Abstract
Prior clinical studies suggest that chronic treatment with atypical antipsychotic medications increase erythrocyte and postmortem prefrontal cortex (PFC) omega-3 fatty acid composition in patients with schizophrenia (SZ). However, because human tissue phospholipid omega-3 fatty acid composition is potentially influenced by multiple extraneous variables, definitive evaluation of this putative mechanism of action requires an animal model. In the present study, we determined the effects of chronic treatment with the atypical antipsychotic risperidone (RISP, 3.0 mg/kg/d) on erythrocyte and PFC omega-3 fatty acid composition in rats maintained on a diet with or without the dietary omega-3 fatty acid precursor, alpha-linolenic acid (ALA, 18:3n-3). Chronic RISP treatment resulted in therapeutically-relevant plasma RISP and 9-OH-RISP concentrations (18±2.6 ng/ml), and significantly increased erythrocyte docosahexaenoic acid (DHA, 22:6n-3, +22%, p=0.0003) and docosapentaenoic acid (22:5n-3, +18%, p=0.01) composition, and increased PFC DHA composition (+7%, p=0.03) in rats maintained on the ALA+ diet. In contrast, chronic RISP did not alter erythrocyte or PFC omega-3 fatty acid composition in rats maintained on the ALA− diet. Chronic RISP treatment did not alter erythrocyte or PFC arachidonic acid (AA, 20:4n-6) composition. These data demonstrate that chronic RISP treatment significantly augments ALA-DHA biosynthesis, and preferentially increases peripheral and central membrane omega-3 fatty acid composition. Augmented omega-3 fatty acid biosynthesis and membrane composition represents a novel mechanism of action that may contribute in part to the efficacy of RISP in the treatment of SZ.
Keywords: Risperidone, 9-OH-RISP, Prefrontal cortex, Erythrocyte, Omega-3 fatty acid, Docosahexaenoic acid, Arachidonic acid, Rat
1. Introduction
Mammalian brain lipids are comprised of a ratio of saturated fatty acids, monounsaturated fatty acids, and polyunsaturated fatty acids (PUFA). Principal PUFAs in brain phospholipids are the omega-3 fatty acid docosahexaenoic acid (DHA, 22:6n-3) and the omega-6 fatty acid arachidonic acid (AA, 20:4n-6). Because mammals are incapable of synthesizing omega-6 and omega-3 fatty acids de novo, they are entirely dependent on dietary sources to procure and maintain adequate peripheral and central tissue levels. The dietary omega-3 fatty acid precursor alpha-linolenic acid (ALA, 18:3n-3) and omega-6 fatty acid precursor linoleic acid (LA, 18:2n-6) are converted into DHA and AA, respectively, following a series of common and competitive desaturation-elongation reactions, and chronic dietary ALA deficiency is associated with reciprocal elevations in peripheral and central omega-6 fatty acid composition (reviewed in Contreras & Rapoport, 2002). In rats, ALA-DHA biosynthesis is predominantly mediated by the liver, and dietary ALA deficiency up-regulates rat hepatic desaturase and elongase expression and activity (Igarashi et al., 2007). In contrast, isotope tracer and dietary studies have found that humans, particularly in males, convert only a small percentage (<1.0%) of dietary ALA to DHA (Arterburn et al., 2006; Pawlowsky et al., 2001a).
An emerging body of evidence suggests that schizophrenia (SZ) is associated with peripheral and central PUFA deficits. DHA and/or AA deficits are observed in postmortem brain tissues of SZ patients (Horrobin et al., 1991; McNamara et al., 2007; Yao et al., 2000), and in erythrocytes (red blood cells) of antipsychotic-naive first- or early-episode psychotic patients (Arvindakshan et al., 2003; Evans et al., 2003; Kale et al., 2008; Khan et al., 2002; Reddy et al., 2004). Consistent with deficits in PUFA biosynthesis from dietary precursors, some studies (Evans et al., 2003; Kale et al., 2008; Reddy et al., 2004), but not all (Khan et al., 2002), have observed normal peripheral ALA or LA composition in SZ patients. However, it is not yet known whether peripheral and central PUFA deficits in SZ are due to dietary insufficiency (Brown et al., 1999; Henderson et al., 2006; Strassnig et al., 2005), or other factors that reduce phospholipid PUFA composition, including cigarette smoking (Hibbeln et al., 2003; Leng et al., 1994), chronic alcohol intake (Pawlosky et al., 2001b), lipid peroxidation (Arvindakshan et al., 2003; Khan et al., 2002), and/or polymorphisms in the PUFA lipogenic genes (Krause et al., 2006; Rzehak et al., 2008; Schaeffer et al., 2006; Williard et al., 2001). Elucidating the mechanisms that confer PUFA deficits in SZ may provide important insight into the underlying pathophysiology.
A potentially important observation is that the erythrocyte PUFA deficits observed in antipsychotic-naive first-episode psychotic patients increase significantly following chronic antipsychotic treatment associated with symptomatic improvement (Arvindakshan et al., 2003; Evans et al., 2003; Khan et al., 2002). Two of these studies combined patients treated with typical and atypical antipsychotic medications (Arvindakshan et al., 2003; Khan et al., 2002), though the majority of patients were treated with atypical antipsychotics, and the third study employed only patients receiving atypical antipsychotics (risperidone or olanzapine)(Evans et al., 2003). Moreover, we found that the PUFA deficits observed in the postmortem PFC of antipsychotic-free SZ patients were greater than those observed in SZ patients treated with atypical (olanzapine, risperidone, clozapine), but not typical (fluphenazine, thiothixene, chlorpromazine, thioridazine, haloperidol), antipsychotic medications (McNamara et al., 2007). Moreover, emerging clinical evidence suggests that atypical antipsychotic drugs augment lipid biosynthesis by increasing lipogenic gene expression (Kaddurah-Daouk et al., 2007; Vik-Mo et al., 2008). Although these data suggest that atypical antipsychotic medications augment PUFA biosynthesis and peripheral and central PUFA composition, multiple extraneous variables including dietary PUFA intake preclude definitive evaluation of a causal relationship. In the present study, we determined the effect of chronic treatment with the atypical antipsychotic risperidone (RISP) on erythrocyte and PFC PUFA composition in rats fed diets fortified with the PUFA precursors ALA and LA, but not preformed DHA or AA or intermediate precursors. As a preliminary evaluation of ALA-DHA biosynthesis, we also investigated in parallel the effects of chronic RISP treatment on erythrocyte and PFC PUFA composition of rats maintained on a diet selectively depleted of ALA.
