Abstract
Schizophrenia is a severe disorder that disrupts the function of multiple brain systems, resulting in impaired social and occupational functioning. The etiology and pathogenesis of schizophrenia appear to involve the interplay of a potentially large number of genetic liabilities and adverse environmental events that disrupt brain developmental pathways. In this Review, we discuss a strategy for determining how particular common and core clinical features of the illness are associated with pathophysiology in certain circuits of the cerebral cortex. The identification of molecular alterations in these circuits is providing critical insights for the rational development of new therapeutic interventions.
Introduction
Schizophrenia is a devastating illness that afflicts 0.5%–1% of the world’s population (1). Individuals diagnosed with schizophrenia have impaired social and occupational functioning that result from the confluence of disturbances in perception, attention, volition, inferential thinking, fluency and production of language, recognition and expression of emotion, and capacity for pleasure. Affected individuals frequently come to clinical attention during late adolescence or early adulthood. Many suffer from comorbid depression and an increased risk of cardiovascular disease as well as excessive nicotine, alcohol, and substance use; 5%–10% commit suicide; and most experience a lifetime of disability and emotional distress (1). When compared with females, males have a higher lifetime risk of developing schizophrenia and tend to have an earlier age of onset and a poorer prognosis. Individuals with schizophrenia are overrepresented among the homeless, unemployed, unmarried, childless, socially isolated, incarcerated, and chronically hospitalized (2). As a result, schizophrenia is also associated with a substantial emotional burden for the family, and it incurs tremendous economic costs for society in terms of medical expenditures and lost productivity. Indeed, in market economies, schizophrenia ranks as one of the leading causes of years of life lost to disability and premature mortality, with a reduction in life expectancy of one to three decades (3).
Clinical features.
Schizophrenia manifests as a wide range of disturbances in perceptual, emotional, cognitive, and motor processes that cluster in three categories (1). The first category is characterized by positive symptoms (i.e., the presence of an abnormal brain function; also referred to as “psychotic symptoms”) including delusions, false beliefs firmly held in the face of contradictory evidence; perceptual disturbances and hallucinations, which may occur in any sensory modality but are most commonly auditory and experienced as hearing voices distinct from one’s own thoughts; abnormalities in the form of thoughts that are usually manifest as loose associations, over-inclusiveness, and/or neologisms; and abnormal psychomotor activity that is usually manifest as grossly disorganized behavior, posturing, and/or catatonia.
In the second category are negative symptoms (i.e., the absence of a normal brain function). These include asociality, which is manifest by withdrawal or isolation from family and friends; avolition (i.e., impaired initiative, motivation, and decision-making); affective disturbances, which reduce capacity to recognize and express emotional states; alogia (i.e., poverty in the amount or content of speech); and anhedonia (i.e., reduced capacity to experience pleasure). The third category of symptoms includes a number of cognitive abnormalities such as disturbances in selective attention, working memory, executive control, episodic memory, language comprehension, and social-emotional processing.
Although positive symptoms are usually the presenting and most striking clinical feature of schizophrenia, disturbances in cognition are now thought to be the core features of the illness for a number of reasons (4, 5). First, a characteristic pattern of cognitive deficits occurs with high frequency, is relatively stable over time, and is independent of psychotic symptoms. Second, cognitive abnormalities have been found throughout the life span of affected individuals, including during childhood and adolescence as well as at the initial onset of psychosis. Third, the unaffected relatives of individuals with schizophrenia also exhibit similar, although milder, cognitive deficits. Last, the degree of cognitive dysfunction is the best predictor of long-term functional outcome (i.e., the ability to engage in educational or occupational activities, the ability to live independently, etc.).
Etiology of schizophrenia.
Schizophrenia exhibits substantial familial aggregation, with the risk of illness directly proportional to the percentage of genes shared with an affected person. For example, the concordance rate for schizophrenia is greater in monozygotic twins than dizygotic twins (6). In addition, adoption studies have shown that the risk of schizophrenia is associated with the presence of the illness in the biological parents and not its presence in the adoptive parents (6). Although the heritability (the proportion of variance in disease liability that is explained by genetic factors) of schizophrenia is estimated to be 80%–85% (7), it is clearly complex and does not conform to a typical mode of inheritance such as autosomal dominant, sex linked, or mitochondrial. Indeed, about two-thirds of individuals with schizophrenia have neither a first- nor a second-degree relative with the illness (6).
Although evidence has accumulated for certain plausible candidate genes for schizophrenia, e.g., neuregulin 1 (NRG1) (8), disrupted-in-schizophrenia 1 (DISC1) (9), and dystrobrevin binding protein 1 (DTNBP1) (10), there is still substantial controversy regarding both the meaning of the positive genetic findings and the best strategies for moving forward (11). Based on recent successes in other diseases, it is hoped that genome-wide association studies will clarify the apparently inconsistent findings from existing studies (12). What is clear, however, is that many of the current leading candidate genes have complex gene structures, encode for proteins that play multiple roles in the nervous system, and have extensive and complicated interactions with multiple other molecules (13, 14).
Twin studies also indicate that a portion of the liability (approximately 11%) for schizophrenia is due to common or shared environmental factors (7). Epidemiological studies have identified a number of adverse events during development that seem to increase the chance that an individual will develop schizophrenia later in life (15). These include severe physical or emotional maternal stress during the first trimester of pregnancy (16), maternal infection with influenza virus during the second trimester of pregnancy (17), labor and delivery complications (18), high population density at the place of birth and rearing (19), frequent cannabis use during adolescence (20), and a personal or family history of immigration, especially to areas with a lower density of people with the same racial or ethnic background (21). A range of infectious agents have also been implicated to varying degrees as potential etiological agents in schizophrenia (22) and may contribute to the widely replicated observation that schizophrenia (and bipolar disorder) have a modest predominance in those born in late winter and early spring (23).
Some studies provide suggestive evidence that interactions between genetic and environmental factors increase the risk that an individual will develop schizophrenia. For example, a functional polymorphism in the gene encoding catechol-O-methyltransferase (COMT), which is thought to affect the availability of dopamine (DA) in the cortex, increases the risk for the later development of psychosis associated with cannabis use during adolescence (24). Similarly, serious obstetric complications have been suggested to interact with variants in genes that are regulated by hypoxia and/or genes that are involved in vascular function to influence risk of developing schizophrenia (25).
Advancing toward rational pharmacological therapies
The principal pharmacological treatment for schizophrenia, antipsychotic medication, reduces the severity of positive symptoms such as hallucinations and delusions. Although antipsychotics have made it possible for many individuals with schizophrenia to live outside hospital settings, limitations in both the effectiveness and tolerability of currently available antipsychotics leave many affected individuals with limited or no remission of symptoms (26). Furthermore, antipsychotics have minimal impact on both negative symptoms and cognitive impairments.
These problems emphasize the need for a new approach to the development of treatments for individuals with schizophrenia. We believe that this approach should be similar to that used in other domains of medicine, where drug development begins with the identification of molecular targets based on their role in the pathophysiology of an illness (27). However, the implementation of this strategy depends upon knowledge of the underlying disease process (Figure 1). In this view of a disease process, the etiology of a brain illness unleashes pathogenetic mechanisms that produce a pathological entity, a conserved set of molecular and cellular disturbances in the brain. The pathological entity then alters the normal circuitry and function of the brain such that the resulting pathophysiology gives rise to the recognized clinical features of the illness (28). This view of the disease process means that rational treatments are designed to normalize the physiology of the affected neural circuits so that the clinical features are ameliorated (Figure 1).
Figure 1. Disease process of schizophrenia.
According to this view, the etiology or cause of schizophrenia unleashes pathogenetic mechanisms that produce specific pathological entities. Each of these conserved sets of molecular and cellular disturbances in the brain alters the normal circuitry and function of the brain so that the resulting pathophysiology gives rise to distinct components of the clinical syndrome recognized as schizophrenia. The bidirectional arrows indicate the following: (a) understanding pathophysiology and pathogenesis is required for the rational identification of novel molecular targets for improving treatment and secondary prevention, respectively, and (b) proof-of-concept validation of compounds with activity at these targets requires normalization of biomarkers for these processes.
As summarized above, the multifaceted clinical syndrome that we call schizophrenia is likely to represent the end point of many different etiologies and pathogenetic paths. One current investigative strategy examines how the genetic and environmental factors associated with the etiology of the illness alter brain circuitry in cell and animal models; space constraints preclude a review of the many interesting findings from these studies. However, given the apparent complexity in the upstream components of the disease process, an alternative strategy for dissecting the illness is to focus downstream on what might be a more constrained set of alterations in brain circuitry (i.e., on the pathological entity) that are distal from the etiology but proximal to the pathophysiology of a specific clinical feature (Figure 1). Such alterations might be expected to be relatively conserved across individuals who share that clinical feature.
