Abstract
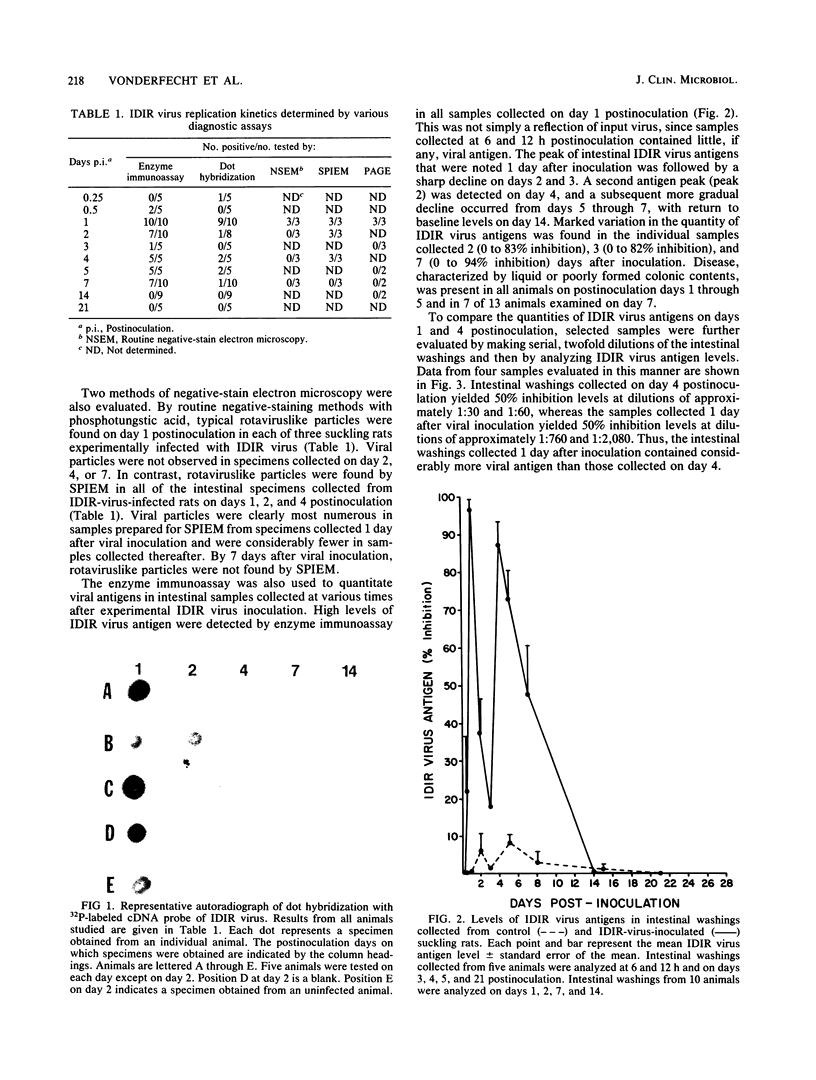
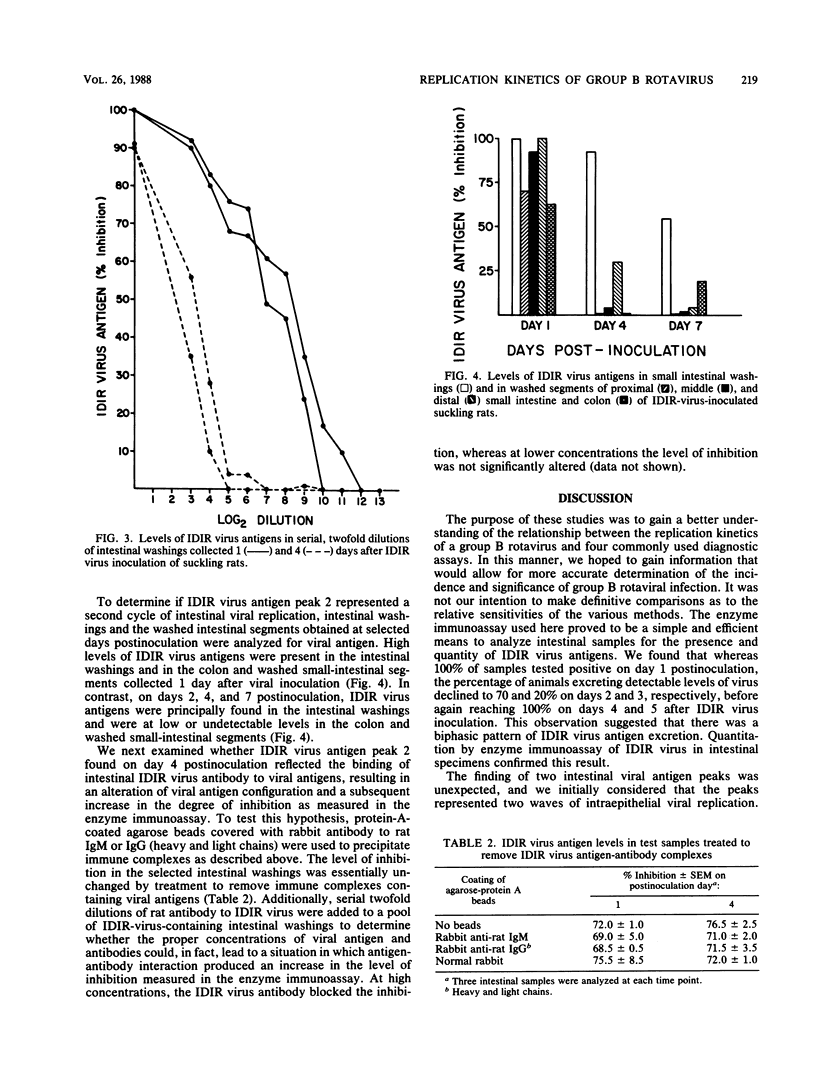
Non-group-A rotaviruses have been implicated with increasing frequency as causes of acute gastroenteritis in humans and other animals. However, the incidence and significance of infection with these agents, as well as appropriate diagnostic strategies for making these determinations, are largely unknown. Studies to make these determinations could be more accurately conducted if the relationship between the viral replication kinetics and the particular diagnostic method used is understood. We thus utilized the murine model of group B rotavirus infection to establish the viral replication kinetics by a variety of commonly used diagnostic methods. Enzyme immunoassay, routine negative-stain electron microscopy, solid-phase immunosorbent electron microscopy, polyacrylamide gel electrophoresis, and a dot hybridization assay were used in these studies. By enzyme immunoassay, 100% of experimentally infected suckling rats tested positive for group B rotaviral antigens at 1, 4, and 5 days postinoculation. However, only 70 and 20% of infected animals tested positive at days 2 and 3 postinoculation, respectively. Dot hybridization with a complementary DNA probe also suggested a biphasic pattern of viral antigen excretion. Evidence of the virus causing infectious diarrhea in infant rats was found only on day 1 postinoculation in samples examined by routine negative-stain electron microscopy and by polyacrylamide gel electrophoresis. Rotaviruslike particles were observed by solid-phase immunosorbent electron microscopy on days 1, 2, and 4 after viral inoculation suckling rats but were clearly the most numerous on day 1. Additionally, the enzyme immunoassay was used to quantitate the kinetics of group B rotaviral replication in the intestines of the experimentally infected animals. Levels of murine group B rotaviral antigens in intestinal samples peaked on days 1 and 4 postinoculation; however, only peak 1 represented actual intraepithelial replication of the virus. These studies thus indicate that early sample collection and selection of the appropriate diagnostic method are critical if the incidence and significance of group B and possibly other non-group-A rotaviral infections are to be accurately assessed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askaa J., Bloch B. Infection in piglets with a porcine rotavirus-like virus. Experimental inoculation and ultrastructural examination. Arch Virol. 1984;80(4):291–303. doi: 10.1007/BF01311220. [DOI] [PubMed] [Google Scholar]
- Bohl E. H., Saif L. J., Theil K. W., Agnes A. G., Cross R. F. Porcine pararotavirus: detection, differentiation from rotavirus, and pathogenesis in gnotobiotic pigs. J Clin Microbiol. 1982 Feb;15(2):312–319. doi: 10.1128/jcm.15.2.312-319.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridger J. C. Detection by electron microscopy of caliciviruses, astroviruses and rotavirus-like particles in the faeces of piglets with diarrhoea. Vet Rec. 1980 Dec 6;107(23):532–533. [PubMed] [Google Scholar]