2. Methods
2.1. Animals and dietary treatments
Immediately following weaning (Postnatal day: P21), male Long-Evans hooded rats (Harlan Farms, Indianapolis, IN) bred in house were randomly assigned to receive a customized research diet that either contained alpha-linolenic acid (ALA+, TD.04285) (n=26) or did not contain ALA (ALA−, TD.04286)(n=22) (Harlan-TEKLAD, Madison, WI). A subset of rats maintained on the ALA+ (n=10) and ALA− (n=10) diets received chronic RISP from P60–P90 (described below). Both ALA+ and ALA− diets were matched for all non-fat nutrients (described in McNamara et al., 2008). Determination of diet fatty acid composition by gas chromatography found that both ALA+ and ALA− diets were matched with the exception of ALA, which was absent from the ALA− diet and represented 4.6% of total fatty acid composition in the ALA+ diet (Table 1). Both diets contained an equivalent amount of the omega-6 fatty acid precursor linoleic acid (18:2n-6), and neither diet contained preformed DHA or AA or intermediate precursors. Rats were housed 2 per cage with food and water available ad libitum, and maintained under standard vivarium conditions on a 12:12 h light:dark cycle. All experimental procedures were approved by the University of Institutional Animal Care and Use Committee, and adhere to the guidelines set by the National Institutes of Health.
Table 1.
Diet Fatty Acid Composition
| Fatty Acid1 | ALA+ | ALA− |
|---|---|---|
| Octanoic acid (8:0) | 4.3 ± 0.1 | 4.6 ± 0.0 |
| Decanoic acid (10:0) | 3.7 ± 0.1 | 3.9 ± 0.0 |
| Lauric acid (12:0) | 29.0 ± 0.4 | 30.5 ± 0.1 |
| Myristic acid (14:0) | 11.0 ± 0.1 | 11.6 ± 0.1 |
| Palmitic acid (16:0) | 8.3 ± 0.0 | 8.5 ± 0.0 |
| Stearic acid (18:0) | 9.4 ± 0.1 | 9.8 ± 0.0 |
| Oleic acid (18:1n-9) | 6.7 ± 0.1 | 5.9 ± 0.1 |
| Linoleic acid (18:2n-6) | 22.7 ± 0.3 | 22.4 ± 0.2 |
| Arachidonic acid (20:4n-6) | - | - |
| α-Linolenic acid (18:3n-3) | 4.6 ± 0.1 | - |
| Docosahexaenoic acid (22:6n-3) | - | - |
Values are means ± SD (mg/100 mg fatty acids).
2.2. Chronic risperidone treatment
A subgroup of rats fed ALA+ (n=10) or ALA− (n=10) diets were randomly assigned to receive chronic RISP from P60 to P90. RISP was administered through the rat’s drinking water in order to obviate daily injection stress and stress associated with the surgical implantation of minipumps, to permit maintenance of drug dose in accordance with age-related increases in body weight, and to mimic oral administration in human patients. For three days prior to drug delivery, 24 h water consumption was determined for each cage using bottle weights (1 g water = 1 ml water), and ml water intake/kg body weight calculated. Based on daily ml/kg water consumption, RISP stock solution (1.0 mg/ml; dissolved in 0.1 M acetic acid, supplied by Ortho-McNeil Janssen Pharmaceuticals) was added to water at a concentration required to deliver a daily dose of 3.0 mg/kg/d. Pilot studies found that this dose was sufficient to produce therapeutically-relevant plasma RISP and 9-OH-RISP concentrations (see below). Red opaque drinking bottles were used to protect RISP from light degradation. Fresh solutions were prepared, and RISP concentration adjusted to body weight, every 3 days. Rats were maintained on RISP until being sacrificed on P90.
2.3. Tissue collection
Rats were sacrificed on P90 by decapitation during the light portion of the cycle, brains extracted and immediately immersed in ice-cold 0.9% NaCl for 2 min. The brain was then dissected on ice to isolate the bilateral PFC, and the olfactory tubercle and residual striatal tissue were removed from the PFC. PFC samples were placed in cryotubes, flash frozen in liquid nitrogen, and stored at −80°C. At the time of sacrifice, trunk blood was collected into 1.5 ml tubes containing 200 μl of EDTA (100 mM, pH 7.5) and centrifuged for 20 min (5,000× g, 4°C). Plasma was isolated for determination of RISP and 9-OH-RISP concentrations (described below). Erythrocytes were isolated and washed 3x with 0.9% NaCl, and stored at −80°C for subsequent determination of fatty acid composition.
2.4. Plasma RISP and 9-OH-RISP concentrations
Plasma RISP and 9-OH-RISP concentrations (ng/ml) were determined using liquid chromatography in tandem with mass spectrometry after solid phase extraction (SPE). 2 ml phosphate buffer 0.1M pH 6.0 was added to the plasma samples (0.1-ml aliquots). Samples were spiked with 100 μl of the stable isotope-labeled internal standards (R215640 and R215639 respectively) in methanol and 100 μl methanol (or calibration standards in methanol for the calibration samples and the independent QC samples, both prepared in same matrix as the study samples). SPE columns (Bond Elut Certify 130 mg, Varian) were conditioned with 3 ml methanol, 3 ml MilliQ water and 1 ml phosphate buffer 0.1M pH 6.0. The samples were transferred to the SPE columns, which were then washed with 3 ml MilliQ water, 1 ml acetic acid 1M and eluted with 3 ml of a mixture methanol/ammonia (98/2 v/v). The eluant was evaporated to dryness under nitrogen at 65°C and redissolved in 300 μl of a mixture 0.01 M ammonium formate (adjusted to pH 4 with formic acid)/acetonitrile 50/50 v/v. A 10 μl aliquot was injected onto a reversed phase column (10 cm × 4.6 mm ID, packed with 3 μm Polaris C18-A) for final separation and quantification. The mobile phase was a mixture of 0.01M ammonium formate (adjusted to pH 4 with formic acid)/acetonitrile and methanol. LC-MS/MS analysis was carried out on an API-3000 MS/MS (Applied Biosystems) operating in the positive ion mode, which was coupled to an HPLC-pump (Agilent) and autosampler (Shimadzu). The quantitation limit, defined as the lowest spiked standard of the calibration curve prepared in the same matrix as the study samples, was 0.5 ng/ml plasma. Linearity of the calibration curve ranged from 0.5 to 1250 ng/ml. All samples were processed by a technician blinded to treatment.