The implementation of this strategy requires selecting a clinical feature of interest, identifying pathological alterations that are associated with it, and determining the pathophysiological mechanisms that link the two. Here, we consider two examples of this approach to dissecting the disease process of schizophrenia, one focused on a common cognitive deficit (impaired working memory) and the other on a common negative symptom (reduced capacity to recognize spoken emotional tone). These symptoms are associated with alterations in the circuitry of the dorsolateral prefrontal cortex (DLPFC) and primary auditory cortex (AI), respectively (Figure 2). For each, we discuss existing evidence for morphological and molecular disturbances in specific neurotransmitter systems and cell types, and place these abnormalities in the context of the neural circuits in which neurophysiological properties are instantiated and from which thought and behavior emerge.
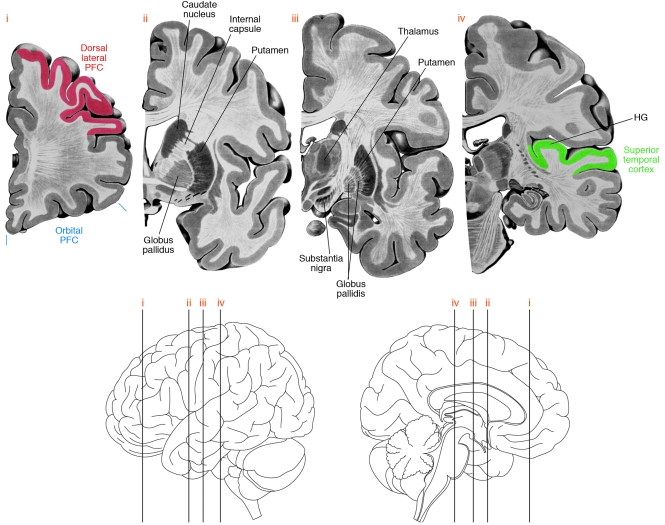
Figure 2. Brain regions involving neural circuitry disturbances in schizophrenia.
Four coronal sections (top) through the left hemisphere of the human brain at the approximate levels shown in the lateral and medial views (bottom). Some of the brain regions implicated in neural circuitry disturbances in schizophrenia are indicated. Note that the AI is located within HG. PFC, prefrontal cortex.
Alterations in DLPFC circuitry in individuals with schizophrenia
Of the cognitive impairments in individuals with schizophrenia, substantial research has focused on working memory, the ability to transiently maintain and manipulate a limited amount of information in order to guide thought or behavior toward a goal. Working memory involves a number of component processes, including temporary storage of information, manipulation of that information, protection from interference by competing information, and maintenance of goal representations (29). Although individuals with schizophrenia exhibit relatively little impairment when performing tasks that depend primarily on the storage of information in working memory, they consistently show impairment in the manipulation of such information and in the maintenance of goal representations (30). These impairments are present in both medicated and unmedicated subjects, in both the early and chronic phases of the illness, and in a manner that cannot be attributed to nonspecific factors such as lack of effort or interest, findings that indicate that these impairments reflect the underlying disease process.
The affected components of working memory are associated with activation of DLPFC circuitry in healthy individuals. This activation is altered in medication-naive individuals with schizophrenia (31) but not in subjects with other psychotic disorders (32). Schizophrenia is not associated with a simple increase or decrease in the degree of DLPFC activation while performing a working memory task; rather, relative to healthy subjects, individuals with schizophrenia exhibit greater DLPFC activation when performing tasks that require low levels of working memory (and they are able to perform these tasks) and reduced DLPFC activation when performing tasks that require substantial use of working memory (and they have an impaired ability to perform these tasks) (33).
Pathology of DLPFC circuitry.
Convergent lines of evidence, albeit with varying degrees of replication, have implicated several components of DLPFC circuitry in the pathology of schizophrenia. First, pyramidal neurons (characterized by a triangular cell body, a single apical dendrite, and multiple basal dendrites, as well as a high density of dendritic spines), which comprise approximately 75% of cortical neurons, utilize the excitatory neurotransmitter glutamate and furnish an axon that projects to other brain regions. Second, interneurons, which comprise approximately 25% of cortical neurons, utilize the inhibitory neurotransmitter GABA, furnish an axon that projects locally, and regulate the activity of pyramidal neurons. Third, axons from neurons in the thalamus and from DA-containing neurons in the mesencephalon innervate targets in the DLPFC (Figure 3).
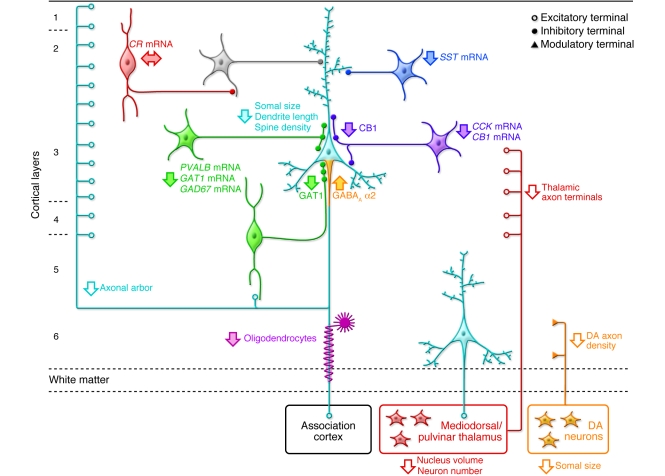
Figure 3. Schematic summary of putative alterations in DLPFC circuitry in schizophrenia.
Pyramidal neurons (light blue) in deep layer 3 have smaller somal size, shorter basilar dendrites, lower dendritic spine density, and a reduced axonal arbor in schizophrenia. Altered GABA neurotransmission by PVALB-containing neurons (green) is indicated by expression deficits in several gene products as well as by lower levels of GAT1 protein in the terminals of chandelier neurons and upregulated GABAA receptor α2 subunits at their synaptic targets, the axon initial segments of pyramidal neurons. Expression of the neuropeptide somatostatin (SST) is decreased in GABA neurons (dark blue) that target the distal dendrites of pyramidal neurons. Decreased cholecystokinin (CCK) and cannabinoid receptor 1 (CB1) mRNA levels and lower CB1 protein in axon terminals suggest altered regulation of GABA neurotransmission in a subset of basket neurons (purple) that target the cell body and proximal dendrites of pyramidal neurons. Gene expression does not seem to be altered in calretinin-containing (CR-containing) GABA neurons (red) that primarily target other GABA neurons (gray). Putative alterations in thalamic and DA cell bodies and their projections to the DLPFC are also shown. Some studies indicate that the number and/or gene expression in oligodendrocytes is also altered (119). Not all of the circuitry alterations shown here have been sufficiently replicated or demonstrated to be specific to the disease process of schizophrenia to be considered established facts; filled arrows indicate abnormalities supported by convergent and/or replicated observations. Figure adapted with permission from Neuron (1).
Defects in pyramidal neurons.
Pyramidal neurons are the principal source of excitatory axon terminals in the cortex. They receive excitatory inputs from other pyramidal neurons (in the same and other cortical regions) and from the thalamus onto their dendritic spines. Because the total number of DLPFC neurons is not altered in schizophrenia (34), findings of increased neuronal density have been interpreted as evidence of a reduction in the number of axon terminals and dendritic spines that occupy the space between neurons (35); that is, the same number of neurons is distributed in a smaller volume, since the number of axon terminals and spines per neuron is lower. Consistent with this interpretation, synaptophysin protein (a marker of axon terminals; ref. 36), gene transcripts that encode proteins present in axon terminals (37), and dendritic length and the density of dendritic spines on pyramidal neurons (38, 39) are all lower in the DLPFC of subjects with schizophrenia. The reduction in spine density is most marked on the basilar dendrites of pyramidal neurons located in deep layer 3 (39, 40). Consistent with these observations, the somal volume of deep layer 3 pyramidal neurons, which correlates with the size of the dendritic tree and axonal arbor of a neuron, is smaller in subjects with schizophrenia (39, 41, 42).
Together these findings suggest that the number of excitatory inputs to deep layer 3 pyramidal neuron basilar dendrites is reduced in schizophrenia. These findings might reflect a reduced number of thalamic afferents, since the excitatory projections from the thalamus to the DLPFC synapse primarily on dendritic spines of pyramidal neurons in deep layers 3 and 4 (43). Studies of the total number of neurons in the mediodorsal thalamic nucleus, a major source of thalamic projections to the DLPFC, have produced mixed results. Initial studies reported lower numbers in individuals with schizophrenia, but subsequent studies with larger sample sizes failed to detect a difference (reviewed in ref. 44). However, several studies have found smaller regional volume and reduced neuron number in the pulvinar, a thalamic association nucleus that also projects to the DLPFC, in individuals with schizophrenia (45, 46). These alterations in the pulvinar are supported by congruent MRI observations (47).
