Abstract
How Ca2+-dependent signaling effectors are regulated in cardiomyocytes, given the extreme cytoplasmic Ca2+ concentration changes that underlie contraction, remains unknown. Cardiomyocyte plasma membrane Ca2+-ATPase (PMCA) extrudes Ca2+ but has little effect on excitation-contraction coupling, suggesting its potential role in controlling Ca2+-dependent signaling effectors such as calcineurin. We generated cardiac-specific inducible PMCA4b transgenic mice that displayed normal global Ca2+ transient and cellular contraction levels and reduced cardiac hypertrophy following transverse aortic constriction (TAC) or phenylephrine/Ang II infusion, but showed no reduction in exercise-induced hypertrophy. Transgenic mice were protected from decompensation and fibrosis following long-term TAC. The PMCA4b transgene reduced the hypertrophic augmentation associated with transient receptor potential canonical 3 channel overexpression, but not that associated with activated calcineurin. Furthermore, Pmca4 gene–targeted mice showed increased cardiac hypertrophy and heart failure events after TAC. Physical associations between PMCA4b and calcineurin were enhanced by TAC and by agonist stimulation of cultured neonatal cardiomyocytes. PMCA4b reduced calcineurin nuclear factor of activated T cell–luciferase activity after TAC and in cultured neonatal cardiomyocytes after agonist stimulation. PMCA4b overexpression inhibited cultured cardiomyocyte hypertrophy following agonist stimulation, but much less so in a Ca2+ pumping–deficient PMCA4b mutant. Thus, Pmca4b likely reduces the local Ca2+ signals involved in reactive cardiomyocyte hypertrophy via calcineurin regulation.
Introduction
Cardiac hypertrophy is typically characterized by an enlargement of the heart associated with an increase in cardiomyocyte cell volume. Hypertrophy occurs during postnatal development, in response to physiologic stimuli such as exercise, and in response to diverse pathophysiologic stimuli such as hypertension, ischemic heart disease, valvular insufficiency, infectious agents, or mutations in sarcomeric genes (1). Pathologic hypertrophic growth of the myocardium is thought to temporarily preserve pump function, although prolongation of the hypertrophic state is a leading predictor for the development of arrhythmias and sudden death as well as dilated cardiomyopathy and heart failure (2, 3). In general, the hypertrophic growth of the myocardium is regulated by endocrine, paracrine, and autocrine growth factors that activate membrane-bound receptors, resulting in signal transduction that culminates in altered gene transcription and protein accumulation as part of the hypertrophic program (4). This hypertrophic growth program is regulated by nodal intracellular signal transduction pathways such as MAPK, calcineurin/nuclear factor of activated T cells (calcineurin/NFAT), IGF-I/PI3K/Akt/PKB, and many others (4).
Ca2+ signaling has been suggested to initiate cardiac hypertrophy, even though it remains unclear how Ca2+ is sensed by select intracellular signaling factors in the heart, given the backdrop of Ca2+ cycling associated with excitation contraction-coupling (ECC) (5). Specialized pools of Ca2+ that are location specific or somehow buffered from cytosolic Ca2+ have been evoked to account for the regulation of Ca2+-sensitive signaling proteins such as calcineurin or Ca2+/calmodulin-activated protein kinase II (CaMKII). Indeed, CaMKII is regulated in cardiomyocytes by a peri-nuclear Ca2+ pool associated with inositol triphosphate receptor (InsP3R) activity (6). The voltage-gated L-type Ca2+ channel, which normally triggers Ca2+-induced Ca2+ release and contraction, has also been shown to localize to specialized lipid raft–containing membrane domains that are known to serve as signal transduction organizing centers (7). Finally, another paradigm has suggested that reactive Ca2+ in myocytes is associated with the transient receptor potential canonical (TRPC) channels that mediate store-, receptor-, and stretch-operated Ca2+ entry (8–11). For example, TRPC channel overexpression in the mouse heart promoted calcineurin activation and cardiac hypertrophy (12–14). Interestingly, distinct plasma membrane lipid domains or caveolar domains, which contain high concentrations of cholesterol and sphingolipids, have been suggested to provide a platform for the assembly of Ca2+ signaling complexes including GPCRs and TRPCs (15).
The plasma membrane Ca2+ ATPase (PMCA) family of pumps is expressed in the heart, with PMCA1 and PMCA4 most represented, where they are thought to specifically reduce Ca2+ in subsarcolemmal microdomains associated with lipid rafts and caveoli (16). However, PMCA activity is not significantly involved in ECC, as the amount of Ca2+ extruded by this pump in adult myocytes is negligible (less than 1%) (17). Indeed, PMCA4b has been suggested to regulate a membrane regional pool of Ca2+ involved in signaling to NOS1 in cardiomyocytes (18, 19). Transgenic rats that overexpress PMCA4b in the heart have been described, and these animals did not show alterations in ECC but instead showed defects in signaling to NOS1 (18, 19). More recently, Oceandy et al. generated PMCA4b transgenic mice using the myosin light chain 2v (MLC2v) promoter, and once again myocytes from these hearts showed no alterations in the Ca2+ transient or in ECC (20). Instead, myocytes from these hearts showed a reduction in β-adrenergic receptor pathway–mediated augmentation in contractility through a NOS1-dependent mechanism (20). In HEK cells, PMCA4b was shown to inhibit the calcineurin/NFAT pathway via interaction with the calcineurin A catalytic subunit by the pump itself (21). While these results suggest that PMCA isoforms might be regulating signaling in select microdomains by reducing Ca2+, PMCA4b transgenic mice were actually reported to have enhanced cardiac hypertrophy following 7 days of isoproterenol infusion (20). Here we show that PMCA4b overexpression in mice reduced calcineurin/NFAT signaling in the heart and was anti-hypertrophic, whereas gene-targeting of Pmca4 rendered the heart more susceptible to hypertrophy following pressure overload stimulation.
Results
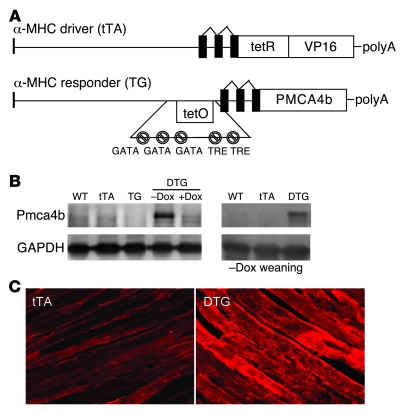
To better understand how region-specific alterations in Ca2+ might affect the cardiac growth response, we generated transgenic mice with inducible overexpression of Pmca4b in the heart. Heart-specific and inducible expression was achieved with a binary α-myosin heavy chain (α-MHC) promoter–based transgene strategy (22). The responder transgene permitted expression of PMCA4b in the heart only in the presence of the driver transgene encoding the tetracycline transactivator (tTA) protein and in the absence of tetracycline/doxycycline (tetracycline/Dox) (Figure 1A). While a number of responder lines were generated, 2 showed faithful inducible expression and were used here. Western blotting for Pmca4b protein in the hearts of one line showed abundant expression in double transgenic (DTG) mice in the absence of Dox but inhibition of expression in the presence of Dox (Figure 1B). For all subsequent experiments, litters of mice were kept on Dox-containing food until weaning (4 weeks) to prevent expression of Pmca4b during heart maturation, reducing the likelihood of developmental effects. By 8–12 weeks of age, Dox cleared the animal’s system and abundant Pmca4b expression was observed in the heart (Figure 1B). Immunolocalization of PMCA4b in the hearts of DTG mice showed mostly sarcolemmal and T-tubular expression, a pattern that was essentially the same for endogenous Pmca4, albeit at substantially lower levels (Figure 1C).