2.5. Gas chromatography
Total (triglyceride, phospholipid, and cholesteryl ester) fatty acid composition was determined using the saponification and methylation methods originally described by Mecalfe et al (1966). PFC and erythrocyte samples were placed in a 20 ml glass vial into which 4 ml of 0.5N methanolic sodium hydroxide was added, and the sample heated at 80°C for 5 min. Following a 10 min cooling period, 3 ml of boron trifluoride in methanol was added to methylate the sample. After an additional five minutes of heating in the water bath (80°C), the sample vial was allowed to cool, and 2 ml of a saturated solution (6.2 M) of sodium chloride and 10 ml of hexane was added. The samples were then mixed by vortex for one minute. The hexane fraction was then transferred into a 20 ml vial containing 10 mg of sodium sulfate to dry the sample. The hexane solution was then removed for GC analysis. Samples were analyzed with a Shimadzu GC-2014 equipped with an auto-injector (Shimadzu Scientific Instruments Inc., Columbia MD). The column was a DB-23 (123-2332): 30m (length), I.D. 0.32 mm wide bore, film thickness of 0.25 μM (J&W Scientific, Folsom CA). The GC conditions were: column temperature ramping by holding at 120°C for one minute followed by an increase of 5°C/min from 120–240°C. The temperature of the injector and flame ionization detector was 250°C. A split (8:1) injection mode was used. The carrier gas was helium with a column flow rate of 2.5 ml/min. Fatty acid identification was determined using retention times of authenticated fatty acid methyl ester standards (Matreya LLC Inc., Pleasant Gap PA). Analysis of fatty acid methyl esters is based on areas calculated with Shimadzu Class VP 4.3 software. Fatty acid composition data are expressed as weight percent of total fatty acids (mg fatty acid/100 mg fatty acids, wt % total). We have previously demonstrated that wt % total data are highly correlated with total mass data (nmol/g)(r=0.995, p≤0.0001)(McNamara et al., 2008). Samples were processed by a technician blinded to treatment.
2.6. Statistical analyses
To reduce the number of comparisons and the associated risk of Type I errors, we restricted our primary analysis to the principal omega-3 fatty acids (22:5n-3, 22:6n-3) and omega-6 fatty acids (18:2n-6, 20:4n-6). Effects of RISP and diet on erythrocyte and PFC fatty acid composition were determined with a two-way ANOVA, with Treatment (water, RISP) and Diet (ALA+, ALA−) as main factors. Pairwise comparisons were made with unpaired t-tests (two-tail, α=0.05). Plasma RISP and 9-OH-RISP concentrations were analyzed with an unpaired t-test (two-tail, α=0.05). In the case of zero values, paired t-tests (two-tail) were used. All statistical analyses were performed with GB-STAT software (Dynamic Microsystems, Inc., Silver Springs MD).
3. Results
3.1. Body weight
There was a significant main effect of Treatment on body weight, F(1,44)=27.1, p≤0.0001, and the main effect of Diet, F(1,44)=0.048, p=0.827, and the Diet × Treatment Interaction, F(1,44)=0.366, p=0.549, were not significant. Chronic RISP treatment significantly reduced body weight in RISP-ALA+ (385±28 gm) and RISP-ALA− (377±24 gm) rats relative to untreated ALA+ (431±34 gm, p≤0.01) and ALA− (435±38 gm, p≤0.01) rats, respectively. The body weight of RISP-ALA+ and RISP-ALA− rats (p>0.05) and untreated ALA+ and ALA− rats (p>0.05) did not differ significantly.
3.2. Plasma RISP and 9-OH-RISP concentrations
There were no significant differences in plasma RISP, 9-OH-RISP, or RISP+9-OH-RISP concentrations between RISP-ALA+ (n=10) and RISP-ALA− (n=10) rats (Table 2). The 9-OH-RISP:RISP ratio did not differ between diet groups, suggesting that both diet groups metabolized RISP to 9-OH-RISP at a similar rate. The overall (N=20) mean plasma RISP+9-OH-RISP concentration (17.7 ± 2.6 ng/ml) was at the lower end of the human therapeutic range (15–60 ng/ml)(Mauri et al., 2007).
Table 2.
Plasma RISP and 9-OH-RISP Concentrations
| ALA+ (n=10) | ALA− (n=10) | p-value2 | |
|---|---|---|---|
| RISP1 | 3.77 ± 1.29 | 5.15 ± 1.74 | 0.535 |
| 9-OH-RISP | 14.2 ± 4.33 | 12.1 ± 2.94 | 0.699 |
| RISP+9-OH-RISP | 18.0 ± 4.47 | 17.3 ± 2.71 | 0.891 |
| 9-OH-RISP/RISP | 5.44 ± 1.27 | 6.08 ± 2.47 | 0.815 |
Data are expressed as ng/ml
Unpaired t-test (two-tail)
3.3. Erythrocyte PUFA composition
For LA (18:2n-6) composition, the main effects of Diet, F(1,44)=68.7, p≤0.0001, and Treatment, F(1,44)=10.2, p=0.003, and the Diet × Treatment interaction, F(1,44)=4.28, p=0.044, were significant. LA composition was significantly lower in RISP-ALA+ (−16%, p=0.005), untreated ALA(−32%, p≤0.0001), and RISP-ALA− (−36%, p≤0.0001) rats relative to untreated ALA+ rats (Fig. 1A). For AA (20:4n-6) composition, the main effect of Diet was significant, F(1,44)=49.4, p≤0.0001, and the main effect of Treatment, F(1,44)=2.25, p=0.141, and the Diet × Treatment interaction, F(1,44)=0.241, p=0.625, were not significant. AA composition was significantly greater in untreated ALA− (+8%, p≤0.0001) and RISP-ALA− (+10%, p≤0.0001) rats relative to untreated ALA+ rats (Fig. 1B). For the AA:LA ratio, the main effects of Diet, F(1,44)=111.7, p≤0.0001, and Treatment, F(1,44)=9.84, p=0.003, were significant, and the Diet × Treatment interaction, F(1,44)=0.80, p=0.374, was not significant. The AA:LA ratio was significantly greater in RISP-ALA+ (+17%, p=0.008), untreated ALA− (+37%, p≤0.0001), and RISP-ALA− (+41%, p≤0.0001) rats relative to untreated ALA+ rats (Fig. 1C).