Alternatively, the smaller somal volume and lower spine density in deep layer 3 pyramidal neurons in individuals with schizophrenia could reflect abnormalities intrinsic to this class of cell that are accompanied by altered axonal arbors. Because the local axon collaterals of pyramidal neurons are the largest source of excitatory synapses in a cortical region, abnormalities in these cells could also contribute to the deficits in axon terminal markers in the DLPFC (Figure 3). Molecular mechanisms for structural abnormalities in these neurons, such as alterations in the expression of proteins that regulate spine size and maintenance (48, 49), are under investigation.
The alterations in excitatory inputs to deep layer 3 pyramidal neurons might be developmental in nature given that the number of excitatory synapses and dendritic spines decline during adolescence in primate DLPFC, with the changes most marked in deep layer 3 (50–52). In humans, this synaptic pruning is thought to underlie the decrease in cortical gray matter thickness that occurs normally during adolescence and, to an exaggerated degree, in individuals with schizophrenia (53). Current hypotheses hold that either the exuberant excitatory synapses present before adolescence somehow compensate for a functional abnormality in excitatory transmission in individuals with schizophrenia, and that this abnormality is then revealed with normal synaptic pruning, or that the mechanisms of adolescence-related synapse elimination are disturbed, resulting in excessive synapse pruning and decreased spine number in individuals with schizophrenia (54).
In concert, these findings suggest that the excitatory inputs to DLPFC deep layer 3 pyramidal neurons, mediated by the actions of glutamate on α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) and NMDA receptors located on the dendritic spines of pyramidal neurons, are reduced in individuals with schizophrenia. This interpretation might be consistent with findings that NMDA receptor antagonists (such as phencyclidine [PCP] and ketamine) replicate clinical aspects of schizophrenia in humans (55). Furthermore, NMDA receptor antagonists disrupt working memory in rats (56), and their direct application to the DLPFC impairs working memory performance in monkeys (57). However, alterations in mRNA or protein levels of NMDA receptor subunits in the DLPFC in postmortem studies of individuals with schizophrenia seem to be limited in magnitude and not always replicated (37, 58, 59). Thus, it may be that other regulators of NMDA receptor signaling are affected in individuals with the illness (e.g., modulation of NMDA receptor function by NRG1 signaling pathways has been reported to be reduced in the postmortem DLPFC from subjects with schizophrenia; ref. 60) or that the psychomimetic effects of NMDA antagonists are mediated through circuits involving other brain regions.
Defects in interneurons.
Deficient excitatory input to, and consequently reduced excitatory output from, DLPFC pyramidal neurons might be expected to reduce GABA neurotransmission since expression of the 67-kDa isoform of glutamic acid decarboxylase (GAD67), an enzyme that regulates GABA synthesis, depends on the amount of excitatory activity received by GABA neurons (61). Consistent with this prediction, a reduced level of mRNA encoding GAD67 in the DLPFC is perhaps the most widely and consistently replicated observation in postmortem studies of individuals with schizophrenia (reviewed in ref. 62). At the cellular level, mRNA encoding GAD67 is not detectable in approximately 30% of GABA neurons in subjects with schizophrenia, suggesting that they have substantially reduced amounts of GABA, but the remaining GABA neurons exhibit normal levels of this mRNA (63, 64). Furthermore, levels of mRNA encoding GABA transporter 1 (GAT1), a protein responsible for reuptake of released GABA into nerve terminals, is also decreased in the DLPFC in schizophrenia (65), and this decrease is restricted to a similar minority of, and presumably the same, GABA neurons (66). These findings suggest that both the synthesis and re-uptake of GABA are lower in a subset of DLPFC interneurons in individuals with schizophrenia.
The affected interneurons include the approximately 25% of primate DLPFC GABA neurons (Figure 3) that express the calcium-binding protein parvalbumin (PVALB), exhibit fast-spiking firing properties, and receive a high number of excitatory inputs from the local axon collaterals of DLPFC deep layer 3 pyramidal neurons (67). In individuals with schizophrenia, expression of PVALB mRNA is reduced (68), although the number of PVALB neurons in the DLPFC seems to be unchanged (68, 69). In addition, in individuals with schizophrenia, approximately half the neurons that contain PVALB mRNA lack detectable levels of mRNA encoding GAD67 (68). Among PVALB-containing interneurons in individuals with schizophrenia, the chandelier neurons have been found to express decreased levels of GAT1 in their axon terminals (70), which target the initial segments of the axons of pyramidal neurons (Figure 3). In contrast, immunoreactivity for the GABAA receptor α2 subunit, which is present in most GABAA receptors in the axon initial segment of layer 2–3 pyramidal neurons, is markedly increased in individuals with schizophrenia (71). Several lines of evidence suggest that these pre- and postsynaptic changes (decreased expression of PVALB and GAT1, and increased expression of GABAA receptor α2 subunits, respectively) in individuals with schizophrenia are compensatory responses to deficient GABA release from chandelier neurons (28, 67). For example, lowering PVALB levels augments GABA release (72). Thus, the deficit in mRNA encoding GAD67 in DLPFC interneurons in individuals with schizophrenia might be a more primary component of the disease process and not merely a response to reduced excitatory neurotransmission.
Similar reductions in GAD67, PVALB, and GAT1 may also be present in the subset of PVALB-containing interneurons known as basket neurons, whose axons target the cell body and proximal dendrites of pyramidal neurons (73). The changes in PVALB-containing basket and chandelier neurons might be specific to the disease process of schizophrenia because they are not attributable to antipsychotic medications or other potential confounds (64, 66, 68, 71). Other populations of DLPFC GABA interneurons, such as those that express the neuropeptide somatostatin (74) or those that express the neuropeptide cholecystokinin (CCK) and cannabinoid receptor 1 (CB1) (75), also seem to be disturbed in individuals with schizophrenia. The affected neurons have distinct influences on the function of DLPFC pyramidal neurons. For example, both CCK/CB1R- and PVALB-containing basket cells provide convergent sources of perisomatic inhibition to pyramidal neurons, but they play complementary roles in shaping the activity of pyramidal neurons (76). In contrast, the approximately 50% of GABA interneurons that express the calcium-binding protein calretinin seem to be unaffected in individuals with schizophrenia (68) (Figure 3).
Defects in neurons that project to the DLPFC.
The activity of both pyramidal and GABA interneurons is modulated by inputs from DA neurons located in the ventral mesencephalon. Because normal working memory performance depends on optimal activation of DA D1 receptors in the DLPFC, a deficit in DA signaling could contribute to working memory impairments in individuals with schizophrenia (77). Several factors might reduce DA signaling in the DLPFC. First, DA innervation of the DLPFC seems to be decreased in individuals with schizophrenia, as indicated by lower levels of expression of markers of DA axons, such as tyrosine hydroxylase (TH; the rate-limiting enzyme in DA synthesis) and the DA transporter (DAT) (78). Although midbrain DA neurons are apparently not altered in number in individuals with schizophrenia, some studies suggest that they have smaller somal volumes (79) and lower levels of TH protein (80). Together, these findings suggest that cortical DA signaling might be diminished in individuals with schizophrenia because there is a decrease in the number of axons and/or a decrease in the DA content per axon. Second, excitatory projections from DLPFC pyramidal neurons are thought to be an essential source of glutamate-mediated excitation to midbrain DA neurons that project to the DLPFC (81). Thus, reduced excitatory output from DLPFC pyramidal neurons in individuals with schizophrenia could lead to persistently decreased activation of DA cells. Third, the availability of extracellular DA in the DLPFC might be reduced in schizophrenia. Inhibition of the DA-degrading enzyme COMT markedly increases prefrontal DA levels in rats (82). Although levels of COMT mRNA and protein do not seem to be altered in schizophrenia (83), a COMT allelic variant (Val158Met) encodes an enzyme with markedly increased catalytic activity (84). Healthy subjects homozygous for the Val allele have less robust cognitive performance, presumably due to lower DLPFC DA levels, since performance improved with an amphetamine-induced increase in DA release (33). However, studies of the association between COMT allelic variants and schizophrenia have been inconclusive (85).
The combination of decreased DA innervation of the DLPFC, DA cell hypoactivity, and increased DA turnover in the DLPFC of individuals with schizophrenia could lead to reduced extracellular DA levels, deficient DA D1 receptor stimulation, and possibly a compensatory upregulation of these receptors. Consistent with this hypothesis, a PET study found increased binding of the DA D1 receptor ligand NNC112 in the DLPFC of drug-free and drug-naive subjects with schizophrenia (86). However, other studies using different ligands have not replicated these results (87, 88). Interestingly, preclinical studies indicate that sustained DA depletion differentially affects binding of these ligands and elevates the in vivo binding of NNC112 (89). Furthermore, the degree of D1 receptor upregulation in individuals with schizophrenia was inversely related to working memory performance (86), consistent with the idea that D1 upregulation is a compensatory, but insufficient, response to DA deficit in the DLPFC.
