Abstract
The Arterial Blood Supply to an orthotopic hepatic allograft is usually provided by an end to end anastomosis between the recipient common hepatic artery and the donor celiac axis (1). Anomalies of the graft arterial supply and anomalies or inadequacies of recipient hepatic arteries may require that the basic technique be modified. The frequency and variety of the anomalies encountered and the technical options used to deal with them during the performance of 123 consecutive orthotopic liver transplantations in 97 recipients at the University of Pittsburgh Health Center from February 1981 to February 1983 are described herein.
INADEQUATE RECIPIENT HEPATIC ARTERY
Incidence and causes of problem
No identifiable hepatic artery could be found in two of the 97 liver recipients. A third patient had poor flow from the transected common hepatic artery and a catheter threaded into the aorta through this hepatic artery measured a mean pressure in the celiac axis 45 millimeters of mercury lower than that measured in the aorta. A fourth patient also had poor flow from the cut end of the common hepatic artery at the time of retransplantation, with a mean pressure 40 millimeters of mercury lower than that measured simultaneously through a femoral artery cannula.
In two other patients, subintimal dissection of the common hepatic artery occurred during mobilization of this vessel. The dissections propagated proximally into the celiac axis and made the vessels unusable. We have seen this accident occur at least eight times over the last 20 years during hepatic transplantation or in the course of pancreacticoduodenectomy. Usually, the dissection starts from the ligated gastroduodenal artery, apparently because of a fracture of a disruption of the intima at the site of the tie. To our knowledge, this serious complication of hepatic artery mobilization with gastroduodenal artery ligation has not been reported previously.