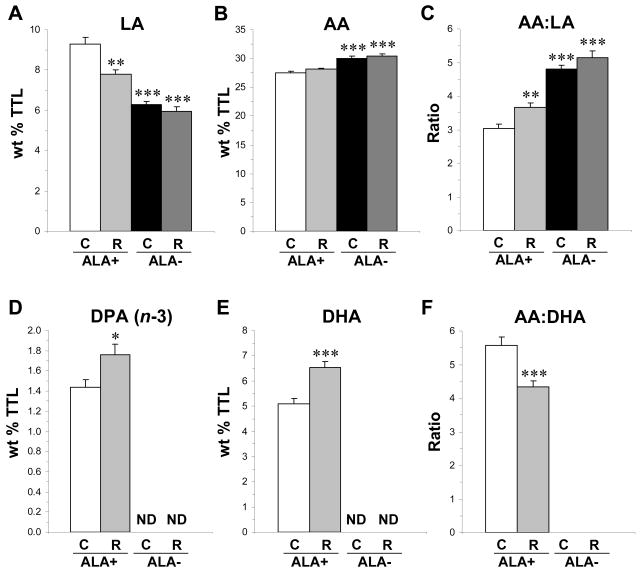
Fig. 1.
Effects of chronic RISP treatment on erythrocyte linoleic acid (LA, A), arachidonic acid (AA, B), the AA:LA ratio (C), docosapentaenoic acid (DPA, 22:5n-3, D), DHA (E), and the AA:DHA ratio (F) in untreated (C, control) and RISP (R)-treated rats maintained on ALA+ or ALA− diets (n=10–14/group). Note: (1) ND in D and E indicate that fatty acids were not detectable, and (2) the AA:DHA ratio was not calculated for untreated ALA− and RISP-ALA− rats due to zero DHA values. Values are group means ± S.E.M. *P≤ 0.05, **P≤0.01, ***P≤0.001 vs. ALA+., not detectable.
For docosapentaenic acid (DPA, 22:5n-3) composition, the main effects of Diet, F(1,44)=606, p≤0.0001, and Treatment, F(1,44)=6.10, p=0.018, and the Diet × Treatment interaction, F(1,44)=6.10, p=0.018, were significant. Relative to untreated ALA+ rats, DPA composition was significantly greater in RISP-ALA+ rats (+18%, p=0.018), and was depleted in untreated ALA− (p≤0.0001) and RISP-ALA− (p≤0.0001) rats (Fig. 1D). For docosahexaenoic acid (DHA, 22:6n-3) composition, the main effects of Diet, F(1,44)=1015, p≤0.0001, and Treatment, F(1,44)=15.7, p=0.003, and the Diet × Treatment interaction, F(1,44)=15.7, p=0.003, were significant. Relative to untreated ALA+ rats, DHA composition was significantly greater in RISP-ALA+ rats (+22%, p=0.0003), and was depleted in untreated ALA− (p≤0.0001) and RISP-ALA− (p≤0.0001) rats (Fig. 1E). For the DPA:DHA ratio, the main effect of Diet, F(1,44)=409, p=0.0001, was significant, and the main effect of Treatment, F(1,44)=0.04, p=0.84, and the Diet × Treatment interaction, F(1,44)=0.04, p=0.84, were not significant (data not shown). For the AA:DHA ratio, the main effects of Diet, F(1,44)=568, p≤0.0001, and Treatment, F(1,44)=8.52, p=0.005, and the Diet × Treatment interaction, F(1,44)=8.52, p=0.005, were significant. Relative to untreated ALA+ rats, the AA:DHA ratio was significantly lower in RISP-ALA+ rats (−22%, p=0.005)(Fig. 1F) and was not calculated for untreated ALA− and RISP-ALA− rats due to zero DHA values.
3.4. PFC PUFA composition
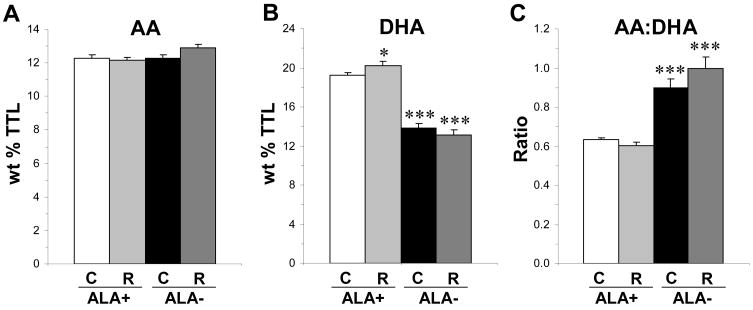
For AA composition, the main effect of Diet was significant, F(1,44)=5.59, p=0.024, and the main effect of Treatment, F(1,44)=1.76, p=0.192, and the Diet × Treatment interaction, F(1,44)=3.51, p=0.069, were not significant (Fig. 2A). For DHA composition, the main effects of Diet, F(1,44)=173, p≤0.0001, and the Diet × Treatment interaction, F(1,44)=5.79, p=0.021, were significant, and the main effect of Treatment was not significant, F(1,44)=0.68, p=0.882. Relative to untreated ALA+ rats, DHA composition was significantly greater in RISP-ALA+ rats (+7%, p=0.027), and significantly lower in untreated ALA− (−28%, p≤0.0001) and RISP-ALA− (−32%, p≤0.0001) rats (Fig. 2B). For the AA:DHA ratio, the main effect of Diet was significant, F(1,44)=86.7, p≤0.0001, and the main effect of Treatment, F(1,44)=0.80, p=0.38, and the Diet × Treatment interaction, F(1,44)=3.59, p=0.07, were not significant. Relative to untreated ALA+ rats, the AA:DHA ratio was significantly greater in untreated ALA− (+29%, p≤0.0001) and RISP-ALA− (+36%, p≤0.0001) rats (Fig. 2C).
Fig. 2.
