Abstract
Nociceptin, also known as orphanin FQ, is a opioid-like neuropeptide that mediates its effects at the nociceptin receptor, a member of the G protein-coupled receptor superfamily. In mammals, nociceptin produces analgesia after spinal administration, however the role of nociceptin and nociceptin receptors in the modulation of noxious stimuli in non-mammalian species has not been examined. In an amphibian pain model using the acetic acid test with Rana pipiens, nociceptin and nociceptin1–13 amide produced dose-dependent antinociception (1–100 nmol), blocked by the nociceptin antagonist, [Nphe1]-nociceptin1–13 amide (30 nmol), but not the opioid antagonist, naltrexone (100 nmol/g, s.c.). Conversely, the antinociceptive effects of mu, delta, and kappa opioid receptor agonists were not blocked by the nociceptin antagonist. Nociceptin and nociceptin1–13 amide were the least potent of the opioid agonists tested. These studies demonstrate that spinal nociceptin receptors and not opioid receptors mediate the antinociceptive effect of nociceptin. Considered with previous findings, these behavioral data supports a role for nociceptin inhibition of spinal nociception in amphibians and perhaps all vertebrates.
Keywords: nociceptin, nociceptin receptor, opioid receptor, antinociception, amphibian
1. Introduction
Nociceptin (also called orphanin FQ) is a 17 amino acid neuropeptide found in brain extracts that was implicated in the modulation of nociceptive transmission, along with other roles (Meunier et al, 1995; Reinscheid et al, 1995). In concert with its name, nociceptin produced hyperalgesia after intracerebroventricular (i.c.v.) administration to mice (Meunier et al., 1995; Suaudeau et al., 1998; Zhu et al., 1997). Conversely, nociceptin administered by the spinal route produced analgesia (Wang et al., 1999a; Yamamoto et al., 1997). However, even this difference in the effect of nociceptin explained by route of administration was not consistent among all studies (Mogil and Pasternak, 2001; Yamamoto et al., 1999). It is now thought that the hyperalgesic effects of supraspinal nociceptin were due to reversal of stress-induced analgesia and that nociceptin administered to the spinal cord of mammals produces a classical analgesic response (Lambert, 2008; Zeilhofer and Calò, 2003).
The pronociceptin precursor protein that yields nociceptin is closely related to the family of endogenous opioid propeptides; proopiomelanocortin, proenkephalin and prodynorphin. Pronociceptin has similar gene structure (e.g. conserved intron/exon boundaries, CYS residues and dibasic cleavage sites) as opioid peptide precursors, especially prodynorphin (Darland et al., 1998; Henderson and Mcknight, 1997; Meunier, 1997). Pronociceptin mRNA and nociceptin immunoreactivity is localized throughout the CNS of mammals within neuronal pathways that function in the processing of pain (Mogil and Pasternak, 2001). Like endogenous opioid peptides, especially high densities of both nociceptin markers are found in the dorsal horn of mammalian spinal cord (Darland et al., 1998; Lai et al., 1997; Pettersson et al., 2002; Schuligoi et al., 1997).
The receptor for nociceptin (NOP) was discovered before its endogenous ligand (hence the ‘orphan’ in its previous name and the abbreviation, ORL) by low-stringency hybridization screening using opioid receptor cDNA probes or by selective amplification of genomic DNA using degenerate primers (Meunier, 1997; Mollereau et al., 1994). The sequence of the NOP is homologous with that of classical mu (MOR), delta (DOR) and kappa (KOR) opioid receptor proteins. Thus the gene for NOP is the fourth member of the opioid receptor gene family. Cell lines expressing NOP receptors display a high-affinity for nociceptin binding, and binding of nociceptin to the NOP receptor stimulates the binding of GTPγS, and activates a pertussis-sensitive Gαi/o signal transduction pathway leading to inhibition of cAMP formation, a decrease in calcium conductance and an increase in potassium channel conductance (Dooley and Houghten, 2000; Fawzi et al., 1997; Hawes et al., 2000; Ikeda et al., 1997). The net effect of these intracellular sequelae is inhibition of neurotransmitter release and neuronal activity. Thus, functionally as well as structurally, the nociceptin receptor is homologous to the classic opioid receptors by acting through similar signal transduction pathways (Fawzi et al., 1997). With the exception of lofentanil and buprenorphine, most opioids do not have appreciable affinity for the nociceptin receptor (Bloms-Funke et al., 2000; Butour et al., 1997; Yamamoto et al., 2006; Zaveri et al., 2001).
Since the original discovery of endogenous nociceptin, other peptide analogs were synthesized including the agonist, nociceptin1–13 amide (Butour et al., 1997) and the antagonist, [Nphe1]-nociceptin1–13-amide (Calò et al., 2000; Pheng et al., 2000; Xu et al., 2002). Nociceptin1–13 amide displaces tritiated nociceptin with the same affinity as the native peptide, nociceptin, in rat brain homogenates and after transfection of NOP receptor in CHO cell membranes (Calò et al., 2002). Like nociceptin, the agonist activity of nociceptin1–13 amide was shown by stimulating the binding of GTPγS, and inhibiting cAMP formation, decreasing calcium conductance and increasing potassium channel conductance (Salvadori et al., 1999). The nociceptin antagonist, [Nphe1]-nociceptin1–13 amide, also has a high affinity for the NOP receptor and the spinal analgesic effect of nociceptin1–13-amide in rodent models (Butour et al., 1997) was blocked by co-administration of [Nphe1]-nociceptin1–13-amide (Pheng et al., 2000; Xu et al., 2002).