- Chasey D., Banks J. The commonest rotaviruses from neonatal lamb diarrhoea in England and Wales have atypical electropherotypes. Vet Rec. 1984 Sep 29;115(13):326–327. doi: 10.1136/vr.115.13.326. [DOI] [PubMed] [Google Scholar]
- Chasey D., Bridger J. C., McCrae M. A. A new type of atypical rotavirus in pigs. Arch Virol. 1986;89(1-4):235–243. doi: 10.1007/BF01309892. [DOI] [PubMed] [Google Scholar]
- Dimitrov D. H., Estes M. K., Rangelova S. M., Shindarov L. M., Melnick J. L., Graham D. Y. Detection of antigenically distinct rotaviruses from infants. Infect Immun. 1983 Aug;41(2):523–526. doi: 10.1128/iai.41.2.523-526.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden J., Vonderfecht S., Theil K., Torres-Medina A., Yolken R. H. Genetic and antigenic relatedness of human and animal strains of antigenically distinct rotaviruses. J Infect Dis. 1986 Dec;154(6):972–982. doi: 10.1093/infdis/154.6.972. [DOI] [PubMed] [Google Scholar]
- Eiden J., Vonderfecht S., Yolken R. H. Evidence that a novel rotavirus-like agent of rats can cause gastroenteritis in man. Lancet. 1985 Jul 6;2(8445):8–11. doi: 10.1016/s0140-6736(85)90057-1. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., Puerto F., Soler C., González N. Characterization of a human pararotavirus. Infect Immun. 1984 Apr;44(1):112–116. doi: 10.1128/iai.44.1.112-116.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring A. J., Inglis N. F., Ojeh C. K., Snodgrass D. R., Menzies J. D. Rapid diagnosis of rotavirus infection by direct detection of viral nucleic acid in silver-stained polyacrylamide gels. J Clin Microbiol. 1982 Sep;16(3):473–477. doi: 10.1128/jcm.16.3.473-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung T., Chen G. M., Wang C. G., Yao H. L., Fang Z. Y., Chao T. X., Chou Z. Y., Ye W., Chang X. J., Den S. S. Waterborne outbreak of rotavirus diarrhoea in adults in China caused by a novel rotavirus. Lancet. 1984 May 26;1(8387):1139–1142. [PubMed] [Google Scholar]
- McNulty M. S., Allan G. M., Todd D., McFerran J. B., McCracken R. M. Isolation from chickens of a rotavirus lacking the rotavirus group antigen. J Gen Virol. 1981 Aug;55(Pt 2):405–413. doi: 10.1099/0022-1317-55-2-405. [DOI] [PubMed] [Google Scholar]
- Nakata S., Estes M. K., Graham D. Y., Loosle R., Tao H., Wang S. H., Saif L. J., Melnick J. L. Antigenic characterization and ELISA detection of adult diarrhea rotaviruses. J Infect Dis. 1986 Sep;154(3):448–455. doi: 10.1093/infdis/154.3.448. [DOI] [PubMed] [Google Scholar]
- Nakata S., Petrie B. L., Calomeni E. P., Estes M. K. Electron microscopy procedure influences detection of rotaviruses. J Clin Microbiol. 1987 Oct;25(10):1902–1906. doi: 10.1128/jcm.25.10.1902-1906.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas J. C., Cohen J., Fortier B., Lourenco M. H., Bricout F. Isolation of a human pararotavirus. Virology. 1983 Jan 15;124(1):181–184. doi: 10.1016/0042-6822(83)90302-1. [DOI] [PubMed] [Google Scholar]