Effects of chronic RISP treatment on PFC AA (A) and DHA (B) composition, and the AA:DHA ratio (C), in untreated (C, control) and RISP (R)-treated rats maintained on ALA+ or ALA− diets (n=10–14/group). Values are group means ± S.E.M. *P≤0.05, ***P≤0.001 vs. ALA+.
4. Discussion
Based on prior clinical observations that treatment with atypical antipsychotic medications increase erythrocyte and PFC DHA and/or AA composition, it was predicted that chronic RISP treatment would increase rat erythrocyte and PFC DHA and AA composition under controlled dietary conditions. In support of this prediction, we found that chronic RISP treatment resulting in therapeutically-relevant plasma concentrations significantly increased DHA composition in erythrocytes (+22%) and PFC (+7%) of rats maintained on the ALA+ diet. In contrast, chronic RISP treatment did not significantly increase erythrocyte or PFC DHA composition in rats maintained on the ALA− diet. Together, these findings provide evidence that chronic RISP augments ALA-DHA biosynthesis. Moreover, chronic RISP treatment did not significantly alter erythrocyte or PFC AA composition, and was associated with a significant reduction in the AA:DHA ratio. Collectively, these data provide proof-of-concept evidence that chronic RISP treatment significantly augments ALA-DHA biosynthesis, and preferentially increases peripheral and central membrane DHA composition.
This study has the following limitations. First, this study was conducted in male rats, and gender differences in omega-3 fatty acid biosynthesis have been reported (female > male)(Childs et al., 2008). However, males were selected because prior clinical studies have found that male SZ patients, but not female SZ patients, exhibit significant PUFA deficits in erythrocytes (Kale et al., 2008) and postmortem PFC (McNamara et al., 2007). Second, only one dose of RISP was used, precluding evaluation of dose-response effects. However, this dose was selected to produce plasma RISP and 9-OH-RISP concentrations that are within the human therapeutic range (Mauri et al., 2007). Third, only one treatment duration was examined (30 d), and longer exposure may have led to larger effects on fatty acid composition. Fourth, only one type and class of antipsychotic was investigated, and other antipsychotic medications may be associated with different effects. It is noteworthy, however, that treatment with atypical antipsychotic medications increase erythrocyte (Evans et al., 2003) and PFC (McNamara et al., 2007) DHA composition, whereas typical antipsychotic medications are less effective. Fifth, only one brain region was investigated, and regional brain differences in DHA loss and accrual rates have been reported (Xiao et al., 2005). However, the rat PFC exhibits a predictable change in DHA composition in response to dietary omega-3 fatty acid depletion and fortification (Xiao et al., 2005), and it was in the human PFC that we observed apparent normalizing effects of atypical antipsychotics on DHA composition (McNamara et al., 2007). Finally, we did not examine indices of oxidative stress to determine the relationship with fatty acid composition. However, a prior study found that chronic (45 – 90 d) RISP (2.5 mg/kg) treatment did not significantly alter indices of oxidative stress in rat brain (Parikh et al., 2003).
In the present study, chronic RISP treatment reduced body weight by approximately 12% irrespective of dietary ALA composition. The absence of significant weight gain following chronic RISP treatment is consistent with a previous rat study using a lower daily dose of RISP (0.5 mg/kg) administered via intraperitoneal injection (Fell et al., 2007). These findings suggest that rats are less vulnerable to the significant weight gain observed in SZ patients following chronic RISP treatment (Allison et al., 1999). However, it is important to note that in the present and prior preclinical studies rats are maintained on standardized and balanced diets fortified with macronutrients, whereas the diets of SZ patients vary considerably and are commonly higher in fat and lacking macronutrients (Brown et al., 1999; Henderson et al., 2006; Strassnig et al., 2005). Nevertheless, the observation that RISP-treated rats maintained on ALA+ diet exhibited elevated erythrocyte and PFC DHA composition would suggest that RISP treatment did not significantly reduce ALA intake despite reductions in body weight.
The present preclinical data are consistent with prior clinical studies finding that chronic atypical antipsychotic treatment increases erythrocyte DHA and DPA (22:5n-3) composition in antipsychotic-naïve psychotic patients (Evans et al., 2003). The present findings further reveal that this increase in erythrocyte omega-3 fatty acid composition is dependent on the presence of ALA in the diet, indicating that chronic RISP increases erythrocyte omega-3 fatty acid composition by augmenting biosynthesis rather than by decreasing phospholipid turnover. Although erythrocyte AA composition increased significantly in response to ALA depletion in untreated rats, chronic RISP treatment did not significantly increase erythrocyte AA composition in rats maintained on either ALA+ or ALA− diets. This finding suggests that elevated omega-3 fatty acid composition following chronic RISP treatment limits AA membrane phospholipid deposition, and suggest that chronic RISP treatment preferentially increases rat erythrocyte membrane omega-3 fatty acid composition.
Compared to the DHA increase observed in erythrocytes (+22%), chronic RISP treatment produced a relatively small increase in PFC DHA composition (+7%) in rats on the ALA+ diet. This difference is consistent with prior studies finding that DHA loss and recuperation occurs at faster rate in erythrocytes than brain (Connor et al., 1990), and suggests that a longer duration of RISP treatment would be associated with a larger increase in PFC DHA composition. By comparison, reintroducing dietary ALA for 30 d (P60–P90) is associated with significant elevations in PFC DHA composition (+18%, p=0.0003, unpublished observations). Consistent with previous studies, untreated rats maintained on the ALA− diet from P21–P90 exhibited significantly lower PFC DHA composition (−28%), and the magnitude of this PFA DHA deficit was not significantly altered by chronic RISP treatment (−32%). Moreover, chronic RISP treatment increased PFA DHA composition in rats maintained on the ALA+ diet, but not in rats maintained on the ALA− diet. Together, these findings suggest that that chronic RISP does not significantly influence DHA turnover rates in PFC phospholipids, and increases PFC DHA composition via elevated biosynthesis from dietary ALA. As observed in erythrocytes, chronic RISP treatment did not significantly increase PFC AA composition under either dietary condition. This may be due in part to reduced AA turnover mediated by RISP-mediated blockade of dopamine D2 receptors (Myers et al., 2001; Trzeciak et al., 1995). Nevertheless, these data suggest that chronic RISP treatment preferentially increases DHA composition in PFC phospholipids.
