Abstract
Objective
To determine whether elevated levels of hemostatic factors are associated with the subsequent development of subclinical cardiovascular disease.
Methods
Fibrinogen, factors VII (FVII) and VIII (FVIII), and von Willebrand factor (vWF) were measured in 1396 participants in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Coronary artery calcification (CAC) and carotid intimal/medial thickness (CIMT) were determined 13 years later. The adjusted prevalence of CAC and mean CIMT across the quartiles of each hemostatic factor was computed for the total sample and for each race and gender group.
Results
The age, race, and gender-adjusted prevalences of CAC with increasing quartiles of fibrinogen were 14.4%, 15.2%, 20.0%, and 29.1% (p<0.001 for trend). This trend persisted after further adjustment for body mass index (BMI), smoking, educational level, center, systolic blood pressure (BP), diabetes, antihypertensive medication use, total and high density lipoprotein (HDL) cholesterol, and CRP. A similar trend was observed for CIMT (age, race and gender-adjusted, p<0.001; multivariable-adjusted, p=0.014). Further analyses of race and gender subgroups showed that increasing quartiles of fibrinogen were associated with CAC and CIMT in all subgroups except black men. The prevalence of CAC was not associated with increasing quartiles of FVII, FVIII or vWF, suggesting they may be less involved in plaque progression.
Conclusion
An elevated fibrinogen concentration in persons aged 25 to 37 is independently associated with subclinical cardiovascular disease in the subsequent decade.
Keywords: hemostatic factors, coronary calcium, carotid thickness, fibrinogen, atherosclerosis
The pathogenesis of atherosclerosis is complex, involving the interactions of many genetic and environmental influences. Hemostatic factors appear to be involved early in atherogenesis. More than 20 years ago, the Northwick Park Heart Study1, a prospective study of middle-aged men, reported that elevated levels of fibrinogen, factor VII (FVII), Factor VIII (FVIII), and von Willebrand factor (vWF) predicted future coronary events. Over the ensuing two decades, several investigations have supported this prediction, especially in regard to fibrinogen, and less often for FVII, FVIII, and vWF. Repeated meta-analyses and reviews have shown that increased concentrations of fibrinogen are associated with the development or presence of atherothrombotic disease2,3,4,5. In middle-aged men and women, Bielak et al6 noted a significant association between fibrinogen and coronary artery calcification (CAC), a marker of subclinical coronary atherosclerosis. Another marker of subclinical atherosclerosis, increased carotid intima-media thickness (CIMT), has also been associated with fibrinogen concentration7. There have been other studies reporting that elevated levels of FVII8 and FVIII9, as well as fibrinogen, are associated with a variety of cardiac risk factors, incident cardiovascular disease (CVD), and death. Fibrinogen and vWF were reported to be independent predictors of acute coronary syndromes in patients with angina pectoris10 and have been associated with CIMT and microalbuminuria (p<0.01) after adjustment for other CVD risk factors and inflammatory markers11.
Whether the increases in these hemostatic factors promote plaque formation, or are a consequence of atherosclerosis, is uncertain. For example, hemostatic factors may be increased in patients with vascular disease because of associated inflammation, either in the vessel itself or elsewhere12, 13. To resolve this controversy, Reitsma14 suggests study of hemostasis in persons prior to evidence of vascular inflammation. To this end, we performed measurements of hemostatic factors in healthy young adults, aged 25 to 37. Thirteen years later, we examined these same individuals for evidence of subclinical atherosclerosis, and determined whether there were associations between their original clotting factor levels and the presence of subclinical CVD.
Methods
Study Participants
Participants in this study were from the Coronary Artery Risk Development in Young Adults (CARDIA) study, a multi-center longitudinal study designed to investigate the evolution of CVD risk factors and sub-clinical atherosclerosis. Details of the study design have been published previously15. Briefly, the cohort included 5115 black and white adults aged 18-30 years at baseline (1985-1986) recruited from 4 centers (Birmingham, Ala; Chicago, Ill; Minneapolis, Minn; and Oakland, Calif). Age, race, gender, and education were roughly balanced by the sample design. To date, six follow-up examinations have been completed at years 2, 5, 7, 10, 15 and 20 (2005-2006). As a substudy, hemostatic factors were measured at year 7 (1992-1993, aged 25-37) in participants from the Chicago and Minneapolis centers. The current study includes data from this hemostatic factor substudy and the years 7 and 20 examinations. Of the 2004 participants examined at year 7 from the Chicago and Minneapolis centers, 1828 (91.2%) had hemostatic factor assays. Of these persons, 1436 (78.6%) had CAC or CIMT measurements at the year-20 follow-up. We excluded pregnant women at year 7 or year 20 (n=25), persons with coronary artery disease at year 7 (having physician-diagnosed heart attack, angina, stroke, or transient ischemic attack, n=3), or persons with missing values on the key variables of interest (n=7). In addition, 5 participants who were on lipid-lowering medications were excluded from the cohort because of small numbers in this category. The final sample for analysis was composed of 1396 participants (81.3% of all participants from Chicago and Minneapolis at the year-20 follow-up). Individuals who were lost to follow-up or who had missing data from the Chicago and Minneapolis centers were more likely to be black, male, younger, less educated, and smokers than participants in the study sample.