Figure 1. Generation of cardiac-specific, inducible Pmca4b transgenic mice.
(A) Schematic of the bi-transgenic inducible expression system used to regulate Pmca4b in the mouse heart. tetR-VP16 is a fusion protein that constitutes the tetracycline activator protein (tTA). GATA, GATA transcription factor binding site; TRE, thyroid response element; tetO, tTA binding site. (B) Pmca4b protein expression in controls that were WT, single tTA, PMCA4b transgenic (TG), or DTG mice in the presence or absence of Dox (left panels). The right panels show Pmca4b protein expression in DTG mice in young adulthood after Dox removal at weaning. (C) Immunofluorescence of Pmca4b protein (red) in hearts from tTA control mice and PMCA4b DTG mice in the induced state. Original magnification, ×600.
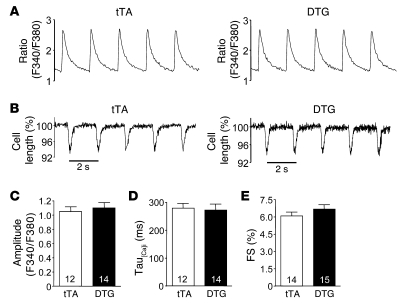
Overexpression of PMCA4b in the hearts of DTG mice did not alter Ca2+ handling. The amplitude of the Ca2+ transient, Ca2+ reuptake rates, and fractional shortening of individual myocytes was identical between single transgenic control (tTA) and DTG mice (Figure 2, A–E). These results indicate that PMCA4b overexpression does not alter global Ca2+ handling in the heart, further suggesting that endogenous Pmca4 likely functions in a more specialized manner to regulate region-specific Ca2+.
Figure 2. PMCA4b overexpression does not alter the Ca2+ transient or fractional shortening.
(A) Representative 0.5-Hz Ca2+ transients from adult cardiomyocytes isolated from the hearts of tTA controls (12 cells) or PMCA4b DTG mice (14 cells). (B) Fractional shortening of isolated ventricular myocytes from tTA and PMCA4b DTG mice. (C) Quantitative analysis of the peak of Ca2+ transients in myocytes from tTA and PMCA4b DTG mice. (D) Quantitative analysis of relaxation rate (Tau) for the Ca2+ transients in A. (E) Quantitative analysis of cell shortening from B (n = 3–5 mice in each group). FS, fractional shortening. Numbers within the bars indicate the number of cells counted.
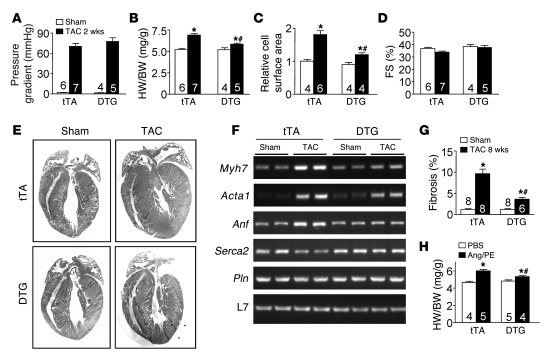
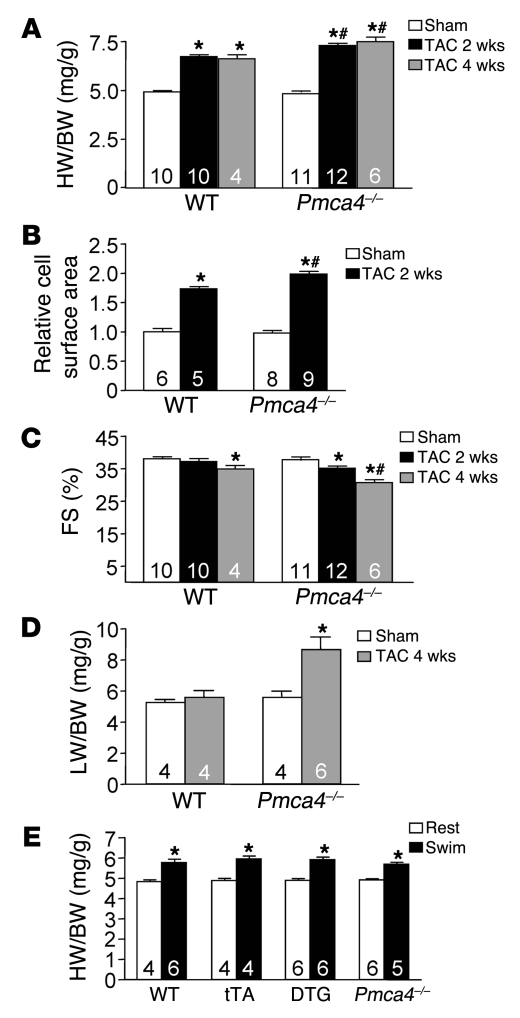
We hypothesized that PMCA4b overexpression might reduce Ca2+ concentration in select microdomains involved with reactive hypertrophic signaling, hence altering the cardiac growth response to stress stimulation. To examine this hypothesis, 10- to 12-week-old tTA control and PMCA4b DTG mice (Dox removed at weaning) were subjected to pressure overload stimulation by transverse aortic constriction (TAC), which produced similar pressure gradients across the constriction (Figure 3A). Remarkably, DTG mice showed significantly less cardiac hypertrophy after 2 and 4 weeks of TAC stimulation compared with control mice (Figure 3B, Table 1, and Supplemental Figure 1A; supplemental material available online with this article; doi:10.1172/JCI36693DS1). Measurement of cellular surface areas from histological sections of control and DTG mice subjected to TAC also showed less cellular hypertrophy associated with PMCA4b overexpression (Figure 3C). PMCA4b overexpression also reduced the magnitude of atrial natriuretic factor (Anf), skeletal α-actin (Acta1), and β-myosin heavy chain (Myh7) mRNA upregulation following 4 weeks of TAC, while sarcoplasmic reticulum (SR) Ca2+ ATPase 2 (Serca2a) mRNA levels did not decrease after TAC compared with tTA controls (Figure 3F). PMCA4b overexpression also significantly reduced cardiac hypertrophy following 2 weeks of phenylephrine/Ang II (PE/Ang II) infusion using osmotic minipumps (Figure 3H). These results strongly suggest that PMCA4b overexpression antagonizes the cardiac growth response following both pressure overload and neuroendocrine agonist stimulation.
Figure 3. Pmca4b overexpression attenuates stress-induced cardiac hypertrophy.
(A) Aortic pressure gradients across the constriction in control (tTA) and DTG (PMCA4b overexpression) TAC-operated mice. (B) Measurement of heart weight normalized to body weight (HW/BW) in control and DTG mice after 2 weeks of TAC or a sham procedure. *P < 0.05 versus sham, #P < 0.05 versus tTA TAC. (C) Cardiomyocyte surface areas from histological sections of tTA or DTG mice after 2 weeks of TAC normalized to sham surface areas (*P < 0.05 versus sham, #P < 0.05 versus tTA TAC; n = 400 cells from 4–6 hearts). (D) Cardiac fractional shortening in tTA and DTG mice after 2 weeks of TAC or sham procedure. (E) Representative H&E-stained heart sections from tTA and DTG mice after 2 weeks of TAC or a sham procedure. (F) mRNA levels for the indicated genes from hearts of the indicated mice after 4 weeks of TAC. L7 is a control for mRNA content and integrity. Myh7, β-myosin heavy chain; Acta1, skeletal α-actin; Pln, phospholamban. (G) Quantitative analysis of fibrosis area in hearts of the indicated groups 8 weeks after TAC or a sham procedure (*P < 0.05 versus sham, #P < 0.05 versus tTA TAC). (H) Heart weight normalized to body weight in control and DTG mice after 2 weeks of Ang/PE infusion or PBS vehicle treatment. *P < 0.05 versus PBS, #P < 0.05 versus tTA Ang/PE). The number of mice used is shown within the bars of the various graphs.
