Abstract
Purpose
Tear lipocalin (Tlc) is a major lipid binding protein in tears and is thought to have an important role in stabilizing the Meibomian lipid layer by transferring lipids to it from the aqueous layer or ocular surface, or by adsorbing to it directly. These possible roles have been investigated in vitro using human Tlc.
Methods
Tlc was purified from human tears by size exclusion chromatography followed by ion exchange chromatography. Three additional samples of the Tlc were prepared by lipidation, delipidation, and relipidation. The lipids extracted from the purified Tlc were analyzed by HPLC-MS followed by fragmentation. Adsorption of these different forms of Tlc to a human Meibomian lipid film spread on the surface of an artificial tear buffer in a Langmuir trough were observed by recording changes in the pressure with time (∏-T profile) and monitoring the appearance of the film microscopically. These results were compared with similar experiments using a bovine Meibomian lipid film.
Results
The results indicated that Tlc binds slowly to a human Meibomian lipid film compared with lysozyme or lactoferrin, even at 37°C. The adsorption of Tlc to a human Meibomian lipid film was very different from its adsorption to a bovine Meibomian lipid film, indicating the nature of the lipids in the film is critical to the adsorption process. Similarly, the different forms of Tlc had quite distinct adsorption patterns, as indicated both by changes in ∏-T profiles and the microscopic appearance of the films.
Conclusions
It was concluded that human Tlc was capable of adsorbing to and penetrating into a Meibomian lipid layer, but this process is very complex and depends on both the types of lipids bound to Tlc and the lipid complement comprising the Meibomian lipid film.
Human tear lipocalin (Tlc) is the third most abundant protein found in tears and is able to bind a broad range of lipid ligands that include fatty acids, cholesterol, fatty alcohol, phospholipids, glycolipids, and retinoids.1,2 A major function attributed to Tlc is the scavenging of lipids from the ocular surface.3 However, Tlc also contributes to the biophysical properties of tears by reducing their surface tension4,5 and increasing their viscosity,6 both of which help in stabilization of the tear film on the ocular surface. The effects of Tlc on viscosity vary depending on whether or not other tear proteins are present,6 and there are strong indications that Tlc may be strongly interacting with lysozyme (LZ) and lactoferrin (LF) in the tear film.7,8 In terms of its effects on surface tension, this varied depending on whether apo-Tlc or holo-Tlc were used. Holo-Tlc (2 to 4 μg/mL) has been shown to adsorb less readily than apo-Tlc to an air-aqueous buffer interface at ∼34°C.9 A similar result was obtained at ∼24°C using ∼24 μg/mL Tlc.10 Tlc has also been shown to adsorb to phospholipid films held at pressures up to ∼30 mN/m, and more readily adsorb to those held at lower initial surface pressures. Previously, we have shown that apo-Tlc penetrated more readily than holo-Tlc into a bovine Meibomian lipid film held at 10 mN/m at 20°C.11 These data all point to Tlc adsorbing to and penetrating into the Meibomian lipid layer of a tear film. Although indicative of Tlc having a role at the surface of the tear film, a better understanding could be achieved by using conditions that emulate the human tear film. This has been attempted in this study where adsorption of Tlc to a human Meibomian lipid film at 20°C has been examined and compared with adsorption of Tlc to films held at 37°C. Similar experiments were then carried out using delipidated Tlc and Tlc that had been lipidated with a fluorescent cholesterol derivative (NBD-cholesterol) to investigate possible release of lipids to the Meibomian lipid film.
Materials and Methods
Polyacrylamide gels (10%) were used for sodium dodecyl sulfate- polyacrylamide gel electrophoresis (SDS-PAGE) and molecular weight markers were precision plus kaleidoscope (Bio-Rad, Gladesville, NSW, Australia). Polyacrylamide was purchased from Bio-Rad, and all other chemicals used for SDS-PAGE were of analytical grade and were purchased from Sigma-Aldrich (Castle Hill, NSW, Australia). Coomassie Blue R-250 (Bio-Rad) or silver nitrate (Sigma-Aldrich) was used for staining polyacrylamide gels.
NBD-cholesterol (25-{N-[(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-methyl-]amino}-27-norcholesterol) and NBD-phosphatidylcholine (1-acyl-2-[12-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino] dodecanoyl]-sn-glycero-3-phospho-choline) were obtained from Avanti Polar Lipids Inc. (Alabaster, AL). Chloroform used for dissolving lipids was HPLC grade (Lab-Scan, Kings Park, NSW, Australia). There was no surface activity associated with this solvent. Ion exchange purified water (Milli-Q; Millipore, Billerica, MA) with a resistance of 18.2 MΩ was used in all experiments. An artificial tear (AT) buffer that emulates ion concentrations in human tears12 was prepared (NaCl, 6.6 g/L; KCl, 1.7 g/L; NaHCO3, 1.4 g/L; CaCl2 · 2H2O, 0.15 g/L, NaH2PO4 · H2O, 0.1 g/L; MOPS, 4.18 g/L [pH 7.4]) and used as the subphase in the surface pressure experiments described below.
Collection of Meibomian Lipids
The research protocol for collection of human Meibomian lipids included informed consent and was approved by the local (Biomedical) Human Research Ethics Advisory Panel reference number 074012. The protocol was in accordance with the World Medical Association Declaration of Helsinki for “Ethical Principles for Medical Research Involving Human Subjects” (October 2000). Subjects had no signs or symptoms of ocular disease, nor were the subjects on any medication. Human Meibomian lipids were expressed from eyelids by applying pressure using two opposed cotton buds. This is known as hard expression and the Meibomian lipids appear as a paste on the eyelid margin. It should be noted that others have reported that gentle squeezing of Meibomian glands from disease-free patients results in an oily secretion, and a viscous solution from patients with Meibomianitis.13,14 The pasty excretion acquired in our case is not to be confused with the latter and represents the normal appearance of Meibomian lipids that are hard expressed from the glands of normal patients. The extracted Meibomian lipids were collected from the lid margin with a stainless steel spatula and dissolved in chloroform. A number of different samples were collected from three subjects until a total of ∼5 mg was obtained over a two-week period. These samples were pooled to provide enough for all experiments in this study. Before pooling, samples were kept at −80°C. The pooled sample was dried, weighed and reconstituted at 1 mg/mL in chloroform. Bovine Meibomian lipids were collected as previously described15,16 and was in accordance with the ARVO Animal Statement.
Purification of Tlc from Human Tears, Its Lipidation, Delipidation, and Relipidation
Collection of tears was approved by the local human ethics committee and was in accordance with the Declaration of Helsinki for human experimentation. After obtaining informed consent, reflex tears were collected from a single healthy pre-menopausal female volunteer over a number of separate occasions during an eight-week period, at different times during the day. Typically, 100 to 300 μL of reflex tears were collected in a single session and these samples were stored at −80°C until a volume of 500 μL had been collected, after which they were processed to minimize deterioration during storage. A total of 6 mL of tears were used for these experiments. The procedure for purification of Tlc from this sample was similar to that described previously.1,9,17,18 Tear proteins were initially separated by size exclusion chromatography using a cross-linked agarose based column (Superose 12 10/300 GL column using an AKTA FPLC system; GE Healthcare, Rydalmere, NSW, Australia). The mobile phase was 50 mM sodium phosphate buffer/0.15 M NaCl (pH 7). Before loading onto the FPLC column, tear samples were centrifuged at 4000g for 5 minutes and the supernatant was loaded onto the column via a 0.22 μm filter (Millipore). Chromatography was performed at a flow rate of 0.3 mL/min and 0.5 mL fractions were collected. This meant that the protein was under relatively low pressure through the column and less likely to denature. Absorbance was measured at 280 nm. Fraction analysis by SDS-PAGE revealed that Tlc eluted earlier than expected based on molecular weight, and always co-eluted with lactoferrin (LF) and lysozyme (LZ; Fig. 1). Appropriate fractions were pooled and concentrated using a 3 kDa ultrafilter (Vivaspin; Sartorius, Aubagne, France). Ion exchange chromatography was carried out using a mini H spin column (Vivapure S; Sigma-Aldrich). The column was equilibrated with 25 mM sodium phosphate buffer (pH 7.2) by loading 400 μL onto the spin column and centrifuging at 2000g for 5 minutes. A protein sample (400 μL) was loaded onto the equilibrated spin column and spun at 2000g for 5 minutes, and the wash containing Tlc was collected. This was repeated until the entire sample was loaded. The column was washed twice with 400 μL of 25 mM sodium phosphate buffer (pH 7.2) to obtain any further Tlc. To release and recover proteins such as LZ and LF, which adsorb to the ion exchange membrane, 25 mM sodium phosphate (pH 7.2) containing 1M NaCl was applied to the column and centrifuged at 2000g for 5 minutes.