Prior rodent studies have found that semi-chronic (21 d) treatment with haloperidol (1 mg/kg/d) or clozapine (20 mg/kg/d) (Levant et al., 2006), or chronic (45 or 90 d) treatment with haloperidol (2 mg/kg/d), RISP (2.5 mg/kg/d), olanzapine (10 mg/kg/d), or clozapine (20 mg/kg/d) (Parikh et al., 2003) do not alter whole brain DHA composition. The apparent discrepancy between these and the present findings may be attributable in part to the relatively small increase in PFC DHA composition (+7%), which may have been masked by regional differences in DHA accrual (Xiao et al., 2005). Moreover, the Parikh et al (2003) study, which also investigated chronic RISP treatment, incorporated a 4-day drug withdrawal period prior to sacrifice, used a different rat strain (Wistar) and diet (Purina Rat Chow), and treated with a slightly lower dose of RISP (2.5 mg/kd/d). Although the present findings suggest that the rat PFC is more sensitive than whole brain to elevations in DHA composition in response to chronic RISP treatment, additional studies will be required to determine whether this effect is unique to the PFC and/or RISP.
Increasing omega-3 fatty acid biosynthesis and erythrocyte and PFC omega-3 fatty acid composition following chronic RISP treatment may contribute in part to its clinical efficacy. This is supported by some clinical trials (Berger et al., 2007; Emsley et al., 2002; Peet et al., 2001), but not all (Fenton et al., 2001), demonstrating that chronic adjunctive treatment with omega-3 fatty acids augment the therapeutic efficacy of antipsychotic medications in SZ patients. For example, Berger et al. (2007) found that first-episode psychotic patients treated with omega-3 fatty acids required a significantly lower dose of atypical antipsychotic relative to patients treated with antipsychotic medications alone. Moreover, chronic RISP treatment (McGorry et al., 2002) and omega-3 fatty acid monotherapy (Amminger et al., 2007) are both efficacious in preventing or delaying psychosis onset in high-risk subjects. Augmented omega-3 fatty acid biosynthesis may additionally contribute to the relatively neutral effect of RISP on serum triglyceride levels (reviewed in Meyer & Koro, 2004). Specifically, chronic omega-3 fatty acid intake has repeatedly been found to significantly reduce serum triglyceride levels in healthy adults (reviewed in Harris, 1989), and adjunctive omega-3 fatty acid treatment significantly reduces elevated serum triglyceride levels in clozapine-treated SZ patients (Caniato et al., 2006). Similarly, augmented omega-3 fatty acid biosynthesis may also contribute to the relatively benign effect of RISP on glucose regulation (Henderson et al., 2005; Nettleton & Katz, 2005).
In summary, we present preclinical evidence that chronic RISP treatment augments ALA-DHA biosynthesis, and preferentially increases erythrocyte and PFC omega-3 fatty acid composition. These preclinical findings are consistent with prior clinical observations, and provide novel evidence that this effect is predominantly mediated by augmented biosynthesis. It may be relevant, therefore, that the principal human PUFA elongase gene HELO1 is localized to chromosome 6p21.1-p12.1 (Leonard et al., 2000), a putative susceptibility locus for SZ (Lewis et al., 2003), and polymorphisms in the PUFA lipogenic genes are associated with membrane PUFA deficits (Krause et al., 2006; Rzehak et al., 2008; Schaeffer et al., 2006; Williard et al., 2001). Moreover, ALA-DHA biosynthesis is limited in healthy males relative females due in part to estrogenic effects (Bakewell et al., 2006; Giltay et al., 2004), and male, but not female, SZ patients were found to exhibit erythrocyte (Kale et al., 2008) and postmortem PFC (McNamara et al., 2007) DHA deficits relative to same-gender controls. Finally, emerging clinical evidence suggests that atypical antipsychotic drugs augment lipid biosynthesis by increasing lipogenic gene expression (Kaddurah-Daouk et al., 2007; Vik-Mo et al., 2008). Collectively, these findings suggest that impairments in lipid biosynthesis may contribute in part to the pathophysiology of SZ, and suggests that increasing omega-3 fatty acid biosynthesis may represent a novel mechanism of action of atypical antipsychotic medications.
Acknowledgments
This work was supported in part by National Institute of Mental Health grants MH073704 and MH074858 to R.K.M., and DK59630 to P.T. Risperidone was generously supplied by Ortho-McNeil Janssen Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, Weiden PJ. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156:1686–1696. doi: 10.1176/ajp.156.11.1686. [DOI] [PubMed] [Google Scholar]
- Amminger GP, Schaefer MR, McGorry PD. Omega-3 fatty acids reduce the risk of early transition to psychosis in ultra-high risk individuals: A double-blind randomized, placebo-controlled treatment study. International Conference of Schizophrenia Research 2007 [Google Scholar]
- Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of omega-3 fatty acids in humans. Am J Clin Nutr. 2006;83(6 Suppl):1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- Arvindakshan M, Sitasawad S, Debsikdar V, Ghate M, Evans D, Horrobin DF, Bennett C, Ranjekar PK, Mahadik SP. Essential polyunsaturated fatty acid and lipid peroxide levels in never-medicated and medicated schizophrenia patients. Biol Psychiatry. 2003;53:56–64. doi: 10.1016/s0006-3223(02)01443-9. [DOI] [PubMed] [Google Scholar]
- Bakewell L, Burdge GC, Calder PC. Polyunsaturated fatty acid concentrations in young men and women consuming their habitual diets. Br J Nutr. 2006;96:93–99. doi: 10.1079/bjn20061801. [DOI] [PubMed] [Google Scholar]