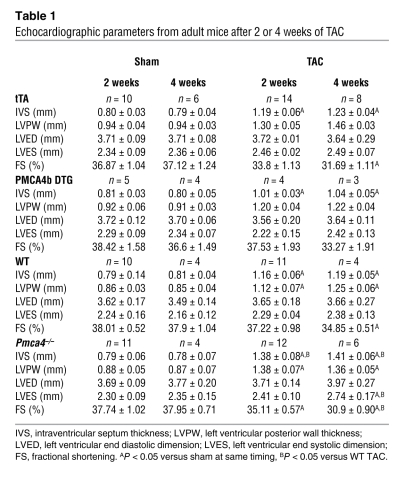
Table 1 .
Echocardiographic parameters from adult mice after 2 or 4 weeks of TAC
While PMCA4b overexpression significantly reduced cardiac hypertrophy following pressure overload, it did not otherwise compromise the heart, as fractional shortening measured by echocardiography after 2 and 4 weeks of TAC was not reduced (Figure 3D, Supplemental Figure 1B, and Table 1). PMCA4b-overexpressing hearts also did not dilate or show any other signs of pathology (Figure 3E and Table 1). Moreover, PMCA4b overexpression actually protected against decompensation following 4 and 8 weeks of TAC stimulation, such that fractional shortening was maintained compared with controls, pulmonary edema was not observed, and interstitial fibrosis was dramatically reduced (Figure 3G; Supplemental Figure 1, C and D; and Table 1).
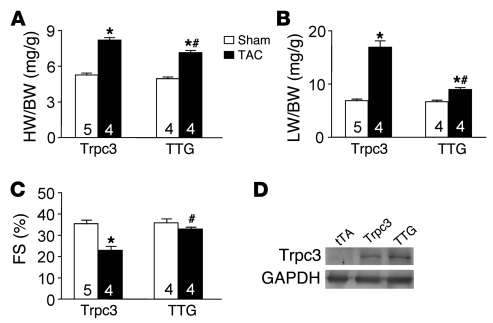
In agreement with the hypothesis that Pmca4b likely reduces cardiac hypertrophic potential by removing Ca2+ from discrete sarcolemmal microdomains, increasing Ca2+ influx through TRPC channels is known to sensitize the heart to greater hypertrophy following TAC (12). TRPC channels are thought to also regulate Ca2+ levels within sarcolemmal microdomains without altering ECC (23). To examine this hypothetical Ca2+ microdomain mechanism in greater detail, we crossed Trpc3 transgenic mice with PMCA4b DTG mice to produce triple transgenic (TTG) mice and performed TAC stimulation. Remarkably, the augmented profile of TAC-induced hypertrophy associated with the Trpc3 transgene was significantly reduced by PMCA4b overexpression (Figure 4A). As described previously, pressure overload stimulation of Trpc3 transgenic mice similarly induced ventricular decompensation and pulmonary edema (12), which was prevented by simultaneous PMCA4b overexpression (Figure 4, B and C). Importantly, the 2 transgenes associated with the PMCA4b overexpression strategy did not alter Trpc3 protein levels driven by the same α-MHC promoter (Figure 4D). Thus, Pmca4b antagonizes the deleterious growth response associated with increased Trpc3 expression and its known ability to augment Ca2+ influx during stress stimulation.
Figure 4. Pmca4b overexpression attenuates Trpc3-enhanced cardiac hypertrophy.
(A) Heart weight normalized to body weight in Trpc3 transgenic and TTG mice containing the Trpc3, tTA, and Pmca4b transgenes (*P < 0.05 versus sham, #P < 0.05 versus Trpc3 TAC). (B) Lung weight normalized to body weight (LW/BW) in Trpc3 and TTG mice after 2 weeks of TAC or a sham procedure (*P < 0.05 versus sham, #P < 0.05 versus Trpc3 TAC). (C) Cardiac fractional shortening in Trpc3 and TTG mice after 2 weeks of TAC or a sham procedure (*P < 0.05 versus sham, #P < 0.05 versus Trpc3 TAC). (D) Western blot for Trpc3 protein expression levels in Trpc3 single transgenic and TTG hearts. GAPDH is shown as a loading control. The number of mice used is shown within the bars of the various graphs.
The results presented above suggested a mechanism whereby a Pmca4b proximal microdomain of Ca2+ could affect the cardiac hypertrophic response. However, it is equally important to determine whether endogenous Pmca4 regulates the cardiac growth response. To this end, adult Pmca4–/– mice and strain-matched littermate controls (FVBN) were subjected to TAC stimulation for 2 weeks. As predicted by the results in Pmca4b-overexpressed mice, Pmca4–/– mice showed a small but significant increase in cardiac hypertrophy following pressure overload stimulation (Figure 5A). Pressure gradients across the aortic constriction were not different between the groups, indicating equal stimulation (data not shown). Histological analysis of myocyte surface area also showed significantly greater growth in Pmca4–/– mice compared with WT controls (Figure 5B), and Pmca4–/– mice showed signs of decompensation following only 2 weeks of TAC stimulation, which was not yet observed in WT control mice (Figure 5C). These experiments were repeated with 4 weeks of TAC in an independent cohort, and once again Pmca4–/– mice showed significantly greater cardiac hypertrophy at the whole organ as well as even greater decompensation, as measured by echocardiography (Figure 5, A and C, and Table 1). Pulmonary edema was also observed in Pmca4–/– mice after 4 weeks of TAC compared with no change in lung weight in WT controls (Figure 5D). Interestingly, an analysis of baseline heart weights in 1-year-old Pmca4–/– mice (n = 4) showed a small (12%) but significant (P < 0.05) increase compared with WT controls (n = 3). Thus, loss of Pmca4 renders the heart slightly more sensitive to pathologic hypertrophic growth following pressure overload stimulation and more prone to heart failure. These results further suggest that Pmca4 helps regulate reactive signaling in the heart through a mechanism involving Ca2+ microdomains. However, overexpression of PMCA4b in transgenic mice or its loss in gene-deleted mice did not alter the hypertrophy response of the heart following exercise stimulation, which consisted of 23 days of forced swimming (Figure 5E). These results strengthen the argument that PMCA4 regulates a Ca2+-dependent signaling microdomain for effectors such as calcineurin, especially since calcineurin/NFAT signaling is also not affected by physiologic stimulation (see Discussion).
Figure 5. Pmca4–/– mice show enhanced pressure overload–induced cardiac hypertrophy.
