Abstract
Fibroblast growth factor (FGF)-activated signaling regulates an array of cellular processes ranging from embryonic development to tissue repair. A recent paper by Murakami et al. identifies a potentially important role for FGF signaling in maintenance of endothelial barrier homeostasis through the regulation of adherens junctions.
The fibroblast growth factor (FGF) family comprises 22 members in the human and mouse with pleiotropic functions including cell migration, proliferation, differentiation, and survival. Many FGF-mediated signaling events are initiated through the classic FGF-FGFR axis in which binding of FGF to high-affinity cell surface tyrosine kinase receptors (FGFRs) leads to receptor dimerization and trans-autophosphorylation (reviewed in Itoh and Ornitz, 2004). The four FGFRs and their splice isoforms display different ligand-binding specificities, allowing them to produce differential cellular responses depending on the context. Although the role of FGFs in angiogenesis is well known, the importance of the FGF system in regulating the endothelial barrier function of the vessel had not been addressed until now. In an article in the Journal of Clinical Investigation, Murakami et al. (2008) present the first evidence for a critical role of FGF signaling in maintenance of vascular integrity due to its ability to anneal adherens junctions (AJs).
The authors use two complementary approaches to examine the functional role of FGF signaling in adult mouse vasculature: expression of soluble FGF receptor traps and a cytoplasmic truncated form of FGFR1IIIc that acts as a dominant-negative by heterodimerizing with all other FGFR isoforms. The dominant-negative approach addresses the overall role of FGFR signaling, while soluble traps provide information about the contribution of individual ligands. The expression of the dominant-negative receptor caused loss of endothelial cell-cell adhesion in both arterial and venous vascular beds. Systemic expression of the sFGFR1IIIc and sFGFR3IIIc traps, both of which affect a wide range of FGF family members, also resulted in the loss of integrity of tracheal microvessels, but this was not the case with the sFGFR3IIIb trap. Based on the ligand-binding specificities of these different traps, FGF2, FGF4, and FGF8, which are specific for both FGFR1IIIc and FGFR3IIIc, appear to be the key mediators responsible for maintenance of endothelial barrier homeostasis. The question of whether all three factors are involved remains to be addressed. FGF2 and FGF1 are known to induce angiogenesis in vivo, but neither single nor double knockout of these factors in mice causes any defect in vascular development (Miller et al., 2000), suggesting that other family members compensate for the absence of FGF1 and FGF2. By contrast, genetic deletion of either fgf4 or fgf8 is early embryonic lethal (Feldman et al., 1995; Sun et al., 1999). A powerful approach for future studies examining the relative contributions of FGF2, FGF4, and FGF8 to maintenance of junctional integrity would be to generate endothelial-specific conditional deletions of the corresponding genes.
One potential explanation for the observed loss of vascular integrity is that FGF signaling is required for regulation of endothelial cell proliferation and survival. However, the authors ruled this out by showing that disruption of FGF signaling did not decrease the cell density in endothelial monolayers or induce apoptosis (Murakami et al., 2008). Instead, their results suggest that FGF signaling is directly required for maintenance of interendothelial adhesion. Suppression of FGF signaling led to dissociation of junctional adhesions in both arterial and venous vascular beds. VE-cadherin-based AJs and claudin-based tight junctions (TJs) form a semipermeable endothelial barrier between the vessel lumen and stroma. Although both junctions contribute to maintenance of tissue fluid homeostasis, AJs are predominant while TJs are poorly developed in endothelial barriers (with the exception being the blood-brain barrier). In addition, VE-cadherin-mediated adhesion induces the expression of the TJ adhesive molecule claudin-5, and therefore acts “upstream” of TJs (Taddei et al., 2008). AJs composed of VE-cadherin, α-catenin, β-catenin, and p120-catenin are indispensable in the regulation of vascular integrity and endothelial barrier function. The association of p120-catenin at the VE-cadherin juxtamembrane domain is known to inhibit VE-cadherin internalization by interfering with its binding to adaptor proteins of the clathrin-mediated endocytic pathway (Miyashita and Ozawa, 2007). Murakami et al. (2008) found that stimulation of endothelial monolayers with FGF1 increased p120-catenin-VE-cadherin interaction, whereas inhibition of FGF signaling induced un-coupling of p120-catenin from VE-cadherin, leading to VE-cadherin internalization. Therefore, the loss of endothelial barrier integrity in the absence of FGF signaling could be explained by destabilization of VE-cadherin homophilic adhesion and subsequent dissociation of AJs.
