Abstract
Background:
Previous studies have reported intracranial aneurysm (IA) occurring at young ages in subsequent generations. These studies did not correct for duration of follow-up. Second-generation members who would have their ruptured IA late in life may not be detected due to shorter follow-up time than the first generation. We examined families in which ruptured IA occurred in two consecutive generations for the hypothesis that the second generation (F1) was more likely to have a rupture at a younger age than the older generation (F0).
Methods:
The Familial Intracranial Aneurysm (FIA) Study is a multicenter, international study recruiting families of ruptured and unruptured IA. All available family members are interviewed. Cox proportional hazards regression models and Kaplan-Meier curves were used to examine differences by generation.
Results:
Although we found that the F1 generation was more likely to have an aneurysm rupture at a younger age than the F0 generation, we found that this was largely because of a lack of follow-up time in the F1 generation. The F1 generation had 50% the rupture rate of the prior generation. When analyzed by Kaplan-Meier curves, we found a tendency to have a slightly later rupture rate in the F1 generation once time to follow-up was included in the analysis model.
Conclusions:
Families of ruptured intracranial aneurysm (IA) do not appear to demonstrate “anticipation.” Our finding suggests that genetic epidemiology of ruptured IA should examine all types of variations such as single base-pair changes, deletions, insertions, and other variations that do not demonstrate anticipation.
GLOSSARY
- FIA
= familial intracranial aneurysm;
- IA
= intracranial aneurysm;
- SAH
= subarachnoid hemorrhage.
We examined families in which ruptured intracranial aneurysm (IA) occurred in two consecutive generations to test the hypothesis that the second generation (F1) was more likely to have a rupture at a younger age than the older generation (F0).
One of the major pitfalls of determining differences in age at onset by generation is that cases in subsequent generations that occur at a later age may not have been identified at the time of study because of lack of an adequate follow-up period. In addition, knowledge of a family member having an IA (particularly at a certain age) may have led to earlier screening for IA among family members.
To address these issues, we used the phenotype of “ruptured IA” and examined all persons in each of two generations among families in which at least one person in each generation had a ruptured IA. We generated Kaplan-Meier curves and developed Cox proportional hazards models to determine whether the second generation (F1) was more likely to have a rupture earlier in life than the prior generation (F0).
METHODS
The Familial Intracranial Aneurysm (FIA) Study methodology has been previously published.1 Briefly, the FIA Study is an international, multicenter study, including 26 clinical centers representing 41 recruitment sites in North America, New Zealand, and Australia. The FIA Study has been approved by the institutional review boards and ethics committees at each of the study centers.
Intracranial aneurysm was defined as a berry-like defect in the wall of an intracranial artery within the brain. For the purposes of anticipation analysis, we included only those subjects with ruptured IA. At the time of analysis, 429 families with definite or probable IA had been recruited. Exclusion criteria include a fusiform-shaped unruptured IA of an intracranial artery; an IA that is part of an arteriovenous malformation; a family history of polycystic kidney disease, Ehlers-Danlos syndrome, Marfan syndrome, fibromuscular dysplasia, or Moya-Moya syndrome; or failure to obtain informed consent.
All medical records and the phone screen of probands and family members with a reported history of IA, subarachnoid hemorrhage (SAH), or intracerebral hemorrhage are reviewed by a verification committee. This committee consists of study neurologists at the University of Cincinnati and the Mayo Clinic. Two neurologists independently review the records and decide whether the subject meets all of the inclusion and exclusion criteria. In cases of disagreement, a third neurologist is used to resolve the case diagnosis. Each potential affected subject is ranked as follows:
Definite: Documented aneurysm on angiogram, operative report, autopsy, or a noninvasive imaging report (MR angiogram, CT angiogram) demonstrates an IA measuring >7 mm.
Probable: Death certificate mentions probable IA. Death certificate mentions SAH without aneurysm and a phone screen consistent with a ruptured IA. MR angiogram documents IA <7 mm but >3 mm.
Possible: Noninvasive imaging report documents an IA measuring between 2 and 3 mm. SAH is noted on death certificate, without any supporting documentation. Death certificate lists “aneurysm” without specifying cerebral location or accompanying SAH.
Not an affected case: There is no supporting information for a possible IA.
In addition, each case is examined for “ruptured” and “date of rupture,” which is used to define our primary phenotype of interest, “ruptured IA” and “age at rupture.”
Every new case was screened for a family history of IA or intracranial hemorrhage. Hypertension was defined as a history of hypertension prior to the rupture of IA. Smoking history was defined as pack-years prior to the rupture of IA. Frequent alcohol use was defined as greater than two drinks per day on average. For the purposes of the current analysis, we included parent–child or aunt/uncle–niece/nephew pairs with ruptured IA.
Statistical methods.