(A) Heart weight normalized to body weight in WT and Pmca4–/– mice after 2 or 4 weeks of TAC or a sham procedure (*P < 0.05 versus sham, #P < 0.05 versus WT TAC). (B) Cardiomyocyte surface areas from histological sections of WT or Pmca4–/– mice after 2 weeks of TAC normalized to sham (*P < 0.05 versus sham, #P < 0.05 versus WT TAC; n = 400 cells from 5–9 hearts). (C) Cardiac fractional shortening in WT and Pmca4–/– mice after 2 or 4 weeks of TAC or a sham procedure (*P < 0.05 versus sham, #P < 0.05 versus WT TAC). (D) Lung weight normalized to body weight after 4 weeks of TAC in the indicated groups of mice (*P < 0.05 versus WT TAC). (E) Heart weight normalized to body weight in the indicated groups of mice at rest or following 23 days of swimming exercise (*P < 0.05 versus rest). The number of mice used is shown within the bars.
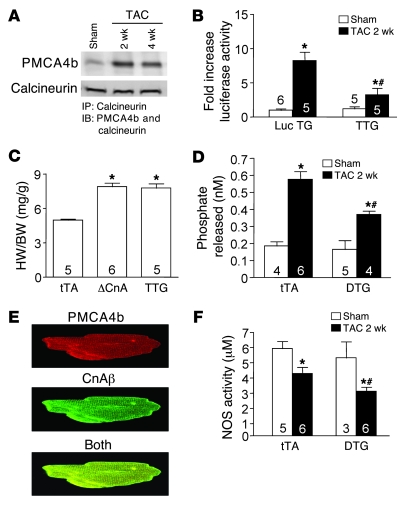
Additional evidence that PMCA4 might regulate a pathologic/pro-hypertrophic Ca2+ signaling microdomain was suggested by the observation that calcineurin could physically associate with PMCA4b (21). Calcineurin is a Ca2+-activated protein phosphatase that regulates the cardiac hypertrophic response through dephosphorylation and subsequent nuclear translocation of NFAT transcription factors (24). Here we observed that pressure overload stimulation dramatically augmented the association between Pmca4b and calcineurin using an immunoprecipitation procedure from DTG mouse hearts subjected to 2 or 4 weeks of TAC (Figure 6A). Consistent with this result, we also crossed the NFAT–luciferase reporter transgene into the DTG PMCA4b background (TTG mice) to assess calcineurin/NFAT signaling capacity in vivo. TAC stimulation for 2 weeks produced a 7- to 8-fold increase in NFAT-luciferase activity in the hearts of single transgenic mice, but only a 2-fold increase in the presence of PMCA4b overexpression (Figure 6B). These results suggest that PMCA4b can alter calcineurin/NFAT signaling capacity in vivo following stress stimulation, providing a potential downstream mechanistic association with the observed reduction in hypertrophy through PMCA4b. Importantly, PMCA4b overexpression did not antagonize the hypertrophy response driven by the activated calcineurin transgene in these TTG mice (Figure 6C), This latter result is especially informative, since the activated calcineurin transgene is no longer Ca2+ activated, further suggesting that Pmca4-regulated hypertrophy is proximally associated with endogenous calcineurin signaling. The lack of attenuated hypertrophy associated with the activated calcineurin transgene also served as an important control to demonstrate that PMCA4b was not simply compromising the growth potential of the heart in a more generalized and nonspecific manner. PMCA4b overexpression also did not alter the distribution of calcineurin between the cytosol and membranes (data not shown).
Figure 6. Pmca4b regulates calcineurin/NFAT signaling in vivo.
(A) Immunoprecipitation of calcineurin from PMCA4b-overexpressing DTG hearts of TAC or sham-operated mice, after which the extracts were Western blotted for PMCA4b or calcineurin protein. (B) NFAT-luciferase activity from the hearts of NFAT-luciferase transgenic mice or NFAT-luciferase transgenic mice (Luc TG) containing the tTA and PMCA4b transgenes (TTG) after 2 weeks of TAC or a sham procedure (*P < 0.05 versus sham, #P < 0.05 versus NFAT-luciferase TAC). (C) Heart weight normalized to body weight in controls (tTA), single calcineurin (ΔCnA) transgenic, or TTG (tTA, PMCA4b, and ΔCnA transgenes) (*P < 0.05 versus tTA). (D) Calcineurin phosphatase activity from the hearts of mice in the indicated groups (*P < 0.05 versus sham, #P < 0.05 versus tTA TAC). (E) Immunocytochemistry of adult rat myocytes for PMCA4b and calcineurin Aβ (CnAβ). Original magnification, ×600. (F) Total NOS activity from the hearts of mice in the indicated groups (*P < 0.05 versus sham, #P < 0.05 versus tTA TAC). The number of mice used is shown within the bars.
We also directly measured calcineurin enzymatic activity after 2 weeks of TAC stimulation from hearts of DTG mice, which showed significantly less induction in activity compared with tTA control mice (Figure 6D). Indeed, overexpression of PMCA4b and calcineurin Aβ in adult rat cardiomyocytes by adenoviral gene transfer showed prominent colocalization at the z lines/T-tubular region (Figure 6E). PMCA4b has also been shown to negatively regulate NOS1 activity in the heart through a direct interaction (see Discussion), suggesting an additional effector pathway that could alter the cardiac hypertrophic response. Indeed, we observed a greater decrease in total cardiac NOS activity in the hearts of DTG mice after 2 weeks of TAC compared with tTA control mice, suggesting that PMCA4b overexpression could further antagonize NOS activity in vivo (Figure 6F).
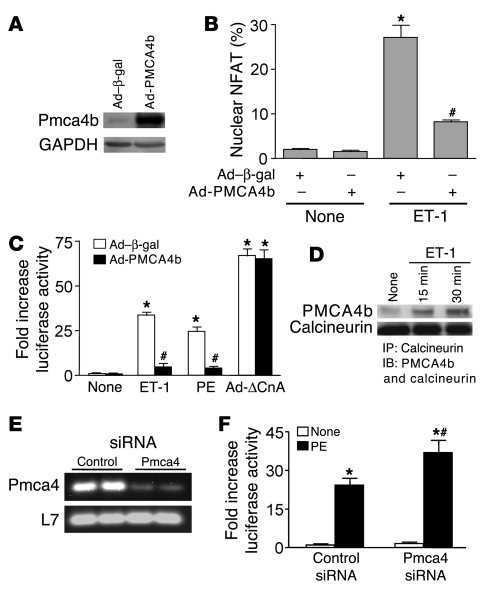
To further examine the association between Pmca4 activity and the regulation of cardiac hypertrophy through calcineurin, we generated a PMCA4b recombinant adenovirus (Ad-PMCA4b) for studies in cultured neonatal rat cardiomyocytes (Figure 7A). Consistent with the results described for the NFAT-luciferase transgenic mice presented above, Ad-PMCA4b infection of neonatal myocytes reduced NFAT nuclear translocation and transcriptional activation following agonist stimulation (Figure 7, B and C). Ad-PMCA4b infection reduced both endothelin-1–induced (ET-1–induced) and PE-induced NFAT activity but had no effect on NFAT activity induced by an activated calcineurin mutant adenovirus (Ad-ΔCnA), which is Ca2+ independent in its mode of regulation (Figure 7C). Mechanistically, ET-1 stimulation increased the association between endogenous calcineurin and overexpressed PMCA4b in neonatal cardiomyocytes after 15 and 30 minutes of stimulation (Figure 7D). These results are similar to the increase in association between calcineurin and PMCA4b observed in vivo after TAC stimulation, collectively suggesting that Pmca4 might proximally regulate calcineurin signaling by altering a common microdomain of Ca2+. Finally, inhibition of endogenous Pmca4 in neonatal myocytes with siRNA dramatically reduced Pmca4 mRNA compared with control siRNA-transfected cells (Figure 7E), and it significantly increased PE-induced NFAT-luciferase activity (Figure 7F). These results further suggest that endogenous Pmca4 quells reactive Ca2+ that induces calcineurin activation after agonist stimulation.