- Berger GE, Proffitt TM, McConchie M, Yuen H, Wood SJ, Amminger GP, Brewer W, McGorry PD. Ethyl-eicosapentaenoic acid in first-episode psychosis: a randomized, placebo-controlled trial. J Clin Psychiatry. 2007;68(12):1867–1875. doi: 10.4088/jcp.v68n1206. [DOI] [PubMed] [Google Scholar]
- Brown S, Birtwistle J, Roe L, Thompson C. The unhealthy lifestyle of people with schizophrenia. Psychol Med. 1999;29:697–701. doi: 10.1017/s0033291798008186. [DOI] [PubMed] [Google Scholar]
- Caniato RN, Alvarenga ME, Garcia-Alcaraz MA. Effect of omega-3 fatty acids on the lipid profile of patients taking clozapine. Aust N Z J Psychiatry. 2006;40:691–697. doi: 10.1080/j.1440-1614.2006.01869.x. [DOI] [PubMed] [Google Scholar]
- Childs CE, Romeu-Nadal M, Burdge GC, Calder PC. Gender differences in the omega-3 fatty acid content of tissues. Proc Nutr Soc. 2008;67:19–27. doi: 10.1017/S0029665108005983. [DOI] [PubMed] [Google Scholar]
- Connor WE, Neuringer M, Lin DS. Dietary effects on brain fatty acid composition: the reversibility of omega-3 fatty acid deficiency and turnover of docosahexaenoic acid in the brain, erythrocytes, and plasma of rhesus monkeys. J Lipid Res. 1990;31:237–247. [PubMed] [Google Scholar]
- Contreras MA, Rapoport SI. Recent studies on interactions between n-3 and n-6 polyunsaturated fatty acids in brain and other tissues. Curr Opin Lipidol. 2002;13(3):267–272. doi: 10.1097/00041433-200206000-00006. [DOI] [PubMed] [Google Scholar]
- Emsley R, Myburgh C, Oosthuizen P, van Rensburg SJ. Randomized, placebo-controlled study of ethyl-eicosapentaenoic acid as supplemental treatment in schizophrenia. Am J Psychiatry. 2002;159:1596–1598. doi: 10.1176/appi.ajp.159.9.1596. [DOI] [PubMed] [Google Scholar]
- Evans DR, Parikh VV, Khan MM, Coussons C, Buckley PF, Mahadik SP. Red blood cell membrane essential fatty acid metabolism in early psychotic patients following antipsychotic drug treatment. Prostaglandins Leukot Essent Fatty Acids. 2003;69:393–399. doi: 10.1016/j.plefa.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Fell MJ, Anjum N, Dickinson K, Marshall KM, Peltola LM, Vickers S, Cheetham S, Neill JC. The distinct effects of subchronic antipsychotic drug treatment on macronutrient selection, body weight, adiposity, and metabolism in female rats. Psychopharmacology. 2007;194:221–231. doi: 10.1007/s00213-007-0833-9. [DOI] [PubMed] [Google Scholar]
- Fenton WS, Dickerson F, Boronow J, Hibbeln JR, Knable M. A placebo-controlled trial of omega-3 fatty acid (ethyl eicosapentaenoic acid) supplementation for residual symptoms and cognitive impairment in schizophrenia. Am J Psychiatry. 2001;158:2071–2074. doi: 10.1176/appi.ajp.158.12.2071. [DOI] [PubMed] [Google Scholar]
- Garman PM, Ried LD, Bengtson MA, Hsu C, McConkey JR. Effect on lipid profiles of switching from olanzapine to another second-generation antipsychotic agent in veterans with schizophrenia. J Am Pharm Assoc. 2007;47:373–378. doi: 10.1331/JAPhA.2007.06090. [DOI] [PubMed] [Google Scholar]
- Giltay EJ, Gooren LJ, Toorians AW, Katan MB, Zock PL. Docosahexaenoic acid concentrations are higher in women than in men because of estrogenic effects. Am J Clin Nutr. 2004;80:1167–1174. doi: 10.1093/ajcn/80.5.1167. [DOI] [PubMed] [Google Scholar]
- Harris WS. Fish oils and plasma lipid and lipoprotein metabolism in humans: a critical review. J Lipid Res. 1989;30:785–807. [PubMed] [Google Scholar]
- Henderson DC, Cagliero E, Copeland PM, Borba CP, Evins E, Hayden D, Weber MT, Anderson EJ, Allison DB, Daley TB, Schoenfeld D, Goff DC. Glucose metabolism in patients with schizophrenia treated with atypical antipsychotic agents: a frequently sampled intravenous glucose tolerance test and minimal model analysis. Arch Gen Psychiatry. 2005;62:19–28. doi: 10.1001/archpsyc.62.1.19. [DOI] [PubMed] [Google Scholar]
- Henderson DC, Borba CP, Daley TB, Boxill R, Nguyen DD, Culhane MA, Louie P, Cather C, Eden Evins A, Freudenreich O, Taber SM, Goff DC. Dietary intake profile of patients with schizophrenia. Ann Clin Psychiatry. 2006;18:99–105. doi: 10.1080/10401230600614538. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR, Makino KK, Martin CE, Dickerson F, Boronow J, Fenton WS. Smoking, gender, and dietary influences on erythrocyte essential fatty acid composition among patients with schizophrenia or schizoaffective disorder. Biol Psychiatry. 2003;53:431–441. doi: 10.1016/s0006-3223(02)01549-4. [DOI] [PubMed] [Google Scholar]
- Horrobin DF, Manku MS, Hillman H, Iain A, Glen M. Fatty acid levels in the brains of schizophrenics and normal controls. Biol Psychiatry. 1991;30:795–805. doi: 10.1016/0006-3223(91)90235-e. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Ma K, Chang L, Bell JM, Rapoport SI. Dietary omega-3 PUFA deprivation for 15 weeks upregulates elongase and desaturase expression in rat liver but not brain. J Lipid Res. 2007;48:2463–2470. doi: 10.1194/jlr.M700315-JLR200. [DOI] [PubMed] [Google Scholar]
- Kaddurah-Daouk R, McEvoy J, Baillie RA, Lee D, Yao JK, Doraiswamy PM, Krishnan KR. Metabolomic mapping of atypical antipsychotic effects in schizophrenia. Mol Psychiatry. 2007;12:934–945. doi: 10.1038/sj.mp.4002000. [DOI] [PubMed] [Google Scholar]
- Kale A, Joshi S, Naphade N, Sapkale S, Raju MS, Pillai A, Nasrallah H, Mahadik SP. Opposite changes in predominantly docosahexaenoic acid (DHA) in cerebrospinal fluid and red blood cells from never-medicated first-episode psychotic patients. Schizophr Res. 2008;98:295–301. doi: 10.1016/j.schres.2007.09.036. [DOI] [PubMed] [Google Scholar]
- Khan MM, Evans DR, Gunna V, Scheffer RE, Parikh VV, Mahadik SP. Reduced erythrocyte membrane essential fatty acids and increased lipid peroxides in schizophrenia at the never-medicated first-episode of psychosis and after years of treatment with antipsychotics. Schizophr Res. 2002;58:1–10. doi: 10.1016/s0920-9964(01)00334-6. [DOI] [PubMed] [Google Scholar]