Data Collection
In the CARDIA study, all data collection technicians were centrally trained and certified. The CARDIA Coordinating Center and the CARDIA Quality Control Committee monitored data collection throughout the study. Informed consent was obtained from each participant at each examination. Participants' age, race, gender, and cigarette use were assessed by questionnaire. Anthropometric variables included height and weight, body mass index (BMI), and blood pressure (BP). Height and weight were measured using a balance beam scale and a vertical ruler, respectively, with the participant wearing light clothing and no shoes. BMI was calculated as the weight (kg) divided by the height in meters squared (m2). The resting BP was measured at each examination in the right arm using a random-zero sphygmomanometer with the participant seated and after a 5 minute rest. Systolic and diastolic BP were recorded as phase I and phase V Korotkoff sounds. Hypertension was defined as systolic BP ≥ 140 mmHg, or diastolic BP ≥ 90 mmHg, or current use of antihypertensive medication. Biochemical variables included total cholesterol, high density lipoprotein (HDL) and low density lipoprotein (LDL) cholesterol, triglycerides, C-reactive protein (CRP), fasting glucose, and insulin. Total cholesterol and triglyceride measurements were made using enzymatic methods16, HDL levels were quantified after dextran sulphate-magnesium precipitation17, and LDLcholesterol was estimated using the Friedewald equation18. Fasting insulin and glucose were measured by a radioimmunoassay after overnight equilibrium incubation and using the hexokinase-ultraviolet method.
Coronary artery calcium (CAC) and carotid intimal-medial thickness (CIMT)
CAC was measured at the year 20 examination by computerized tomography (CT) of the chest19. Electron beam CT (Chicago) and multidetector CT (Minneapolis) scanners were used to obtain 40 contiguous 2.5- to 3 mm-thick transverse images from the root of the aorta to the apex of the heart in 2 sequential scans. Participants were scanned over a hydroxyl-apatite phantom to allow monitoring of image brightness and noise and adjust for scanner differences. Data from both scans were transmitted electronically to the CARDIA CT Reading Center. A calcium score in Agatston units was calculated for each calcified lesion and the scores were summed across all lesions within a given artery and across all arteries (left anterior descending, left main, circumflex, and right coronary) to obtain the total calcium score for the patient.
High-resolution B-mode ultrasonography was used to capture images of the bilateral common carotid (CC) and internal carotid (IC) arteries using a Logiq 700 ultrasound machine (General Electric Medical Systems). One longitudinal image of the CC and three longitudinal images of the IC were acquired. Measurements of the maximal CIMT were made at a central reading center by readers blinded to all clinical information. The maximum IMT of the CC, the bulb/IC was defined as the mean of the IMT of the near and far wall on both the left and right sides. The number of measurements that were available for averaging ranged from 1 to 4 for the CC and 1 to 16 for the IC20. A normalized composite CIMT measure was derived from combining the maximal CC IMT and the bulb/IC IMT (consisting of the arithmetic average of all IC and bulb measurements) by averaging these two measurements after standardization (subtraction of the mean and division by the standard deviation for the measurement).
Hemostasis Factor Measurements
Blood was drawn between 7 AM and 10 AM from participants who were asked to fast for 8 hours. Five ml was placed into each of two vacutainer tubes containing 3.8% sodium citrate, mixed by repeated inversion, and spun at 3,000 × g for 20 minutes at 4° C in a refrigerated centrifuge. This centrifugation was performed within 10 min of collection, and within one hour the plasma was placed in a -70° C freezer.
To avoid cold activation of FVII, the plasma was never held at 4° C except during the brief period of centrifugation prior to freezing. After storage for a maximum of four months, samples were analyzed in batches. They were thawed at 37° C and kept at room temperature until tested. Samples collected in Minneapolis were sent to Chicago by overnight express packed in dry ice. The blood collection and processing system described is virtually identical to that of the ARIC study21 in which this method of sample preparation and overnight storage on dry ice did not alter the concentrations of fibrinogen, FVII, FVIII, or vWF.
Fibrinogen was assessed by the method of Clauss22 using reagents from Dade Division, Baxter Healthcare Corporation, Deerfield, Ill. The assay is calibrated with standard normal plasma (SNP reagent, Dade) which is referenced to the World Health Organization (WHO) standard, and the results calculated using the data management system of the MLA-Electra 800 clot timer.
FVII and FVIII coagulant activities were assayed by a one-stage system using reagents from Pacific Hemostasis, Huntersville, North Carolina and George King Biomedical, Inc., Overland Park, Kansas. The standard curve was prepared using Universal Reference Plasma (URP) from Curtin Matheson Scientific, Woodale, Illinois and the results calculated as a percentage of the standard with the data management system of the MLA-Electra 800. URP is referenced to the WHO standard.
vWF antigen was measured by an ELISA assay23 obtained from American Bioproducts Company, Parsippany, New Jersey. In brief, a plastic support coated with specific rabbit anti-human vWF antibodies binds the factor in the test plasma. Rabbit anti-vWF antibody coupled with peroxidase binds to the remaining free antigenic determinants of the factor and the bound peroxidase is revealed by its activity on ortho-phenylenediamine in the presence of hydrogen peroxide. A standard curve was prepared using URP and the results reported as a percentage of the standard. vWF activity was measured using the ristocetin cofactor assay24. Dilutions of test and reference plasma are mixed in an aggegometer cuvette, and the change in optical density recorded after addition of ristocetin. A standard curve was prepared using URP and the results reported as a percentage of the standard.
The technical errors of the assays were determined by internal and external testing. The internal quality control was performed by obtaining blood from 5 volunteers, splitting each sample into two parts, aliquotting each into 4 parts and storing the aliquots at -20° C. Each week for 4 weeks, samples were retrieved and a complete set of assays performed by technicians blinded to the identity of the samples. The technical error as a percent of the mean for fibrinogen was 5.6%±2.4, for FVII 5.0%±2.2, for FVIII 6.0%±2.3, and for vWF antigen 7.6%±1.5.
In addition, external quality control was assessed using split samples derived from 8% of the study participants (123 pairs). The duplicate samples were given bogus identification numbers, stored for varying periods of time, and assayed as much as four months apart. The technical error for fibrinogen was within 10% of the mean; for FVII and FVIII it was 13-14% of the mean, and for vWF, 17% of the mean.