Technical solutions
For a number of years, our policy has been to harvest vascular grafts from all cadaveric organ donors (2). Segments are taken of the abdominal aorta, inferior vena cava, iliac arteries and iliac veins. The vascular grafts are placed into large sterile test tubes and immersed in tissue culture medium for refrigeration at 5 degrees C. Although most of these vessel grafts are subsequently discarded, their availability may be critical in the event of unexpected problems of revascularization in the recipient. If the probability of such a problem is known in advance, the donor team can remove the thoracic and upper abdominal aorta in continuity with the celiac axis and also with the superior mesenteric artery if the latter is found to supply a branch of the liver.
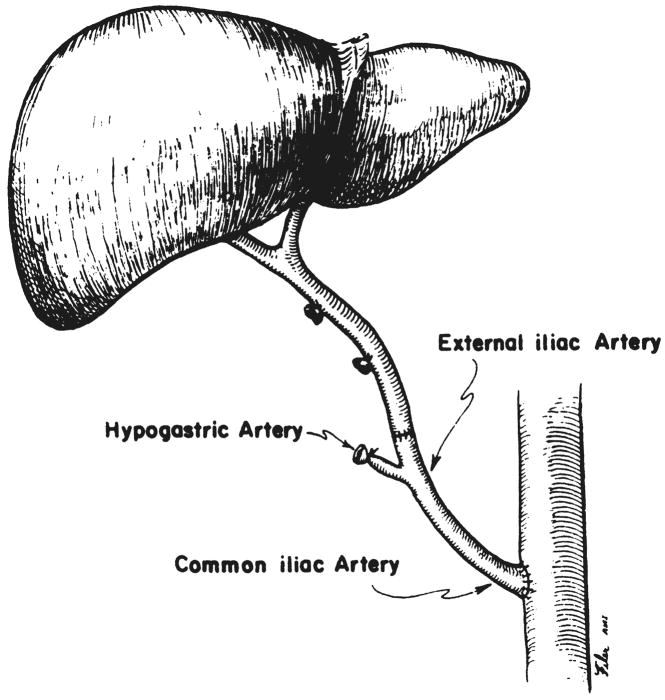
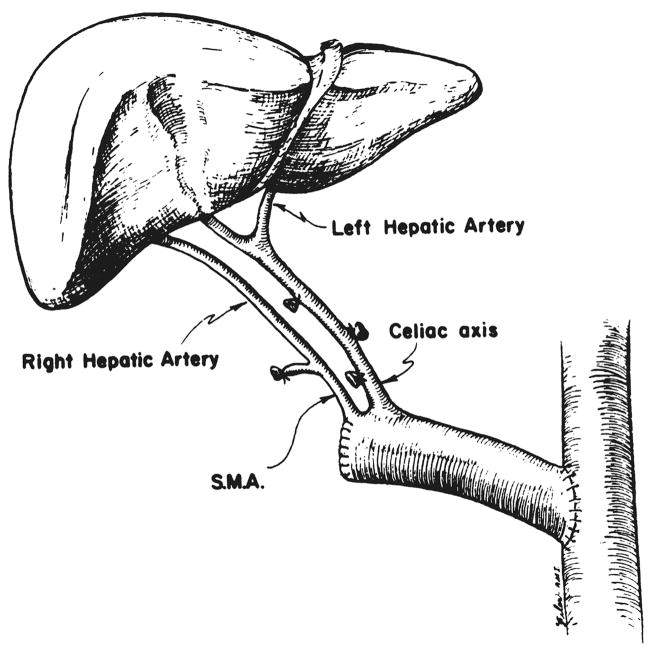
In four of the six recipients who had inadequate hepatic arteries, iliac artery grafts were anastomosed to the distal abdominal aorta of the recipient (Fig. 1). The common iliac artery was used for the aortic anastomosis and the external iliac artery was anastomosed to the celiac axis of the donor liver.
Fig. 1.
Use of iliac arterial graft between recipient aorta and homograft celiac axis.
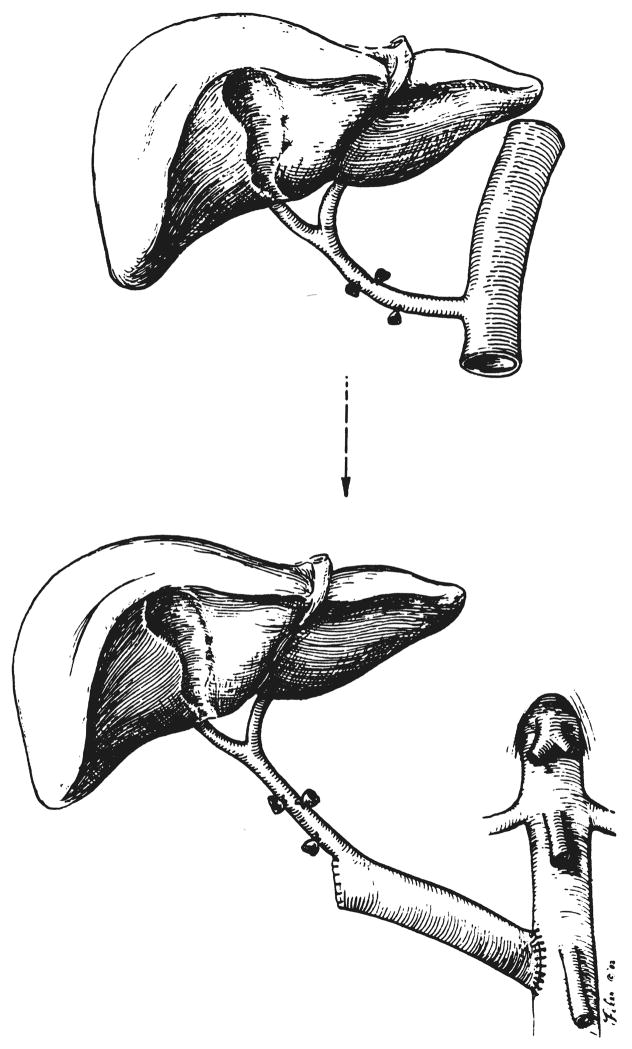
In two other patients, the thoracic aorta, in continuity with the celiac axis, was rotated 180 degrees and anastomosed to the distal abdominal aorta of the recipient (Fig. 2). The other end of the graft aorta was tied off or closed by sutures (Fig. 2). The procedure has been almost identical to that originally developed in dogs 25 years ago (3).