Figure 7. PMCA4b overexpression blunts calcineurin/NFAT activity in cultured neonatal rat cardiomyocytes.
(A) Western blot for PMCA4b protein after Ad-PMCA4b infection of neonatal cardiomyocytes in culture. GAPDH was used as a loading control. (B) Quantitation of NFATc1-GFP nuclear occupancy (Ad–NFAT-GFP infection) in cultured cardiomyocytes with Ad–β-gal (control) or Ad-PMCA4b co-infection at baseline or after ET-1 stimulation (*P < 0.05 versus no stimulation, #P < 0.05 versus Ad–β-gal + ET-1). (C) Quantitation of NFAT-luciferase activity (Ad–NFAT-luciferase reporter infection) in cultured cardiomyocytes with Ad–β-gal (control) or Ad-PMCA4b co-infection at baseline or after ET-1, PE stimulation, or Ad-ΔCnA co-infection (*P < 0.05 versus no stimulation, #P < 0.05 versus Ad–β-gal with ET-1 or PE). (D) Western blot for PMCA4b and calcineurin association after immunoprecipitation of calcineurin at baseline or with ET-1 stimulation. Data were summed from 4–6 independent experiments. (E) RT-PCR from neonatal cardiomyocytes transfected with control or anti-Pmca4 siRNA. L7 was used as a loading and RT-PCR control. (F) Quantitation of NFAT-luciferase activity (Ad–NFAT-luciferase reporter infection) in cultured cardiomyocytes, followed by transfection with a control siRNA or an anti-Pmca4 siRNA at baseline or after PE stimulation (*P < 0.05 versus no stimulation, #P < 0.05 versus control siRNA + PE).
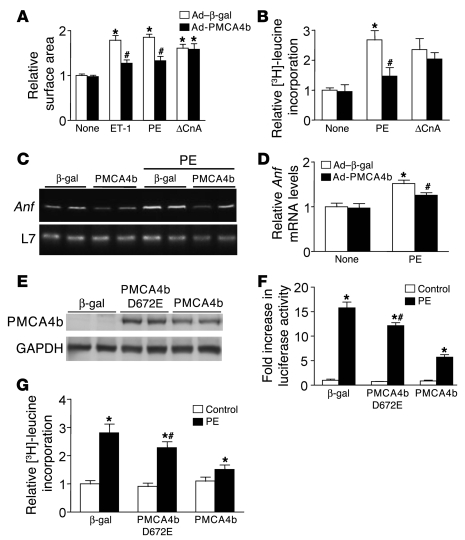
Consistent with the observed inhibition of calcineurin/NFAT signaling, overexpression of PMCA4b in cultured neonatal cardiomyocytes also significantly blunted the hypertrophic increase in myocyte surface area to ET-1 and PE, but not Ad-ΔCnA (Figure 8A). Protein synthesis rates following PE stimulation were also significantly reduced by Ad-PMCA4b infection, but not in combination with Ad-ΔCnA (Figure 8B). Ad-PMCA4b infection also blocked Anf mRNA upregulation following PE stimulation (Figure 8, C and D). These results further suggest that Pmca4b can serve an anti-hypertrophic regulatory role in cardiomyocytes in association with inhibition of calcineurin/NFAT signaling. However, it is not entirely clear whether PMCA4b overexpression exerts its anti-hypertrophic effect by reducing Ca2+ within signaling microdomains in cardiomyocytes, because proper Ca2+ imaging techniques for these regions have not been developed. Moreover, Pmca4b can directly bind a number of signaling proteins, and hence potentially inhibit their activity simply by sequestration when overexpressed. To at least rule out that sequestration was not the anti-hypertrophic mechanism in play, we generated a mutant Ad-PMCA4b in which Asp672 was mutated to Glu, rendering the pump with only 15% of WT activity (25). Expression of this mutant protein after adenoviral infection of neonatal cardiomyocytes showed equivalent levels of protein expression to WT Ad-PMCA4b infection (Figure 8E). The mutant PMCA4b only mildly inhibited PE-induced NFAT-luciferase activity and hypertrophy of neonatal cardiomyocytes compared with robust inhibition by WT PMCA4b (Figure 8, F and G). Considering that the mutant PMCA4b still retained 15% Ca2+ pumping activity, these results strongly suggest that inhibition of NFAT signaling and myocyte hypertrophy at the hands of PMCA4b is not due to a scaffold sequestration effect, but to removal of Ca2+, further supporting the microdomain hypothesis.
Figure 8. PMCA4b overexpression blunts agonist-induced hypertrophy in cultured neonatal cardiomyocytes.
(A) Cardiomyocyte surface area by immunocytochemistry staining of α-actinin in control (Ad–β-gal) or Ad-PMCA4b–overexpressing conditions (*P < 0.05 versus no stimulation, #P < 0.05 versus Ad–β-gal + ET-1 or PE). (B) Relative [3H]-leucine incorporation in cultured neonatal cardiomyocytes infected with the indicated adenoviruses and co-stimulants (*P < 0.05 versus no stimulation, #P < 0.05 versus Ad–β-gal + PE). (C) DNA amplification from RT-PCR reactions for Anf mRNA, and subsequent quantitation (D) in control (Ad–β-gal) and Ad-PMCA4b–overexpressing cultured cardiomyocytes at baseline or after PE treatment (*P < 0.05 versus no stimulation, #P < 0.05 versus Ad–β-gal + PE). (E) Western blot for PMCA4b from adenoviral-infected neonatal cardiomyocytes expressing the indicated proteins. GAPDH was used as a protein loading control. (F) Fold increase in NFAT-luciferase activity from neonatal cardiomyocytes infected with Ad–NFAT-luciferase and Ad–β-gal, Ad–PMCA4b D672E, or Ad-PMCA4b (*P < 0.05 versus control, #P < 0.05 versus PMCA4b + PE). (G) Relative [3H]-leucine incorporation in cultured neonatal cardiomyocytes infected with the indicated adenoviruses and co-stimulants (*P < 0.05 versus control, #P < 0.05 versus PMCA4b + PE). Data were summed from 4–8 independent experiments.
Discussion
Ca2+ handling involved in programming cardiomyocyte contraction is regulated by a highly specialized system of ion channels, pumps, and exchangers (17). The contractile cycle begins by depolarization of the sarcolemma and activation of the voltage-dependent L-type Ca2+ channel, which induces Ca2+ influx that directly stimulates adjacent ryanodine receptors embedded within the SR. This priming Ca2+ from the L-type channel induces a much larger release of Ca2+ from ryanodine receptors that together increase intracellular Ca2+ concentration by more than 10-fold to induce contraction. During relaxation, Ca2+ is removed from the cytoplasm by re-sequestration back into the SR through the action of SERCA, as well as extrusion from the cytoplasm to outside the cell through the action of the Na+/Ca2+ exchanger (NCX) within the sarcolemma. While PMCA isoforms serve as the main mediator of Ca2+ extrusion in non-excitable cell types (16), they likely serve little to no role in removing bulk cytoplasmic Ca2+ from cardiomyocytes during relaxation, given the overwhelming effect of the NCX (17). Indeed, overexpression of PMCA4b in the hearts of transgenic rats or mice had no effect on ECC (18–20). This observation, along with other correlative data whereby Pmca isoforms associate with signaling effectors, suggests the hypothesis that Pmca only regulates Ca2+ in membrane microdomains. However, one large limitation in proving this hypothesis is an inability to directly measure Ca2+ itself in these presumed microdomains in excitable cells such as cardiomyocytes, especially if they end up being nothing more than channel-associated complexes such as InsP3R with CaMKII (see below).