Pathophysiological consequences.
Understanding how these alterations in DLPFC circuitry (summarized in Figure 3) could interact to give rise to the pathophysiology of working memory deficits in schizophrenia remains a challenge. Several interpretations have been suggested. One idea is that hypofunction of NMDA receptors selectively present on PVALB-containing interneurons might lead to reduction in GAD67 expression and decreased GABA production, disinhibition of pyramidal neurons, and excess glutamate at non-NMDA receptors, disrupting cortical circuit function (90). Although aspects of this hypothesis require further explanation (e.g., why NMDA receptor function might be selectively affected on PVALB-containing interneurons) (67), the hypothesis provided the rationale for the development of novel compounds with agonist activity at metabotropic glutamate receptors that reduces glutamate release through a presynaptic mechanism (91). A recent clinical trial demonstrated antipsychotic efficacy of such a compound in individuals with schizophrenia, although its effects on cognitive deficits were not reported (92).
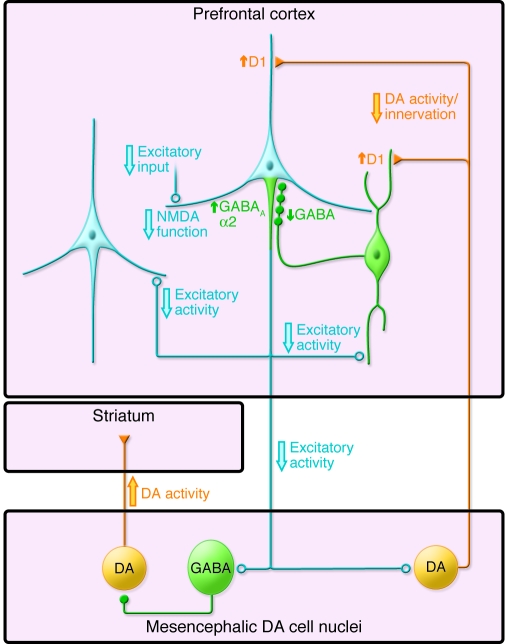
A second interpretation (Figure 4) is that a deficit in excitatory inputs to pyramidal neurons, resulting from fewer dendritic spines and/or hypofunctional NMDA (and/or AMPA) receptors on these spines, might lead to reduced excitatory output from the cortex. Deficient excitatory input to DA neurons projecting to the DLPFC would cause sustained hypoactivity and, consequently, both morphological and biochemical changes in these DA neurons. This in turn would lead to decreased DA innervation of the DLPFC and compensatory, but functionally insufficient, upregulation of DA D1 receptors by pyramidal neurons, interneurons, or both. Because DA D1 receptor activation increases the activity of PVALB-containing interneurons, reduced DA D1 receptor–mediated signaling might reduce the activity of these neurons and contribute to activity-dependent downregulation of GABA synthesis. Because DLPFC pyramidal neurons indirectly inhibit mesostriatal DA cells through activation of GABA neurons in the mesencephalon, the reduction in DLPFC excitatory activity might lead to a functional excess of DA activity at DA D2 receptors in the striatum that could contribute to the psychotic features of schizophrenia (93).
Figure 4. Schematic illustration of putative functional alterations in DLPFC circuitry in schizophrenia.
In one view, the excitatory activity of cortical pyramidal neurons (light blue) is thought to be reduced in schizophrenia due to lower excitatory inputs, NMDA receptor hypofunction, and/or decreased DA neurotransmission through D1 receptors. Similar alterations may contribute to reduced GABA synthesis in PVALB chandelier (green) neurons and a compensatory increase in GABAA receptors containing α2 subunits in the axon initial segment of pyramidal neurons. Decreased excitatory input to neurons in the mesencephalon would lead to increased DA activity in the striatum and decreased DA activity in the cortex, with compensatory but functionally insufficient upregulation of D1 receptors. See text for additional details. Figure adapted with permission from Nature Medicine (125).
A third suggestion is that the deficit in expression of mRNA encoding GAD67 is highly conserved and thus a central feature of DLPFC pathology in schizophrenia (28). Because the activity of DLPFC interneurons is essential for normal working memory function in monkeys (94), reduced GABA signaling from PVALB-containing interneurons to DLPFC pyramidal neurons might contribute to the pathophysiology of working memory dysfunction. This idea is supported by a number of findings. First, networks of PVALB-containing interneurons seem to be specialized to synchronize the activity of local populations of pyramidal neurons so that they fire together at a certain frequency termed the γ-band (30–80 Hz) (67). Second, γ-band oscillations in the human DLPFC increase in proportion to working memory load (95). Third, the capacity to increase extracellular GABA predicts DLPFC γ-band power during a working memory task in humans (96). Last, prefrontal γ-band oscillations are reduced during this task in subjects with schizophrenia (97). Thus, a deficit in the synchronization of pyramidal cell firing, resulting from impaired regulation of pyramidal cell networks by PVALB-containing interneurons, could contribute to reduced levels of induced γ-band oscillations and, consequently, to impaired working memory in individuals with schizophrenia (28). This hypothesis is supported by recent findings that a novel compound designed to augment GABA neurotransmission selectively from the PVALB-containing chandelier neuron inputs to pyramidal neurons improved both working memory function and prefrontal γ-band oscillations in subjects with schizophrenia (98).
Alterations in auditory cortex circuitry
Impaired prosody.
Difficulty in recognizing and expressing spoken emotional tone is a prominent negative symptom in individuals with schizophrenia that impairs the ability to recognize and convey important cues for successful social interactions (99). Emotional tone is represented by acoustic features of speech (prosody). Recognizing prosody is also essential for successful interpretation of spoken content such as distinguishing between statements and questions, appreciating stressed words, recognizing sincere versus sarcastic intent, and even identifying the sex of the speaker (100).
Prosody is largely conveyed by variations in speech pitch; that is by changing the predominant acoustic frequency of spoken words (100). Individuals with schizophrenia have a reduced ability to discriminate tones of differing frequencies, and this impairment predicts problems in distinguishing spoken emotions (99). Impaired tone discrimination is evident even in the absence of an inter-tone interval (101), indicating that this abnormality is unlikely to be a consequence of cognitive dysfunction such as impaired working memory. Tone discrimination depends on the circuitry of the AI.
Pathology of AI circuitry.
The AI in humans is located in Heschl’s gyrus (HG). Most in vivo MRI studies have found reduced HG gray matter volume in subjects with schizophrenia (including those with a first episode of psychosis) relative to normal controls and to subjects with affective psychosis (102). Subjects with schizophrenia also have an accelerated rate of gray matter volume loss in HG after first presentation of psychosis (102). Alterations in several components of AI circuitry might contribute to both the volume deficits and functional impairments observed in schizophrenia. For example, in deep layer 3 of the AI, the mean somal volume of pyramidal neurons is smaller (103), and the densities of markers of dendritic spines (104) and of axon boutons (105) are lower. Because the densities of dendritic spines and axon boutons were highly correlated within subjects (104), the combined findings suggest a structural deficit in excitatory transmission.
Pathophysiological consequences.
The cortical circuit supporting auditory processing of frequency discrimination is summarized in Figure 5. Neurons in the AI are organized tonotopically; that is, they are arranged such that their spatial location varies according to the frequency that generates maximal response (106). This tonotopic arrangement reflects the organization of the projections from the ventral subdivision of the medial geniculate nucleus of the thalamus to layer 4 and deep layer 3. The broad thalamic frequency representation is then refined within the AI. Activation following the thalamic input spreads through layer 3 via interlaminar and horizontal projections (107, 108). The horizontal projections in the AI, formed by the long-range axon collaterals of layer 3 pyramidal neurons, reciprocally connect cortical patches with similar characteristic frequency responses (107, 108). It is primarily these reciprocal connections of layer 3 pyramidal neurons that define frequency tuning, selectively enhancing the preferred frequency (109, 110).
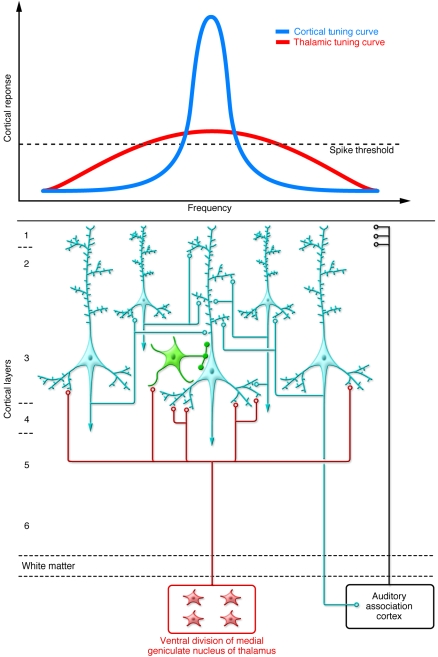
Figure 5. Schematic summary of auditory cortex circuitry relevant to impaired frequency discrimination in schizophrenia.