Fig. 2.
Use of incontinuity aortic graft for anastomosis to recipient aorta.
With the use of either a free arterial graft or of the aorta in continuity with the celiac axis, the most easily accessible place for anastomosis to the recipient aorta is at or just above the level of the origin of the inferior mesenteric artery (Fig. 2). The duodenum, ascending colon and hepatic flexure are mobilized and reflected medially. In patients with severe portal hypertension, the dissection in the retroperitoneal space requires meticulous attention to hemostasis but can be accomplished quite readily. In addition, this dissection has proved to be easier, and the exposure obtained is better than that possible when using the more proximal subdiaphragmatic aorta as we recommended previously (1).
GRAFT HEPATIC ARTERY FROM SUPERIOR MESENTERIC ARTERY
Incidence of problem
If recognized early in the course of a donor hepatectomy, many of the arterial anomalies encountered will not necessitate major modifications of the technique for revascularization (1). An example is an arterial supply to the left lateral segment which arises from the left gastric artery. This branch courses from left to right within the gastrohepatic ligament. Although care must be taken in the donor procedure to avoid injury or inadvertent ligation of the anomalous artery, its presence presents no problem in the recipient operation since the left gastric artery arises from the celiac axis and a single trunk can be developed for anastomosis to the recipient artery. In nine of 123 consecutive liver grafts (7.3 per cent), a more complicated situation was encountered in that the posterior segment of the right lobe or the entire right lobe was supplied by a branch from the superior mesenteric artery.
Technical solutions
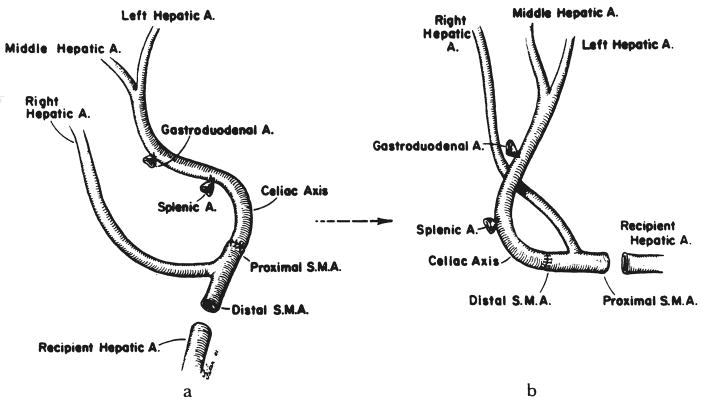
We previously advocated creating a single trunk by anastomosing the hepatic branch from the superior mesenteric artery to a branch of the celiac axis, most commonly the splenic artery (1). However, a more satisfactory solution has been to perform an anastomosis, on a back table with the liver still immersed in an iceslush solution, between the superior mesenteric artery (either proximal or distal to the take off of the hepatic branch) and the celiac axis as it arises from the aorta (Fig. 3). The donor to recipient connection is then made between the open end of the superior mesenteric artery of the donor and the hepatic artery or celiac axis of the recipient (Fig. 3). The anastomoses are performed in an orientation which allows for the best positioning of vessels without kinking or twisting as shown in Figure 3.
Fig. 3.
Reconstruction which can be used if the hepatic arterial supply comes from both the celiac axis and superior mesenteric artery. a and b, These versions differ only in that the opposite ends of the superior mesenteric artery are used for the anastomosis to the recipient vessels.
Among these nine donor organs with major hepatic arteries arising from the superior mesenteric artery, five were revascularized as shown in Figure 3a and three as shown in Figure 3b. This type of reconstruction was not necessary in the ninth donor liver because the entire hepatic arterial supply arose from the superior mesenteric artery.