Statistical Analyses
Year 7 (baseline) characteristics were compared between black and white men and women separately with significant tests by χ2 for categorical variables or t-tests for continuous variables. Participants were divided into quartiles according to their year 7 hemostatic factor levels based on the entire cohort. An overall measure of hemostatic factor level was defined a priori by combining the factors and categorized participants into one of three groups according to their year 7 quartiles of fibrinogen, FVII, FVIII, and vWF antigen levels. Group 1: none of the hemostatic measures in the highest quartile; Group 2: one hemostatic measure in the highest quartile; Group 3: two or more hemostatic measures in the highest quartile. Tests relating hemostatic factors to CAC or CIMT were performed separately for individual hemostatic factors and for the hemostatic factor groups. The presence of CAC was defined as an Agatston score > 0 with alternative cut points (10 and 20) also examined. Continuous CAC scores were not investigated due to the low prevalence of CAC in this young and early middle-aged cohort (aged 38-50) and the skewness of the data. The adjusted prevalence (percentage) of CAC across the quartiles of each hemostatic factor was computed for the total sample and for each race-sex group using linear regression and the test for linear trend was based on logistic regression. Multivariable linear regression was used to model the mean of CIMT across the quartiles of each hemostatic factor for the total sample and for each race-sex group and the test for linear trend was performed with continuous hemostatic variables in the general linear model. Analyses were repeated adjusting for age, gender, race (white/black), BMI, current cigarette smoking (yes, no), educational level, center, systolic BP, diabetes, antihypertensive medication use, total and HDL cholesterol level. Other risk factors (e.g. fasting glucose, CRP, and low-density lipoprotein cholesterol) were examined in additional analyses. We tested for effect modification by sex and race for each hemostatic factor by entering product terms into the linear regression models. None of the interaction terms we tested were significant (p>0.10 for all tests for interactions). However, results were stratified by race and sex for 3 reasons: 1) the CARDIA population was designed to be balanced by age, sex, race and education when recruited at baseline; 2) large sex differences in the prevalence of CAC; and 3) the relationship between risk factors and CAC (or CIMT) may be different for whites and blacks.
Of the 1828 participants who had hemostatic factor measurements in the year 7 exam (baseline) from Chicago and Minneapolis, 377 did not participate in the year 20 exam and 55 were excluded because of pregnancy, coronary disease, or missing data. To investigate the impact of selection bias introduced by these losses to follow-up, we accounted for the missing information by using response probability models to weight the respondents25. Logistic regression was used to model the probability to respond to the year 20 exam separately for each race-sex group and the entire cohort, adjusting for year 7 covariates (age, gender, race, education level, center, BMI, systolic BP, current cigarette smoking, total and HDL cholesterol level). The weights applied to individual responses were the inverse of this response probability (inverse probability weighting [IPW]) and essentially restored the year 7 eligible sample. We used the bootstrap method to estimate the variance of the prevalence of CAC and mean CIMT scores computed with the weighted adjustments by drawing 1000 independent samples, each of the same size as the original sample. We then estimated the prevalence of CAC and mean CIMT for each sample and the confidence intervals from the 1000 samples26. All analyses were conducted using SAS statistical software, version 9.1 (SAS Institute Inc, Cary, NC).
Results
Selected characteristics of the CARDIA participants at the year 7 examination are shown in Table 1. There were more whites and women than blacks and men. The average age of the subjects was 32 and whites were slightly older and had more education than blacks. The mean BMI of the group was 26.5 kg/m2, and was higher in black women than white women. One quarter of the participants, and more blacks than whites, were current smokers. Systolic BP was higher in blacks but hypertension was infrequent (41 participants) and the use of antihypertensive medications was uncommon (1% of participants). HDL cholesterol waslower in white men than in black men. CRP was lower in whites than in blacks, and in men than in women.
Table 1. Year 7 Characteristics of Participants, the CARDIA Study, 1992-1993.
| Characteristics | Total (n=1396) |
Black men (n=217) |
White men (n=426) |
Black women (n=313) |
White women (n=440) |
|---|---|---|---|---|---|
| Age (years) | 32.4 (3.5) | 31.6 (3.7)*** | 32.7 (3.2)*** | 31.6 (3.9)*** | 33.0 (3.1)*** |
| Body mass index (kg/m2) | 26.5 (5.8) | 26.8 (5.2) | 26.1 (4.2) | 28.8 (7.5)*** | 24.9 (5.4)*** |
| Education (years) | 14.6 (2.5) | 13.1 (2.1)*** | 15.5 (2.6)*** | 13.4 (1.9)*** | 15.4 (2.4)*** |
| Current smoking (%) | 27.8 | 41.9*** | 19.9*** | 36.1*** | 22.5*** |
| Diabetes (%) | 1.1 | 1.4 | 0.9 | 0.6 | 1.4 |
| Systolic blood pressure (mmHg) | 108.0 (11.3) | 112.7 (10.7)* | 110.9 (10.1)* | 108.9 (12.7)*** | 102.2 (9.0)*** |
| Antihypertensive medication (%) | 1.0 | 0.5 | 1.2 | 1.6 | 0.7 |
| Total cholesterol (mg/dL) | 177 (33) | 178 (35) | 180 (37) | 174 (32) | 175 (30) |
| HDL cholesterol (mg/dL) | 52 (15) | 52 (14)*** | 46 (14)*** | 57 (16) | 56 (13) |
| C-reactive protein μg/mL, median (range) | 0.98 (0.15-76.4) n=1380 |
1.10 (0.15-46.1)*** n=213 |
0.77 (0.15-23.6)*** n=423 |
1.79 (0.15-76.4)*** n=310 |
0.96 (0.15-40.0)*** n=434 |
Data shown are means (SD) unless otherwise indicated. Diabetes was defined as fasting glucose ≥ 126 mg/100ml or current use of diabetic medication.
P<0.001 compared between blacks and whites within gender.
P<0.01 compared between blacks and whites within gender.
P<0.05 compared between blacks and whites within gender.