To date, all of the behavioral studies of nociceptin were done using mammalian species. Given the variable results on nociceptin effects in mammals and for purposes of contributing data to a larger comparative dataset on the pharmacology of opioids and vertebrate opioid receptor proteins, the present study examined the pharmacology of nociceptin following spinal administration in amphibians. The amphibian model for assessing opioid effects is an alternative or adjunct model for pain and analgesia research (Stevens, 1992; 2004). Previous studies examined the relative antinociceptive potency of non-selective and selective opioid agonists after systemic, spinal, and supraspinal routes of administration (Mohan and Stevens, 2006; Stevens et al. 1994, 1996, 2007b; Stevens and Rothe, 1997). Non-opioid analgesics and adrenergic agents were also tested using the amphibian model (Brenner et al., 1994; Stevens et al., 2001; Stevens and Brenner, 1996).
The present study examined the effects of nociceptin and nociceptin1–13 amide following spinal administration in amphibians. For comparison, the antinociceptive effects of the MOR agonist, fentanyl (Janssen et al., 1963), the DOR agonist, [D-Pen2-D-Pen5]-enkephalin (DPDPE; (Mosberg et al., 1983) and the KOR agonist, U50488 (VonVoigtlander et al., 1983) were also tested. Finally, the receptor selectivity of nociceptin’s effect was examined by the use of the non-selective opioid receptor antagonist, naltrexone, and the selective NOP receptor antagonist, [Nphe1]-nociceptin1–13-amide.
2. Methods
All procedures were approved by the Institutional Animal Use Committee (IACUC) and adhere to the National Institutes of Health (U.S.A.) and the European Community guidelines on the use of animals for biomedical research.
2.1 Animals
Northern grass frogs, Rana pipiens (Charles Sullivan, Inc., Nashville, TN, USA) with a mean weight of 28 g were kept in groups of 48 in a flow-through, stainless steel enclosure at room temperature with running water after arrival. They were maintained with a 12-hour photoperiod (lights on 0700) and were fed live crickets twice a week. At least two days before experiments, animals were transferred to the laboratory and placed in individual plastic pans with an adequate amount of tap water. On the day of experimental study, water was adjusted to a depth such that the dorsal surface of the frog’s thigh was exposed for testing. Baseline nociceptive thresholds (see below) were obtained 2 h after the water level was adjusted on the morning of the experiment and post-treatment nociceptive thresholds at 1 and 3 h after drug administration. Previous studies of analgesics administered by the intraspinal route in amphibians demonstrated that maximal effects were obtained within this time period.
2.2 Drugs and drug administration
Naltrexone, fentanyl, DPDPE, U50488H, and nociceptin1–13 amide were obtained from the National Institute on Drug Abuse, Drug Supply Program (with generous assistance from Mr. Kevin Gormley of the Research Technology Branch). Nociceptin was obtained from Bachem, Inc. (Torrance, CA) and the antagonist, [Nphe1]-nociceptin1–13-amide, was purchased from Tocris Cookson, Inc. (Ellisville, MO). Drugs were mixed in saline to give nmol/μl solutions of the peptide or free base. Spinal administration was done using a Hamilton microsyringe and was made between the articulation of the lumbar vertebrate in a volume of 5μl (Stevens, 1996). Saline-injected control animals were co-run with agonist and antagonist experiments, and there were no significant changes from baseline values. The nociceptin antagonist was given by concurrent spinal injection with the agonist using doses determined in pilot experiments. For opioid antagonism experiments, systemic naltrexone (100 nmol/g) or saline was administered 1h before the spinal administration of agonists. Systemic administration was made by bolus injection into the dorsal lymph sac at a volume of 10 μl/g body weight (Stevens et al., 1994). Treatment groups consisted of six to eight animals per dose. Each animal was used only once.
2.3 The acetic acid test for determining nociceptive thresholds in amphibians
The acetic acid test to determine the nociceptive threshold (NT) in frogs consists of eleven concentrations of acetic acid diluted in half-log steps from glacial acetic acid. The concentrations are given a code number from 0 to 10 with the lowest code number equal to the lowest concentration of acetic acid (Pezalla, 1983). Nociceptive testing is done by placing, with a Pasteur pipette, a single drop of acid on the dorsal surface of the frog’s thigh. Testing begins with the lowest concentration and proceeds with increasing concentrations until the NT is reached. The NT is defined as the code number of the lowest concentration of acid that causes the frog to vigorously wipe the treated leg. The nociceptive response of the animal is directly dependent on the pH of the acid solution applied as the noxious stimulus (Hamamoto et al., 2000). To prevent tissue damage, the acetic acid is immediately wiped off with a gentle stream of distilled water once the animal responds or after four seconds. If the animal fails to respond, testing continues on the opposite hindlimb. An animal that fails to respond to the highest concentration (#10) is assigned the cut-off of 11. Each NT determination consists of a single trial of the acetic acid solutions. The acetic acid test was done to obtain baseline NT before drug treatment and post-treatment NT at various times after treatment. Baseline NT was obtained 30 min before drug administration. The raw NT data (code number of acetic acid solution) was converted to maximum percent effect (MPE) by the following formula:
MPE data was plotted for treatment groups as the time course after administration, and the maximum MPE value over that time course was pooled from individual animals at the same treatment dose for construction of dose-response curves. Pharmacological software (Pharmacological Calculation Systems, PCS v. 4.0, MicroComputer Specialists, Philadelphia, PA) was used to calculate the median effective dose (ED50) and 95% confidence interval, the slope and 95% confidence interval, and for statistical testing of the significant differences between treatment groups. Antagonist data were analyzed by a one-way ANOVA followed by the post-hoc Newman-Keuls test. Significant effects were considered at the p < 0.05 level.
