Abstract
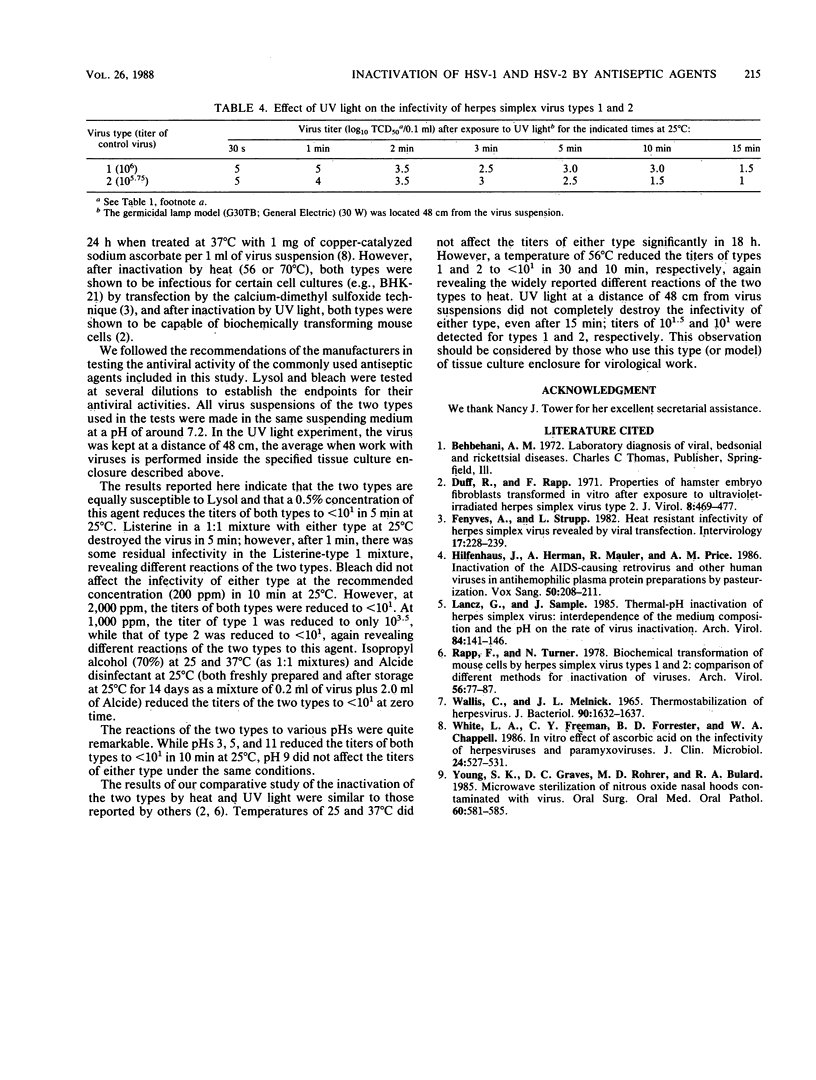
A comparative study of the different reactions of herpes simplex virus types 1 and 2 to Lysol, Listerine, bleach, rubbing alcohol, Alcide disinfectant (Alcide Corp., Westport, Conn.), and various pHs, temperatures, and UV light exposures was performed. Both types of stock virus (titers of approximately 10(6) and 10(5.5) for types 1 and 2, respectively) were inactivated by 0.5% Lysol in 5 min; by Listerine (1:1 mixtures) in 5 min; by 2,000 ppm (2,000 microliters/liter) of bleach in 10 min; by rubbing alcohol (1:1 mixtures) at zero time; by Alcide disinfectant (0.2 ml of virus plus 2.0 ml of Alcide) at zero time; by pHs 3, 5, and 11 in 10 min; and by a temperature of 56 degrees C in 30 min. A germicidal lamp (model G30TB; General Electric Co., Schenectady, N.Y.) (30 W) at a distance of 48 cm failed to completely inactivate the two types in 15 min. Type 1 showed slightly more resistance to Listerine and bleach and significantly more resistance to heat; moreover, pH 9 did not affect the infectivity of either type after 10 min.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Duff R., Rapp F. Properties of hamster embryo fibroblasts transformed in vitro after exposure to ultraviolet-irradiated herpes simplex virus type 2. J Virol. 1971 Oct;8(4):469–477. doi: 10.1128/jvi.8.4.469-477.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenyves A., Strupp L. Heat-resistant infectivity of herpes simplex virus revealed by viral transfection. Intervirology. 1982;17(4):228–239. doi: 10.1159/000149293. [DOI] [PubMed] [Google Scholar]
- Hilfenhaus J., Herrmann A., Mauler R., Prince A. M. Inactivation of the AIDS-causing retrovirus and other human viruses in antihemophilic plasma protein preparations by pasteurization. Vox Sang. 1986;50(4):208–211. doi: 10.1111/j.1423-0410.1986.tb04882.x. [DOI] [PubMed] [Google Scholar]
- Lancz G., Sample J. Thermal-pH inactivation of herpes simplex virus: interdependence of the medium composition and the pH on the rate of virus inactivation. Brief report. Arch Virol. 1985;84(1-2):141–146. doi: 10.1007/BF01310561. [DOI] [PubMed] [Google Scholar]
- Rapp F., Turner N. Biochemical Transformation of mouse cells by herpes simplex virus types 1 and 2: comparison of different methods for inactivation of viruses. Arch Virol. 1978;56(1-2):77–87. doi: 10.1007/BF01317284. [DOI] [PubMed] [Google Scholar]
- Wallis C., Melnick J. L. Thermostabilization and thermosensitization of herpesvirus. J Bacteriol. 1965 Dec;90(6):1632–1637. doi: 10.1128/jb.90.6.1632-1637.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White L. A., Freeman C. Y., Forrester B. D., Chappell W. A. In vitro effect of ascorbic acid on infectivity of herpesviruses and paramyxoviruses. J Clin Microbiol. 1986 Oct;24(4):527–531. doi: 10.1128/jcm.24.4.527-531.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. K., Graves D. C., Rohrer M. D., Bulard R. A. Microwave sterilization of nitrous oxide nasal hoods contaminated with virus. Oral Surg Oral Med Oral Pathol. 1985 Dec;60(6):581–585. doi: 10.1016/0030-4220(85)90355-x. [DOI] [PubMed] [Google Scholar]