Figure 1.
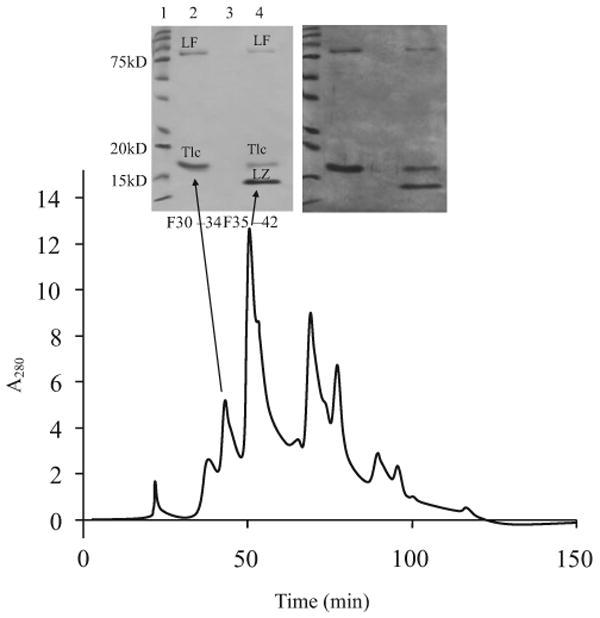
Elution profile of whole tears from sized exclusion chromatography with corresponding Coomassie blue (left) and silver stained (right) gels of selected pooled 0.5mL fractions. Arrows link the absorbance spectrum and pooled fractions to their lane in the gel. The different bands are lactoferrin (LF), lysozyme (LZ), and tear lipocalin (Tlc).
SDS-PAGE of the purified Tlc sample was carried out under reducing conditions. Two-dimensional gel electrophoresis was done using an isoelectric focusing system (IPGphor; GE Healthcare) and IPG strips (pH 3 to 10, length 7 cm; Sigma-Aldrich). The gels were stained with Coomassie blue or silver.
To microscopically monitor the performance of Tlc at an air liquid interface, various forms of Tlc were prepared by loading it with a fluorescently tagged form of cholesterol (NBD-cholesterol). Cholesterol was chosen because Tlc has been shown to bind cholesterol1 and cholesterol is a neutral lipid. For the purposes of these experiments, the purified Tlc was assumed to be carrying a mixture of lipids from the tears and will be referred to as purified Tlc. This was loaded with NBD-cholesterol without prior delipidation using previously described methods.6,19 This sample will be referred to as lipidated Tlc. The expectation was that there would be some apo-Tlc in this sample and it would become loaded with NBD-cholesterol, and some of the lipids in holo-Tlc would be replaced by NBD-cholesterol. Another sample of the purified Tlc was delipidated by extraction with a mixture of chloroform:methanol (2:1).1 This will be known as delipidated Tlc. The lipid extracts were dried and stored at −80°C for analysis. Some of the delipidated Tlc was loaded with NBD-cholesterol using the same method as used for the lipidation above; this will be known as relipidated Tlc.
Analysis of the Lipid Extracts from Human Tlc
The extracts were reconstituted in an n-hexane:propan-2-ol (1:1, v/v) solvent mixture (HP) flushed with nitrogen, and stored in a glass vial at −80°C. The samples were readily soluble in the solvent and no precipitate was observed, indicating no salts, peptides, or carbohydrates were present in the samples. Analyses were performed as described previously.20,21
Two separation procedures were used: the first was optimized for nonpolar lipids, and the second was designed to separate polar lipids. The nonpolar lipids were separated by normal phase (NP) high pressure liquid chromatography using a 3.2 × 150 mm, 5 μm column (Lichrosphere Diol; Phenomenex, Torrance, CA). An HPLC system (Alliance 2695 HPLC Separations Module; Waters Corp., Milford, MA) was used throughout the study. Isocratic elution of the compounds was performed at 30°C and a flow rate of 0.3 mL/min using a n-hexane: propan-2-ol:acetic acid (HPA; 949:50:1, v/v/v) solvent mixture. The whole effluent was directed to an ion trap mass spectrometer (LCQ Deca XP Max; Thermo Electron Corp., San Jose, CA) using an atmospheric pressure chemical ionization (APCI) ion source and analyzed in both positive and negative ion modes. The system was controlled using data system software (XCalibur; Thermo Electron Corp.). The retention times and m/z values of the analytes were compared with those of authentic lipid standards. Between 1 and 30 μL of the sample solutions were injected. No attempts to quantify the analytes were made.
The lipid components of the extracts were also separated by NP HPLC using a 3.2 × 150 mm, 5 μm column (Lichrosphere Si-60 silica gel HPLC; Supelco, Bellefonte, PA). The elution of polar lipids (both authentic standards and unknowns in the sample) was detected using electrospray ionization (ESI) mass spectrometry in the positive ion mode. The separation was performed using gradient elution of the analytes by n-hexane/propan-2-ol/5mM aqueous ammonium formate mixtures at 30°C. For structural elucidation of the putative lipid components, samples were analyzed using direct infusion protocols.20,21
Adsorption of Tlc to Human and Bovine Meibomian Lipid Films
A dual barrier Langmuir trough (NIMA 102M; Nima Technology Ltd, Coventry, UK) was used for the protein adsorption experiments. The principle of this instrumentation (see Ref. 22 for more details of this technique) is that the interaction or the presence of surface active molecules can be measured by an increase in surface pressure over that of water (or buffer) alone. Typically, a small amount of the surfactant of interest (Meibomian lipids in this case) is spread on an aqueous buffer subphase between two movable barriers which are at maximum distance from each other. Initially the number of molecules spread on the surface between the barriers is such that there is no interaction between these molecules and so there is no change in surface pressure. The two barriers are then moved toward each other, decreasing the surface area between the barriers. The consequence is that the surfactant molecules are pushed closer and closer together and start to interact with each other. This is seen as an increase in pressure (∏) which is measured using a Wilhelmy plate (Whatman, Chr 1 filter paper) connected to a Wilhelmy balance. For these experiments, 25 μL of human Meibomian lipids dissolved in chloroform (1 mg/mL) were initially spread between the barriers onto a subphase (∼80 mL of AT buffer, which had had the surface swept clean). The barriers were then moved toward each other from their maximum surface area (80 cm2) until a surface pressure of 10 mN/m was achieved. Although other initial surface pressures could have been used, this was chosen for comparison with previous protein adsorption experiments.11,16 This surface area was kept constant during the adsorption experiments. To test protein adsorption 50 μL of Tlc (1.2 mg/mL in 10 mM PBS; 0.9% NaCl [pH 7.4]) was injected into the subphase outside the barriers, and ∏ monitored over time (∏-T). The expectation was that as more molecules adsorbed to the surface, the molecules on the surface would rearrange and hence ∏ would gradually increase until equilibrium was reached.
After an equilibrium pressure (∏eq) was attained, a series of isocycles were carried out on the protein adsorbed Meibomian lipid film. This is done by moving the barriers toward each other (compressing the film) and then moving them apart (expanding the film) while recording ∏-A profiles. Each experiment was repeated at least three times. The maximum pressure (∏max) when the barriers were closest together was always within ± 0.5 mN/m if the same volume of a particular sample were added to the trough. The temperature of the trough was maintained by a circulatory water jacket connected to a thermostat. The relative humidity in the laboratory ranged from 35% to 55%, but no differences were found when the same experiments were carried out at different humidity.
Fluorescence Microscopy and Photography of the Surface Films
To microscopically examine changes in the Meibomian lipid film due to adsorption of Tlc, Meibomian lipids were doped with a fluorescently tagged phosphatidylcholine (1% NBD-PC). No significant differences were found in the ∏-T profiles with or without the fluorescent tag. Similarly, for adsorption of delipidated Tlc to Meibomian lipid film, NBD-PC was mixed with Meibomian lipids. For monitoring lipidated and relipidated Tlc adsorption, Meibomian lipid films without any fluorescent tag were used. In these cases, any fluorescence observed in the film was the result of NBD-cholesterol which had been loaded into the Tlc. Films were observed at 400× using an epifluorescence microscope (Leica Microsystems GmbH, Wetzlar, Germany) with appropriate excitation and barrier filters. Digital images of the surface films were recorded using an EMCCD camera (back-illuminated, DV887ECS-BV; Andor Ixon, South Windsor, CT) with a shutter speed of 10−2 s or less.