3. Results
3.1 Time course of nociceptin and nociceptin1–13 amide antinociceptive effects
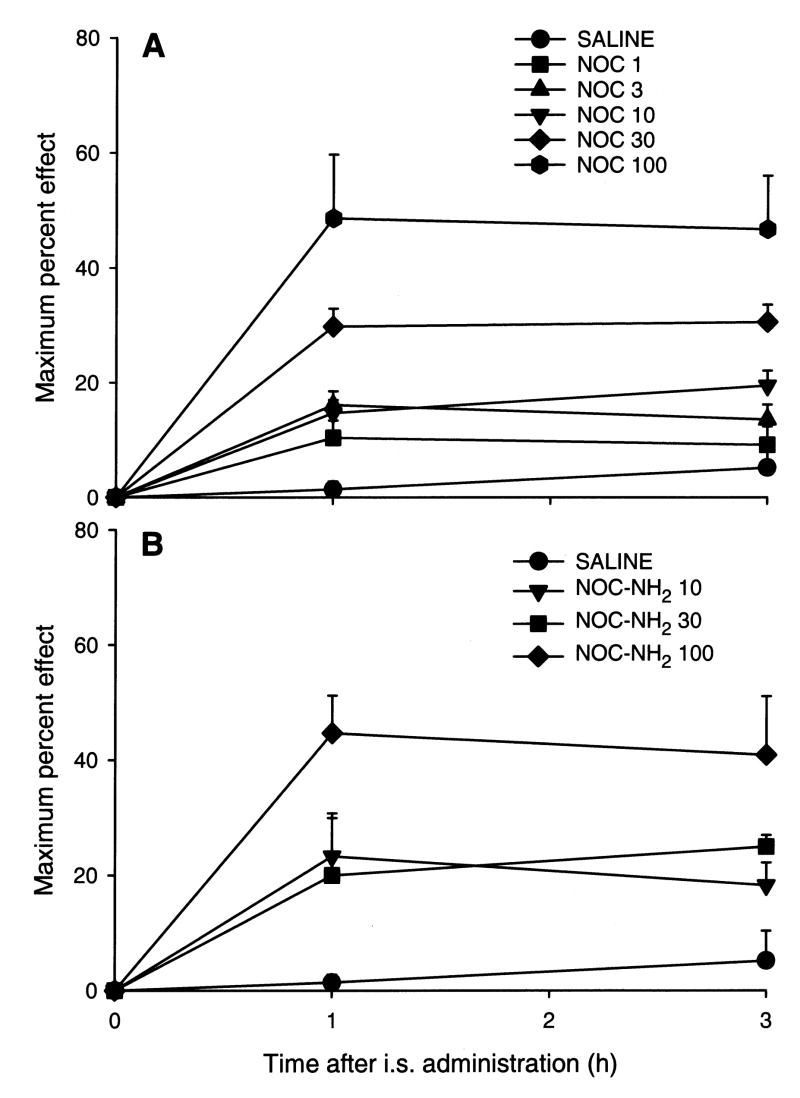
Nociceptin and nociceptin1–13 amide produced dose-dependent antinociceptive effects over the 3 h time course with doses from 1 to 100 nmol/frog (Fig. 1A,B). At 300 nmol/frog of nociceptin, hindlimb dysfunction was noted in 4 out of 6 animals precluding assessment of nociceptin effects at that high dose. Consistent with other analgesic agents administered to amphibians, the antinociceptive effects lasted at least 3 h, however the animals returned to normal nociceptive thresholds the following day.
Fig. 1.
Time course of the antinociceptive effect of nociceptin (NOC, panel A) or noiceptin1–13 amide (NOC-NH2, panel B) after spinal administration. Doses were 1–100 nmol/frog for nociceptin and 10–100 nmol/frog for noiceptin1–13 amide. N=6–8 animals per treatment dose and saline-injected controls.
3.2 Relative antinociceptive potency of nociceptin and nociceptin1–13 amide
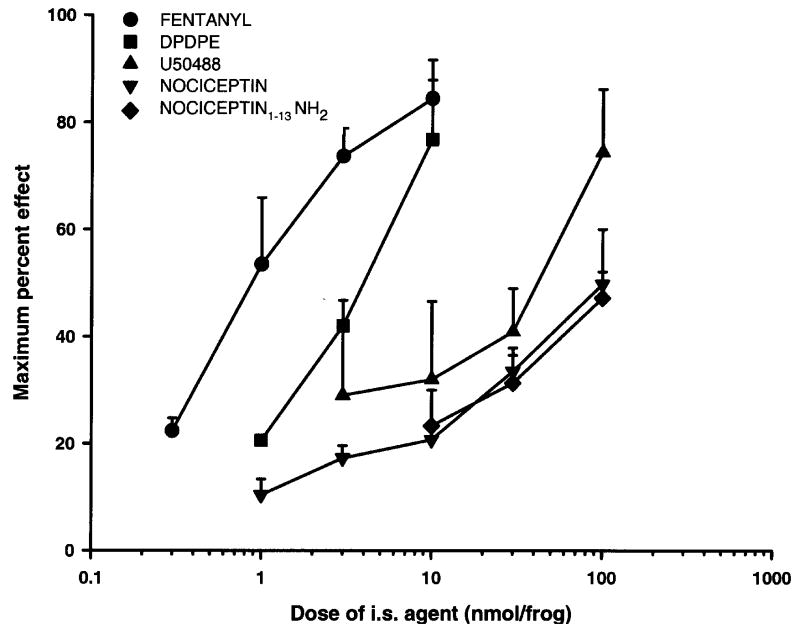
The dose-response curves of antinociceptive effect of nociceptin and nociceptin1–13 amide following spinal administration in amphibians is in Fig. 2. For comparison, the dose-response curves of the selective MOR (fentanyl), DOR (DPDPE) or KOR (U50488) agonists were also obtained. The ED50 values and 95% confidence intervals are given in Table 1. Nociceptin and nociceptin1–13 amide had ED50 values of 211.3 and 160.5 nmol/animal, respectively. The relative potency of nociceptin and nociceptin1–13 amide was 0.04 and 0.06 compared to fentanyl (set at 1.00). With the exception of nociceptin1–13 amide, all agents had a significant slope (i.e. the 95% confidence interval of the slope did not include 0) indicating dose-dependent responses. The selective MOR opioid receptor agonist, fentanyl, was the most potent agonist, followed by the DOR and KOR agonists; with nociceptin and nociceptin1–13 amide the least potent.
Fig. 2.