- Obert G., Gloeckler R., Burckard J., van Regenmortel M. H. Comparison of immunosorbent electron microscopy, enzyme immunoassay and counterimmunoelectrophoresis for detection of human rotavirus in stools. J Virol Methods. 1981 Sep;3(2):99–107. doi: 10.1016/0166-0934(81)90006-9. [DOI] [PubMed] [Google Scholar]
- Rodger S. M., Bishop R. F., Holmes I. H. Detection of a rotavirus-like agent associated with diarrhea in an infant. J Clin Microbiol. 1982 Oct;16(4):724–726. doi: 10.1128/jcm.16.4.724-726.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif L. J., Bohl E. H., Theil K. W., Cross R. F., House J. A. Rotavirus-like, calicivirus-like, and 23-nm virus-like particles associated with diarrhea in young pigs. J Clin Microbiol. 1980 Jul;12(1):105–111. doi: 10.1128/jcm.12.1.105-111.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass D. R., Herring A. J., Campbell I., Inglis J. M., Hargreaves F. D. Comparison of atypical rotaviruses from calves, piglets, lambs and man. J Gen Virol. 1984 May;65(Pt 5):909–914. doi: 10.1099/0022-1317-65-5-909. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Chen G. M., Hung T., Beards G. M., Brown D. W., Flewett T. H. Effects of two negative staining methods on the Chinese atypical rotavirus. Arch Virol. 1987;94(3-4):305–308. doi: 10.1007/BF01310723. [DOI] [PubMed] [Google Scholar]
- Theil K. W., Saif L. J., Moorhead P. D., Whitmoyer R. E. Porcine rotavirus-like virus (group B rotavirus): characterization and pathogenicity for gnotobiotic pigs. J Clin Microbiol. 1985 Mar;21(3):340–345. doi: 10.1128/jcm.21.3.340-345.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil K. W., Saif Y. M. Age-related infections with rotavirus, rotaviruslike virus, and atypical rotavirus in turkey flocks. J Clin Microbiol. 1987 Feb;25(2):333–337. doi: 10.1128/jcm.25.2.333-337.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonderfecht S. L., Eiden J. J., Torres A., Miskuff R. L., Mebus C. A., Yolken R. H. Identification of a bovine enteric syncytial virus as a nongroup A rotavirus. Am J Vet Res. 1986 Sep;47(9):1913–1918. [PubMed] [Google Scholar]
- Vonderfecht S. L., Huber A. C., Eiden J., Mader L. C., Yolken R. H. Infectious diarrhea of infant rats produced by a rotavirus-like agent. J Virol. 1984 Oct;52(1):94–98. doi: 10.1128/jvi.52.1.94-98.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonderfecht S. L., Miskuff R. L., Eiden J. J., Yolken R. H. Enzyme immunoassay inhibition assay for the detection of rat rotavirus-like agent in intestinal and fecal specimens obtained from diarrheic rats and humans. J Clin Microbiol. 1985 Nov;22(5):726–730. doi: 10.1128/jcm.22.5.726-730.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]