Results
SDS-PAGE and 2D Electrophoresis of Tlc
SDS-PAGE of purified Tlc showed a single band at ∼18 kDa (Fig. 2) and there was no indication of a band at ∼36 kDa even in the silver stained gels, which would have indicated a dimeric form of the protein. Two-dimensional gel electrophoresis showed that there were probably two isoforms and that the protein had an isoelectric point of ∼pH 5 (Fig. 2B), which is consistent with the literature.23 Western blot was also used to confirm that this band was Tlc (data not shown).
Figure 2.
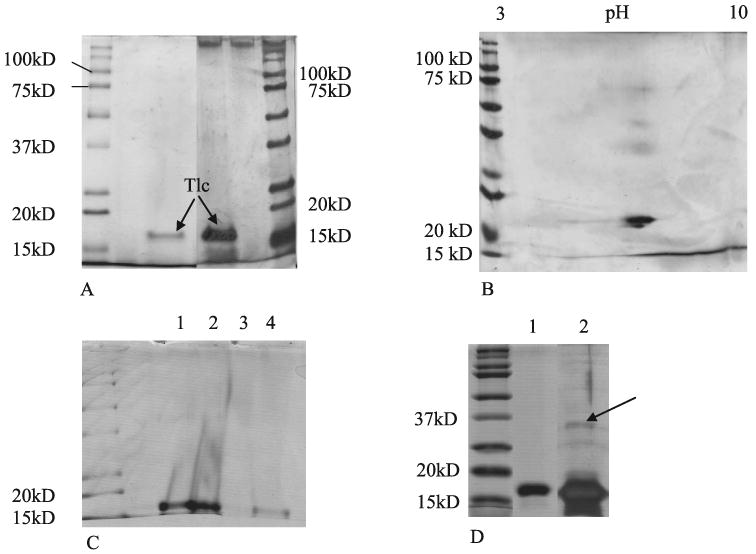
SDS-PAGE of purified Tlc sample separated after ion exchange chromatography and size exclusion chromatography. (A) Comparison of Coomassie blue stained gel (left) with Silver stained gel (right). (B) Silver stained 2D electrophoresis gel of purified Tlc. (C) Coomassie stained gel of Tlc (lane 1) lipidated Tlc (lane 2) delipidated Tlc (lane 3) and relipidated Tlc (lane 4). (D) Delipidated Tlc. A large quantity of delipidated Tlc was applied with extra glycerol in the loading buffer. Lane 1 is Coomassie blue stained and lane 2 silver stained. Arrow indicates a possible dimeric form of the protein.
SDS-PAGE was also performed on the lipidated, delipidated, and relipidated forms of Tlc. After delipidation (Fig. 2C, lane 3), delipidated Tlc floated out of the loading pocket in the gel, which caused the apparent distortions in lanes 1 and 2. A second gel was made, and a much higher concentration of glycerol and protein was used in the loading buffer. In this case, besides the band at ∼18 kDa, a faint band could be seen at ∼34 kDa in the overloaded silver stained gel that could indicate a dimeric form of Tlc (Fig. 2D). It should be noted that the possible dimeric forms mentioned here and above would be due to noncovalent dimerization, because the gel was run under reducing conditions. Similarly, under these conditions dimeric forms of Tlc due to cystine formation would not have been detected.
Lipids Obtained from Delipidation of Tlc
Under the conditions of NP HPLC APCI analysis of the lipid extract, a single main HPLC peak with a retention times (RT) of ∼4.4 minutes (Fig. 3A) was produced. Its elution time was in the region of tri- and di-acyl glycerols rather than wax esters (RT ∼3.2 min), cholesterol esters (RT ∼3.4 min) and free cholesterol (RT ∼7.4 min), which are typical components of Meibomian lipids.20,21 Using positive ion mode the main ions m/z were 663.3, 637.1 (minor), 607.4, 593.5, 579.4, 565.4, 551.4, and 537.4 (Fig. 3B). To elucidate their possible structures, these peaks were fragmented, and some had either unexpected or very complex fragmentation patterns. For instance, a m/z of 663 could have been an ion of dehydrated diarchidoylglycerol ([M − H2O + H]+). However, sequentional MSn fragmentation in the positive ion mode at the relative collision energy of 40 V produced a series of fragments differing by 56 atomic mass units (amu): 663 (MS1) → 607 (MS2) → 551 (MS3) → 495 (MS4) → 439 (MS5) → 383 (MS6) → 327 (MS7). This was attributed to a potential neutral loss of six [(CH3)2C=CH2] fragments consistent with fragmentation studies of Irgafos24 (a contaminant from plasticware) rather than diarachidonoylglycerol. This was confirmed in a second sample, in which the lipids of the Tlc sample had been extracted in plasticware rather than glass. In this case, the strongest m/z signal was 663; there was also a strong signal at 637. Fragmentation of these gave the same pattern as above (not shown). The peaks 663 and 495 co-eluted, indicating that the 495 peak was also derived from Irgafos. Similarly, in the negative ion mode, the peak displayed a spectrum with a prominent ion at m/z 473.5 oxidized Irgafos (data not shown).
Figure 3.
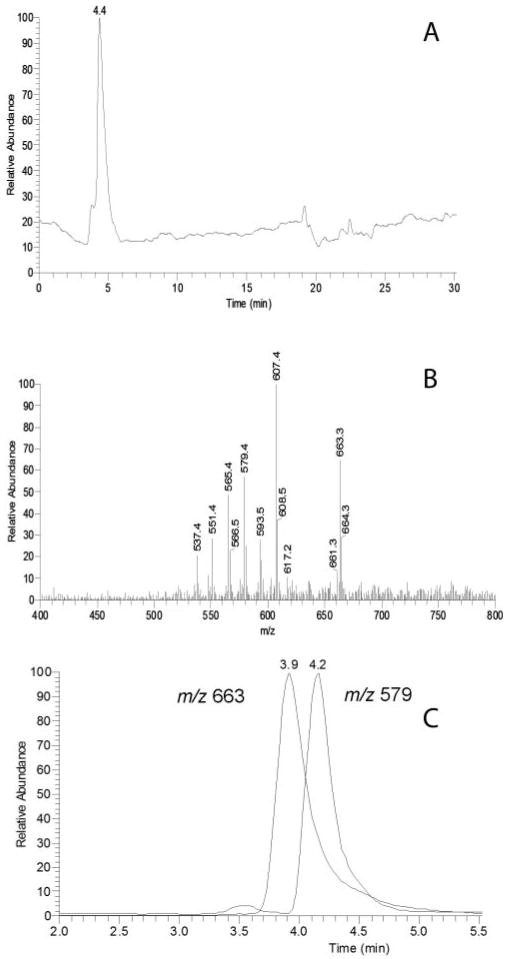
Chromatographic separation and mass spectra of the analytes present in lipid extracts from Tlc. (A) Total ion chromatogram after separation on a Diol column in the HPA solvent. Detection was by APCI MS in the positive ion mode. (B) Integrated MS spectrum of peak with RT ∼4 min. (C) Single ion chromatograms of ions with m/z values of 663 and 579. The ion intensities are normalized.