In non-excitable cells, controlled elevations in Ca2+ directly activate Ca2+-dependent signaling factors such as PKC, CaMKII, and the Ca2+-activated protein phosphatase calcineurin. However, it remains a mystery how these same signaling factors, which are each known to affect cardiac hypertrophy and/or physiology, are regulated in a cardiomyocyte, given the overwhelming fluxes in Ca2+ that occur during each contractile cycle throughout the entire cytoplasm. One reasonable hypothesis to explain this mystery involves the existence of membrane microdomains whereby elevations in Ca2+ are sensed by macromolecular signaling complexes in confined areas outside of bulk cytoplasmic Ca2+ that controls contraction (5). For example, Bers and colleagues showed that CaMKII is regulated by a perinuclear Ca2+ pool associated with the InsP3R, which upon activation regulated translocation of histone deacetylase 5 (HDAC5) out of the nucleus to presumably permit hypertrophic gene expression (6). PMCA isoforms are also attractive candidates as selective modulators of Ca2+ within microdomains of cardiomyocytes to control reactive signaling. Indeed, PMCA isoforms have been previously localized to caveolae (26–28), which may constitute a Ca2+ microdomain, given the known enrichment of signaling proteins in these lipid raft-like structures.
PMCA isoforms interact with a number of signaling effectors, such as NOS1, Ras-associated factor-1, α1-syntrophin, 14-3-3ε, Ca2+-calmodulin-dependent serine protein kinase (CASK), and calcineurin (29). Thus, PMCA isoforms may serve a more specialized role in cardiomyocytes to regulate those signaling effectors that are either physically associated with, or within the same general region of, the sarcolemma by conditioning the proximal Ca2+ microenvironment. It should also be noted that PMCA4b becomes quiescent once Ca2+ is removed in its microenvironment (it is Ca2+ activated), thus high levels of overexpression may not be detrimental or even unphysiologic in the transgenic approach employed here. However, the fact that PMCAs can directly bind various signaling effectors suggests that they might also function strictly as a scaffold, such that overexpression inhibits signaling through a sequestration mechanism. To address this issue, we generated a mutant Ad-PMCA4b in which Ca2+ pump activity was reduced by 85% (25), which correlated with a severe reduction in the ability of this mutant to reduce NFAT-luciferase activity and the hypertrophic response, suggesting that it is the Ca2+-pumping effect of Pmca4b that predominates in reducing calcineurin/NFAT signaling, further supporting the microdomain hypothesis.
PMCA4b was previously shown to interact with NOS1 and thereby reduce its activity, consequently blunting the cardiac inotropic response to β-adrenergic stimulation (20). In this same study, PMCA4b-overexpressing transgenic mice were shown to have an enhanced hypertrophic response to isoproterenol infusion, presumably by affecting NOS1 and β-adrenergic receptor signaling (20). On the surface, these results suggest that overexpression of PMCA4b might have a pro-hypertrophic effect on the heart. However, isoproterenol is a rather toxic agent to the myocardium, and the manner in which it signals the hypertrophic response is unknown. Moreover, PMCA4b-overexpressing transgenic rats have also been previously reported, and hearts from these animals showed a reduction in stress-responsive gene expression following ET-1 infusion (30). This latter result suggests that PMCA4b overexpression might serve an anti-hypertrophic regulatory role. Indeed, we showed that PMCA4b overexpression in cultured neonatal cardiomyocytes blocked hypertrophic enlargement following PE or ET-1 stimulation. Our PMCA4b transgenic mice also showed blunted cardiac hypertrophy at multiple time points after pressure overload stimulation and in response to PE/Ang II infusion. Moreover, Pmca4–/– mice showed a small but statistically significant increase in cardiac growth after 2 and 4 weeks of TAC (Figure 5A), further suggesting that Pmca4 is a bona fide negative regulator of reactive hypertrophic signaling in the heart. The seemingly discordant results observed by Oceandy et al. may reflect either the rather nonspecific effects associated with isoproterenol or the characteristics of their transgenic approach (20). Oceandy et al. used a constitutive Pmca4b transgene driven by the MLC2v promoter, which is expressed throughout development and maturation of the heart, while our transgene was only induced in the heart during young adulthood, bypassing the possibility of developmental effects.
While PMCA4b was previously shown to interact with calcineurin and to inhibit calcineurin/NFAT activity in HEK cells (21), here we show that hypertrophic agonist stimulation enhanced the interaction between calcineurin and Pmca4b in cultured cardiomyocytes and in the adult heart. This increase in association during hypertrophic stimulation may simply reflect a movement of calcineurin/NFAT to select membrane regions where it can come in contact with Pmca, or it may reflect a change in Pmca4b conformation that allows greater binding of calcineurin. Indeed, calcineurin activity itself was reduced by PMCA4b overexpression after pressure overload stimulation in mouse hearts in vivo. Regardless of the mechanism, we observed a strong correlation between Pmca4b inhibition of hypertrophy and a change in calcineurin/NFAT signaling, both in cultured myocytes and in the adult heart, although our results are only a correlation and we cannot prove that Pmca4b regulates the cardiac growth response directly through inhibition of calcineurin. Despite this qualification, PMCA4b DTG mice and Pmca4–/– mice showed a cardiac hypertrophic response to exercise stimulation equivalent to that of control mice, suggesting that Pmca4 only regulates pathologic hypertrophy, possibly through calcineurin/NFAT signaling. Indeed, we previously showed that calcineurin/NFAT signaling does not participate in physiologic cardiac hypertrophy (31). These results further strengthen the argument that Pmca4b functions at the level of a Ca2+ signaling microdomain in the heart to control calcineurin/NFAT.
Another issue to consider is that cardiomyocytes also express the Pmca1 gene in addition to Pmca4, and we did not determine whether calcineurin might also be regulated by Pmca1. Thus, Pmca1 could partially compensate for loss of Pmca4 in regulating microdomain Ca2+ levels and reactive signaling events, especially if it also binds calcineurin. Final analysis of the true requirement of Pmca in attenuating reactive signaling in the heart may have to wait until Pmca1 conditionally targeted mice are generated, so that both isoforms can be deleted simultaneously.
Methods
Generation of transgenic mice and animal use.
All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the Cincinnati Children’s Hospital and University of Cincinnati Institutional Animal Care and Use Committee. Pmca4–/– mice were described previously (32). A cDNA encoding human Pmca4b was cloned into the inducible α-MHC promoter expression vector (a gift from Jeffrey Robbins, Cincinnati Children’s Hospital) to permit Dox-regulated expression in combination with a cardiac-specific tTA-expressing transgene (22). NFAT-luciferase, ΔCnA, and Trpc3 transgenic mice were described previously (12, 24, 31). The tTA, Pmca4b, NFAT-luciferase, and Trpc3 transgenic mice were all on the FVBN background, as were the Pmca4–/– mice (32). The ΔCnA transgenic mice were on the B6/C3 background, but only F1 littermates were analyzed when crossed with PMCA4b DTG mice, ensuring an identical genetic background from this cross.
Echocardiography and surgical models.