Log dose-response curves of the antinociceptive effect of nociceptin and noiceptin1–13 amide, and fentanyl, DPDPE, and U50488, after spinal administration in amphibians. N=6–8 animals per treatment dose.
Table 1.
Analysis of nociceptin and opioid antinociceptive dose-response curves after spinal administration in amphibians.
| Agent | ED50a | 95% C.I.b | Slope | 95% C.I. | R.P.c |
|---|---|---|---|---|---|
| fentanyl | 9.8 | (6.7–14.1) | 38.7 | (23.8–53.7) | 1.00 |
| DPDPE | 11.1 | (5.93–20.80) | 40.5 | (13.7–67.2) | 0.88 |
| U50488 | 48.2 | (20.9–111.5) | 36.5 | (7.0–65.8) | 0.20 |
| nociceptin | 211.3 | (75.5–590.9) | 18.5 | (12.7–24.3) | 0.04 |
| nociceptin1–13 amide | 160.5 | (7.9–3252.8) | 22.4 | (−12.2–56.9) | 0.06 |
in nmol/animal, spinal administration;
95% confidence interval;
relative potency compared to fentanyl by the ratio of the ED50 fentanyl/ED50 other agent.
3.3 Effects of naltrexone on nociceptin1–13 amide and fentanyl antinociception
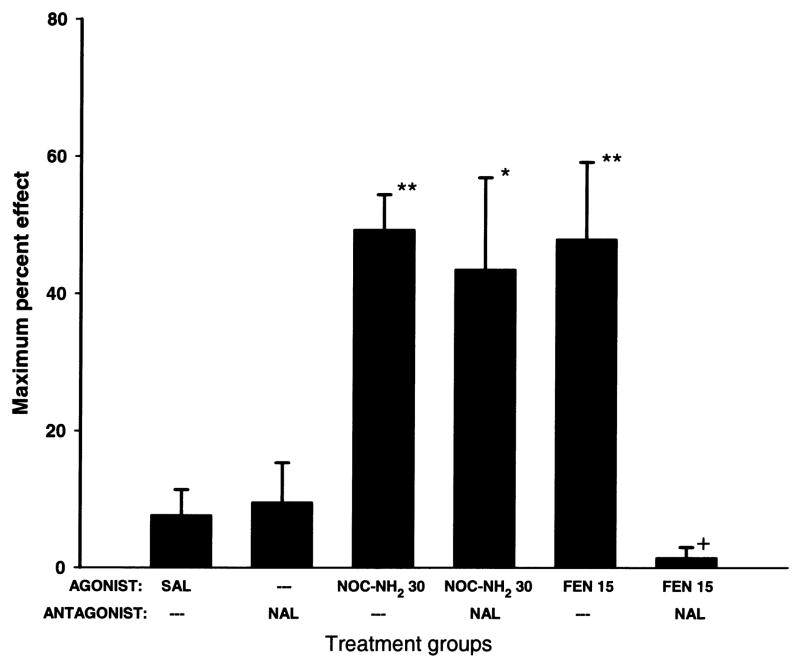
The effect of pretreatment with the opioid antagonist, naltrexone (100 nmol/g, s.c.), on the antinociceptive action of fentanyl or nociceptin1–13 amide is shown in Fig. 3. Saline or pretreatment with naltrexone alone did not significantly affect the nociceptive threshold in amphibians. Nociceptin1–13 amide (30 nmol/animal, i.s.) or fentanyl (15 nmol/animal, i.s.) alone produced significant antinociceptive effects. Pretreatment with naltrexone did not affect the antinociception produced by spinal administration of nociceptin1–13 amide, but significantly blocked the antinociceptive effect of fentanyl.
Fig. 3.
Naltrexone pretreatment blocks fentanyl but not noiceptin1–13 amide antinociceptive effects in amphibians. Naltrexone was given by the systemic route (NAL; 100 nmol/g, s.c.) 1 h before spinal administration of noiceptin1–13 amide (NOC-NH2; 30 nmol/frog) or fentanyl (FEN; 15 nmol/frog). Asterisks indicate significantly greater thresholds than SAL alone (*=p<0.05, **=p<0.01). Plus (+) indicates significantly reduced thresholds (p<0.01) compared to FEN alone treatment group.
3.4 Effects of [NPhe1]-nociceptin1–13 amide on nociceptin and opioid antinociception
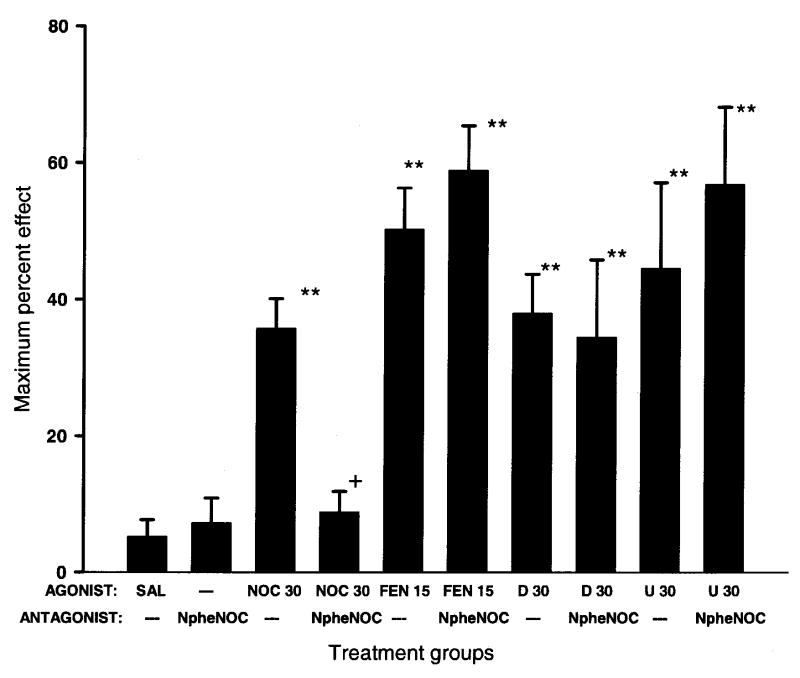
The effects of concurrent spinal administration of the nociceptin receptor antagonist, [Nphe1]-nociceptin1–13 amide, with nociceptin, fentanyl, DPDPE, or U50488 is shown in Fig. 4. Spinal administration of saline or [Nphe1]-nociceptin1–13 amide (30 nmol/animal) alone did not significantly alter the nociceptive threshold in amphibians. The nociceptin antagonist, [Nphe1]-nociceptin1–13 amide (30 nmol/animal) blocked the antinociceptive effects of nociceptin but not fentanyl, DPDPE, or U50488.
