Abstract
The kidney kallikrein-kinin system plays important roles in inflammation, coagulation, angiogenesis, and regulation of vessel tone and permeability. In this issue of the JCI, Liu et al. provide data that suggest a protective role for kallikrein in animal models of anti–glomerular basement membrane (GBM) antibody–induced nephritis, an experimental model of Goodpasture disease (see the related article beginning on page 911). Furthermore, human systemic lupus erythematosus and lupus nephritis were shown to be associated with kallikrein 1 (KLK1) and the KLK3 promoter. The authors suggest that kallikrein genes are involved in the development of SLE and lupus nephritis and may exert a renoprotective role. It is possible, however, that the kallikrein-kinin system may play dual roles: protecting the kidney against ischemia and interstitial fibrosis while also mediating vasodilation, inflammation, and activation of the innate immune response.
The kallikrein-kinin system
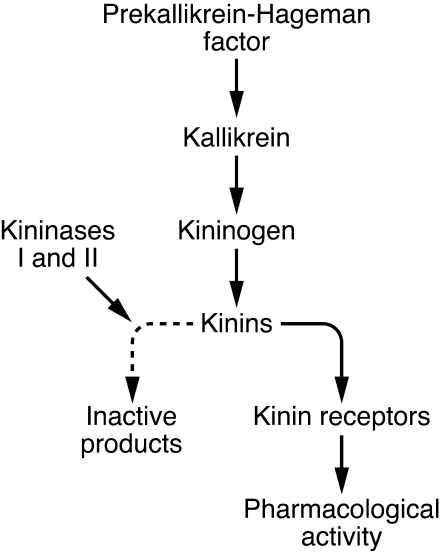
The kallikrein-kinin system comprises proteins that play a role in inflammation, coagulation, and regulation of vessel tone and permeability via the production of small peptides called kinins. The system originates from prekallikrein, a serum protease that, after being activated by factor XIIa (also known as Hageman factor), is cleaved to form kallikrein. Kallikreins are serine peptidases (kininogenases) that rapidly release kinins in the plasma by cleaving kininogens — multifunctional proteins derived mainly from α2-globulins. Tissue kallikrein 1 (KLK1) is synthesized in many organs, including kidney and arteries, where it can generate the vasodilators bradykinin and kallidin, which are rapidly hydrolyzed to inactive products by a group of peptidases known as kininases (Figure 1).
Figure 1. Simplified overview of the kallikrein-kinin system.
Kallikreins originate from prekallikrein, which is cleaved to form kallikrein after being activated by factor XIIa (Hageman factor). Kallikreins are enzymes that cleave kininogens (proteins derived mainly from α2-globulins) into peptides called kinins. In turn, kinins may be cleaved by kininases to form inactive final products or may bind to their receptors and exert pharmacological activity.
Kallikreins are encoded by a variable number of genes in different mammalian species. The human tissue kallikreins are encoded by a cluster of 15 genes located on chromosome 19q13.4, a position analogous to that of the kallikrein gene family on mouse chromosome 7 (1, 2). Among these genes, the principal kinin-generating enzyme, KLK1, is encoded by KLK1.
Complement activation may trigger the kinin cascade and vice versa. There is crosstalk between the two systems, which leads to the generation of activation products that ultimately affect endothelial functions (3).
Kallikreins, hypertension, and renal function
Experimental and clinical studies have shown an inverse correlation between urinary kallikrein levels and blood pressure (4–6). Further studies have shown that the tissue kallikrein-kinin system can inhibit apoptosis, proliferation, hypertrophy, and fibrosis and can promote angiogenesis in different experimental animal models (7). Moreover, kallikrein gene delivery or kallikrein protein infusion improves cardiac, renal, and neurological function without reducing blood pressure (7). A main player involved in these effects is likely klk1. Studies of KLK1-deficient mice and human subjects partially deficient in KLK1 have revealed a critical role for KLK1 in arterial function in both species and prompted a search for a possible genetic link between kallikrein gene polymorphisms and blood pressure regulation (8). In an effort to identify a genetic basis for differential urinary kallikrein excretion, Yu et al. (6) found that the KLK1 promoter is uniquely polymorphic, with a poly-G–length polymorphism coupled with multiple single-base substitutions. These authors also found a significant association between the 12 G allele (the longest of the length locus alleles) and arterial hypertension and end-stage renal disease in African Americans (6). These findings suggested that kallikrein/kinin may serve as new drug targets for the prevention and treatment of the systemic vasculopathy associated with arterial hypertension.