For echocardiography, mice were anesthetized with 2% isoflurane and hearts were visualized using a Hewlett Packard Sonos 5500 instrument and a 15-MHz transducer (33). Cardiac ventricular dimensions were measured on M-mode 3 times in a single session for the number of animals indicated in Table 1. Mice undergoing TAC were also subjected to Doppler echocardiography at the level of the constricted aorta to measure pressure gradients. Pathologic hypertrophy was induced by TAC, whereby the aorta was visualized through a median sternotomy and a 7-0 silk ligature was tied around the aorta and a 27-gauge wire between the right brachiocephalic and left common carotid arteries, after which the wire was removed to generate a defined constriction (31). Alzet miniosmotic pumps (model 1002; Durect Corp.) containing a mixture of PE (100 mg/kg/d) and Ang II (432 μg/kg/d), or PBS (vehicle control) were surgically inserted dorsally and subcutaneously in mice under isoflurane anesthesia. Swimming exercise for 23 days was described previously (31).
Generation of adenovirus.
The same 3.6-kb cDNA encoding Pmca4b was inserted into the P-shuttle vector followed by linearization, electroporation, and amplification to generate Ad-Pmca4b using the AdEasy kit (Stratagene). NFAT-luciferase adenovirus (Ad–NFAT-luciferase) and Ad–NFATc1-GFP were described previously (31). Calcineurin Aβ adenovirus was generated with a mouse cDNA for this gene, and the Ad–PMCA4b-D672E mutant was generated from a cDNA in which this amino acid was mutated with the QuickChange Site-Directed Mutagenesis Kit (Stratagene).
Isolation of adult cardiomyocytes and Ca2+ measurements.
Adult myocytes were isolated as described previously (12), except that 2,3-butanedione monoxime was withdrawn from all solutions to avoid its adverse effect on Ca2+ release and cell contraction. Briefly, hearts were rapidly excised and cannulated via the aorta, followed by 3–5 min normal media perfusion and 8–10 min perfusion with liberase. The hearts were then manually dissected, minced, and filtered to generate individual myocytes. For measurements of Ca2+ transients, myocytes were loaded with 5 μM Fura 2 for 20 min and de-esterified for 15 min. All the data were acquired at room temperature using a Delta Scan dual-beam spectrofluorophotometer (Photon Technology International), operating at an emission wavelength of 510 nm and excitation wavelengths of 340 nm and 380 nm (12).
Western blotting, immunoprecipitation, calcineurin activity, and NOS activity.
The Western blotting and immunoprecipitation were done as described previously (33). The antibodies used in this study were anti-Pmca4b monoclonal antibody and anti-Trpc3/6/7 polyclonal antibody (Santa Cruz Biotechnology Inc.); anti–calcineurin A polyclonal antibody (Millipore), anti–α-actinin monoclonal, and anti-GAPDH antibody (Sigma-Aldrich). Calcineurin activity was measured using an assay kit (Biomol) according to the manufacturer’s instructions. Cardiac extracts were prepared and calcineurin activity was measured as the dephosphorylation rate of a synthetic phosphopeptide substrate (RII peptide). The amount of PO4 released was determined spectrophotometrically with the BIOMOL GREEN reagent. NOS activity was measured using a colorimetric assay kit (Calbiochem) according to the manufacturer’s instructions. Cardiac extracts were prepared, and the concentrations of final NO metabolites (nitrite and nitrate) were measured as an index of NOS activity.
Histology, immunocytochemistry, and cell surface area measurement.
Histological analysis of hypertrophy and fibrosis was performed by fixing hearts overnight in 10% phosphate-buffered formalin and processed into paraffin blocks for sectioning. Serial 5-μm sections were cut and stained with H&E, Masson’s trichrome, or lectin-FITC conjugate (50 μg/ml) to visualize cell membranes for measuring myocyte cross-sectional areas; at least 400 cells/heart from 4–5 independent mice were measured. Immunohistochemistry for Pmca4b from control and DTG hearts was performed from fresh frozen heart histological sections prepared in OCT (Tissue-Tek) and sectioned at 7 μm and stained with Pmca4b antibody (Santa Cruz Biotechnology Inc.) at 1:100 dilution. Adult rat myocytes were labeled with Pmca4b antibody and rabbit polyclonal calcineurin Aβ antibody (Millipore) 24 hours after recombinant adenoviral infection for both Pmca4b and calcineurin Aβ.
RT-PCR mRNA, NFAT-luciferase assay, [3H]-leucine incorporation, and siRNA.
The luciferase reporter assay, [3H]-leucine incorporation assays, and RT-PCR were done as described previously (31, 34). Knockdown of Pmca4 was performed with a pool of 4 independent siRNAs from Dharmacon (Thermo Scientific; catalog L-082306-10-0020), while the control was a non-targeting pool of siRNAs (catalog D-001206-14-20). These siRNA pools were transfected into neonatal myocytes with Lipofectamine (Invitrogen) 2 h after Ad–NFAT-luciferase infection. Twelve hours later, the cells were stimulated with PE for another 48 h before harvest.
Statistics.
All data were expressed as means ± SEM. Differences between experimental groups were evaluated for statistical significance using the Student’s t test for unpaired data or for multiple groups using both 2-tailed t test and 2-way ANOVA. The data were distributed normally in all cases. P values of less than 0.05 were considered to be statistically significant.
Supplementary Material
Acknowledgments
This work was supported by the NIH (to J.D. Molkentin, J. Robbins, and G.E. Shull). This work was also supported by an international grant in heart failure research for the Fondation Leducq and the Howard Hughes Medical Institute (J.D. Molkentin). X. Wu was supported by an American Heart Association Postdoctoral Fellowship (08255690).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: Ad-ΔCnA, ΔCnA adenovirus; Ad–NFAT-luciferase, NFAT-luciferase adenovirus; Ad-PMCA4b, PMCA4b adenovirus; ANF, atrial natriuretic factor; CaMKII, Ca2+/calmodulin-activated protein kinase II; ΔCnA, activated calcineurin mutant; Dox, doxycycline; DTG, double transgenic; ECC, excitation contraction-coupling; ET-1, endothelin-1; InsP3R, inositol triphosphate receptor; LTCC, L-type Ca2+ channel; α-MHC, myosin heavy chain; NFAT, nuclear factor of activated T cell; PE, phenylephrine; PMCA, plasma membrane Ca2+ ATPase; SR, sarcoplasmic reticulum; TAC, transverse aortic constriction; TRPC, transient receptor potential canonical; tTA, tetracycline transactivator; TTG, triple transgenic.