A satisfactory technical result was obtained in six of the eight recipients of organs requiring this type of reconstruction (Fig. 4). Thrombosis of one of the lobar arteries occurred in two patients, both of whom required retransplantation and one of whom later died. The alternative solution of keeping the double blood supply in continuity with the aorta and performing an aorta to aorta anastomosis (Fig. 5), was not used in this recent series. However, this latter procedure was carried out six years ago upon a nine month old child with biliary atresia who is still alive.
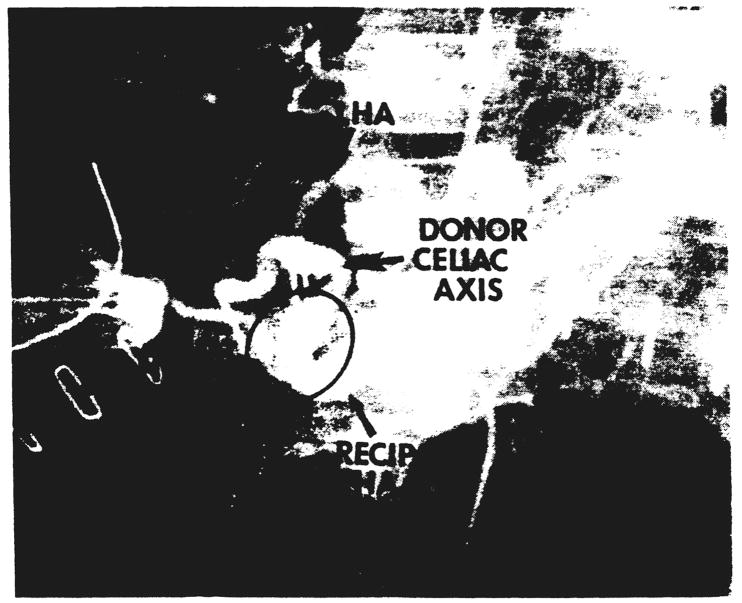
Fig. 4.
Hepatic arteriogram of a liver reconstructed by the technique shown in Figure 3a. The arrows show the two anastomoses.
Fig. 5.
The use of an incontinuity donor aortic graft in a patient with a double hepatic arterial supply.
It is noteworthy that the incidence of a double hepatic artery supply to the diseased recipient livers was nine of 97 (9 per cent), almost the same as in the donors. The larger of the two vessels is usually used for rearterialization of the donor liver. This proved to be the superior mesenteric artery in eight of these nine recipients.
SUMMARY
During the course of performing 123 consecutive orthotopic liver transplantations in a two year period, a total of 220 livers were encountered (123 donors and 97 recipient organs), 24 of which had inadequacies or anomalies of hepatic arterial supply which required modifications of the basic techniques of arterial reconstruction. The various methods used to deal with these various situations are described herein. The use of vessel grafts from the donor has proved quite satisfactory over a number of years. The routine procurement of various vessel grafts can provide a multiplicity of options for arterial reconstruction, only a few of which have been mentioned herein.
Acknowledgments
This study was supported by research grants from the Veterans Administration; by project grant No. AM-29961 from the National Institutes of Health, and by grant No. RR-00084 from the General Clinical Research Centers Program of the Division of Research Resources, National Institutes of Health.
References
- 1.Starzl TE. Experience in Hepatic Transplantation. Philadelphia: W. B. Saunders; 1969. (With the assistance of C. W. Putman) [Google Scholar]
- 2.Starzl TE, Halgrimson CG, Koep LJ, et al. Vascular homografts from cadaveric organ donors. Surg Gynecol Obstet. 1979;149:737. [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE, Kaupp HA, Brock DR, et al. Reconstructive problems in canine liver homotransplantation with special reference to the postoperative role of hepatic venous flow. Surg Gynecol Obstet. 1961;11:733–743. [PMC free article] [PubMed] [Google Scholar]