Auditory cortical processing is initiated by projections from the medial geniculate nucleus of the thalamus. These projections are arranged tonotopically (i.e., along a frequency gradient that is broadly tuned). The subsequent activation of a reciprocally connected isofrequency network of pyramidal cells (light blue) within layer 3 selectively amplifies a narrower preferred frequency, refining the thalamic tuning curve. Densities of dendritic spines and axonal boutons are reduced in deep layer 3 of subjects with schizophrenia, potentially limiting activation and current flow in the pyramidal cell network. Pyramidal neurons are co-tuned (i.e., they receive concurrent stimulation from thalamic or cortical projection neurons) with local inhibitory neurons (green), leading to a stereotyped excitatory-inhibitory sequence of postsynaptic potentials (123), which increases the temporal precision of depolarization and enhances phasic activity of the pyramidal neuron network (124). Thus, though alterations in GABA neurons in the AI (as described in DLPFC of subjects with schizophrenia; see Figure 2) are currently understudied, if conserved across regions (65), they may further contribute to impaired activation of isofrequency pyramidal neuron networks.
Frequency discrimination in the AI has been evaluated at the level of single neurons and neuronal populations using paradigms in which an auditory stimulus of one frequency is repeatedly presented at a fixed interval, interspersed with stimuli of a different frequency, which occur at a lower probability of presentation (111). The event-related potential, mismatch negativity (MMN), represents the difference in magnitude of the responses generated by the two stimuli, recorded intracortically or at the scalp. Consistent with the mechanisms of frequency tuning described above, intracortical recordings in monkeys have indicated that MMN arises in the AI after the initial depolarizing thalamic volley, during a phase of increased multi-unit activity within deep layer 3 (111, 112).
MMN is reduced in subjects with schizophrenia (102, 113), and the reduction correlates with the degree of impairment in tone discrimination (114). Reduced MMN in subjects with schizophrenia appears to represent an inability to generate maximum current flow in these layer 3 pyramidal circuits (112). For example, reductions of MMN similar to those in subjects with schizophrenia can be modeled by infusing NMDA receptor antagonists into the auditory cortex of animals (112), an observation paralleled by systemic administration of NMDA antagonists in human subjects without psychiatric illness (115). Thus, impairments in pitch discrimination and detection of prosody in subjects with schizophrenia might reflect a deficit in the activation of pyramidal neuron networks in layer 3 of the AI due to reduced numbers of excitatory synaptic connections among these neurons. This model is consistent with the observation that impairments in MMN progress in the early stages of the illness and correlate with the magnitude of progressive reductions in gray matter volume of HG (102).
Important unresolved questions remain. For example, are reductions in numbers of excitatory synapses themselves sufficient to diminish the spread of excitation and the generation of current flow in layer 3 of the AI, or are additional impairments of glutamate function required, such as deficits in NMDA receptor expression within these synapses? Similarly, GABA neuron populations, which regulate phasic activity of layer 3 pyramidal neuron networks in the AI (Figure 5), have not been investigated in this region in subjects with schizophrenia.
Other alterations in cortical circuitry in individuals with schizophrenia
Certain cortical circuitry alterations in individuals with schizophrenia seem to be relatively widespread. For example, lower dendritic spine density, smaller pyramidal cell somal volumes, and fewer markers of axon terminals have been found in other cortical regions in addition to the DLFPC and AI (73). Pyramidal neurons in deep layer 3 (but not deep layer 5) of the auditory association cortex also have a smaller mean somal volume (116) and reduced dendritic spine density (104), although not a concurrent reduction in density of axon boutons (105), in individuals with schizophrenia.
Similarly, a recent study found the same pattern of altered GABA-related gene expression in the DLPFC, anterior cingulate, primary visual cortex, and primary motor cortex of individuals with schizophrenia (65). Disturbances in γ-band oscillations in schizophrenia have also been observed in a number of cortical regions under different task or stimulus conditions (117, 118). Thus, a conserved alteration in GABA neurotransmission across cortical regions could underlie a common abnormality in γ-band oscillations that is associated with different clinical features of schizophrenia depending upon the cortical circuits affected.
It is also important to note that alterations in cortical circuitry in schizophrenia are unlikely to be restricted to those discussed above. For example, oligodendrocytes, critical mediators of white matter myelination as well as neuronal development and support, may be dysfunctional and/or reduced in number in individuals with schizophrenia (119) (Figure 3). These disturbances might contribute to the evidence from functional and structural imaging studies that the connectivity among cortical regions is altered in individuals with schizophrenia (120). In addition, other neurotransmitter systems, such as cholinergic signaling through nicotinic receptors, are disrupted in at least some cortical regions (121).
Translating an understanding of disease process into novel therapeutics
The findings discussed in this Review suggest that impaired working memory and auditory information processing in schizophrenia are attributable, at least in part, to a complex set of alterations in cortical circuitry in the DLPFC and AI, respectively. However, the emergence of working memory and prosody depends upon more distributed cortical networks, and thus alterations within local circuits must be considered within the broader organization of the cortex and its connections with subcortical structures. Although the frequency of alterations in specific components of these cortical circuits are common enough to be consistently detected in different cohorts of subjects identified by a common set of diagnostic criteria, the extent to which these alterations are restricted to only certain types of individuals with schizophrenia remains to be determined; this knowledge is essential for considering the opportunities for personalized medicine in the treatment of schizophrenia.
Rational pharmacological treatments for schizophrenia are designed to normalize the pathophysiology that mediates the clinical feature of interest (Figure 1). Thus, any molecule identified as a drug target must be understood in the context of the pathological circuit, and ideally the effects of compounds with the desired activity at that target are evaluated using both direct assessments of pathophysiology (e.g., EEG, functional MRI, and PET) and sensitive and specific measures of the clinical feature, as well as broader and more standard measures of neuropsychological function and symptomatology. Indeed, recent studies suggest that broad measures employing neuropsychological test batteries, although advantageous for clinical trials because of their psychometric properties (e.g., test-retest reliability), are prone to practice effects that might obscure the therapeutic effects of novel drugs (122).
Any given pathological entity in a disease process could represent a cause (an upstream factor related to the disease pathogenesis), a consequence (a deleterious effect of a cause), or a compensation (a response to either cause or consequence that helps restore homeostasis) (73). Although understanding these distinctions is clearly necessary for drug design, as it determines the required mode of action of the drug, making these distinctions requires an understanding of the functional properties of the cortical circuitry in which they are embedded. For example, the idea that GABAA receptors containing α2 subunits are upregulated in pyramidal neurons due to a deficit in GABA input from chandelier neurons led to the use of a novel positive allosteric modulator of this receptor subtype that improved both working memory function and prefrontal γ-band oscillations in a small randomized controlled trial of subjects with schizophrenia (98). Similarly, the idea that DA D1 receptors are upregulated to compensate for a deficient DA innervation of the DLPFC has motivated attempts to develop selective approaches for modulating activity at cortical DA D1 receptors (77). In this regard, PET-based assessments of the degree of DA D1 receptor upregulation in individual patients may help in guiding therapy to maximize the likelihood of obtaining optimal levels of DA D1 receptor stimulation.
Finally, analyses of pathological circuits might lead to the future identification and validation of new types of therapeutic targets beyond the manipulation of neurotransmitter systems. For example, spine-specific kinases, whose activity regulates spine size, number, and function, might be of potential value as novel targets (48, 49). If the adolescence-related pruning of dendritic spines is, as discussed above, critical in the emergence of the clinical features of schizophrenia, then such compounds might provide a means for secondary prevention through early intervention in high-risk individuals.
Acknowledgments
Cited work conducted by the authors was supported by NIH grants MH043784, MH045156, MH084053, and MH071533 from the National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the NIH. The authors thank Mary Brady for preparation of the figures, Lindsay Karr for preparation of the manuscript, and Guillermo Gonzalez-Burgos and David Volk for helpful comments.
Footnotes
Conflict of interest: David A. Lewis receives research support from the Bristol-Myers Squibb Foundation, Merck, Pfizer, and Curridium Limited and in 2006–2008 served as a consultant to Bristol-Myers Squibb, Hoffman-Roche, Lilly, Merck, Neurogen, Pfizer, Sepracor, and Wyeth.
Nonstandard abbreviations used: AI, primary auditory cortex; COMT, catechol-O-methyltransferase; DA, dopamine; DLPFC, dorsolateral prefrontal cortex; GAD67, 67-kDa isoform of glutamic acid decarboxylase; GAT1, GABA transporter 1; HG, Heschl’s gyrus; MMN, mismatch negativity; PVALB, parvalbumin.