Fig. 4.
Concurrent spinal administration of the nociceptin antagonist, [Nphe1]-nociceptin1–13 amide (NpheNOC, 30 nmol/frog) blocks the antinociceptive effects of nociceptin (NOC, 30 nmol/frog) but not fentanyl (FEN, 15 nmol/frog), DPDPE (D, 30 nmol/frog), or U50488 (U, 30 nmol/frog) in amphibians. Asterisks indicate significantly greater nociceptive thresholds than SAL alone (**=p<0.01). Plus (+) indicates significantly reduced thresholds (p<0.05) than nociceptin (NOC) alone.
4. Discussion
The present data are the first to show that nociceptin and nociceptin1–13 amide produces significant antinociception in a non-mammalian vertebrate species, the amphibian Rana pipiens. Nociceptin administered to the spinal cord of amphibians produced a dose-dependent analgesic effect with an ED50 of 211 nmol/frog. Nociceptin was a weak analgesic agent, only 0.04 times as potent as fentanyl. Nociceptin 1–13 amide had a similar weak analgesic potency, although the dose-response curve was too shallow to give a significant slope (Table 1). Among the selective opioid agonists tested to date in the amphibian model, the potency of nociceptin falls within the range of most KOR opioid agonists (Stevens, 1996; 2004). This is not surprising as the amino acid sequence of the nociceptin precursor, pronociceptin, is most similar to prodynorphin, and among all vertebrate opioid receptors, the NOP receptor sequence is most similar to that of the KOR protein (Stevens et al., 2007a).
Comparison of the antinociceptive potency of spinally administered nociceptin or nociceptin1–13 amide in amphibians to similar studies in rodents is problematic due to the surprising lack of published ED50 values. Additionally, previous studies did not directly compare the relative analgesic potency of nociceptin with selective opioid agonists. However, in mammalian studies where spinal nociceptin produced an analgesic effect, the highest dose tested did not produce as potent an analgesic effect as typically observed following morphine or other opioid agonists (Candeletti et al., 2000; Corradini et al., 2001; King et al., 1997; Ko et al., 2006; Nazzaro et al., 2006; Wang et al., 1999a; Wang et al., 1999b). The low potency nature of nociceptin and nociceptin 1–13 amide has led to the search for more potent NOP agonists (Gunduz et al., 2006).
The antinociceptive effects of spinal nociceptin in amphibians were blocked by the concurrent administration of the NOP antagonist, [Nphe1]-nociceptin1–13 amide. This is in concert with the results from mammalian studies whereby the analgesic effects of nociceptin after spinal administration were blocked by co-administration of [Nphe1]-nociceptin1–13 amide in normal (Lu et al., 2001; Xu et al., 2002) and neuropathic rodents (Corradini et al., 2001; Obara et al., 2005). The non-selective MOR, DOR, and KOR opioid antagonist, naltrexone, did not block the antinociceptive effect of nociceptin1–13 amide after spinal administration in amphibians in the present study. Comparison of these results with the mammalian literature is less convergent as some studies report naloxone or naltrexone blockade of nociceptin analgesic effects whereas others report no effect of opioid antagonists on nociceptin effects (Xu et al., 2000). More recently, two papers strengthened the case for the spinal antinociceptive effects of nociceptin by anatomically and functionally linking nociceptin action to the inhibition of substance P release from primary afferent fibers (Inoue et al., 2003; Mika et al., 2003).
The putative target of nociceptin action is the NOP receptor in amphibian spinal cord. Although numerous studies showed that NOP receptors were expressed in mammalian brain and spinal cord (see above), there is only a single report of [3H]-nociceptin1–13 amide binding in amphibians using brain tissue homogenates from the European water frog, Rana esculenta (Benyhe et al., 1999). These results showed that the nociceptin analog bound to a single, high-affinity site with an affinity of 0.55 nM and a density of 180 fmol/mg protein. More recently, the amphibian NOP receptor was cloned from two different species, and was shown to be expressed in amphibian brain and spinal cord (Stevens et al., 2007a; Walthers et al., 2005). These results, along with the present pharmacological data using NOP and opioid receptor antagonists, strongly suggest that the antinociceptive effects of nociceptin and its agonist analogues are mediated by NOP receptors in amphibian spinal cord.
The relatively weak antinociceptive effect of spinal nociceptin compared to opioid agonists appears to be conserved in amphibians and mammals. With regard to differences in the vertebrate opioid receptors, comparative bioinformatics of all vertebrate opioid-like sequences suggests that the NOP receptor is the most ancestral of the four members of the opioid receptor family, with the mu opioid receptor (MOR) being the most derived (Stevens et al., 2007a). In this sense, the vertebrate nociceptin system may represent a primitive endogenous analgesic system later surpassed by the endogenous opioid system with its more potent opioid receptors. There is some support for this notion given that the early-evolved sturgeon fish, Acipenser transmontanus, referred to as a ‘living fossil’, expresses a hybrid opioid- and nociceptin-like pronociceptin protein (Danielson et al., 2001).