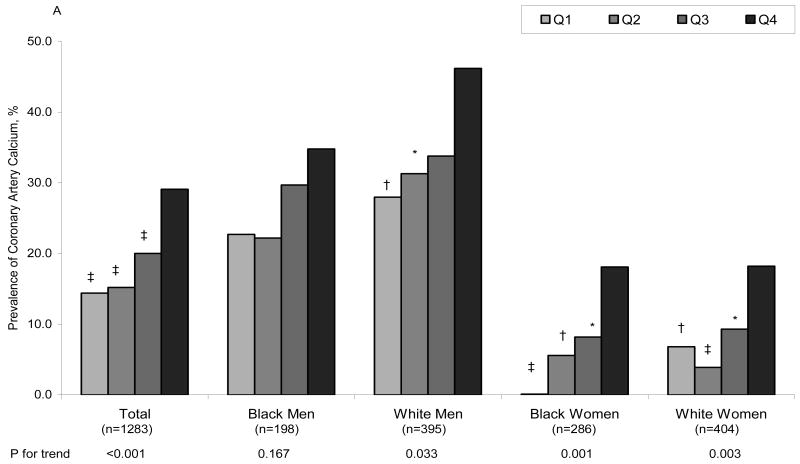
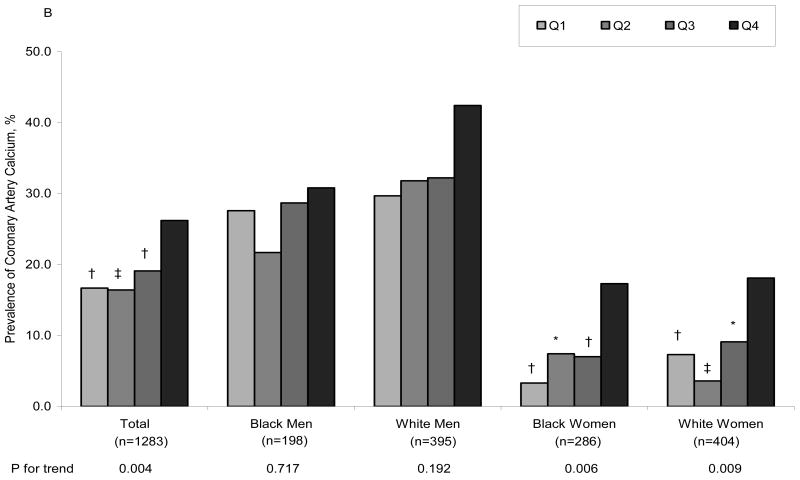
Figure 1A displays the age-adjusted prevalence of CAC at year 20, by fibrinogen quartiles at year 7, in the total group and in each race and gender subgroup. Note that there is a significant graded prevalence of CAC with increasing quartiles of fibrinogen levels for the total group and for each race/gender subgroup except for black men. Figure 1B shows that the association of CAC with fibrinogen levels persists after adjusting for age, BMI, smoking, education, center, systolic BP, diabetes, antihypertensive medication use, total and HDL cholesterol in the total group and in the subgroups of women. Neither FVII, FVIII, vWF activity nor vWF antigen were associated with CAC. Similar relationships were observed with alternative CAC cut points (data not shown).
Figure 1.
Adjusted prevalence of coronary artery calcium after 13 years of follow-up by year 7 fibrinogen level. A= Adjusted for age, B= Adjusted for age, BMI, smoking, education, center, systolic BP, diabetes, antihypertensive medication use, total and HDL cholesterol. Adjusted model for the total sample also included race and sex. * p<0.05, † p<0.01, ‡ p<0.001 compared to the reference group (Q4, the highest quartile).
Table 2 shows adjusted mean CIMT scores by year 7 quartiles of hemostatic factors after 13 years of follow-up, for the total group and for each race and gender subgroup. There is a significant increase in age-adjusted scores with increasing quartiles of fibrinogen levels for the total group and for each race/gender subgroup except for black men. After further adjusting for BMI, smoking, education, center, systolic BP, diabetes, antihypertensive medication use, total and HDL cholesterol, the associations remained significant for the total group (p=0.014) and for black women (p<0.001), but not for men or white women. Age-adjusted CIMT scores were significantly associated with FVII levels, but after multivariable adjustment an association was seen only in white women (p=0.021). In white men, vWF antigen was associated with age-adjusted (p=0.010) and multivariable adjusted (p=0.035) CIMT. No associations of CIMT scores with FVIII or vWF activity were observed. Similar results were observed with the use of alternative risk factors (LDL cholesterol instead of total cholesterol), and addition of fasting glucose, cholesterol-lowering medication, or CRP in the models (data not shown).
Table 2. Adjusted Mean Carotid Intima-media Thickness (CIMT) by Year 7 Hemostatic Factor Quartiles after 13 Years of Follow-up, the CARDIA Study, 1992-2006.