Citation for this article: J. Clin. Invest. 119:706–716 (2009). doi:10.1172/JCI37335
References
- 1.Lewis D.A., Lieberman J.A. Catching up on schizophrenia: natural history and neurobiology. Neuron. 2000;28:325–334. doi: 10.1016/S0896-6273(00)00111-2. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter W.T., Koenig J.I. The evolution of drug development in schizophrenia: past issues and future opportunities. Neuropsychopharmacology. 2008;33:2061–2079. doi: 10.1038/sj.npp.1301639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parks, J., Svendsen, D., Singer, P., and Foti, M. 2006. Morbidity and mortality in people with serious mental illness. National Association of State Mental Health Program Directors (NASMHPD) Medical Directors Council. Alexandria, Virginia, USA. http://www.bu.edu/cpr/resources/wellness-summit/documents/morbidity-and-mortality_nasmhpd.ppt. [Google Scholar]
- 4.Gold J.M. Cognitive deficits as treatment targets in schizophrenia. Schizophr. Res. 2004;72:21–28. doi: 10.1016/j.schres.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Green M.F. What are the functional consequences of neurocognitive deficits in schizophrenia? Am. J. Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 6.Gottesman, I.I. 1991. Schizophrenia genesis: the origins of madness. W.H. Freeman. New York, New York, USA. 296 pp. [Google Scholar]
- 7.Sullivan P.F., Kendler K.S., Neale M.C. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch. Gen. Psychiatry. 2003;60:1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- 8.Stefansson H., et al. Neuregulin 1 and susceptibility to schizophrenia. . Am. J. Hum. Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blackwood D.H., et al. Schizophrenia and affective disorders — cosegregation with a translocation at chromosome 1q42 that directly disrupts brain-expressed genes: clinical and P300 findings in a family. Am. J. Hum. Genet. 2001;69:428–433. doi: 10.1086/321969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Straub R.E., et al. Genetic variation in the 6p22.3 gene DTNBP1, the human ortholog of the mouse dysbindin gene, is associated with schizophrenia. Am. J. Hum. Genet. 2002;71:337–348. doi: 10.1086/341750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abbott A. Psychiatric genetics: the brains of the family. Nature. 2008;454:154–157. doi: 10.1038/454154a. [DOI] [PubMed] [Google Scholar]
- 12.Sanders A.R., et al. No significant association of 14 candidate genes with schizophrenia in a large European ancestry sample: implications for psychiatric genetics. Am. J. Psychiatry. 2008;165:497–506. doi: 10.1176/appi.ajp.2007.07101573. [DOI] [PubMed] [Google Scholar]
- 13.Mei L., Xiong W.C. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat. Rev. Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chubb J.E., Bradshaw N.J., Soares D.C., Porteous D.J., Millar J.K. The DISC locus in psychiatric illness. Mol. Psychiatry. 2008;13:36–64. doi: 10.1038/sj.mp.4002106. [DOI] [PubMed] [Google Scholar]
- 15.Lewis D.A., Levitt P. Schizophrenia as a disorder of neurodevelopment. Ann. Rev. Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- 16.Susser E., et al. Schizophrenia after prenatal famine: Further evidence. Arch. Gen. Psychiatry. 1996;53:25–31. doi: 10.1001/archpsyc.1996.01830010027005. [DOI] [PubMed] [Google Scholar]
- 17.Mednick, S.A., Machon, R.A., and Huttunen, M. 1989. Disturbances of fetal neural development and adult schizophrenia. In Schizophrenia: scientific progress. S.C. Schultz and C.A. Tamminga, editors. Oxford University Press. New York, New York, USA. 69–77. [Google Scholar]
- 18.Geddes J.R., Lawrie S.M. Obstetric complications and schizophrenia: A meta-analysis. Br. J. Psychiatry. 1995;167:786–793. doi: 10.1192/bjp.167.6.786. [DOI] [PubMed] [Google Scholar]
- 19.Pedersen C.B., Mortensen P.B. Evidence of a dose-response relationship between urbanicity during upbringing and schizophrenia risk. Arch. Gen. Psychiatry. 2001;58:1039–1046. doi: 10.1001/archpsyc.58.11.1039. [DOI] [PubMed] [Google Scholar]
- 20.Moore T.H., et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- 21.Veling W., et al. Ethnic density of neighborhoods and incidence of psychotic disorders among immigrants. Am. J. Psychiatry. 2008;165:66–73. doi: 10.1176/appi.ajp.2007.07030423. [DOI] [PubMed] [Google Scholar]
- 22.Yolken R.H., Torrey E.F. Are some cases of psychosis caused by microbial agents? A review of the evidence. Mol. Psychiatry. 2008;13:470–479. doi: 10.1038/mp.2008.5. [DOI] [PubMed] [Google Scholar]
- 23.Torrey E.F., Miller J., Rawlings R., Yolken R.H. Seasonality of births in schizophrenia and bipolar disorder: A review of the literature. Schizophr. Res. 1997;28:1–38. doi: 10.1016/S0920-9964(97)00092-3. [DOI] [PubMed] [Google Scholar]
- 24.Caspi A., et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol. Psychiatry. 2005;57:1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 25.Nicodemus K.K., et al. Serious obstetric complications interact with hypoxia-regulated/vascular-expression genes to influence schizophrenia risk. Mol. Psychiatry. 2008;13:873–877. doi: 10.1038/sj.mp.4002153. [DOI] [PubMed] [Google Scholar]
- 26.Lieberman J.A., et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N. Engl. J. Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 27.Insel T.R., Scolnick E.M. Cure therapeutics and strategic prevention: raising the bar for mental health research. Mol. Psychiatry. 2006;11:11–17. doi: 10.1038/sj.mp.4001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis D.A., Hashimoto T., Volk D.W. Cortical inhibitory neurons and schizophrenia. Nat. Rev. Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 29.Barch D.M., Smith E. The cognitive neuroscience of working memory: relevance to CNTRICS and schizophrenia. Biol. Psychiatry. 2008;64:11–17. doi: 10.1016/j.biopsych.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barch D.M. What can research on schizophrenia tell us about the cognitive neuroscience of working memory? Neuroscience. 2006;139:73–84. doi: 10.1016/j.neuroscience.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 31.MacDonald A.W., 3rd, Pogue-Geile M.F., Johnson M.K., Carter C.S. A specific deficit in context processing in the unaffected siblings of patients with schizophrenia. Arch. Gen. Psychiatry. 2003;60:57–65. doi: 10.1001/archpsyc.60.1.57. [DOI] [PubMed] [Google Scholar]
- 32.MacDonald A.W., 3rd, et al. Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. Am. J. Psychiatry. 2005;162:475–484. doi: 10.1176/appi.ajp.162.3.475. [DOI] [PubMed] [Google Scholar]
- 33.Tan H.Y., Callicott J.H., Weinberger D.R. Dysfunctional and compensatory prefrontal cortical systems, genes and the pathogenesis of schizophrenia. Cereb. Cortex. 2007;17(Suppl. 1):i171–i181. doi: 10.1093/cercor/bhm069. [DOI] [PubMed] [Google Scholar]
- 34.Thune J.J., Uylings H.B.M., Pakkenberg B. No deficit in total number of neurons in the prefrontal cortex in schizophrenics. J. Psychiatr. Res. 2001;35:15–21. doi: 10.1016/S0022-3956(00)00043-1. [DOI] [PubMed] [Google Scholar]
- 35.Selemon L.D., Goldman-Rakic P.S. The reduced neuropil hypothesis: A circuit based model of schizophrenia. Biol. Psychiatry. 1999;45:17–25. doi: 10.1016/S0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- 36.Glantz L.A., Lewis D.A. Reduction of synaptophysin immunoreactivity in the prefrontal cortex of subjects with schizophrenia: regional and diagnostic specificity. Arch. Gen. Psychiatry. 1997;54:943–952. doi: 10.1001/archpsyc.1997.01830220065010. [DOI] [PubMed] [Google Scholar]
- 37.Mirnics K., Middleton F.A., Marquez A., Lewis D.A., Levitt P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron. 2000;28:53–67. doi: 10.1016/S0896-6273(00)00085-4. [DOI] [PubMed] [Google Scholar]
- 38.Garey L.J., et al. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J. Neurol. Neurosurg. Psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glantz L.A., Lewis D.A. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch. Gen. Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 40.Kolluri N., Sun Z., Sampson A.R., Lewis D.A. Lamina-specific reductions in dendritic spine density in the prefrontal cortex of subjects with schizophrenia. Am. J. Psychiatry. 2005;162:1200–1202. doi: 10.1176/appi.ajp.162.6.1200. [DOI] [PubMed] [Google Scholar]
- 41.Pierri J.N., Volk C.L.E., Auh S., Sampson A., Lewis D.A. Decreased somal size of deep layer 3 pyramidal neurons in the prefrontal cortex of subjects with schizophrenia. Arch. Gen. Psychiatry. 2001;58:466–473. doi: 10.1001/archpsyc.58.5.466. [DOI] [PubMed] [Google Scholar]