By contrast, the fragmentation of the peak with an m/z value of 579.4 gave: 579 (MS1) → 561 (579 − H2O) + 327 + 267 + 239 + 221 (239 − H2O). When plotted as two single ion chromatograms detected in one HPLC MS experiment, ions 663 and 579 eluted as two clearly different HPLC peaks (Fig. 3C). The m/z peak of 607 gave a complex pattern indicating that it was clearly composed of two different isobaric compounds, one which produced ions m/z 551 and 495 (loss of 56 amu described above consistent with it being an Irgafos contaminant) while the other gave strong signals m/z 327, 285, 267 (285 − H2O), and 249, and a much weaker signal m/z 589 (607 − H2O). Signals consistent with phospholipids were not detected in the samples using HPLC-MS and direct infusion MS protocols described previously.20,21
Compression and Expansion Isocycles of Tlc Samples (Purified, Lipidated, Delipidated, and Relipidated) Applied to the Surface of the Subphase
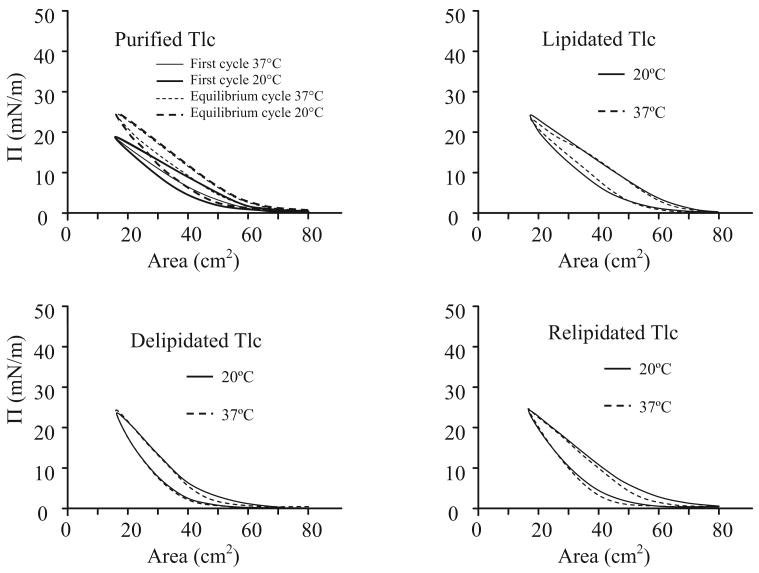
Purified Tlc (35 μL) dissolved in AT buffer (1 mg/mL) was spread on the buffer surface (no lipids present) between the barriers and allowed to equilibrate for five minutes. Isocycles were then carried out until there was no further change in the ∏-A curve (equilibrium). Irrespective of the temperature, the equilibrium ∏-A curve (after 100 cycles) compared with the initial curve showed an increase in ∏max (maximum pressure recorded during compression) from 18 mN/m to 25 mN/m, and take-off (the area where ∏ first increased above 0 mN/m during compression) increased. There was little difference in the overall shape of the initial and equilibrium curves. Similar studies were done with lipidated, delipidated and relipidated Tlc (Fig. 4). In all cases, the ∏-A curves were very similar, being relatively linear during compression, and there was more hysteresis during expansion at 20°C than at 37°C. This latter finding would be expected with increased molecular mobility at the higher temperature; the molecules rearrange more quickly resulting in decreased viscosity. Notably, the lipidated and relipidated samples show more hysteresis than the delipidated sample. The overall lack of differences in these curves indicate that there was little unfolding or rearrangement of the molecules on the surface.
Figure 4.
The compression and expansion isocycles at 20°C and 37°C of human Tlc in various forms (purified, lipidated, delipidated, and relipidated) at an air-AT buffer interface. For purified Tlc, the first and equilibrium isocycles are also shown.
Compression and Expansion Isocycles of Meibomian Lipid Films
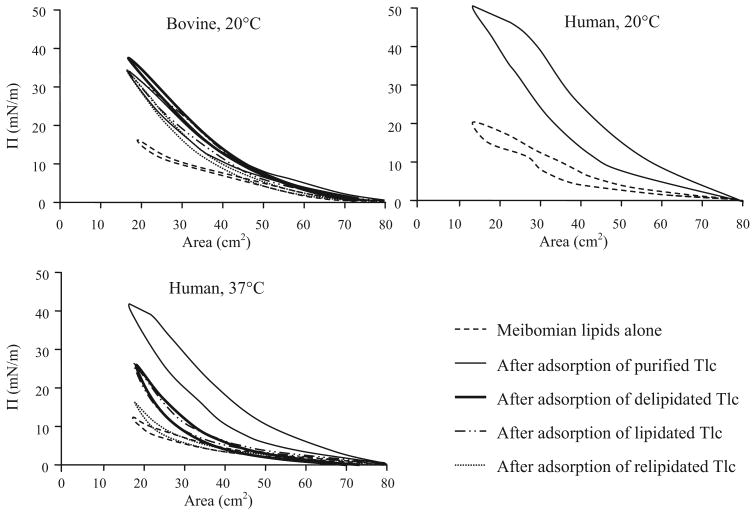
Isocycles of a human Meibomian lipid film at 20°C differed substantially from those obtained with an equivalent amount of bovine Meibomian lipids (Fig. 5). ∏max was consistently higher for human Meibomian lipids, and there was some hysteresis on expansion of the human Meibomian lipid film. The apparently small difference in ∏max between human and bovine Meibomian lipids (20 mN/m versus 18 mN/m) was consistently observed. To obtain a ∏max of 20 mN/m with bovine lipids, 40 μL of 1 mg/mL had to be spread on the surface (nearly double the number of molecules). Seeding the film with NBD-PC did not alter these ∏-A curves. At 37°C, ∏max was reduced and there was less hysteresis in the human Meibomian lipid film (Fig. 5).
Figure 5.
Compression and expansion isocycles of bovine and human Meibomian lipid films before and after adsorption of different forms of Tlc. After adsorption, ∏max and hysteresis are always greater and more marked for human Meibomian lipid films adsorbed with purified Tlc.
Microscopically, human Meibomian lipid films were relatively amorphous, and there were no obvious differences at 20°C versus 37°C apart from more mobility in the film at the higher temperature. The film initially appeared amorphous and progressively small dark patches and “gray” regions appeared with increasing pressure (Fig. 6, ∏=7). These gray patches coalesced and condensed into nonfluorescent patches. At ∏max, the film was again relatively amorphous interlaced with nonfluorescent wispy patches (Fig. 6).
Figure 6.
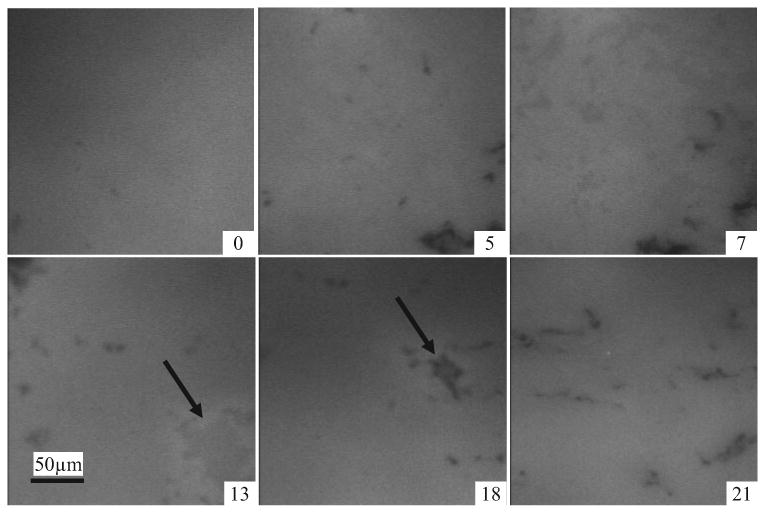
Micrographs of a human Meibomian lipid film doped with a small amount of fluorescently tagged phospholipid. These are at 20°C and the films held at increasing surface pressures (∏ = 0 – 21 mN/m). Lighter areas are due to fluorescence of the marker lipid. Notable is that between ∏ = 13 mN/m and ∏ = 18 mN/m, a gray amorphous zone becomes a condensed dark region (arrows). In these and other micrographs, the width of one panel is ∼250 μM.
Adsorption of Various Forms of Tlc to Meibomian Lipid Films
This section is complex because of the various combinations and permutations of Tlc and lipid films that were used. A brief explanation is given here for clarification. In essence, the adsorption of purified Tlc (regarded as holo-Tlc loaded with a range of lipids from the tear film) into either a human Meibomian lipid film or a bovine Meibomian lipid film was compared with adsorption of delipidated Tlc (apo-Tlc). To monitor the adsorption of purified Tlc or delipidated Tlc microscopically, the Meibomian lipid films themselves were seeded with NBD-PC (the fluorescent tag was in the Meibomian lipid film, not on the Tlc). The adsorption to a bovine Meibomian lipid film was done at 20°C, because similar experiments have been done at this temperature previously.11,16 Experiments using human Meibomian lipid films were done at both 20°C and 37°C, the latter being more physiological.
Similar experiments were carried out using either purified human Tlc which had been lipidated with fluorescently tagged cholesterol (NBD-cholesterol; lipidated Tlc) or delipidated Tlc which had similarly been loaded with NBD-cholesterol (relipidated Tlc). When lipidated or relipidated Tlc was used, no fluorescent tag was used in the Meibomian lipids, hence the fluorescence observed microscopically came from the NBD-cholesterol that was loaded into the Tlc. Theoretically, this could enter the film either bound to Tlc or as free cholesterol.