| Year 7 hemostatic factor level (range) | Mean normalized composite CIMT score§ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age-adjusted † | Multivariable-adjusted † | |||||||||
| Total | Black men | White men | Black women | White women | Total | Black men | White men | Black women | White women | |
| Fibrinogen (mg/dl) | ||||||||||
| N | 1249 | 181 | 389 | 275 | 404 | 1249 | 181 | 389 | 275 | 404 |
| Q1 (108-220) | -0.136 | 0.126 | 0.030 | -0.157 | -0.412 | -0.051 | 0.190 | 0.086 | -0.123 | -0.289 |
| Q2 (221-254) | -0.065 | 0.319 | 0.118 | -0.001 | -0.442 | -0.001 | 0.358 | 0.149 | 0.002 | -0.310 |
| Q3 (255-299) | 0.065 | 0.344 | 0.252 | -0.06 | -0.182 | 0.021 | 0.322 | 0.173 | -0.035 | -0.246 |
| Q4 (>299) | 0.204 | 0.347 | 0.403 | 0.261 | -0.116 | 0.093 | 0.246 | 0.303 | 0.270 | -0.309 |
| P trend | <0.001 | 0.122 | 0.003 | <0.001 | 0.005 | 0.014 | 0.461 | 0.100 | <0.001 | 0.481 |
| Factor VII (%) | ||||||||||
| N | 1248 | 180 | 390 | 273 | 405 | 1248 | 180 | 390 | 273 | 405 |
| Q1 (28-64) | -0.019 | 0.273 | 0.074 | -0.005 | -0.243 | 0.049 | 0.378 | 0.150 | -0.033 | -0.126 |
| Q2 (65-75) | -0.059 | 0.321 | 0.011 | 0.032 | -0.357 | -0.004 | 0.301 | 0.091 | 0.052 | -0.276 |
| Q3 (76-86) | -0.003 | 0.349 | 0.114 | 0.164 | -0.382 | -0.022 | 0.297 | 0.125 | 0.180 | -0.385 |
| Q4 (>86) | 0.139 | 0.211 | 0.383 | 0.172 | -0.158 | 0.036 | 0.171 | 0.220 | 0.159 | -0.340 |
| P trend | 0.005 | 0.953 | 0.003 | 0.404 | 0.234 | 0.610 | 0.425 | 0.560 | 0.453 | 0.021 |
| Factor VIII (%) | ||||||||||
| N | 1251 | 181 | 390 | 275 | 405 | 1251 | 181 | 390 | 275 | 405 |
| Q1 (22-75) | -0.009 | 0.224 | 0.192 | 0.094 | -0.392 | 0.006 | 0.176 | 0.169 | 0.148 | -0.331 |
| Q2 (76-95) | -0.020 | 0.006 | 0.154 | 0.066 | -0.303 | -0.008 | 0.047 | 0.200 | 0.035 | -0.289 |
| Q3 (96-121) | 0.032 | 0.282 | 0.075 | 0.140 | -0.197 | 0.033 | 0.337 | 0.072 | 0.107 | -0.202 |
| Q4 (>121) | 0.051 | 0.451 | 0.193 | 0.056 | -0.254 | 0.023 | 0.425 | 0.164 | 0.064 | -0.335 |
| P trend | 0.256 | 0.144 | 0.615 | 0.823 | 0.121 | 0.758 | 0.105 | 0.434 | 0.916 | 0.896 |
| Von Willebrand antigen (%) | ||||||||||
| N | 1221 | 178 | 380 | 271 | 392 | 1221 | 178 | 380 | 271 | 392 |
| Q1 (28-77) | 0.076 | 0.370 | 0.303 | 0.104 | -0.352 | 0.054 | 0.282 | 0.272 | 0.102 | -0.356 |
| Q2 (78-100) | -0.028 | 0.126 | 0.104 | 0.081 | -0.302 | -0.023 | 0.130 | 0.088 | 0.137 | -0.278 |
| Q3 (101-128) | -0.049 | 0.287 | 0.021 | 0.003 | -0.293 | -0.010 | 0.362 | 0.080 | -0.040 | -0.258 |
| Q4 (>128) | 0.047 | 0.378 | 0.083 | 0.108 | -0.200 | 0.025 | 0.360 | 0.084 | 0.100 | -0.282 |
| P trend | 0.612 | 0.291 | 0.010 | 0.980 | 0.140 | 0.687 | 0.240 | 0.035 | 0.987 | 0.377 |
| Von Willebrand activity (%) | ||||||||||
| N | 845 | 124 | 264 | 200 | 257 | 845 | 124 | 264 | 200 | 257 |
| Q1 (17-69) | 0.001 | 0.428 | 0.111 | 0.109 | -0.418 | 0.030 | 0.421 | 0.123 | 0.179 | -0.379 |
| Q2 (70-90) | -0.006 | 0.190 | 0.205 | 0.180 | -0.451 | 0.001 | 0.199 | 0.207 | 0.205 | -0.451 |
| Q3 (91-116) | 0.028 | 0.242 | 0.073 | 0.168 | -0.235 | -0.011 | 0.335 | 0.082 | 0.058 | -0.286 |
| Q4 (>116) | -0.001 | 0.458 | 0.137 | 0.105 | -0.428 | 0.003 | 0.378 | 0.105 | 0.111 | -0.412 |
| P trend | 0.624 | 0.485 | 0.509 | 0.692 | 0.486 | 0.997 | 0.682 | 0.456 | 0.963 | 0.856 |
Age-adjusted models for the total sample also included race and sex. Multivariable models were also adjusted for BMI, smoking, education, center, systolic blood pressure, diabetes, antihypertensive medication use, total and HDL cholesterol.
A normalized composite measure (see method section for details).
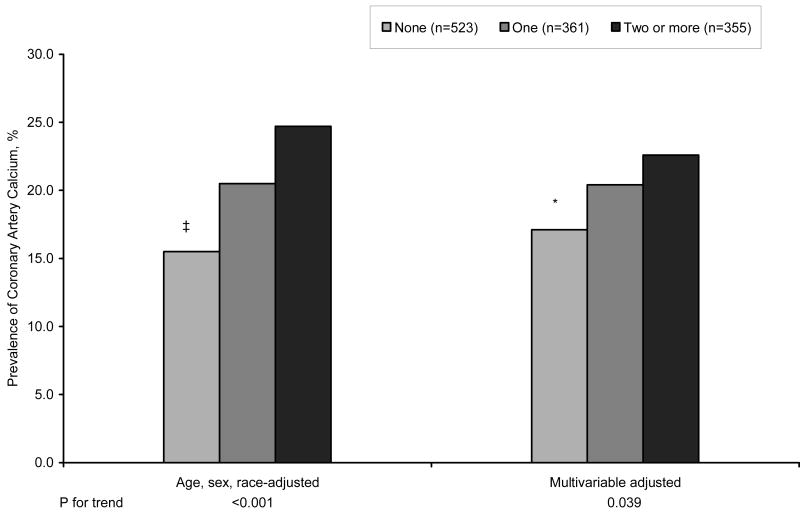
To determine whether the prevalence of CAC or an increased CIMT score would be greater in persons with more than one elevated hemostatic factor, participants were placed into one of three groups based on whether they had none, one, or two or more hemostatic factors in the highest quartile. In age, race, gender, and multivariable-adjusted analyses, the hemostatic group classification was associated linearly with the prevalence of CAC (Figure 2). Table 3 shows that age, sex, and race-adjusted CIMT scores also trended to be higher in persons with two or more hemostatic values in the highest quartile (p for trend 0.001).