Acknowledgments
Supported in part by a research grant from the U.S. National Institutes of Health, NIDA 012448 (CWS) and a summer medical student scholarship from the Auxiliary to the Oklahoma Osteopathic Association (BWS). This paper is dedicated to the memory of Peace Corps Nepal volunteer, Phil Cyr, killed in the line of duty.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benyhe S, Monory K, Farkas J, Toth G, Guerrini R, Salvadori S, et al. Nociceptin binding sites in frog (Rana esculenta) brain membranes. Biochem Biophys Res Commun. 1999;260:592–6. doi: 10.1006/bbrc.1999.0907. [DOI] [PubMed] [Google Scholar]
- Bloms-Funke P, Gillen C, Schuettler AJ, Wnendt S. Agonistic effects of the opioid buprenorphine on the nociceptin/OFQ receptor. Peptides. 2000;21:1141–6. doi: 10.1016/s0196-9781(00)00252-7. [DOI] [PubMed] [Google Scholar]
- Brenner GM, Klopp AJ, Deason LL, Stevens CW. Analgesic potency of alpha adrenergic agents after systemic administration in amphibians. J Pharmacol Exp Ther. 1994;270:540–5. [PubMed] [Google Scholar]
- Butour J-L, Moisand C, Mazarguil H, Mollereau C, Meunier J-C. Recognition and activation of the opioid receptor-like ORL1 receptor by nociceptin, nociceptin analogs and opioids. Eur J Pharmacol. 1997;321:97–103. doi: 10.1016/s0014-2999(96)00919-3. [DOI] [PubMed] [Google Scholar]
- Calò G, Guerrini R, Bigoni R, Rizzi A, Marzola G, Okawa H, et al. Characterization of [Nphe1]nociceptin(1–13)NH2, a new selective nociceptin receptor antagonist. Br J Pharmacol. 2000;129:1183–93. doi: 10.1038/sj.bjp.0703169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calò G, Rizzi A, Bigoni R, Guerrini R, Salvadori S, Regoli D. Pharmacological profile of nociceptin/orphanin FQ receptors. Clin Exp Pharmacol Physiol. 2002;29:223–8. doi: 10.1046/j.1440-1681.2002.03633.x. [DOI] [PubMed] [Google Scholar]
- Candeletti S, Guerrini R, Calò G, Romualdi P, Ferri S. Supraspinal and spinal effects of [PHE1 ψ(ch2-nh)gly2]-nociceptin(1–13)-NH2 on nociception in the rat. Life Sci. 2000;66:257–64. doi: 10.1016/s0024-3205(99)00588-3. [DOI] [PubMed] [Google Scholar]
- Corradini L, Briscini L, Ongini E, Bertorelli R. The putative OP4 antagonist, [Nphe1]nociceptin(1–13)NH2, prevents the effects of nociceptin in neuropathic rats. Brain Res. 2001;905:127–33. doi: 10.1016/s0006-8993(01)02520-3. [DOI] [PubMed] [Google Scholar]
- Danielson PB, Hoversten MT, Fitzpatrick M, Schreck C, Akil H, Dores RM. Sturgeon orphanin, a molecular “fossil” that bridges the gap between the opioids and orphanin FQ/nociceptin. J Biol Chem. 2001;276:22114–9. doi: 10.1074/jbc.M011741200. [DOI] [PubMed] [Google Scholar]
- Darland T, Heinricher MM, Grandy DK. Orphanin FQ/nociceptin: a role in pain and analgesia, but so much more. Trends Neurosci. 1998;21:215–21. doi: 10.1016/s0166-2236(97)01204-6. [DOI] [PubMed] [Google Scholar]
- Dooley CT, Houghten RA. Orphanin FQ/nociceptin receptor binding studies. Peptides. 2000;21:949–60. doi: 10.1016/s0196-9781(00)00231-x. [DOI] [PubMed] [Google Scholar]
- Fawzi A, Zhang H, Weig B, Hawes B, Graziano M. Nociceptin activation of the human ORL1 receptor expressed in Chinese hamster ovary cells: Functional homology with opioid receptors. Eur J Pharmacol. 1997;336:233–42. doi: 10.1016/s0014-2999(97)01227-2. [DOI] [PubMed] [Google Scholar]
- Gunduz O, Rizzi A, Baldisserotto A, Guerrini R, Spagnolo B, Gavioli EC, et al. In vitro and in vivo pharmacological characterization of the nociceptin/orphanin FQ receptor ligand Ac-RYYRIK-ol. Eur J Pharmacol. 2006;539:39–48. doi: 10.1016/j.ejphar.2006.03.075. [DOI] [PubMed] [Google Scholar]
- Hamamoto DT, Forkey MW, Davis WL, Kajander KC. The role of pH and osmolarity in evoking the acetic acid-induced wiping response in a model of nociception in frogs. Brain Res. 2000;862:217–29. doi: 10.1016/s0006-8993(00)02138-7. [DOI] [PubMed] [Google Scholar]
- Hawes B, Graziano M, Lambert DG. Cellular actions of nociceptin: transduction mechanisms. Peptides. 2000;21:961–7. doi: 10.1016/s0196-9781(00)00232-1. [DOI] [PubMed] [Google Scholar]
- Henderson G, Mcknight AT. The orphan opioid receptor and its endogenous ligand-nociceptin/orphanin FQ. Trends Pharmacol Sci. 1997;18:293–300. [PubMed] [Google Scholar]
- Ikeda K, Kobayashi K, Kobayashi T, Ichikawa T, Kumanishi T, Kishida H, et al. Functional coupling of the nociceptin/orphanin FQ receptor with the G-protein-activated K+ (GIRK) channel. Mol Brain Res. 1997;45:117–26. doi: 10.1016/s0169-328x(96)00252-5. [DOI] [PubMed] [Google Scholar]
- Inoue M, Kawashima T, Takeshima H, Calò G, Inoue A, Nakata Y, et al. In vivo pain-inhibitory role of nociceptin/orphanin FQ in spinal cord. J Pharmacol Exp Ther. 2003;305:495–501. doi: 10.1124/jpet.102.046326. [DOI] [PubMed] [Google Scholar]
- Janssen PAJ, Niemegeers CJE, Dony JGH. The inhibitory effect of fentanyl and other morphine-like analgesics on the warm water induced tail withdrawal reflex in rats. Arzneim-Forsch. 1963;13:502–7. [PubMed] [Google Scholar]