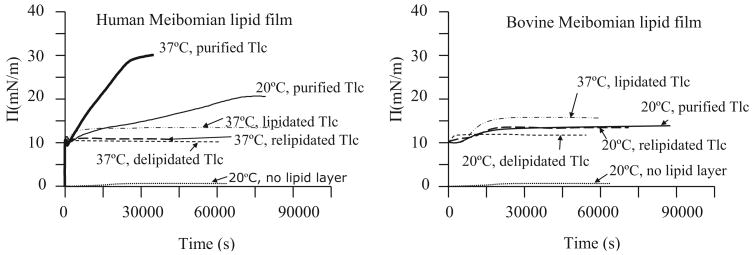
Purified Tlc injected into the subphase outside the barriers caused little change to ∏ inside the barriers when there was no lipid film on the surface (Fig. 7). A bovine Meibomian lipid film spread between the barriers and held at ∏init = 10 mN/m showed a little change in surface pressure if purified Tlc were injected into the subphase outside the barriers. By contrast, adsorption to a human Meibomian lipid film at 20°C or 37°C held at ∏init = 10 mN/m was substantial leading to a ∏eq = 20 mN/m and 30 mN/m respectively. Adsorption was relatively slow at 20°C, but faster at 37°C, and the adsorption curve in both cases was linear. Once equilibrium was attained, ∏-A isocycles were performed. These showed much greater ∏max and much greater hysteresis than for films of Meibomian lipids alone (Fig. 5). The ∏max obtained at 20°C was much greater than that obtained at 37°C.
Figure 7.
∏-T curves for the adsorption of various forms of Tlc (purified, lipidated, delipidated, and relipidated) to a human Meibomian lipid film or a bovine Meibomian lipid film. The Meibomian lipid films were compressed to have ∏init = 10 mN/m before injecting proteins into AT subphase. These curves are compared with adsorption of purified Tlc to an air-AT interface (bottom curve, no lipid layer).
Changes to the appearance of Meibomian films were monitored microscopically during adsorption of purified Tlc and during isocycles after adsorption. For human Meibomian lipid films, the changes to the appearance of the film were similar at 20°C and 37°C as Tlc penetrated the film, hence only data from 37°C are shown. In these micrographs, the brighter areas are more fluorescent and the darker or black areas are less fluorescent. Before the penetration of Tlc, the human Meibomian lipid film held at ∏ = 10 mN/m was relatively amorphous, exhibiting a few small dark (nonfluorescent) patches. After injection of Tlc into the subphase, the film became patchy, presumably as the Tlc (nonfluorescent) penetrated the surface. There was a simultaneous increase in ∏. With further time, dark patches appeared in the film that became progressively larger. Once equilibrium was reached, the barriers were moved toward each other which reduced the surface area and increased ∏. As this happened, the brighter areas of the film appeared to condense so that at ∏max the film looked fuzzy and gel like (Fig. 8). Important from these data is that the film is not homogenous, but comprises domains which presumably contain higher concentrations of lipids and other domains that contain higher concentrations of proteins.
Figure 8.
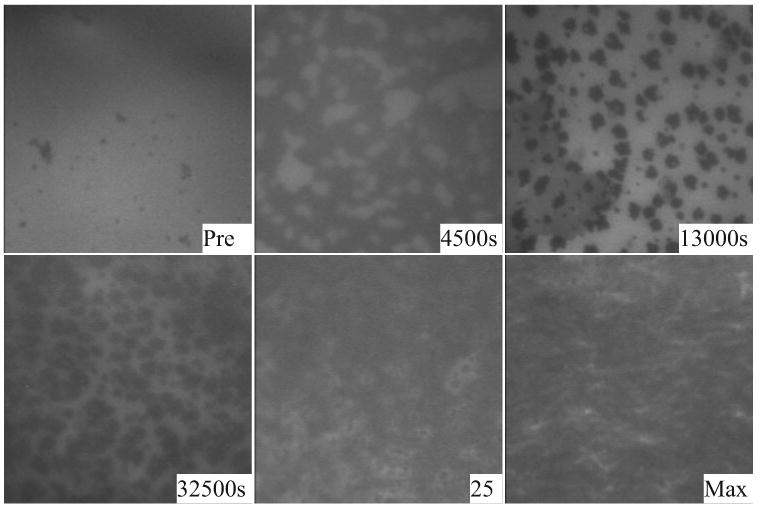
Penetration at 37°C of Tlc into a human Meibomian lipid film initially held at 10 mN/m. The micrographs show the appearance of the film before penetration (Pre) and at various times after penetration (4500 s, 13,000 s). After equilibrium was reached (32,500 s), the film was compressed and its appearance during (∏ = 25 mN/m) and at maximum compression (∏ = 41 mN/m) are shown.
Delipidation was expected to remove all or some of the lipids from Tlc and therefore result in a lower ∏eq after adsorption compared with that obtained with purified Tlc. This was the case for both bovine and human Meibomian lipid films (Fig. 7). By contrast, lipidation of purified Tlc was expected to add NBD-cholesterol to Tlc and/or replace some of the lipids already bound to Tlc with NBD-cholesterol. Therefore, it was expected that more molecules would be added to surface during adsorption, resulting in a higher ∏eq. This was observed with adsorption to bovine Meibomian lipids, but not with adsorption to human Meibomian lipids. Indeed, ∏eq was substantially less (Fig. 7).
Theoretically, relipidation of delipidated Tlc should have loaded it with NBD-cholesterol, hence a ∏eq similar to that of purified Tlc was expected after adsorption. This was observed with adsorption to a bovine Meibomian lipid film, but not with human Meibomian lipid film (Fig. 7). Adsorption of relipidated Tlc to human Meibomian lipid film resulted in a ∏eq that was comparable with that obtained with delipidated Tlc (Fig 7), and far lower than that obtained with purified Tlc.
After ∏eq had been reached, isocycles were performed on the protein adsorbed Meibomian lipid films. There were no significant differences with respect to different forms of Tlc (purified, delipidated, lipidated, and relipidated) for the isocycles of bovine Meibomian lipid films (Fig. 5). However, there were large differences for human Meibomian lipid films (Fig. 5). Hysteresis was less and it was similar to that of Meibomian lipid films alone. ∏max was quite low compared with the purified Tlc adsorbed film. ∏max for lipidated and delipidated Tlc adsorbed films were similar, and for relipidated Tlc adsorbed films, it was further reduced (Fig. 5).
Like purified Tlc, the adsorption of delipidated Tlc to a human Meibomian lipid film was markedly different from the adsorption to a bovine Meibomian lipid film (Figs. 9 and 10). Adsorption to a human Meibomian lipid film initially gave dark small patches in the film followed by larger dark areas at ∏eq. Isocycles caused the dark patches to become condensed and then distinct dark gray and bright areas were seen at higher pressures. Adsorption of delipidated Tlc to a bovine Meibomian lipid film initially resulted in the formation of a few very dark regions and gray zones. The dark regions disappeared and the gray zones coalesced to form squiggly patterns in the film. During isocycles, these squiggly patterns remained even at high pressures (Fig. 11) which was different from the equivalent human Meibomian lipid film in which, during isocycles, small dark patches appeared in the film (Fig. 9).
Figure 9.
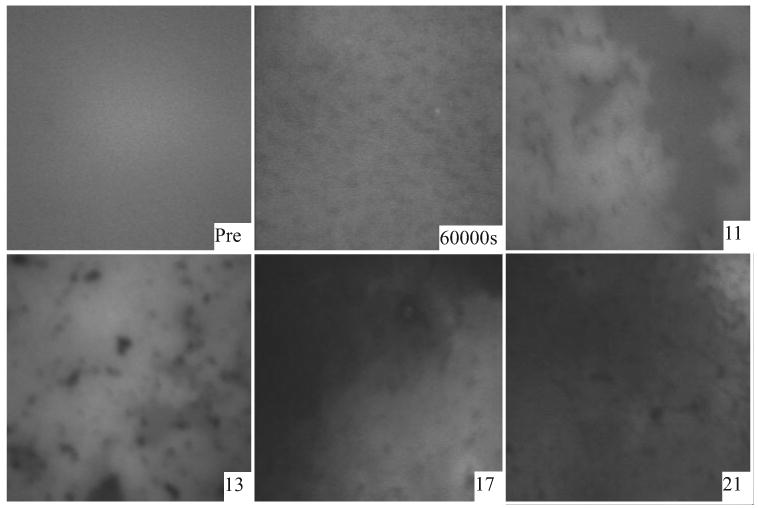
Microscopic appearance of human Meibomian film before (Pre) and after (60,000 s) adsorption with delipidated Tlc at 37°C, and the appearance of the film at different pressures during isocycles after adsorption.