Figure 2.
Adjusted prevalence of having coronary artery calcium after 13 years of follow-up by year 7 hemostatic group. Multivariable model was also adjusted for BMI, smoking, education, center, systolic BP, diabetes, antihypertensive medication use, total and HDL cholesterol. * p<0.05, ‡ p<0.001 compared to the reference group (Two or more groups in the highest quartile).
Table 3. Adjusted Mean Carotid Intima-media Thickness (CIMT) by Year 7 Hemostatic Group after 13 Years of Follow-up, the CARDIA Study, 1992-2006.
| Number of year 7 hemostatic measures in the highest quartile | Mean normalized composite CIMT score § | |
|---|---|---|
| Age, sex, and race-adjusted | Multivariable-adjusted † | |
| None (n=508) | -0.063 | -0.003 |
| One (n=343) | 0.014 | 0.001 |
| Two or more (n=360) | 0.121 | 0.049 |
| P trend | 0.001 | 0.334 |
Data shown are adjusted means unless otherwise indicated. Year 7 hemostatic measures included fibrinogen, FVII, FVIII, and vWF antigen.
A normalized composite measure (see method section for details).
Multivariable model was also adjusted for BMI, smoking, education, center, systolic blood pressure, diabetes, antihypertensive medication use, total and HDL cholesterol.
Lastly, we applied a weighting method used in survey research to reduce bias from loss of participants. The response probability models determined that the important effect of censoring due to losses to follow-up was not observed (data not shown). In general, the two sets of estimates (non-response adjustment and complete-case) did not differ significantly. The weighting altered the prevalence rates, means and confidence intervals only to a small degree, thus providing assurance that the results were likely robust despite some loss of participants due to dropout (non-response).
Discussion
The main finding from this study is that elevated levels of fibrinogen in persons aged 25 to 37 were associated with an increased prevalence of CAC and mean CIMT after 13 years of follow-up (ages 38 to 50). These associations persisted after adjustment for standard cardiovascular risk factors and were thus independently associated with subclinical CV disease. When the data were analyzed according to race and gender, CAC and CIMT were associated with fibrinogen levels in all race/gender subgroups except black men. No associations were observed between CAC and the other hemostatic factors measured (FVII, FVIII, vWF antigen and vWF activity). There were also no associations between CIMT scores and FVIII or vWF activity. However, multivariable-adjusted CIMT scores were associated with factor VII levels in white women, and with vWF antigen in white men. Some of these latter findings may be chance associations due to extreme values While Taylor et al27 did not find an association between fibrinogen and progression of CAC, their sample size was small and their window of observation was only 4.2 years.
Fibrinogen contributes to blood viscosity28, platelet aggregation29, fibrin formation, and modulates subsequent coagulation activation and fibrinolysis30. Furthermore, fibrinogen participates directly in atherogenesis. Many years ago, Smith and Crosbie31 showed that as plaques develop they incorporate fibrinogen, fibrinogen fragments, and fibrin. The fibrin then provides a scaffold for smooth muscle cell migration and proliferation and is a source of fibrin degradation products that are mitogenic for macrophages. Smooth muscle cells and macrophages are the major cellular components of the atheromatous plaques associated with coronary and carotid disease. More recent work has shown that as the concentration of fibrinogen rises above physiologic levels, clots become more resistant to lysis; the balance between clotting and fibrinolysis is shifted toward the former30. Persistence of fibrin in areas of denuded endothelium may accelerate plaque formation, accounting for the coronary artery calcification and carotid intimal-medial thickening observed in our relatively young population with high fibrinogen levels.
Fibrinogen is a 340 kDA glycoprotein that circulates in the plasma as a dimer composed of three pairs of polypeptide chains designated Aα, Bβ, and γ. These chains are encoded by the fibrinogen alpha (FGA), beta (FGB), and gamma (FGG) genes. Polymorphisms in the promoter region of FGB, G455→A and C148→T, are associated with a sustained but relatively small increase in fibrinogen levels (0.12 g/L in heterozygotes and 0.24 g/L in homozygotes)32. Our observation that elevated fibrinogen levels in young adulthood may presage atheromatous changes later in life raises the question of whether genetically-programmed increases in fibrinogen are associated with coronary disease. Many investigators have examined this possibility, but a recent meta-analysis of 20 individual studies reported that the risk ratio for FGB and myocardial infarction was 1.00 (confidence interval, 0.95-1.04)33. Therefore, it seems unlikely that the small increases in fibrinogen associated with FGB are atherogenic, but the pathogenicity of higher concentrations is not excluded.
Fibrinogen is an acute-phase protein, participating in the systemic response to inflammation34. Inflammation plays a key role in the development of the atheromatous plaque13. Monocytes infiltrating the plaque differentiate into macrophages that release cytokines, such as interleukin-6, that increase plasma fibrinogen35. CRP is also a marker of atherosclerosis, but we observed that the significant association of fibrinogen with subclinical disease persisted after adjustment for CRP. Fibrinogen, by virtue of its role in platelet crosslinking, thrombus formation, and increased blood viscosity, may enhance plaque progression. 12 On the other hand, the absence of associations with other hemostatic factors that we studied, such as FVII, FVIII, and vWF, suggests that these proteins are less involved than fibrinogen in early plaque development, and are more related to events (angina, myocardial infarction, stroke)10,11,36,37.