Kallikreins, SLE, and lupus nephritis
Kallikreins can also act as proinflammatory mediators and play a role in several autoimmune diseases. SLE is a prototypic systemic autoimmune disease of unknown etiology in which immune complex deposition and complement activation lead to inflammation and tissue damage. The kidney is a target organ of such processes, and immune-mediated nephritis is a common complication of SLE. The SLE autoimmune response involves abnormal expansion of autoreactive T and B cells. An important role is also played by innate immune effectors, which trigger additional local mechanisms of inflammation and tissue damage (9–11) (Figure 2).
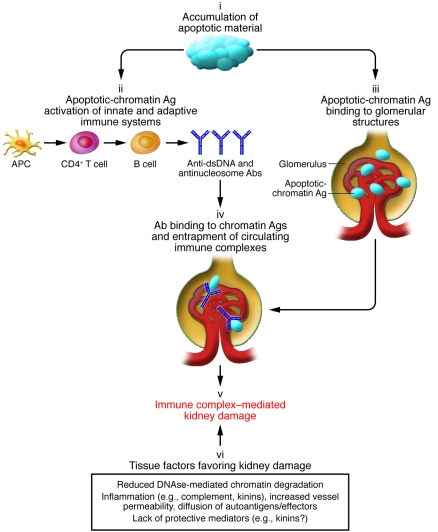
Figure 2. The main mechanisms mediating kidney damage in lupus nephritis.
SLE is characterized by an accumulation of apoptotic material due to its poor clearance (9) (i). This leads to an immune response against chromatin (ii) and to increased expression and binding of apoptotic-chromatin antigens to kidney glomerular structures (iii). Antibodies against apoptotic-chromatin antigens (in particular anti-dsDNA and anti-nucleosome antibodies) may form immune complexes in the kidney with their specific planted antigens or may be entrapped on glomerular structures as immune complexes preformed in the circulation (iv). Immune complex deposition/formation eventually leads to immune-mediated tissue inflammation and damage (v). Local factors may also increase the susceptibility of renal tissue to damage (vi): Chromatin antigens may accumulate in the kidney tissue because of reduced DNAse-mediated chromatin degradation (11), and immune-mediated inflammation may itself increase vessel permeability, consequently increasing diffusion of the autoantigens themselves and of the soluble and cellular mediators of the autoimmune response. In this context, the lack of the potentially protective effect of kinins may represent an additional mechanism contributing to renal tissue damage. Ag, antigen.
Theoretically, abnormalities in angiotensin-converting enzyme (ACE), a kininase that breaks down kallikrein-produced kinins, might favor perpetuation of autoimmune inflammation and progression of renal disease in SLE. Some studies have suggested an association between SLE and an insertion/deletion (I/D) polymorphism in ACE. Individuals homozygous for the insertion polymorphism display higher plasma levels of ACE and show significantly increased activity of lupus nephritis and SLE, suggesting a possible pathogenic role of kallikreins in SLE (12). However, other studies could not confirm such an association, and a meta-analysis of 2,962 patients showed a lack of association of the ACE I/D polymorphism with SLE and lupus nephritis (13).
In line with the role of kallikreins as proinflammatory mediators, higher plasma levels of high-MW kininogen, low-MW kininogen, and kallikrein were found in 30 patients with active lupus nephritis compared with age- and sex-matched controls (14). In addition, plasma and urinary activities of tissue kallikrein and kininase II were also significantly increased in these patients. These data suggest a role for the kallikrein-kinin system in the acute manifestations of lupus nephritis.
KLK1 and KLK3 are disease genes in lupus and anti-GBM antibody–induced nephritis
Anti–glomerular basement membrane (GBM) antibodies have been used to induce experimental nephritis in mice (15). It should be noted, however, that inbred mouse strains differ in their susceptibility to anti-GBM antibody–induced nephritis and lupus nephritis. In this issue of the JCI, Liu et al. set out to investigate whether kallikreins play a role in this differential susceptibility to renal disease. The authors report that inbred mouse strains with upregulated expression of renal and urinary kallikreins exhibited less susceptibility to anti-GBM antibody–induced and lupus nephritis (16). The authors also showed that the administration of kallikreins dampened anti-GBM antibody–induced nephritis, strongly suggesting a link between kallikreins and immune complex–mediated kidney damage. In line with their demonstration that Klk genes from one of the nephritis-sensitive mouse strains conferred increased nephritis susceptibility in disease-resistant mice, the authors found that SLE and lupus nephritis in human patients were also associated with genes homologous to murine Klk genes, particularly KLK1 and the KLK3 (also called KLKB1) promoter. Taken together, these results suggest that kallikrein genes KLK1 and KLK3 are disease genes in lupus and anti-GBM antibody–induced nephritis (16).
Kallikreins in lupus nephritis: a dual role?