Figure 10.
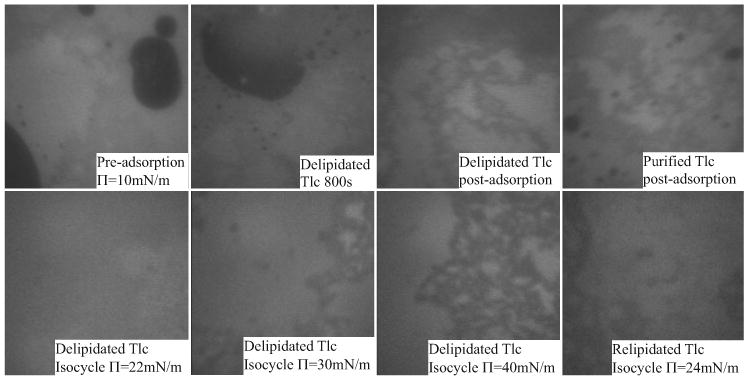
The top row shows the microscopic appearance of a bovine Meibomian lipid film before and after adsorption with delipidated Tlc. After adsorption the film is very similar in appearance to a film with purified Tlc adsorbed (top right). The bottom row shows the appearance of the film during isocycles after adsorption with delipidated Tlc. The appearance of the film is very similar to a film with relipidated Tlc adsorbed (bottom right).
Figure 11.
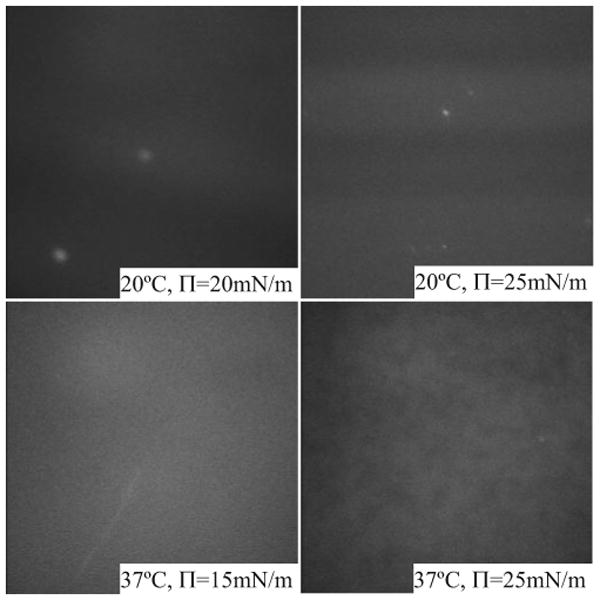
Appearance of lipidated Tlc (NBD-cholesterol loaded) applied directly to the AT buffer surface (no lipids present). At 20°C, the film showed a banded appearance that was more intense at higher pressure. At 37°C, the film was very mobile and diffuse at low pressure, and at high pressure weakly fluorescent patches could be seen that were far less mobile. The brighter fluorescent dots might be crystals of free cholesterol.
As a control for the appearance of films after adsorption of lipidated Tlc, lipidated Tlc was applied directly to the surface of the trough (Fig. 11) and compressed. At 20°C this gave very faint fluorescent banding that was more intense at a higher pressure. At 37°C, the fluorescence was weak and diffuse at low pressures, but at high pressure aggregations of the fluorescence could be seen. The surface was very mobile at 37°C. In both cases, there were small numbers of brightly fluorescent spots, which most probably were crystals of free unbound cholesterol.25
For some of the adsorption experiments using lipidated and relipidated Tlc, the appearance of the surface outside the barriers (at an air buffer interface with no lipid) was observed. The fluorescence was very weak, and the film was very mobile and had a slightly stripy appearance (Figs. 12 and 13), similar to that described above. A striking feature of adsorption of lipidated Tlc to a Meibomian lipid film held at 10 mN/m was that the appearance of the human Meibomian lipid film was very different from the bovine Meibomian lipid film. A main feature of adsorption to a human Meibomian lipid film was the appearance of diffuse bright spots (not crystals) which became smaller with time (Fig. 12). This was not seen during adsorption to a bovine Meibomian lipid film (Fig. 13), nor with adsorption of relipidated Tlc to a human Meibomian lipid film (Fig. 12). During isocycles, these spots were not present at low pressures, but reappeared at higher pressures. Adsorption to a bovine Meibomian lipid film by lipidated Tlc caused a type of clumping of fluorescent areas. During isocycles, these clumps were pushed together at higher pressures to form larger aggregations, and these broke up into smaller aggregates at lower pressure. For relipidated Tlc, the fluorescence was diffuse for the bovine Meibomian lipid film and there were distinct regions of fluorescence. For a human Meibomian lipid film, adsorption of relipidated Tlc resulted in a very diffuse film and at ∏eq the film was honeycombed with less intense fluorescent regions. This was very similar in appearance to the film at the end of adsorption with delipidated Tlc (Fig. 9). On compression of a human Meibomian lipid film after adsorption of relipidated Tlc, diffuse spots, similar to those obtained for lipidated Tlc, could be seen in some regions (Fig. 12).
Figure 12.
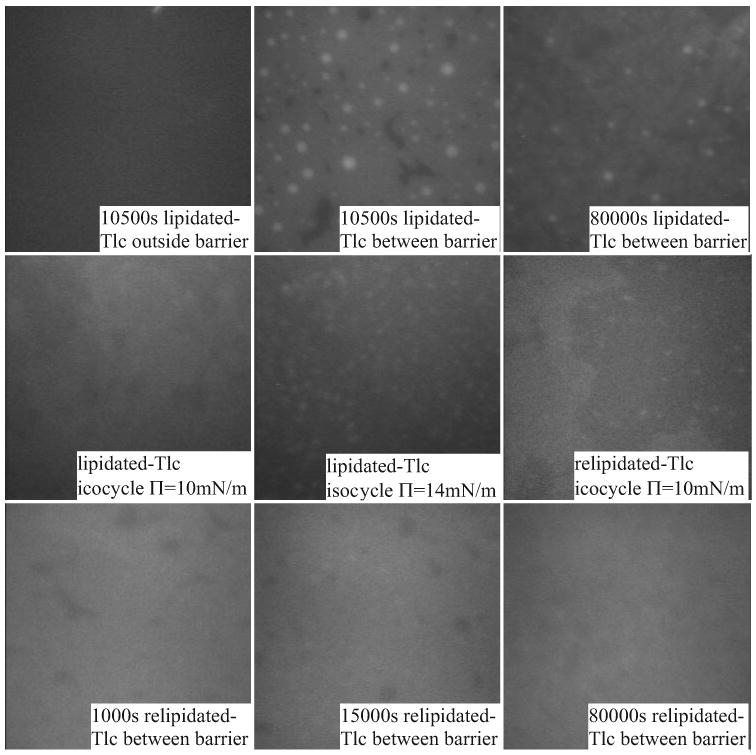
Microscopic appearance of film during adsorption and isocycles after adsorption of lipidated and relipidated Tlc to a human Meibomian lipid film at 37°C. At higher pressures distinct, small bright patches appear.
Figure 13.
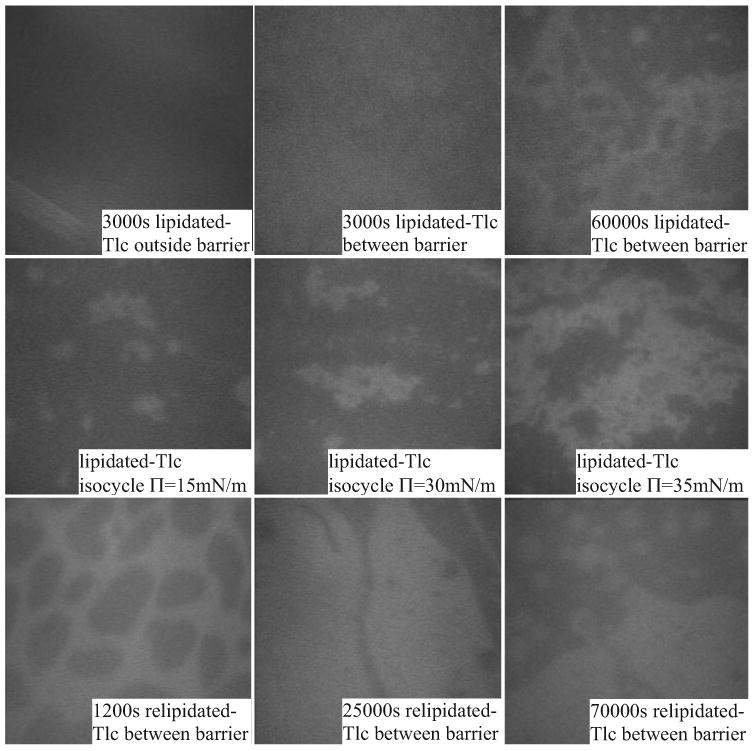
The appearance of adsorption of lipidated and relipidated Tlc to a bovine Meibomian lipid film. The top row shows that adsorption of lipidated-Tlc to the AT buffer surface (outside barrier) is different to adsorption of the lipid layer. The middle row shows the appearance at different pressures during isocycles. The bottom row shows a less condensed appearance of the film when relipidated Tlc is adsorbed.