- 42.Rajkowska G., Selemon L.D., Goldman-Rakic P.S. Neuronal and glial somal size in the prefrontal cortex: A postmortem morphometric study of schizophrenia and Huntington disease. Arch. Gen. Psychiatry. 1998;55:215–224. doi: 10.1001/archpsyc.55.3.215. [DOI] [PubMed] [Google Scholar]
- 43.Erickson S.L., Lewis D.A. Cortical connections of the lateral mediodorsal thalamus in cynomolgus monkeys. J. Comp. Neurol. 2004;473:107–127. doi: 10.1002/cne.20084. [DOI] [PubMed] [Google Scholar]
- 44.Dorph-Petersen K.-A., Pierri J.N., Sun Z., Sampson A.R., Lewis D.A. Stereological analysis of the mediodorsal thalamic nucleus in schizophrenia: Volume, neuron number, and cell types. J. Comp. Neurol. 2004;472:449–462. doi: 10.1002/cne.20055. [DOI] [PubMed] [Google Scholar]
- 45.Byne W., et al. Postmortem assessment of thalamic nuclear volumes in subjects with schizophrenia. Am. J. Psychiatry. 2002;159:59–65. doi: 10.1176/appi.ajp.159.1.59. [DOI] [PubMed] [Google Scholar]
- 46.Danos P., et al. Volumes of association thalamic nuclei in schizophrenia: a postmortem study. Schizophr. Res. 2003;60:141–155. doi: 10.1016/s0920-9964(02)00307-9. [DOI] [PubMed] [Google Scholar]
- 47.Gilbert A.R., et al. Thalamic volumes in patients with first-episode schizophrenia. Am. J. Psychiatry. 2001;158:618–624. doi: 10.1176/appi.ajp.158.4.618. [DOI] [PubMed] [Google Scholar]
- 48.Hill J.J., Hashimoto T., Lewis D.A. Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia. Mol. Psychiatry. 2006;11:557–566. doi: 10.1038/sj.mp.4001792. [DOI] [PubMed] [Google Scholar]
- 49.Penzes P., Jones K.A. Dendritic spine dynamics - a key role for kalirin-7. Trends Neurosci. 2008;31:419–427. doi: 10.1016/j.tins.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson S.A., Classey J.D., Condé F., Lund J.S., Lewis D.A. Synchronous development of pyramidal neuron dendritic spines and parvalbumin-immunoreactive chandelier neuron axon terminals in layer III of monkey prefrontal cortex. Neuroscience. 1995;67:7–22. doi: 10.1016/0306-4522(95)00051-J. [DOI] [PubMed] [Google Scholar]
- 51.Bourgeois J.-P., Goldman-Rakic P.S., Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb. Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- 52.Huttenlocher P.R., Dabholkar A.S. Regional differences in synaptogenesis in human cerebral cortex. J. Comp. Neurol. 1997;387:167–178. doi: 10.1002/(SICI)1096-9861(19971020)387:2<167::AID-CNE1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 53.Rapoport J.L., Gogtay N. Brain neuroplasticity in healthy, hyperactive and psychotic children: insights from neuroimaging. Neuropsychopharmacology. 2008;33:181–197. doi: 10.1038/sj.npp.1301553. [DOI] [PubMed] [Google Scholar]
- 54.McGlashan T.H., Hoffman R.E. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch. Gen. Psychiatry. 2000;57:637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- 55.Krystal J.H., et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch. Gen. Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 56.Verma A., Moghaddam B. , 1996NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. J. Neurosci. 16373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dudkin K.N., Kruchinin V.K., Chueva I.V. Neurophysiological correlates of delayed visual differentiation tasks in monkeys: the effects of the site of intracortical blockade of NMDA receptors. Neurosci. Behav. Physiol. 2001;31:207–218. doi: 10.1023/A:1005224610354. [DOI] [PubMed] [Google Scholar]
- 58.Kristiansen L.V., Beneyto M., Haroutunian V., Meador-Woodruff J.H. Changes in NMDA receptor subunits and interacting PSD proteins in dorsolateral prefrontal and anterior cingulate cortex indicate abnormal regional expression in schizophrenia. Mol. Psychiatry. 2006;11:737–747. doi: 10.1038/sj.mp.4001844. [DOI] [PubMed] [Google Scholar]
- 59.Akbarian S., et al. Selective alterations in gene expression of NMDA receptor subunits in prefrontal cortex of schizophrenics. J. Neurosci. 1996;16:19–30. doi: 10.1523/JNEUROSCI.16-01-00019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hahn C.G., et al. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat. Med. 2006;12:734–735. doi: 10.1038/nm0706-734. [DOI] [PubMed] [Google Scholar]
- 61.Jones E.G. Cortical development and thalamic pathology in schizophrenia. Schizophr. Bull. 1997;23:483–501. doi: 10.1093/schbul/23.3.483. [DOI] [PubMed] [Google Scholar]
- 62.Hashimoto T., et al. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol. Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Akbarian S., et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch. Gen. Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 64.Volk D.W., Austin M.C., Pierri J.N., Sampson A.R., Lewis D.A. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch. Gen. Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 65.Hashimoto T., et al. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am. J. Psychiatry. 2008;165:479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Volk D.W., Austin M.C., Pierri J.N., Sampson A.R., Lewis D.A. GABA transporter-1 mRNA in the prefrontal cortex in schizophrenia: Decreased expression in a subset of neurons. Am. J. Psychiatry. 2001;158:256–265. doi: 10.1176/appi.ajp.158.2.256. [DOI] [PubMed] [Google Scholar]
- 67.Gonzalez-Burgos G., Lewis D.A. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr. Bull. 2008;34:944–961, 2008. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hashimoto T., et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J. Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Woo T.-U., Miller J.L., Lewis D.A. Schizophrenia and the parvalbumin-containing class of cortical local circuit neurons. Am. J. Psychiatry. 1997;154:1013–1015. doi: 10.1176/ajp.154.7.1013. [DOI] [PubMed] [Google Scholar]
- 70.Woo T.-U., Whitehead R.E., Melchitzky D.S., Lewis D.A. A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 1998;95:5341–5346. doi: 10.1073/pnas.95.9.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Volk D.W., et al. Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb. Cortex. 2002;12:1063–1070. doi: 10.1093/cercor/12.10.1063. [DOI] [PubMed] [Google Scholar]
- 72.Vreugdenhil M., Jefferys J.G., Celio M.R., Schwaller B. Parvalbumin-deficiency facilitates repetitive IPSCs and gamma oscillations in the hippocampus. J. Neurophysiol. 2003;89:1414–1422. doi: 10.1152/jn.00576.2002. [DOI] [PubMed] [Google Scholar]
- 73.Lewis D.A., Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology Reviews. 2008;33:141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- 74.Morris H.M., Hashimoto T., Lewis D.A. Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb. Cortex. 2008;18:1575–1587. doi: 10.1093/cercor/bhm186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eggan S.M., Hashimoto T., Lewis D.A. Reduced cortical cannabinoid 1 receptor messenger RNA and protein expression in schizophrenia. Arch. Gen. Psychiatry. 2008;65:772–784. doi: 10.1001/archpsyc.65.7.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Freund T.F., Katona I. Perisomatic inhibition. Neuron. 2007;56:33–42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 77.Goldman-Rakic P.S., Castner S.A., Svensson T.H., Siever L.J., Williams G.V. Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology (Berl.) 2004;174:3–16. doi: 10.1007/s00213-004-1793-y. [DOI] [PubMed] [Google Scholar]
- 78.Akil M., et al. Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am. J. Psychiatry. 1999;156:1580–1589. doi: 10.1176/ajp.156.10.1580. [DOI] [PubMed] [Google Scholar]
- 79.Bogerts B., Hantsch J., Herzer M.1983. . A morphometric study of the dopamine-containing cell groups in the mesencephalon of normals, Parkinson patients, and schizophrenics. Biol. Psychiatry. 18951–969. [PubMed] [Google Scholar]
- 80.Perez-Costas E., Melendez-Ferro M., Gao X.M., Conley R., Roberts R.C. Decreased expression of tyrosine hydroxylase in the substantia nigra of schizophrenia brains. Schizophr. Bull. 2007;33:266. [Google Scholar]
- 81.Sesack S.R., Carr D.B. Selective prefrontal cortex inputs to dopamine cells: implications for schizophrenia. Physiol. Behav. 2002;77:513–517. doi: 10.1016/S0031-9384(02)00931-9. [DOI] [PubMed] [Google Scholar]
- 82.Tunbridge E.M., Bannerman D.M., Sharp T., Harrison P.J. Catechol-o-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J. Neurosci. 2004;24:5331–5335. doi: 10.1523/JNEUROSCI.1124-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tunbridge E., Burnet P.W., Sodhi M.S., Harrison P.J. Catechol-o-methyltransferase (COMT) and proline dehydrogenase (PRODH) mRNAs in the dorsolateral prefrontal cortex in schizophrenia, bipolar disorder, and major depression. Synapse. 2004;51:112–118. doi: 10.1002/syn.10286. [DOI] [PubMed] [Google Scholar]