- King MA, Rossi GC, Chang AH, Williams L, Pasternak GW. Spinal analgesic activity of orphanin FQ/nociceptin and its fragments. Neurosci Lett. 1997;223:113–6. doi: 10.1016/s0304-3940(97)13414-0. [DOI] [PubMed] [Google Scholar]
- Ko MC, Wei H, Woods JH, Kennedy RT. Effects of intrathecally administered nociceptin/orphanin FQ in monkeys: behavioral and mass spectromentric studies. J Pharmacol Exp Ther. 2006;318:1257–64. doi: 10.1124/jpet.106.106120. [DOI] [PubMed] [Google Scholar]
- Lai CC, Wu SY, Dun SL, Dun NJ. Nociceptin-like immunoreactivity in the rat dorsal horn and inhibition of substantia gelatinosa neurons. Neuroscience. 1997;81:887–91. doi: 10.1016/s0306-4522(97)00251-0. [DOI] [PubMed] [Google Scholar]
- Lambert DG. The nociceptin/orphanin FQ receptor: a target with broad therapeutic potential. Nature Rev Drug Disc. 2008;7:694–710. doi: 10.1038/nrd2572. [DOI] [PubMed] [Google Scholar]
- Lu J-T, Huang Y-H, Palmer PP, Xie G-X, Gabriel A, Grond S, et al. Blockade effects of (Nphe1)Nociceptin(1–13)-NH2 on anti-nociception induced by intrathecal administration of nociceptin in rats. Regul Pept. 2001;101:81–5. doi: 10.1016/s0167-0115(01)00263-4. [DOI] [PubMed] [Google Scholar]
- Meunier J-C. Nociceptin/orphanin FQ and the opioid receptor-like ORL1 receptor. Eur J Pharmacol. 1997;340:1–15. doi: 10.1016/s0014-2999(97)01411-8. [DOI] [PubMed] [Google Scholar]
- Meunier J-C, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour J-L, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–5. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Mika J, Li Y, Weihe E, Schafer M. Relationship of pronociceptin/orphanin FQ and the nociceptin receptor ORL1 with substance P and calcitonin gene-related peptide expression in dorsal root ganglion of the rat. Neurosci Lett. 2003;348:190–4. doi: 10.1016/s0304-3940(03)00786-9. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Pasternak G. The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol Rev. 2001;53:381–415. [PubMed] [Google Scholar]
- Mohan SK, Stevens CW. Systemic and spinal administration of the mu opioid, remifentanil, produces antinociception in amphibians. Eur J Pharmacol. 2006;534:89–94. doi: 10.1016/j.ejphar.2006.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollereau C, Parmentier M, Mailleux P, Butour J-L, Moisand C, Chalon P, et al. ORL1, a novel member of the opioid receptor family: cloning, functional expression and localization. FEBS Lett. 1994;341:33–8. doi: 10.1016/0014-5793(94)80235-1. [DOI] [PubMed] [Google Scholar]
- Mosberg HI, Hurst R, Hruby VJ, Gee K, Yamamura HI, Galligan JJ, et al. Bis-penicillamine enkephalins possess highly improved specificity toward delta opioid receptors. Proc Natl Acad Sci USA. 1983;80:5871–4. doi: 10.1073/pnas.80.19.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazzaro C, Rizzi A, Salvadori S, Guerrini R, Regoli D, Zeilhofer HU, et al. UPF-101 antagonizes the spinal antinociceptive effects of nociceptin/orphanin FQ: Behavioral and electrophysiological studies in mice. Peptides. 2006;28:663–9. doi: 10.1016/j.peptides.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Obara I, Przewlocki R, Przewlocka B. Spinal and local peripheral antiallodynic activity of Ro64-6198 in neurophathic pain in the rat. Pain. 2005;116:17–25. doi: 10.1016/j.pain.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Pettersson LME, Sundler F, Danielsen N. Expression of orphanin FQ/nociceptin and its receptor in rat peripheral ganglia and spinal cord. Brain Res. 2002;945:266–75. doi: 10.1016/s0006-8993(02)02817-2. [DOI] [PubMed] [Google Scholar]
- Pezalla PD. Morphine-induced analgesia and explosive motor behavior in an amphibian. Brain Res. 1983;273:297–305. doi: 10.1016/0006-8993(83)90854-5. [DOI] [PubMed] [Google Scholar]
- Pheng L, Calò G, Guerrini R, Regoli D. [Nphe1]nociceptin-(1–13)NH2 selectively antagonizes nociceptin effects in the rabbit isolated ileum. Eur J Pharmacol. 2000;397:383–8. doi: 10.1016/s0014-2999(00)00300-9. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, et al. Orphanin FQ: a neuropeptide that activates an opioid-like G protein-coupled receptor. Science. 1995;270:792–4. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Salvadori S, Guerrini R, Calò G, Regoli D. Structure-activity studies on nociceptin/orphanin FQ: from full agonist, to partial agonist, to pure antagonist. Il Farmaco. 1999;54:810–25. doi: 10.1016/s0014-827x(99)00108-1. [DOI] [PubMed] [Google Scholar]
- Schuligoi R, Amann R, Angelberger P, Peskar BA. Determination of nociceptin-like immunoreactivity in the rat dorsal spinal cord. Neurosci Lett. 1997;224:136–8. doi: 10.1016/s0304-3940(97)13453-x. [DOI] [PubMed] [Google Scholar]
- Stevens CW. Relative analgesic potency of mu, delta and kappa opioids after spinal administration in amphibians. J Pharmacol Exp Ther. 1996;276:440–8. [PubMed] [Google Scholar]
- Stevens CW. Alternatives to the use of mammals for pain research. Life Sci. 1992;50:901–12. doi: 10.1016/0024-3205(92)90167-n. [DOI] [PubMed] [Google Scholar]