Discussion
Evidence from the biochemical studies indicates that the Tlc sample used in these studies was very pure. In other studies, mixtures of dimeric and monomeric forms of the protein have been reported6,9,26 and there has been a difference of opinion as to whether the dimeric form represents a lipidated or delipidated form of Tlc. Only the monomeric form was found here, although there was a hint of a possible dimeric form under extreme conditions when the gel was overloaded and silver stained, with the possible dimeric band at the limits of silver stain resolution. Since we were unable to find a difference between lipidated and delipidated samples in terms of dimeric versus monomeric forms, the difference in the literature could be due to different isoforms of the protein (only one isoform was found in this study, but six have been reported6), or there was some denaturation of the protein during purification and or delipidation. The latter seems unlikely because the extreme conditions used in this study for delipidation did not lead to any apparent denaturation of the protein, indicating it is very stable.
One of the most surprising results was the marked difference in the interaction of Tlc with human Meibomian lipid films and bovine Meibomian lipid films. This was unexpected because the overall composition of bovine and human Meibomian lipids is reportedly similar, with differences being mainly in the detail, such as the length of the fatty acid or fatty alcohol chains, and differences in their relative polar lipid composition (13.3% in human, 1.7% in bovine) and hydrocarbon composition (7.5% in human and 0.1% in bovine27). It is also unlikely that the method for collecting the Meibomian lipids could account for this difference because in both cases the lipids were collected by hard expression. It should be noted that in preliminary studies to these experiments, our analysis of gentle versus hard expressed lipids from bovine samples or humans (not reported) revealed no difference in composition. Indeed, high levels of phospholipids found in cell membranes would have been expected if the hard expression contained cellular material due the holocrine nature of the glands, but this was not the case.
Extrapolating these results means that although animal Meibomian lipids are excellent for developing experimental methodologies and providing background data, extreme caution has to be used in drawing direct conclusions about human Meibomian lipids from the animal models. It also implies that subtle differences in Meibomian lipid composition between people may result in profound differences in performance of this layer. A strong indication for this comes from the differences in isocycles of human Meibomian compared with the bovine Meibomian lipid films after adsorption of the various forms of Tlc. An initial mechanism for determining the relative importance of particular lipids to the behavior of the lipid films and how they interact with Tlc would be to compare the performance Meibomian lipid films from individuals rather than a pooled sample. Should there be differences between individuals, then it would necessary to carry out detailed lipid analysis on the same samples to identify critical differences. An alternative experiment would be to seed a pooled sample of human Meibomian lipids with different specific lipids—for example, a fatty acid such as oleic acid—in progressively higher ratios.
In contrast to the differences seen using the different lipid layers, temperature had little effect. The small changes in the isocycle curves and Tlc penetration curves could be attributed to a higher kinetic energy at higher temperatures.
HPLC-MS analyses of the lipids extracted from purified Tlc samples showed detectable amounts of nonpolar compounds similar in their HPLC and MS behavior to diacylglycerols (see below). However, samples clearly had varying amounts of contaminations due to contact with plastic-containing products, even when the lipid extraction itself was done in glass/Teflon vials. This indicates some leaching of plastics occurs even in an aqueous environment and that Tlc can adsorb these plastic contaminants. The unique fragmentation pattern of the compounds with m/z values of 663 in the positive ion mode, and a strong signal m/z 473 detected in the negative ion mode clearly identified it as an oxidized form of Irgafos 16824—a common additive used in production of polyethylene and other polymers. The second compound, which had m/z value of 637, is also related to the same group of additives. Without fragmentation of the extracts, it is likely that we would have drawn an erroneous, but nevertheless satisfying conclusion that these were additional diacylglycerols based on expected results.
Our studies of the lipid extracts from Tlc revealed a relatively simple complement of predominantly diacylglycerols (tentatively identified as 1-palmitoyl-2-stearoyl-glycerol and 1,2-distearoyl-glycerol), based on the fragmentation patterns of peaks with m/z values of 607 and 579. This differs from what has been previously published. Glasgow et al.1 found a broad array of lipids including fatty acids, cholesterol, and phospholipids. This could be due to differences in procedure; for example, the lack of phospholipids in our sample could have been due to ion exchange used to separate Tlc from LF and LZ because theoretically it is possible that this also removed ionic lipids such as phospholipids. This needs to be followed up in the future, by examining the lipid composition of the ion exchange resin retentates.
The difference in how the lipidated and relipidated Tlc adsorbed to Meibomian lipid films compared with purified Tlc could be due to the nature of the bound lipids. The lipidated and relipidated forms have predominantly NBD-cholesterol bound. So in addition to the nature of the lipid film being important (bovine versus human), it appears that the interaction of Tlc with the lipid layer strongly depends on the lipids bound. Added to this is the speculation from above that there are different isoforms of Tlc and these may behave quite differently. A limitation of these experiments was that the binding of cholesterol to Tlc was not quantified, and nor was the degree of its binding into the lipid binding pocket of Tlc19 measured. This would be a useful modification for future experiments, as would loading of Tlc with other lipids such as fatty acids. A further complexity to be considered is the interaction with other proteins as well. There is good evidence that Tlc strongly interacts with LF and LZ and may exist, partly at least, as oligomers with these.8 One would expect that this would also affect the adsorption of Tlc to the lipid layer, and this should be examined in future studies.
Compared with isocycles of other proteins (bovine serum albumin, LZ, LF)28 applied to the surface with no lipids present, Tlc was remarkably stable at the surface, as indicated by only minor changes in the ∏-A isocycle curves with time and changes in temperature. This indicates that there is very little change in conformation or in organization of Tlc molecules when it is at the air liquid interface in the absence of a lipid film. Adsorption of Tlc to a Meibomian lipid layer spread on the surface and isocycles after adsorption indicate that Tlc was able to penetrate the film and not just interact with lipids at the aqueous lipid interface. In particular, this was indicated by the increase in ∏max. The temperature at which these adsorption experiments were carried out had little effect on penetration other than some kinetic changes (stiffness of film). The adsorption of purified Tlc to a human Meibomian lipid film is much slower (∼10 times less) than for LZ or LF (Mudgil P, et al. IOVS 2007;48:ARVO E-Abstract 436; time for ∏eq at 37°C for LZ = 1000 s, for LF = 3000 s, but ∏max was less ∼25 mN/m). An interesting mechanistic reason for such slow penetration comes from a recent study which showed that there is an ionic binding site deep within the binding pocket of Tlc.29 This is capable of binding to fatty acids through their carboxyl group, albeit weakly. If it is assumed that free fatty acids are at the lipid aqueous interface, then Tlc might use this ionic interaction with the fatty acids as an initial phase for adsorption. Since this is weak binding, adsorption would be slow. Whether the differences between the rate of adsorption of Tlc to a Meibomian lipid layer and that of LZ or LF have a physiological relevance still needs to be determined because the concentration of these proteins in tears is at least 100 times greater than used here and presumably these proteins are continuously in contact with the lipid layer (do not have to diffuse through the subphase). Moreover, the presence of LZ or LF might also influence the adsorption of Tlc to a Meibomian lipid layer.
The presence of a human Meibomian lipid layer also seemed to facilitate adsorption of purified Tlc, particularly compared with no lipid layer. This was indicated both by the comparative changes in ∏eq with and without lipids on the surface, and the appearance of Tlc outside the barriers compared with its appearance inside the barriers in the presence of a lipid layer. In particular, the changes to the appearance of the films after adsorption of the various forms of Tlc indicate that Tlc itself is adsorbing to the lipid layer and not simply releasing cholesterol in the case of lipidated and relipidated Tlc. If this were the case, we would have expected that during isocycles under high pressure, crystals of cholesterol would form and once formed they would not dissipate at low pressures, and this was not the case.