The elegant study reported by Liu et al. represents an important advance and may pave the way for new diagnostic and therapeutic approaches for lupus nephritis (16). One of the main findings of the study is that effector mechanisms of the innate immune response may play a role in modulating the autoimmune response against a target organ (i.e., the kidney) (16). In fact, the authors raise the possibility that the kallikrein-kinin system activated by the innate immune response enhances the local tissue damage triggered by the autoimmune response.
However, a number of issues remain unresolved. The experimental data are largely derived from evidence that a mouse model of anti-GBM antibody–induced glomerulonephritis may mimic lupus nephritis (16). Of the approximately 25 different molecules that have been specifically examined in the experimental anti-GBM antibody–induced disease model and also in spontaneous lupus nephritis, all were reported to influence both diseases concordantly, suggesting that the experimental model might be a useful tool for unraveling the molecular basis of spontaneous lupus nephritis (15). Although Liu et al. (16) also showed an association between kallikrein genes and human SLE and lupus nephritis, there are still discrepancies between the mouse model and human diseases: (a) the target of anti-GBM antibodies in human anti-GBM antibody–induced disease (also known as Goodpasture disease) is the α3 chain of type IV collagen, while at least nine antigens, of which the most frequent are dsDNA and nucleosomes, have been identified in SLE (17); (b) in Goodpasture disease, there are linear deposits of IgG and C3 along the GBM, while in human lupus nephritis, immunofluorescence shows subendothelial, mesangial, or subepithelial granular deposits of IgG, C3, and C1q, and more rarely of IgA and IgM (18); (c) the initial immunological response in each condition and also the downstream pathogenic cascades are different, as shown by the diverse clinical and histological patterns observed in the two diseases. In particular, in human lupus nephritis, six classes of glomerular lesions, each containing different subclasses, have been recognized (18). A variety of factors may be responsible for such a heterogeneous renal pathology. The morphologic type of glomerulonephritis and its clinical course may depend upon the predominant type of antigen exposed, its persistence, the concurrent degree of activation of complement, the recruitment of polymorphonuclear cells and macrophages, the stimulation of the clotting system, as well as the development of hypertension and the functional response of the kidney to reduced renal mass. Further studies are necessary to assess the relative contribution to disease of polymorphisms in KLK1 or the KLK3 promoter; many other mediators, including C1-inhibitor — the physiological inhibitor of the plasma kallikrein and kinin signaling pathways — may also play a role.
On the other hand, one may wonder whether the involvement of the kallikrein-kinin system is actually beneficial in active lupus nephritis. As discussed above, previous studies have reported a nephroprotective effect of the tissue kallikrein-kinin system (7, 8). However, in the inflammatory response, the kallikrein proteolytic cascade plays a significant role, since it is considered to initiate and maintain systemic inflammatory responses and immune-modulated disorders (3). Moreover, kinins may also facilitate immune complex deposition by vasodilation and may induce the release of other proinflammatory mediators, including cytokines (3, 19). Finally, formation of bradykinin has been reported to activate innate immunity (20), which is actively involved in the pathogenesis of SLE (9). Thus, it seems that in lupus nephritis, the kallikrein-kinin system may operate as either a mediator or modulator of immunomediated inflammation. It might be possible that kallikreins actually protect the kidney in the quiescent phases of the disease, when arterial hypertension and chronic renal failure are responsible for progressive interstitial fibrosis and glomerular sclerosis, but they could be potentially harmful in the acute phases of the disease, when the immune and inflammatory responses take place. Alternatively, KLK downregulation during acute kidney inflammation may represent a form of negative feedback to counteract the tissue damage.
These uncertainties do not reduce the importance of the instructive contribution from Liu et al. in this issue of the JCI (16). Rather, they should stimulate future research toward the goal of better understanding the role of the kallikrein-kinin system in the various phases of lupus nephritis and other forms of autoimmune-mediated glomerulonephritis.
Footnotes
Conflict of interest: C. Ponticelli is a consultant for Novartis, Italy.
Nonstandard abbreviations used: ACE, angiotensin-converting enzyme; GBM, glomerular basement membrane; KLK1, kallikrein 1.
Citation for this article: J. Clin. Invest. 119:768–771 (2009). doi:10.1172/JCI38786
See the related article beginning on page 911.