- Stevens CW. Opioid research in amphibians: an alternative pain model yielding insights on the evolution of opioid receptors. Brain Res Rev. 2004;46:204–15. doi: 10.1016/j.brainresrev.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens CW, Brenner GM. Spinal administration of adrenergic agents produces analgesia in amphibians. Eur J Pharmacol. 1996;316:205–10. doi: 10.1016/s0014-2999(96)00681-4. [DOI] [PubMed] [Google Scholar]
- Stevens CW, Rothe KS. Supraspinal administration of opioids with selectivity for μ–, δ–, and κ–opioid receptors produces analgesia in amphibians. Eur J Pharmacol. 1997;331:15–21. doi: 10.1016/s0014-2999(97)01026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens CW, MacIver DN, Newman LC. Testing and comparison of non-opioid analgesics in amphibians. Contemp Top Lab Anim Sci. 2001;40:23–7. [PMC free article] [PubMed] [Google Scholar]
- Stevens CW, Klopp AJ, Facello JA. Analgesic potency of mu and kappa opioids after systemic administration in amphibians. J Pharmacol Exp Ther. 1994;269:1086–93. [PubMed] [Google Scholar]
- Stevens CW, Brasel CM, Mohan SK. Cloning and bioinformatics of amphibian mu, delta, kappa, and nociceptin opioid receptors expressed in brain tissue: Evidence for opioid receptor divergence in mammals. Neurosci Lett. 2007a;419:189–94. doi: 10.1016/j.neulet.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens CW, Toth G, Borsodi A, Benyhe S. Xendorphin B1, a novel opioid-like peptide determined from a Xenopus laevis brain cDNA library, produces opioid antinociception after spinal administration in amphibians. Brain Res Bull. 2007b;71:628–32. doi: 10.1016/j.brainresbull.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suaudeau C, Florin S, Meunier J-C, Costentin J. Nociceptin-induced apparent hyperalgesia in mice as a result of the prevention of opioid autoanalgesic mechanisms triggered by the stress of an intracerebroventricular injection. Fund Clin Pharm. 1998;12:420–5. doi: 10.1111/j.1472-8206.1998.tb00966.x. [DOI] [PubMed] [Google Scholar]
- VonVoigtlander PF, Lahti RA, Ludens JH. U-50,488: A selective and structurally novel non-mu (kappa) opioid agonist. J Pharmacol Exp Ther. 1983;224:7–12. [PubMed] [Google Scholar]
- Walthers EA, Bradford CS, Moore FL. Cloning, pharmacological characterization and tissue distribution of an ORL1 opioid receptor from an amphibian, the rough-skinned newt, Taricha granulosa. J Mol Endocrinol. 2005;34:247–56. doi: 10.1677/jme.1.01687. [DOI] [PubMed] [Google Scholar]
- Wang JL, Zhu C-B, Cao X-D, Wu G-C. Distinct effect of intracerebroventricular and intrathecal injections of nociceptin/orphanin FQ in the rat formalin test. Regul Pept. 1999a;79:159–63. doi: 10.1016/s0167-0115(98)00161-x. [DOI] [PubMed] [Google Scholar]
- Wang YQ, Zhu C-B, Cao X-D, Wu G-C. Supraspinal hyperalgesia and spinal analgesia by [Phe1ψCH2-NH)Gly2]nociceptin-(1--13)-NH2 in rat. Eur J Pharmacol. 1999b;376:R1–2. doi: 10.1016/s0014-2999(99)00399-4. [DOI] [PubMed] [Google Scholar]
- Xu IS, Grass S, Calò G, Guerrini R, Wiesenfeld-Hallin Z, Xu X-J. Intrathecal [Nphe1]nociceptin(1–13)NH2 selectively reduces the spinal inhibitory effect of nociceptin. Life Sci. 2002;70:1151–1157. doi: 10.1016/s0024-3205(01)01496-5. [DOI] [PubMed] [Google Scholar]
- Xu XJ, Grass S, Hao J, Xu IS, Wiesenfeld-Hallin Z. Nociceptin/orphanin FQ in spinal nociceptive mechanisms under normal and pathological conditions. Peptides. 2000;21:1031–6. doi: 10.1016/s0196-9781(00)00234-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nozaki-Taguchi N, Kimura S. Analgesic effect of intrathecally administered nociceptin, an opioid receptor-like1 receptor agonist in the rat formalin test. Neuroscience. 1997;81:249–54. doi: 10.1016/s0306-4522(97)00166-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nozaki-Taguchi N, Sakashita Y, Kimura S. Nociceptin/orphanin FQ: role in nociceptive information processing. Prog Neurobiol. 1999;57:527–35. doi: 10.1016/s0301-0082(98)00067-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Shono K, Tanabe S. Buprenorphine activates mu and opioid receptor like-1 receptors simultaneously, but the analgesic effect is mainly mediated by mu receptor activation in the rat formalin test. J Pharmacol Exp Ther. 2006;318:206–13. doi: 10.1124/jpet.105.100859. [DOI] [PubMed] [Google Scholar]
- Zaveri N, Polgar WE, Olsen CM, Kelson AB, Grundt P, Lewis JW, et al. Characterization of opiates, neuroleptics, and synthetic analogs at ORL1 and opioid receptors. Eur J Pharmacol. 2001;428:29–36. doi: 10.1016/s0014-2999(01)01282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C-B, Cao X-D, Xu S-F, Wu G-C. Orphanin FQ potentiates formalin-induced pain behavior and antagonizes morphine analgesia in rats. Neurosci Lett. 1997;235:37–40. doi: 10.1016/s0304-3940(97)00704-0. [DOI] [PubMed] [Google Scholar]
- Zeilhofer HU, Calò G. Nociceptin/orphanin FQ and its receptor—potential targets for pain therapy? J Pharmacol Exp Ther. 2003;306:423–29. doi: 10.1124/jpet.102.046979. [DOI] [PubMed] [Google Scholar]