We used survival analysis methods to identify differences in age at onset by generation. The earlier generation was designated the F0 generation, and the subsequent generation was labeled the F1 generation. The outcome of interest was age at ruptured IA censored by age at death from another cause, lost to follow-up, or end of the study period. Initial comparisons were for average age at rupture for F1 subjects compared with F0 subjects, using a nonparametric approach developed by Rabinowitz and Yang.2 All subjects in each generation were then included in a Kaplan-Meier analysis to make unadjusted comparisons of the two generations. Kaplan-Meier plots were generated to provide a visual representation of the differences in age at rupture, with significance assessed by log-rank tests. However, this analytical approach does not account for correlations of outcomes within families, which may cause a bias in the tests of significance.
To account for the correlation of outcomes within families and to adjust estimates for potential confounders, the Cox proportional hazards model with robust sandwich covariance estimators was used.3 This method effectively considers families to be independent units of observation, while observations within families are correlated. This approach may be conservative in that the sandwich estimator is consistent but may overestimate the standard errors of model parameters. Possible confounders that were examined in the Cox models included sex, race, age at enrollment, cigarette smoking, hypertension, education, and alcohol consumption.
The primary analysis followed each generation for their entire duration of observation but accounted for differences in duration of follow-up through survival analysis methods. To further account for differences in age between generations, in secondary analyses we censored the follow-up of each generation at 5-year intervals beginning at age 40. For example, when we censored observations at age 45, any ruptures occurring after age 45 in the F1 or F0 generation would not be counted; i.e., the follow-up would be limited to ages 45 or less in both the F0 and F1 generations. This approach forces the duration of follow-up to be similar between the two generations.
With our sample size of approximately 800 subjects in each generation, we estimated 80% power to detect a hazard ratio of 1.5 for the rupture rates between generations. Power was calculated for a log-rank test assuming a significance level of 0.05.
RESULTS
Of 429 families with definite or probable IA, 54 (12.5%) were found to have parent–offspring (35) or aunt/uncle–niece/nephew (19) pairs. No significant gender concordance was observed. From these families, all siblings of each generation were included into analysis for a total of 1,641 subjects.
The average age at rupture in the F0 generation was 54 years compared with 41 years for the F1 generation (p < 0.0001). This is the standard method used for comparisons in multiple prior reports without consideration of duration of follow-up. However, the Rabinowitz and Yang nonparametric test controlling for duration of follow-up indicated no significant difference in age at rupture. The average age for last follow-up in the F0 generation was 56.9 years (range 14.5–91.1 years) and for the F1 generation was 44.6 years (range = 13.3–81.8 years). Figure 1 shows the Kaplan-Meier plot for the birth to rupture or censor. It is surprising that the F1 generation had their ruptures later in life than the F0 generation; perhaps rupture at an older age was missed in the F1 generation. Figure 2 shows the hazard plot for rupture by age. The figure demonstrates that the difference in rupture is not at the earlier ages but predominantly at the older ages, where the earlier F0 generation has a higher risk for rupture than the later F1 generation.
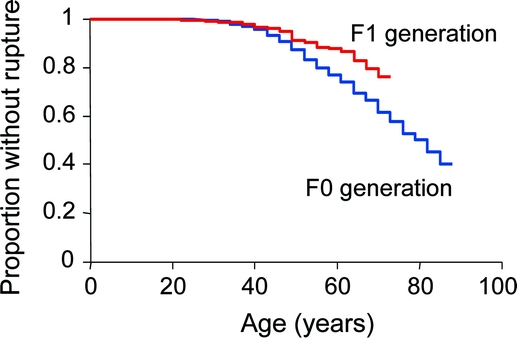
Figure 1 Kaplan-Meier curve of birth to rupture or censor by generation

Figure 2 Hazard plot of birth to rupture or censor by generation
We created six additional analyses in which all subjects were censored between the ages of 40 and 65 and found that the F1 generation had a later age at rupture than the F0 generation in all six cases. Even when all subjects were censored at the early age of 40 years, we found that the F1 generation had a later age at rupture than the F0 generation (adjusted hazard ratio = 0.62, p = 0.03). Among subjects with at least one aneurysm in each generation (includes ruptured and unruptured), 78.8% of the F0 generation and 78.7% of the F1 generation had a single aneurysm.
The unexpected finding of a later onset of ruptured IA among the second generation and the 50% lower prevalence of ruptured IA compared with the older generation prompted an evaluation of the treatment of ruptured IA. It is conceivable that the F1 generation had unruptured IAs treated earlier in life, which prevented later rupture. When the treatment variable was added into the model, only 471 subjects (29.8% of the full cohort) had data available. In that analysis, the difference in age by generation still showed a trend toward older age in the second generation (p = 0.057).