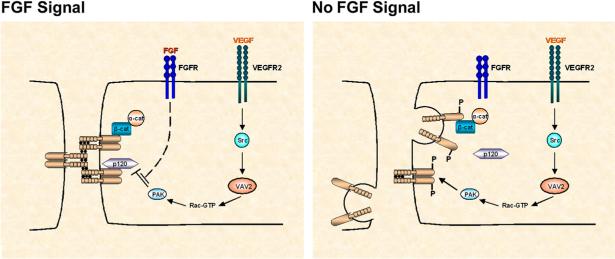
What are the signaling mechanisms by which FGF exerts its effect on AJs? Taking into account the known functions of FGF signaling in angiogenesis, one possible mechanism could involve N-cadherin. N-cadherin is also expressed in endothelial cells, mediates adhesion between endothelium and pericytes or smooth muscle cells, and functions coordinately with VE-cadherin during vascular morphogenesis (Luo and Radice, 2005). Because FGFRs interact directly with N-cadherin and regulate its function, FGF signaling might indirectly control stability of VE-cadherin adhesion, and thereby junctional integrity. Murakami et al. examined this possibility, but found that inhibition of FGF signaling did not affect N-cadherin cell surface expression or N-cadherin-mediated adhesion of endothelial cells to smooth muscle cells. Another model, favored by Murakami et al., is that FGF signaling regulates the stability of AJs by counteracting signals activated by another angiogenic factor, vascular endothelial growth factor (VEGF). In this model (Figure 1), VEGF induces Src-dependent activation of p21-activated kinase (PAK), which in turn phosphorylates VE-cadherin on Ser665 and stimulates its β-arrestin-mediated endocytosis (Gavard and Gutkind, 2006). Although this hypothesis does not as yet have experimental support, the concept of such a crosstalk between FGF and VEGF signaling pathways (Figure 1) is testable. Because FGF2 is known to induce VEGF expression in endothelial cells through paracrine and autocrine mechanisms (Seghezzi et al., 1998), it is also possible that inhibition of the FGF system itself perturbs VEGF signaling.
Figure 1. A Speculative Model for the Role of FGF Signaling in Regulating AJ Integrity through Inhibition of VEGF Signaling.
FGF signaling could regulate the integrity of AJs by counteracting VEGF-mediated VE-cadherin internalization. FGF deficiency or increased VEGF production leads to disruption of AJs and increased endothelial permeability. VEGF-VEGFR2 signaling results in sequential activation of Vav2, Rac1, and PAK in a Src-dependent manner. PAK phosphorylates VE-cadherin, leading to dissociation of p120-catenin from VE-cadherin and VE-cadherin internalization. However, FGF signaling could potentially override this mechanism by inhibiting PAK-mediated phosphorylation of VE-cadherin. p120, p120-catenin; β-cat, β-catenin; α-cat, α-catenin.
Although a number of fundamental questions remain, the study by Murakami et al. (2008) has uncovered a new role of FGF signaling and its possible interplay with VEGF signaling in the maintenance of endothelial junctions and, thus, vascular integrity. It is apparent from this study that unraveling further the details of endothelial barrier function, the most important function of the endothelium, has a great deal to teach us about angiogenesis, the disruption of the barrier in disease states, and how to restore barrier integrity.
REFERENCES
- Feldman B, Poueymirou W, Papaioannou VE, DeChiara TM, Goldfarb M. Science. 1995;13:246–249. doi: 10.1126/science.7809630. [DOI] [PubMed] [Google Scholar]
- Gavard J, Gutkind JS. Nat. Cell Biol. 2006;8:1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- Itoh N, Ornitz DM. Trends Genet. 2004;20:563–569. doi: 10.1016/j.tig.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Luo Y, Radice GL. J. Cell Biol. 2005;169:29–34. doi: 10.1083/jcb.200411127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DL, Ortega S, Bashayan O, Basch R, Basilico C. Mol. Cell. Biol. 2000;20:2260–2268. doi: 10.1128/mcb.20.6.2260-2268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita Y, Ozawa M. J. Biol. Chem. 2007;282:11540–11548. doi: 10.1074/jbc.M608351200. [DOI] [PubMed] [Google Scholar]
- Murakami M, Nguyen LT, Zhang ZW, Moodie KL, Carmeliet P, Stan RV, Simons M. J Clin. Invest. 2008;118 doi: 10.1172/JCI35298. in press.. Published online September 5, 2008. 10.1172/JCI35298.
- Seghezzi G, Patel S, Ren CJ, Gualandris A, Pintucci G, Robbins ES, Shapiro RL, Galloway AC, Rifkin DB, Mignatti P. J. Cell Biol. 1998;141:1659–1673. doi: 10.1083/jcb.141.7.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Meyers EN, Lewandoski M, Martin GR. Genes Dev. 1999;15:1834–1846. doi: 10.1101/gad.13.14.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, Potente M, Daly C, Dimmeler S, Dejana E. Nat. Cell Biol. 2008;10:923–934. doi: 10.1038/ncb1752. [DOI] [PubMed] [Google Scholar]