Our study has some important limitations. We do not have measurements of subclinical atherosclerosis in the participants at the year 7 examination, and therefore cannot rule out the possibility that atheroma were already present at the time of this examination. Such atheroma would likely not have been detected, since only 0.5% of men and 0.6% of women under age 40 have detectable CAC38. However, if such plaques were present in participants with raised fibrinogen levels, during the 13 year observation period they may have progressed and calcified. Another observation was that the level of fibrinogen in the highest quartile of our participants was only modestly increased; however, it was associated with two different aspects of atheromatous disease (coronary calcification and carotid intimal/medial thickening), demonstrating the consistency of the association. Fibrinogen was measured by the modified Clauss method; inadequate mixing with anticoagulant, lipemic plasma, and repeated freezing and thawing could give spurious results; care was taken to minimize these analytic variables. Another limitation is that there may be residual confounding from unmeasured variables, although we included systematic adjustment for established cardiovascular risk factors. Finally, our results are based on a cohort from 2 urban areas that may not be generalizable to other populations.
Previous work in young adults has shown that fibrinogen concentrations are positively associated with BMI, BP, and cigarette smoking, and negatively associated with physical activity and HDL cholesterol, suggesting that the pattern for atherosclerotic cardiovascular disease is set in early adulthood39. Because CAC and CIMT may be precursors to more severe, symptomatic disease, identification of persons with elevated fibrinogen levels and subclinical atherosclerosis may offer an opportunity to initiate interventions to decrease progression and induce regression of this disorder. Our study of the CARDIA participants provides a unique opportunity to examine the longitudinal effects of hemostatic factors linked to atherosclerosis and correlate their evolution with that of other recognized coronary risk factors preceding clinical manifestations of this disease.
Acknowledgments
Source of Funding: This study was supported by grant HL-43758 and contracts NO1-HC-48049 and NO1-HC-95095 from the National Heart, Lung, and Blood Institute, and grant AG032136 from the National Institute on Aging, National Institutes of Health. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript, except as required of all studies supported by the NHLBI. The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Meade TW, Mellows S, Brozovic M, Miller GJ, Chakrabarti RR, North WRS, Haines AP, Stirling Y, Imeson JD, Thompson SG. Haemostatic function and ischaemic heart disease: principal results of the Northwick Park Heart Study. Lancet. 1986;2:533–537. doi: 10.1016/s0140-6736(86)90111-x. [DOI] [PubMed] [Google Scholar]
- 2.Ernst E, Resch KL. Fibrinogen as a cardiovascular risk factor: a meta-analysis and review of the literature. Ann Intern Med. 1993;118:956–963. doi: 10.7326/0003-4819-118-12-199306150-00008. [DOI] [PubMed] [Google Scholar]
- 3.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998;279:1477–1482. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 4.Feinbloom D, Bauer K. Assessment of hemostatic risk factors in predicting arterial thrombotic events. Arterioscler Thromb Vasc Biol. 2005;25:2043–2053. doi: 10.1161/01.ATV.0000181762.31694.da. [DOI] [PubMed] [Google Scholar]
- 5.Fibrinogen Studies Collaboration. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA. 2005;294:1799–1809. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- 6.Bielak LF, Klee GG, Sheedy PF, Turner ST, Schwartz RS, Peyser PA. Association of fibrinogen with quantity of coronary artery calcification measured by electron beam computed tomography. Arterioscler Thromb Vasc Biol. 2000;20:2167–2171. doi: 10.1161/01.atv.20.9.2167. [DOI] [PubMed] [Google Scholar]
- 7.Baldassarre D, de Jong A, Amato M, Werba PJ, Castelnuovo S, Frigerio B, Veglia F, Tremoli E, Sirtori CR. Carotid intima-media thickness and markers of inflammation, endothelial damage and hemostasis. Ann Med. 2008;40:21–44. doi: 10.1080/07853890701645399. [DOI] [PubMed] [Google Scholar]
- 8.Junker R, Heinrich J, Schulte H, van de Loo J, Assmann G. Coagulation factor VII and the risk of coronary heart disease in healthy men. Arterioscler Thromb Vasc Biol. 1997;17:1539–1544. doi: 10.1161/01.atv.17.8.1539. [DOI] [PubMed] [Google Scholar]
- 9.Tracy RP, Arnold AM, Ettinger W, Fried L, Meilahn E, Savage P. The relationship of fibrinogen and factors VII and VIII to incident cardiovascular disease and death in the elderly. Results from the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1999;19:1776–1783. doi: 10.1161/01.atv.19.7.1776. [DOI] [PubMed] [Google Scholar]
- 10.Thompson SG, Kienast J, Pyke SDM, Haverkate F, van de Loo JCW. Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. N Engl J Med. 1995;332:635–641. doi: 10.1056/NEJM199503093321003. [DOI] [PubMed] [Google Scholar]
- 11.Paramo JA, Beloqui O, Colina I, Diez J, Orbe J. Independent association of von Willebrand factor with surrogate markers of atherosclerosis in middle-aged asymptomatic subjects. J Thromb Haemost. 2005;3:662–664. doi: 10.1111/j.1538-7836.2005.01305.x. [DOI] [PubMed] [Google Scholar]