DISCUSSION
We found no significant evidence for a difference in age at onset for ruptured IA by generation. This finding should be contrasted to the existing literature. Prior studies from the Dutch population have identified “anticipation” of IA. Genetic anticipation refers to subsequent generations being affected more severely or at an earlier age than prior generations. Those investigators reported that patients with SAH and a family history of IA were 6.8 years younger than sporadic SAH patients.4 A separate report showed a 23.1-year difference between subsequent generations for proven IA.5 This study included asymptomatic IA, which may have been sought due to parental history, which may lead to an artificial “anticipation.” An additional report examined 20 families with ruptured IA in subsequent generations and identified a 19.8-year difference between generations.6 These studies were all of the same population (Dutch), and few studies outside of this population have been published. It should be noted, however, that the duration of follow-up was not addressed in these prior reports.
Similar methodology was used to demonstrate a lack of anticipation of Crohn disease, whereas prior studies had demonstrated anticipation to occur.7 When comparing generational ages, there was a marked difference noted in the age at diagnosis of Crohn disease for second-generation subjects compared with the first generation. Yet again, the average age of the second generation at the time of capture was younger than the older age, and when included by decade, no significant difference was found in the average age of the second generation than the first.
Genetic anticipation suggests an “accumulation” of risk over generations, the classic example of which is instability of multinucleotide repeats. Single base-pair changes are unlikely to demonstrate anticipation, and the presence of anticipation may argue against a search for genes related to IA by a genome-wide SNP screen. However, ruptured IA is likely to be a complex trait with multiple genetic and environmental risk factors interacting with one another. As such, it is unlikely to demonstrate genetic anticipation. Thus, our findings support examination of all types of variations for association with the phenotype of ruptured IA.
It is also important to note that on a clinical basis, without clear evidence of anticipation, it is challenging to identify an age at which screening for IA is more or less appropriate. If our evidence is correct, then the offset in ages may indicate that the subsequent generation will develop IA but at a later date than the primary generation has.
Our study avoids the issues of increased screening in subsequent generations by using the phenotype of ruptured IA rather than any IA. In addition, our examination included a much larger number of families than previously examined with ruptured intracranial IA (other studies had more total IA but fewer ruptured IA cases). It is difficult to comment on whether earlier diagnosis and treatment of unruptured IA may have avoided ruptures in the second generation leading to the significantly older age at which patients ruptured. Including treatment into the model reduced the finding of a difference in age by generation to a trend, but there was a substantial loss of power, as not everyone in each cohort was screened for IA.
APPENDIX
FIA Study operational centers
Coordinating Center—University of Cincinnati: J. Broderick, principal investigator; D. Kleindorfer, co-principal investigator; L. Sauerbeck, study coordinator; S. Ewing, administrator; J. Sester, research assistant; Genotyping Center—University of Cincinnati: R. Deka, principal investigator; Linkage Analysis— Indiana University: T. Foroud, principal investigator; Imaging Center—Mayo Clinic: J. Huston III, co-principal investigator; D. Kallmes, study neuroradiologist, M. Maronie Smith, MRI study coordinator; Cell Storage Center—Camden, NJ: R Corriveau; National Institute of Neurological Disorders and Stroke: J. Marler; K. Hardey; E. Golanov.
FIA Recruitment centers
University of Alabama at Birmingham: W. Fisher, principal investigator, H. Forson, coordinator; Auckland New Zealand: C. Anderson, principal investigator, E. Mee, co-principal investigator, C. Howe, coordinator, S. Vos, coordinator; Australia: G. Hankey, principal investigator, P. DUrso, principal investigator, N. Knuckey, principal investigator, J. Laidlaw, principal investigator, P. Reilly, principal investigator, N. Dorsch, co-principal investigator, M. Morgan, principal investigator, M. Besser, principal investigator, J. Rosenfeld, principal investigator, K. Athanasiadis, coordinator, A. Claxton, coordinator, J. Davidson, coordinator, V. Dunne, coordinator, J. Griffith, coordinator, S. Pope, coordinator, J. Capstick, coordinator, A. Froelick, coordinator, Katherin Kesketh, coordinator; Brigham & Women’s Hospital: A. Day, principal investigator, B. Brach, coordinator; University of Cincinnati: D. Woo, co-principal investigator, M. Zuccarello, co-principal investigator, A. Ringer, co-principal investigator, H. Yeh, co-principal investigator, K. Franklin, coordinator; Cleveland Clinic Foundation: P. Ramussen, principal investigator, D. Andrews-Hinders, coordinator, T. Wheeler, coordinator; Columbia University: E.S. Connolly, principal investigator, R. Sacco, co-principal investigator, R. Ellsasser, coordinator, P. Yung, coordinator; University of Florida: S.B. Lewis, principal investigator, R. Dettorre, coordinator, A. Royster, coordinator; Indianapolis Neurosurgical Group: T. Payner, principal investigator, N. Miracle, coordinator, K. Redelman, coordinator; London Health Science Center Research Inc.: G. Ferguson, principal investigator, C. Mayer, coordinator, J. Peacock, coordinator; John Hopkins University: K. Murphy, principal investigator, B. Kohler, coordinator; A. Jones, coordinator; Massachusetts General Hospital: C. Ogilvy, principal investigator, D. Buckley, coordinator, T. Taytsel, coordinator, J. Manasal, coordinator; McGill University: G. Rouleau, principal investigator, A. Noreau, coordinator, N. Satge, coordinator, A, Desjarlais, coordinator; University of Maryland: E.F. Aldrich, principal investigator, C. Aldrich, coordinator; K. Booker, coordinator; Mayo Clinic: R.D. Brown, principal investigator, I. Meissner, co-principal investigator; D. Weibers, co-principal investigator; L. Jaeger, coordinator; University of Michigan: L. Morgenstern, principal investigator, L. Lisabeth, co-principal investigator, A. Caveney, coordinator, M. Concannon, coordinator; New Jersey Medical School: A.I. Qureshi, principal investigator, P. Harris-Lane, coordinator; Northwestern University: H. Batjer, principal investigator, C. Getch, co-principal investigator, B. Bendok, co-principal investigator, C. Concannon, coordinator, G. Joven, coordinator, K. Matijevich, coordinator, S. Garbutt, coordinator; University of Ottawa: M.T. Richard, principal investigator, A. Hopper, coordinator; University of Pittsburgh: A.B, Kassam, principal investigator, G. Seever, coordinator, J. Genevro, coordinator, K. Lee, coordinator; University of California, San Francisco: C. Johnston, principal investigator, K. Katsura, coordinator, M. Banach, coordinator; University of Southern California: S. Giannotta, principal investigator, V. Thomson, coordinator, D. Fishback, coordinator; Stanford University Medical Center: G. Steinberg, principal investigator, D. Luu, coordinator; M. Coburn, coordinator; University of Texas at Houston: M. Malkoff, principal investigator, A. Wojner, coordinator; University of Virginia: N. Kassel, principal investigator, B. Worrall, co-principal investigator, S. Cook, coordinator, B. Stoutenger, coordinator, G. Radakovic, coordinator; University of Washington: D. Tirschwell, principal investigator, P. Tanzi, coordinator; University of Manitoba, Winnipeg: A. Kaufmann, principal investigator, D. Gladish, coordinator; Washington University St. Louis: C. Derdeyn, principal investigator, M. Catanzare, coordinator.
Address correspondence and reprint requests to Dr. Daniel Woo, Department of Neurology, University of Cincinnati College of Medicine, 231 Albert Sabin Way ML 0525, Cincinnati, OH 45267-0525 Daniel.Woo@uc.edu
*See the appendix for a list of the Familial Intracranial Aneurysm (FIA) Study operational centers, recruitment centers, and investigators.
Funded by grants from the National Institute of Neurological Disorders and Stroke (R-O1 NS39512).
Disclosure: The authors report no disclosures.
Received April 3, 2008. Accepted in final form November 12, 2008.
REFERENCES
- 1.Broderick JP, Sauerbeck LR, Foroud T, et al. The Familial Intracranial Aneurysm (FIA) study protocol. BMC Med Genet 2005;6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rabinowitz D, Yang Q. Testing for age-at-onset anticipation with affected parent-child pairs. Biometrics 1999;55:834–838. [DOI] [PubMed] [Google Scholar]
- 3.Pfeiffer RM, Goldin LR, Chatterjee N, et al. Methods for testing familial aggregation of diseases in population-based samples: application to Hodgkin lymphoma in Swedish registry data. Ann Hum Genet 2004;68:498–508. [DOI] [PubMed] [Google Scholar]
- 4.Bromberg J, Rinkel G, Algra A, et al. Familial subarachnoid hemorrhage: distinctive features and patterns of inheritance. Ann Neurol 1995;38:929–934. [DOI] [PubMed] [Google Scholar]
- 5.Struycken PM, Pals G, Limburg M, et al. Anticipation in familial intracranial aneurysms in consecutive generations. Eur J Hum Genet 2003;11:737–743. [DOI] [PubMed] [Google Scholar]
- 6.Ruigrok YM, Rinkel GJ, Wijmenga C, Van Gijn J. Anticipation and phenotype in familial intracranial aneurysms. J Neurol Neurosurg Psychiatry 2004;75:1436–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Picco MF, Goodman S, Reed J, Bayless TM. Methodologic pitfalls in the determination of genetic anticipation: the case of Crohn disease. Ann Intern Med 2001;134:1124–1129. [DOI] [PubMed] [Google Scholar]