References
- 1.Evans B.A., et al. Structure and chromosomal localization of the human renal kallikrein gene. Biochemistry. 1988;27:3124–3129. doi: 10.1021/bi00409a003. [DOI] [PubMed] [Google Scholar]
- 2.Riegman P.H., et al. The prostate-specific antigen gene and the human glandular kallikrein-1 gene are tandemly located on chromosome 19. FEBS Lett. 1989;247:123–126. doi: 10.1016/0014-5793(89)81253-0. [DOI] [PubMed] [Google Scholar]
- 3.Bossi F., Bulla R., Tedesco F. Endothelial cells are a target of both complement and kinin system. Int. Immunopharmacol. . 2008;8:143–147. doi: 10.1016/j.intimp.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Song Q., Chao J., Chao L. High level expression of human tissue kallikrein in the circulation induces hypotension in transgenic mice. Immunopharmacology. 1996;32:105–107. doi: 10.1016/0162-3109(95)00065-8. [DOI] [PubMed] [Google Scholar]
- 5.Margolius H.S., Horwitz D., Pisano J.J., Keiser H.R. Urinary kallikrein excretion in hypertensive man. Relationship to sodium intake and sodium-retaining steroids. Circ. Res. 1974;35:820–825. doi: 10.1161/01.res.35.6.820. [DOI] [PubMed] [Google Scholar]
- 6.Yu H., et al. 2002Association of the tissue kallikrein gene promoter with ESRD and hypertensio n . Kidney Int. 611030–1039. 10.1046/j.1523-1755.2002.00198.x [DOI] [PubMed] [Google Scholar]
- 7.Chao J., Bledsoe G., Yin H., Chao L. The tissue kallikrein-kinin system protects against cardiovascular and renal diseases and ischemic stroke independently of blood pressure reduction. Biol. Chem. 2006;387:665–675. doi: 10.1515/BC.2006.085. [DOI] [PubMed] [Google Scholar]
- 8.Pizard A., et al. Genetic deficiency in tissue kallikrein activity in mouse and man: effect on arteries, heart and kidney. Biol. Chem. 2008;389:701–706. doi: 10.1515/BC.2008.081. [DOI] [PubMed] [Google Scholar]
- 9.Rahman A., Isenberg D.A. Systemic lupus erythematosus. N. Engl. J. Med. . 2008;358:929–939. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 10.Allam R., Anders H.J. The role of innate immunity in autoimmune tissue injury. Curr. Opin. Rheumatol. 2008;20:538–544. doi: 10.1097/BOR.0b013e3283025ed4. [DOI] [PubMed] [Google Scholar]
- 11.Zykova S.N., Seredkina N., Benjaminsen J., Rekvig O.P. Reduced fragmentation of apoptotic chromatin is associated with nephritis in lupus-prone (NZB x NZW) F(1) mice. Arthritis Rheum. 2008;58:813–25. doi: 10.1002/art.23276. [DOI] [PubMed] [Google Scholar]
- 12.Parsa A., et al. Association of angiotensin-converting enzyme polymorphisms with systemic lupus erythematosus and nephritis: analysis of 644 SLE families. Genes Immun. 2002;3(Suppl. 1):S42–S46. doi: 10.1038/sj.gene.6363907. [DOI] [PubMed] [Google Scholar]
- 13.Lee Y.H., Rho Y.H., Choi S.J., Ji J.D., Song G.G. Angiotensin-converting enzyme insertion/deletion polymorphism and systemic lupus erythematosus: a metaanalysis. J. Rheumatol. 2006;33:698–702. [PubMed] [Google Scholar]
- 14.Dellalibera-Joviliano R., Reis M.L., Donadi E.A. Kinin system in lupus nephritis. Int. Immunopharmacol. 2001;1:1889–1896. doi: 10.1016/S1567-5769(01)00109-6. [DOI] [PubMed] [Google Scholar]
- 15.Fu Y., Du Y., Mohan C. Experimental anti-GBM disease as a tool for studying spontaneous lupus nephritis. Clin. Immunol. 2007;124:109–118. doi: 10.1016/j.clim.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Liu K., et al. Kallikrein genes are associated with lupus and glomerular basement membrane–specific antibody–induced nephritis in mice and humans. J. Clin. Invest. . 2009;119:911–923. doi: 10.1172/JCI36728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Bavel C.C., Fenton K.A., Rekvig O.P., van der Vlag J., Berden J.H. Glomerular targets of nephritogenic autoantibodies in systemic lupus erythematosus. Arthritis Rheum. 2008;58:1892–1899. doi: 10.1002/art.23626. [DOI] [PubMed] [Google Scholar]
- 18.Weening J.J., et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J. Am. Soc. Nephrol. 2004;15:241–250. doi: 10.1097/01.ASN.0000108969.21691.5D. [DOI] [PubMed] [Google Scholar]
- 19.Bhoola K., et al. Kallikrein and kinin receptor expression in inflammation and cancer. Biol. Chem. 2001;382:77–89. doi: 10.1515/BC.2001.013. [DOI] [PubMed] [Google Scholar]
- 20.Joseph K., Kaplan A.P. Formation of bradykinin: a major contributor to the innate inflammatory response. Adv. Immunol. 2005;86:159–208. doi: 10.1016/S0065-2776(04)86005-X. [DOI] [PubMed] [Google Scholar]