Citation for this article: J. Clin. Invest. 119:976–985 (2009). doi:10.1172/JCI36693
References
- 1.Lorell B.H., Carabello B.A. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. 2000;102:470–479. doi: 10.1161/01.cir.102.4.470. [DOI] [PubMed] [Google Scholar]
- 2.Ho K.K., Levy D., Kannel W.B., Pinsky J.L. The epidemiology of heart failure: The Framingham study. J. Am. Coll. Cardiol. 1993;22:6–13. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones D.M., et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.CIR.0000039105.49749.6F. [DOI] [PubMed] [Google Scholar]
- 4.Molkentin J.D., Dorn G.W., 2nd. Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu. Rev. Physiol. 2001;63:391–426. doi: 10.1146/annurev.physiol.63.1.391. [DOI] [PubMed] [Google Scholar]
- 5.Molkentin J.D. Dichotomy of Ca2+ in the heart: contraction versus intracellular signaling. . J. Clin. Invest. 2006;116:623–626. doi: 10.1172/JCI27824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu X., et al. Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J. Clin. Invest. 2006;116:675–682. doi: 10.1172/JCI27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balijepalli R.C., Foell J.D., Hall D.D., Hell J.W., Kamp T.J. Localization of cardiac L-type Ca(2+) channels to a caveolar macromolecular signaling complex is required for beta(2)-adrenergic regulation. Proc. Natl. Acad. Sci. U. S. A. 2006;103:7500–7505. doi: 10.1073/pnas.0503465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freichel M., et al. V. Store-operated cation channels in the heart and cells of the cardiovascular system. Cell Physiol. Biochem. . 1999;9:270–283. doi: 10.1159/000016321. [DOI] [PubMed] [Google Scholar]
- 9.Hunton D.L., et al. Capacitative calcium entry contributes to nuclear factor of activated T-cells nuclear translocation and hypertrophy in cardiomyocytes. J. Biol. Chem. 2002;277:14266–14273. doi: 10.1074/jbc.M107167200. [DOI] [PubMed] [Google Scholar]
- 10.Hunton D.L., Zou L., Pang Y., Marchase R.B. Adult rat cardiomyocytes exhibit capacitative calcium entry. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H1124–H1132. doi: 10.1152/ajpheart.00162.2003. [DOI] [PubMed] [Google Scholar]
- 11.Uehara A., Yasukochi M., Imanaga I., Nishi M., Takeshima H. Store-operated Ca2+ entry uncoupled with ryanodine receptor and junctional membrane complex in heart muscle cells. Cell Calcium. 2002;31:89–96. doi: 10.1054/ceca.2001.0257. [DOI] [PubMed] [Google Scholar]
- 12.Nakayama H., Wilkins B.J., Bodi I., Molkentin J.D. Calcineurin-dependent cardiomyopathy is activated by TRPC in the adult mouse heart. FASEB J. 2006;20:1660–1670. doi: 10.1096/fj.05-5560com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bush E.W., et al. Canonical transient receptor potential channels promote cardiomyocyte hypertrophy through activation of calcineurin signaling. J. Biol. Chem. 2006;281:33487–33496. doi: 10.1074/jbc.M605536200. [DOI] [PubMed] [Google Scholar]
- 14.Kuwahara K., et al. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J. Clin. Invest. 2006;116:3114–3126. doi: 10.1172/JCI27702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel H.H., Murray F., Insel P.A. G-protein-coupled receptor-signaling components in membrane raft and caveolae microdomains. Handb. Exp. Pharmacol. 2008;186:167–184. doi: 10.1007/978-3-540-72843-6_7. [DOI] [PubMed] [Google Scholar]
- 16.Strehler E.E., Zacharias D.A. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiol. Rev. 2001;81:21–50. doi: 10.1152/physrev.2001.81.1.21. [DOI] [PubMed] [Google Scholar]
- 17.Bers, D.M. 2001. Excitation-contraction coupling and cardiac contractile force. 2nd edition. Kluwer Academic Publishers. Dordrecht, The Netherlands. 427 pp. [Google Scholar]
- 18.Hammes A., et al. Overexpression of the sarcolemmal calcium pump in the myocardium of transgenic rats. Circ. Res. 1998;83:877–888. doi: 10.1161/01.res.83.9.877. [DOI] [PubMed] [Google Scholar]
- 19.Schuh K., Uldrijan S., Telkamp M., Rothlein N., Neyses L. The plasma membrane calmodulin-dependent calcium pump: a major regulator of nitric oxide synthase I. J. Cell Biol. 2001;155:201–205. doi: 10.1083/jcb.200104131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oceandy D., et al. Neuronal nitric oxide synthase signaling in the heart is regulated by the sarcolemmal calcium pump 4b. Circulation. 2007;115:483–492. doi: 10.1161/CIRCULATIONAHA.106.643791. [DOI] [PubMed] [Google Scholar]
- 21.Buch M.H., et al. The sarcolemmal calcium pump inhibits the calcineurin/nuclear factor of activated T-cell pathway via interaction with the calcineurin A catalytic subunit. J. Biol. Chem. 2005;280:29479–29487. doi: 10.1074/jbc.M501326200. [DOI] [PubMed] [Google Scholar]
- 22.Sanbe A., et al. Reengineering inducible cardiac-specific transgenesis with an attenuated myosin heavy chain promoter. Circ. Res. 2003;92:609–616. doi: 10.1161/01.RES.0000065442.64694.9F. [DOI] [PubMed] [Google Scholar]
- 23.Ambudkar I.S., Brazer S.C., Liu X., Lockwich T., Singh B. Plasma membrane localization of TRPC channels: role of caveolar lipid rafts. Novartis Found. Symp. 2004;258:63–70. doi: 10.1002/0470862580.ch5. [DOI] [PubMed] [Google Scholar]
- 24.Molkentin J.D., et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/S0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adamo H.P., Filoteo A.G., Enyedi A., Penniston J.T. Mutants in the putative nucleotide-binding region of the plasma membrane Ca(2+)-pump. A reduction in activity due to slow dephosphorylation. J. Biol. Chem. 1995;270:30111–30114. doi: 10.1074/jbc.270.50.30111. [DOI] [PubMed] [Google Scholar]
- 26.Fujimoto T. Calcium pump of the plasma membrane is localized in caveolae. J. Cell Biol. . 1993;120:1147–1157. doi: 10.1083/jcb.120.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amino K., Honda Y., Ide C., Fujimoto T. Distribution of plasmalemmal Ca(2+)-pump and caveolin in the corneal epithelium during the wound healing process. Curr. Eye Res. 1997;16:1088–1095. doi: 10.1076/ceyr.16.11.1088.5098. [DOI] [PubMed] [Google Scholar]
- 28.Schnitzer J.E., Oh P., Jacobson B.S., Dvorak A.M. Caveolae from luminal plasmalemma of rat lung endothelium: microdomains enriched in caveolin, Ca(2+)-ATPase, and inositol trisphosphate receptor. Proc. Natl. Acad. Sci. U. S. A. 1995;92:1759–1763. doi: 10.1073/pnas.92.5.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oceandy D., Stanley P.J., Cartwright E.J., Neyses L. The regulatory function of plasma-membrane Ca(2+)-ATPase (PMCA) in the heart. Biochem. Soc. Trans. 2007;35:927–930. doi: 10.1042/BST0350927. [DOI] [PubMed] [Google Scholar]
- 30.Piuhola J., et al. Overexpression of sarcolemmal calcium pump attenuates induction of cardiac gene expression in response to ET-1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R699–R705. doi: 10.1152/ajpregu.2001.281.3.R699. [DOI] [PubMed] [Google Scholar]
- 31.Wilkins B.J., et al. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ. Res. 2004;94:110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 32.Okunade G.W., et al. Targeted ablation of plasma membrane Ca2+-ATPase (PMCA) 1 and 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. J. Biol. Chem. 2004;279:33742–33750. doi: 10.1074/jbc.M404628200. [DOI] [PubMed] [Google Scholar]
- 33.Kaiser R.A., et al. Genetic inhibition or activation of JNK1/2 protects the myocardium from ischemia-reperfusion-induced cell death in vivo. . J. Biol. Chem. 2005;280:32602–32608. doi: 10.1074/jbc.M500684200. [DOI] [PubMed] [Google Scholar]
- 34.Xu J., et al. GDF15/MIC-1 functions as a protective and antihypertrophic factor released from the myocardium in association with SMAD protein activation. Circ. Res. 2006;98:342–350. doi: 10.1161/01.RES.0000202804.84885.d0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.