- 84.Chen J., et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am. J. Hum. Genet. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Burmeister M., McInnis M.G., Zollner S. Psychiatric genetics: progress amid controversy. Nat. Rev. Genet. 2008;9:527–540. doi: 10.1038/nrg2381. [DOI] [PubMed] [Google Scholar]
- 86.Abi-Dargham A., et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J. Neurosci. 2002;22:3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Okubo Y., et al. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature. 1997;385:634–636. doi: 10.1038/385634a0. [DOI] [PubMed] [Google Scholar]
- 88.Karlsson P., Farde L., Halldin C., Sedvall G. PET study of D1 dopamine receptor binding in neuroleptic-naive patients with schizophrenia. . Am. J. Psychiatry. 2002;159:761–767. doi: 10.1176/appi.ajp.159.5.761. [DOI] [PubMed] [Google Scholar]
- 89.Guo N., et al. Dopamine depletion and in vivo binding of PET D1 receptor radioligands: implications for imaging studies in schizophrenia. Neuropsychopharmacology. 2003;28:1703–1711. doi: 10.1038/sj.npp.1300224. [DOI] [PubMed] [Google Scholar]
- 90.Moghaddam B. Targeting metabotropic glutamate receptors for treatment of the cognitive symptoms of schizophrenia. Psychopharmacology (Berl.) 2004;174:39–44. doi: 10.1007/s00213-004-1792-z. [DOI] [PubMed] [Google Scholar]
- 91.Moghaddam B., Adams B.W. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- 92.Patil S.T., et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat. Med. 2007;13:1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- 93.Laruelle, M. 2003. Dopamine transmission in the schizophrenic brain. In Schizophrenia. S.R. Hirsch and D. Weinberger, editors. Blackwell Publishing Company. Malden, Massachusetts, USA. 365–386. [Google Scholar]
- 94.Rao S.G., Williams G.V., Goldman-Rakic P.S. Destruction and creation of spatial tuning by disinhibition: GABAA blockade of prefrontal cortical neurons engaged by working memory. . J. Neurosci. 2000;20:485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Howard M.W., et al. Gamma oscillations correlate with working memory load in humans. Cereb. Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- 96.Frankle W.G., et al. Tiagabine increases [(11)C]flumazenil binding in cortical brain regions in healthy control subjects. Neuropsychopharmacology. 2009;34:624–633. doi: 10.1038/npp.2008.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cho R.Y., Konecky R.O., Carter C.S. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lewis D.A., et al. Subunit-selective modulation of GABAA receptor neurotransmission and cognition in schizophrenia. Am. J. Psychiatry. . 2008;165:1585–1593. doi: 10.1176/appi.ajp.2008.08030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Leitman D.I., et al. Sensory contributions to impaired prosodic processing in schizophrenia. Biol. Psychiatry. 2005;58:56–61. doi: 10.1016/j.biopsych.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 100.Moore B.C. Basic auditory processes involved in the analysis of speech sounds. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2008;363:947–963. doi: 10.1098/rstb.2007.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Javitt D.C., Strous R.D., Grochowski S., Ritter W., Cowan N. Impaired precision, but normal retention, of auditory sensory (“echoic”) memory information in schizophrenia. J. Abnorm. Psychol. 1997;106:315–324. doi: 10.1037/0021-843X.106.2.315. [DOI] [PubMed] [Google Scholar]
- 102.Salisbury D.F., Kuroki N., Kasai K., Shenton M.E., McCarley R.W. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch. Gen. Psychiatry. 2007;64:521–529. doi: 10.1001/archpsyc.64.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sweet R.A., et al. Pyramidal cell size reduction in schizophrenia: Evidence for involvement of auditory feedforward circuits. Biol. Psychiatry. 2004;55:1128–1137. doi: 10.1016/j.biopsych.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 104.Sweet R.A., Henteleff R.A., Zhang W., Sampson A.R., Lewis D.A. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 2009;34:374–389. doi: 10.1038/npp.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sweet R.A., et al. Anatomical evidence of impaired feedforward auditory processing in schizophrenia. Biol. Psychiatry. 2007;61:854–864. doi: 10.1016/j.biopsych.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 106.Merzenich M.M., Brugge J.F. Representation of the cochlear partition on the superior temporal plane of the macaque monkey. Brain Res. 1973;50:275–296. doi: 10.1016/0006-8993(73)90731-2. [DOI] [PubMed] [Google Scholar]
- 107.Ojima H., Honda C.N., Jones E.G. Patterns of axon collateralization of identified supragranular pyramidal neurons in the cat auditory cortex. Cereb. Cortex. 1991;1:80–94. doi: 10.1093/cercor/1.1.80. [DOI] [PubMed] [Google Scholar]
- 108.Wallace M.N., Kitzes L.M., Jones E.G. Intrinsic inter- and intra-laminar connections and their relationship to the tonotopic map in cat primary auditory cortex. Exp. Brain Res. 1991;86:527–544. doi: 10.1007/BF00230526. [DOI] [PubMed] [Google Scholar]
- 109.Liu B.H., Wu G.K., Arbuckle R., Tao H.W., Zhang L.I. Defining cortical frequency tuning with recurrent excitatory circuitry. Nat. Neurosci. 2007;10:1594–1600. doi: 10.1038/nn2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kaur S., Rose H.J., Lazar R., Liang K., Metherate R. Spectral integration in primary auditory cortex: laminar processing of afferent input, in vivo and in vitro. Neuroscience. 2005;134:1033–1045. doi: 10.1016/j.neuroscience.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 111.Javitt D.C., Steinschneider M., Schroeder C.E., Vaughan H.G., Arezzo J.C. Detection of stimulus deviance within primate primary auditory cortex: intracortical mechanisms of mismatch negativity (MMN) generation. Brain Res. 1994;667:192–200. doi: 10.1016/0006-8993(94)91496-6. [DOI] [PubMed] [Google Scholar]
- 112.Javitt D.C., Steinschneider M., Schroeder C.E., Arezzo J.C. Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: implications for schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 1996;93:11962–11967. doi: 10.1073/pnas.93.21.11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Javitt D.C., Doneshka P., Grochowski S., Ritter W. Impaired mismatch negativity generation reflects widespread dysfunction of working memory in schizophrenia. Arch. Gen. Psychiatry. 1995;52:550–558. doi: 10.1001/archpsyc.1995.03950190032005. [DOI] [PubMed] [Google Scholar]
- 114.Javitt D.C., Shelley A.M., Ritter W. Associated deficits in mismatch negativity generation and tone matching in schizophrenia. Clin. Neurophysiol. 2000;111:1733–1737. doi: 10.1016/S1388-2457(00)00377-1. [DOI] [PubMed] [Google Scholar]
- 115.Umbricht D., et al. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Arch. Gen. Psychiatry. 2000;57:1139–1147. doi: 10.1001/archpsyc.57.12.1139. [DOI] [PubMed] [Google Scholar]
- 116.Sweet R.A., Pierri J.N., Auh S., Sampson A.R., Lewis D.A. Reduced pyramidal cell somal volume in auditory association cortex of subjects with schizophrenia. Neuropsychopharmacology. 2003;28:599–609. doi: 10.1038/sj.npp.1300120. [DOI] [PubMed] [Google Scholar]
- 117.Spencer K.M., et al. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Uhlhaas P.J., Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 119.Karoutzou G., Emrich H.M., Dietrich D.E. The myelin-pathogenesis puzzle in schizophrenia: a literature review. Mol. Psychiatry. 2008;13:245–260. doi: 10.1038/sj.mp.4002096. [DOI] [PubMed] [Google Scholar]
- 120.Fields R.D. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Martin L.F., Freedman R. Schizophrenia and the alpha7 nicotinic acetylcholine receptor. Int. Rev. Neurobiol. 2007;78:225–246. doi: 10.1016/S0074-7742(06)78008-4. [DOI] [PubMed] [Google Scholar]
- 122.Goldberg T.E., et al. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Arch. Gen. Psychiatry. 2007;64:1115–1122. doi: 10.1001/archpsyc.64.10.1115. [DOI] [PubMed] [Google Scholar]
- 123.Oswald A.M., Schiff M.L., Reyes A.D. Synaptic mechanisms underlying auditory processing. Curr. Opin. Neurobiol. 2006;16:371–376. doi: 10.1016/j.conb.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 124.Wehr M., Zador A.M. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 2003;426:442–446. doi: 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]
- 125.Lewis D.A., Gonzalez-Burgos G. Pathophysiologically based treatment interventions in schizophrenia. Nat. Med. 2006;9:1016–1022. doi: 10.1038/nm1478. [DOI] [PubMed] [Google Scholar]