- 12.Tracy RP. Thrombin, inflammation, and cardiovascular disease: an epidemiologic perspective. Chest. 2003;124:49S–57S. doi: 10.1378/chest.124.3_suppl.49s. [DOI] [PubMed] [Google Scholar]
- 13.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 14.Reitsma PH. Is hypercoagulability an issue in arterial thrombosis? No J Thromb Haemost. 2004;2:692–694. doi: 10.1111/j.1538-7836.2004.00743.x. [DOI] [PubMed] [Google Scholar]
- 15.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Liu K, Savage PJ. CARDIA study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 16.Enzymatic methods for quantification of lipoprotein lipids. Methods Enzymol. 1986;129:101–23. doi: 10.1016/0076-6879(86)29064-3. [DOI] [PubMed] [Google Scholar]
- 17.Warnick GR19, Warnick GT, Benderson J, Albers JJ. Dextran sulphate-mg2+ precipitation procedure for quantitation of high-density-lipoprotein lipids. Clin Chem. 1982;28:1379–1388. [PubMed] [Google Scholar]
- 18.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultrcentrifuge. Clin Chem. 1972;18:499–501. [PubMed] [Google Scholar]
- 19.Carr JJ, Nelson JC, Wong ND, Nitt-Gray M, Arad Y, Jacobs DR, Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234(1):35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 20.O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 21.Papp AC, Hatzakis H, Bracey A, Wu KK. ARIC hemostasis study. 1. Development of a blood collection and processing system suitable for multicenter hemostatic studies. Thromb Haemost. 1989;61:15–19. [PubMed] [Google Scholar]
- 22.Sirridge MS, Shannon R. Laboratory Evaluation of Hemostasis and Thrombosis. 3rd. Lea & Febiger; Philadelphia: 1983. pp. 164–165. [Google Scholar]
- 23.Batlle J, Lopez-Fernandez MF. Laboratory asays for von Willebrand factor. In: Zimmerman TS, Ruggeri ZM, editors. Coagulation and Bleeding Disorders. Marcel Dekkar; New York: 1989. pp. 330–331. [Google Scholar]
- 24.MacFarlane DE, Stibbe J, Kirby EP, Zucker MB, Grant RA, McPherson J. A method for assaying von Willebrand factor (ristocetin cofactor. Thromb Diath Haemorrh. 1975;34:306–308. [PubMed] [Google Scholar]
- 25.Kessler RC, Little RJ, Groves RM. Advances in strategies for minimizing and adjusting for survey nonresponse. Epidemiol Rev. 1995;17(1):192–204. doi: 10.1093/oxfordjournals.epirev.a036176. [DOI] [PubMed] [Google Scholar]
- 26.Lepkowski J. Treatment of wave nonresponse in panel surveys. New York, NY: John Wiley & Sons, Inc.; 1989. [Google Scholar]
- 27.Taylor AJ, Bindeman J, Le TP, Bauer K, Byrd C, Feuerstein IM, Wu H, O'Malley PG. Progression of calcified coronary atherosclerosis: relationship to coronary risk factors and carotid intima-media thickness. Atherosclerosis. 2008;197:339–345. doi: 10.1016/j.atherosclerosis.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 28.Danesh J, Collins R, Peto R, Lowe GDO. Haematocrit, viscosity, erythrocyte sedimentation rate: meta-anlyses of prospective studies of coronary heart disease. Eur Heart J. 2000;21:515–520. doi: 10.1053/euhj.1999.1699. [DOI] [PubMed] [Google Scholar]
- 29.Sinzinger H, Pirich C. Platelet function and fibrinogen. In: Ernst E, Koenig W, Lowe GDO, Meade TW, editors. Fibrinogen: A “New” Cardiovascular Risk Factor. Vienna, Austria: Blackwell-MZV; 1992. pp. 46–50. [Google Scholar]
- 30.Kim PY, Stewart RJ, Lipson SM, Nesheim ME. The relative kinetics of clotting and lysis provide a biochemical rationale for the correlation between elevated fibrinogen and cardiovascular disease. J Thromb Haemost. 2007;5:1250–1256. doi: 10.1111/j.1538-7836.2007.02426.x. [DOI] [PubMed] [Google Scholar]
- 31.Smith EB, Crosbie L. Fibrinogen and fibrin in atherogenesis. In: Ernst E, Koenig W, Lowe GDO, Meade TW, editors. Fibrinogen: A “New” Cardiovascular Risk Factor. Vienna, Austria: Blackwell-MZV; 1992. pp. 4–10. [Google Scholar]
- 32.Smith GD, Harbord R, Milton J, Ebrahim S, Sterne JAC. Does elevated plasma fibrinogen increase the risk of coronary heart disease? Arterioscler Thromb Vasc Biol. 2005;25:2228–2233. doi: 10.1161/01.ATV.0000183937.65887.9c. [DOI] [PubMed] [Google Scholar]
- 33.Keavney B, Danesh J, Parish S, Palmer A, Clark S, Youngman L, Delepine M, Lathop M, Peto R, Collins R. Fibrinogen and coronary heart disease: test of causality by ‘Mendelian randomization’. Intl J Epidemiol. 2006;35:935–943. doi: 10.1093/ije/dyl114. [DOI] [PubMed] [Google Scholar]
- 34.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 35.Heinrich PC, Castell JV, Andus T. Interleukin 6 and the acute phase response. Biochem J. 1990;265:621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zakai NA, Katz R, Jenny NS, Psaty BM, Reiner AP, Schwartz SM, Cushman M. Inflammation and hemostasis biomarkers and cardiovascular risk in the elderly: the Cardiovascular Health Study. J Thromb Haemost. 2007;5:1128–1135. doi: 10.1111/j.1538-7836.2007.02528.x. [DOI] [PubMed] [Google Scholar]
- 37.Catto AJ, Carter AM, Barrett JH, Bamford J, Rice PJ, Grant PJ. Von Willebrand factor and factor VIII:C in acute cerebrovascular disease. Thromb Haemost. 1997;77:1104–1108. [PubMed] [Google Scholar]
- 38.Hoff JA, Chomka EV, Krainik AJ, Daviglus M, Rich S, Kondos GT. Age and gender distributions of coronary artery calcium detected by electron beam tomography in 35,246 adults. Am J Cardiol. 2001;87:1335–1339. doi: 10.1016/s0002-9149(01)01548-x. [DOI] [PubMed] [Google Scholar]
- 39.Folsom AR, Qamhieh HT, Flack JM, Hilner JE, Liu K, Howard BV, Tracy RP. Plasma fibrinogen: levels and correlates in young adults. Am J Epidemiol. 1993;138:1023–36. doi: 10.1093/oxfordjournals.aje.a116821. [DOI] [PubMed] [Google Scholar]