Acknowledgments
Supported by the Australian Government linkage project scheme and Alcon #LP0776482, the University of Western Sydney International Research Initiative Scheme, by an unrestricted grant from Research to Prevent Blindness, Inc. (New York, NY), and NIH core Grant EY01666403.
Footnotes
Disclosure: T.J. Millar, None; P. Mudgil, None; I.A. Butovich, None; C.K. Palaniappan, None
References
- 1.Glasgow BJ, Abduragimov AR, Farahbaksh ZT, Faull KF, Hubbell WL. Tear lipocalins bind a broad array of lipid ligands. Curr Eye Res. 1995;14:363–372. doi: 10.3109/02713689508999934. [DOI] [PubMed] [Google Scholar]
- 2.Redl B. Human tear lipocalin. Biochim Biophys Acta. 2000;1482:241–248. doi: 10.1016/s0167-4838(00)00142-4. [DOI] [PubMed] [Google Scholar]
- 3.Gasymov OK, Abduragimov AR, Prasher P, Yusifov TN, Glasgow BJ. Tear lipocalin: evidence for a scavenging function to remove lipids from the human corneal surface. Invest Ophthalmol Vis Sci. 2005;46:3589–3596. doi: 10.1167/iovs.05-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagyova B, Tiffany JM. Components responsible for the surface tension of human tears. Curr Eye Res. 1999;19:4–11. doi: 10.1076/ceyr.19.1.4.5341. [DOI] [PubMed] [Google Scholar]
- 5.Tiffany JM, Nagyova B. The role of lipocalin in determining the physical properties of tears. Adv Exp Med Biol. 2002;506:581–585. doi: 10.1007/978-1-4615-0717-8_81. [DOI] [PubMed] [Google Scholar]
- 6.Gouveia SM, Tiffany JM. Human tear viscosity: an interactive role for proteins and lipids. Biochim Biophys Acta. 2005;1753:155–163. doi: 10.1016/j.bbapap.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Gasymov OK, Abduragimov AR, Yusifov TN, Glasgow BJ. Interaction of tear lipocalin with lysozyme and lactoferrin. Biochem Biophys Res Comm. 1999;265:322–325. doi: 10.1006/bbrc.1999.1668. [DOI] [PubMed] [Google Scholar]
- 8.Gasymov OK, Abduragimov AR, Yusifov TN, Glasgow BJ. Binding studies of tear lipocalin: the role of the conserved tryptophan in maintaining structure, stability and ligand affinity. Biochim Biophys Acta. 1999;1433:307–320. doi: 10.1016/s0167-4838(99)00133-8. [DOI] [PubMed] [Google Scholar]
- 9.Glasgow BJ, Marshall G, Gasymov OK, Abduragimov AR, Yusifov TN, Knobler CM. Tear lipocalins: potential lipid scavengers for the corneal surface. Invest Ophthalmol Vis Sci. 1999;40:3100–3107. [PubMed] [Google Scholar]
- 10.Sarren-Seppälä H, Jaubiainen M, Tervo TMT, Redl B, Kinnunen PKJ, Holopainen JM. Interaction of purified tear lipocalin with lipid membranes. Invest Ophthalmol Vis Sci. 2005;46:3649–3656. doi: 10.1167/iovs.05-0176. [DOI] [PubMed] [Google Scholar]
- 11.Mudgil P, Millar TJ. Adsorption of apo- and holo- tear lipocalin to a bovine Meibomian lipid film. Exp Eye Res. 2008;86:622–628. doi: 10.1016/j.exer.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Mirejovsky D, Patel AS, Rodriguez DD, Hunt TJ. Lipid adsorption onto hydrogel contact lens materials. Advantages of Nile Red over Oil Red O in visualization of lipids. Optom Vis Sci. 1991;68:858–864. doi: 10.1097/00006324-199111000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Mathers WD, Lane JA, Sutphin JE, Zimmerman MB. Model for ocular tear film function. Cornea. 1996;15:110–119. doi: 10.1097/00003226-199603000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Foulks GN, Bron AJ. Meibomian gland dysfunction: a clinical scheme for description, diagnosis, classification and grading. Ocular Surf. 2003;1:107–126. doi: 10.1016/s1542-0124(12)70139-8. [DOI] [PubMed] [Google Scholar]
- 15.Millar TJ, Tragoulias ST, Anderton PJ, et al. The surface activity of purified ocular mucin at the air-liquid interface and interactions with meibomian lipids. Cornea. 2006;25:91–100. doi: 10.1097/01.ico.0000164779.87795.3c. [DOI] [PubMed] [Google Scholar]
- 16.Mudgil P, Torres M, Millar TJ. Adsorption of lysozyme to phospho-lipid and meibomian lipid monolayers. Colloids Surf B: Biointer-faces. 2006;48:128–137. doi: 10.1016/j.colsurfb.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 17.Sack RA, Tan KO, Tan A. Diurnal tear cycle: evidence for a nocturnal inflammatory constitutive tear fluid. Invest Ophthalmol Vis Sci. 1992;33:626–640. [PubMed] [Google Scholar]
- 18.Sack RA, Beaton A, Sathe S, Morris C, Willcox M, Bogart B. Towards a closed eye model of the pre-ocular tear layer. Prog Retinal Eye Res. 2000;19:649–668. doi: 10.1016/s1350-9462(00)00006-9. [DOI] [PubMed] [Google Scholar]
- 19.Gasymov OK, Abduragimov AR, Yusifov TN, Glasgow BJ. Structural changes in human tear lipocalins associated with lipid binding. Biochim Biophys Acta. 1998;1386:145–156. doi: 10.1016/s0167-4838(98)00092-2. [DOI] [PubMed] [Google Scholar]
- 20.Butovich IA, Uchiyama E, McCulley JP. Lipids of human meibum: mass-spectrometric analysis and structural elucidation. J Lipid Res. 2007;48:2220–2235. doi: 10.1194/jlr.M700237-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Butovich IA, Uchiyama E, Di Pascuale MA, McCulley JP. Liquid chromatography-mass spectrometric analysis of lipids present in human meibomian gland secretions. Lipids. 2007;42:765–776. doi: 10.1007/s11745-007-3080-2. [DOI] [PubMed] [Google Scholar]
- 22.Dynarowicz-Latka P, Dhanabalan A, Oliveira ON., Jr Modern physicochemical research on Langmuir monolayers. Adv Colloid Interface Sci. 2001;91:221–293. doi: 10.1016/s0001-8686(99)00034-2. [DOI] [PubMed] [Google Scholar]
- 23.Fullard RJ, Kissner DM. Purification of the isoforms of tear specific prealbumin. Curr Eye Res. 1991;10:613–628. doi: 10.3109/02713689109013853. [DOI] [PubMed] [Google Scholar]
- 24.Carrott MJ, Jones DCG. Identification and analysis of polymer additives using packed-column supercritical fluid chromatography with APCI mass spectrometric detection. Analyst. 1998;123:1827–1833. [Google Scholar]
- 25.Mudgil P, Dennis GR, Millar TJ. The surface pressure dynamics and appearance of mixed monolayers of cholesterol and different sized polystyrene at an air-water interface. Langmuir. 2005;21:1338–1345. doi: 10.1021/la0482498. [DOI] [PubMed] [Google Scholar]
- 26.Gasymov OK, Abduraqimov AR, Merschak P, REdl B, Glasgow BJ. Oligomeric state of lipocalin-1 (LCN-1) by multiple laser light scattering and fluorescence anisotropy decay. Biochim Biophys Acta. 2007;1774:1307–1305. doi: 10.1016/j.bbapap.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tiffany JM. Physiological functions of the Meibomian glands. Prog Retinal Eye Res. 1995;14:47–74. [Google Scholar]
- 28.Tragoulias ST, Anderton PJ, Dennis GR, Miano F, Millar TJ. Surface pressure measurements of human tears and individual tear film components indicate that proteins are major contributors to the surface pressure. Cornea. 2005;24:189–200. doi: 10.1097/01.ico.0000138837.52694.37. [DOI] [PubMed] [Google Scholar]
- 29.Gasymov OK, Abduragimov AR, Glasgow BJ. Ligand binding site of tear lipocalin contribution of a triconal cluster of charged residues probed by 8-anilino-1-naphthalenesulfonic acid. Biochemistry. 2008;47:1414–1424. doi: 10.1021/bi701955e. [DOI] [PMC free article] [PubMed] [Google Scholar]