-160.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D. W., Parker C. D. Isolation and characterization of monoclonal antibodies to Bordetella pertussis. J Biol Stand. 1984 Oct;12(4):353–365. doi: 10.1016/s0092-1157(84)80060-8. [DOI] [PubMed] [Google Scholar]
- Galanos C., Rietschel E. T., Lüderitz O., Westphal O., Kim Y. B., Watson D. W. Biological activities of lipid A complexed with bovine-serum albumin. Eur J Biochem. 1972 Dec 4;31(2):230–233. doi: 10.1111/j.1432-1033.1972.tb02524.x. [DOI] [PubMed] [Google Scholar]
- Gustafsson B., Holme T. Immunological characterization of Vibrio cholerae O:1 lipopolysaccharide, O-side chain, and core with monoclonal antibodies. Infect Immun. 1985 Aug;49(2):275–280. doi: 10.1128/iai.49.2.275-280.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B., Rosén A., Holme T. Monoclonal antibodies against Vibrio cholerae lipopolysaccharide. Infect Immun. 1982 Nov;38(2):449–454. doi: 10.1128/iai.38.2.449-454.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J. Electrophoretic heterogeneity and interstrain variation of the lipopolysaccharide of Haemophilus influenzae. J Infect Dis. 1983 Sep;148(3):492–499. doi: 10.1093/infdis/148.3.492. [DOI] [PubMed] [Google Scholar]
- KASUGA T., NAKASE Y., UKISHIMA K., TAKATSU K. Studies on Haemophilus pertussis. I. Antigen structure of H. pertussis and its phases. Kitasato Arch Exp Med. 1953 Nov;26(2-3):121–133. [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Le Dur A., Caroff M., Chaby R., Szabó L. A novel type of endotoxin structure present in Bordetella pertussis. Isolation of two different polysaccharides bound to lipid A. Eur J Biochem. 1978 Mar 15;84(2):579–589. doi: 10.1111/j.1432-1033.1978.tb12201.x. [DOI] [PubMed] [Google Scholar]
- Le Dur A., Chaby R., Szabó L. Isolation of two protein-free and chemically different lipopolysaccharides from Bordetella pertussis phenol-extracted endotoxin. J Bacteriol. 1980 Jul;143(1):78–88. doi: 10.1128/jb.143.1.78-88.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau M., Chaby R., Szabo L. Isolation of a trisaccharide containing 2-amino-2-deoxy-D-galacturonic acid from the Bordetella pertussis endotoxin. J Bacteriol. 1982 Apr;150(1):27–35. doi: 10.1128/jb.150.1.27-35.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau M., Chaby R., Szabo L. Structure of the terminal reducing heptasaccharide of polysaccharide 1 isolated from the Bordetella pertussis endotoxin. J Bacteriol. 1984 Aug;159(2):611–617. doi: 10.1128/jb.159.2.611-617.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppler M. S. Two physically and serologically distinct lipopolysaccharide profiles in strains of Bordetella pertussis and their phenotype variants. Infect Immun. 1984 Jan;43(1):224–232. doi: 10.1128/iai.43.1.224-232.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman M. The concept of pertussis as a toxin-mediated disease. Pediatr Infect Dis. 1984 Sep-Oct;3(5):467–486. doi: 10.1097/00006454-198409000-00019. [DOI] [PubMed] [Google Scholar]
- Potter M., Pumphrey J. G., Walters J. L. Growth of primary plasmacytomas in the mineral oil-conditioned peritoneal environment. J Natl Cancer Inst. 1972 Jul;49(1):305–308. [PubMed] [Google Scholar]
- Regan J., Lowe F. Enrichment medium for the isolation of Bordetella. J Clin Microbiol. 1977 Sep;6(3):303–309. doi: 10.1128/jcm.6.3.303-309.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Stainer D. W., Scholte M. J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970 Oct;63(2):211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Pertussis toxin and extracytoplasmic adenylate cyclase as virulence factors of Bordetella pertussis. J Infect Dis. 1984 Aug;150(2):219–222. doi: 10.1093/infdis/150.2.219. [DOI] [PubMed] [Google Scholar]
- Wofsy L. Methods and applications of hapten-sandwich labeling. Methods Enzymol. 1983;92:472–488. doi: 10.1016/0076-6879(83)92040-2. [DOI] [PubMed] [Google Scholar]
- Wolff J., Cook G. H., Goldhammer A. R., Berkowitz S. A. Calmodulin activates prokaryotic adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3841–3844. doi: 10.1073/pnas.77.7.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]