- Krause C, Rosewich H, Thanos M, Gartner J. Identification of novel mutations in PEX2, PEX6, PEX10, PEX12, and PEX13 in Zellweger spectrum patients. Hum Mutat. 2006;27:1157–1165. doi: 10.1002/humu.9462. [DOI] [PubMed] [Google Scholar]
- Leng GC, Horrobin DF, Fowkes FG, Smith FB, Lowe GD, Donnan PT, Ells K. Plasma essential fatty acids, cigarette smoking, and dietary antioxidants in peripheral arterial disease. A population-based case-control study. Arterioscler Thromb. 1994;14:471–478. doi: 10.1161/01.atv.14.3.471. [DOI] [PubMed] [Google Scholar]
- Leonard AE, Bobik EG, Dorado J, Kroeger PE, Chuang LT, Thurmond JM, Parker-Barnes JM, Das T, Huang YS, Mukerji P. Cloning of a human cDNA encoding a novel enzyme involved in the elongation of long-chain polyunsaturated fatty acids. Biochem J. 2000;350:765–770. [PMC free article] [PubMed] [Google Scholar]
- Levant B, Crane JF, Carlson SE. Sub-chronic antipsychotic drug treatment does not alter brain phospholipid fatty acid composition in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:728–732. doi: 10.1016/j.pnpbp.2005.11.034. [DOI] [PubMed] [Google Scholar]
- Lewis, et al. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: Schizophrenia. Am J Hum Genet. 2003;73:34–48. doi: 10.1086/376549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauri MC, Volonteri LS, Colasanti A, Fiorentini A, De Gaspari IF, Bareggi SR. Clinical pharmacokinetics of atypical antipsychotics: a critical review of the relationship between plasma concentrations and clinical response. Clin Pharmacokinet. 2007;46:359–388. doi: 10.2165/00003088-200746050-00001. [DOI] [PubMed] [Google Scholar]
- McGorry PD, Yung AR, Phillips LJ, Yuen HP, Francey S, Cosgrave EM, Germano D, Bravin J, McDonald T, Blair A, Adlard S, Jackson H. Randomized controlled trial of interventions designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Arch Gen Psychiatry. 2002;59(10):921–928. doi: 10.1001/archpsyc.59.10.921. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Jandacek R, Rider T, Tso P, Hahn CG, Richtand NM, Stanford KE. Abnormalities in the fatty acid composition of the postmortem orbitofrontal cortex of schizophrenic patients: Gender differences and partial normalization with antipsychotic medications. Schizophr Res. 2007;91:37–50. doi: 10.1016/j.schres.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Jandacek R, Rider T, Tso P, Stanford KE, Hahn CG, Richtand NM. Deficits in docosahexaenoic acid and associated elevations in the metabolism of arachidonic acid and saturated fatty acids in the postmortem orbitofrontal cortex of patients with bipolar disorder. Psychiatry Res. 2008;160:285–299. doi: 10.1016/j.psychres.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe LD, Schmitz AA, Pelka JR. Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Anal Chem. 1966;38:514–515. [Google Scholar]
- Meyer JM, Koro CE. The effects of antipsychotic therapy on serum lipids: a comprehensive review. Schizophr Res. 2004;70:1–17. doi: 10.1016/j.schres.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Myers CS, Contreras MA, Chang MC, Rapoport SI, Appel NM. Haloperidol downregulates phospholipase A(2) signaling in rat basal ganglia circuits. Brain Res. 2001;896:96–101. doi: 10.1016/s0006-8993(01)02014-5. [DOI] [PubMed] [Google Scholar]
- Nettleton JA, Katz R. n-3 long-chain polyunsaturated fatty acids in type 2 diabetes: a review. J Am Diet Assoc. 2005;105:428–440. doi: 10.1016/j.jada.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Parikh V, Khan MM, Mahadik SP. Differential effects of antipsychotics on expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. J Psychiatr Res. 2003;37:43–51. doi: 10.1016/s0022-3956(02)00048-1. [DOI] [PubMed] [Google Scholar]
- Pawlosky RJ, Hibbeln JR, Novotny JA, Salem N., Jr Physiological compartmental analysis of alpha-linolenic acid metabolism in adult humans. J Lipid Res. 2001a;42:1257–1265. [PubMed] [Google Scholar]
- Pawlosky RJ, Bacher J, Salem N. Ethanol consumption alters electroretinograms and depletes neural tissues of docosahexaenoic acid in rhesus monkeys: nutritional consequences of a low omega-3 fatty acid diet. Alcohol Clin Exp Res. 2001b;25:1758–1765. [PubMed] [Google Scholar]
- Peet M, Brind J, Ramchand CN, Shah S, Vankar GK. Two double-blind placebo-controlled pilot studies of eicosapentaenoic acid in the treatment of schizophrenia. Schizophr Res. 2001;49:243–251. doi: 10.1016/s0920-9964(00)00083-9. [DOI] [PubMed] [Google Scholar]
- Reddy RD, Keshavan MS, Yao JK. Reduced red blood cell membrane essential polyunsaturated fatty acids in first episode schizophrenia at neuroleptic-naive baseline. Schizophr Bull. 2004;30:901–911. doi: 10.1093/oxfordjournals.schbul.a007140. [DOI] [PubMed] [Google Scholar]
- Rzehak P, Heinrich J, Klopp N, Schaeffer L, Hoff S, Wolfram G, Illig T, Linseisen J. Evidence for an association between genetic variants of the fatty acid desaturase 1 fatty acid desaturase 2 ( FADS1 FADS2) gene cluster and the fatty acid composition of erythrocyte membranes. Br J Nutr. 2008;15:1–7. doi: 10.1017/S0007114508992564. [DOI] [PubMed] [Google Scholar]
- Schaeffer L, Gohlke H, Muller M, Heid IM, Palmer LJ, Kompauer I, Demmelmair H, Illig T, Koletzko B, Heinrich J. Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Hum Mol Genet. 2006;15:1745–1756. doi: 10.1093/hmg/ddl117. [DOI] [PubMed] [Google Scholar]
- Strassnig M, Singh Brar J, Ganguli R. Dietary fatty acid and antioxidant intake in community-dwelling patients suffering from schizophrenia. Schizophr Res. 2005;76:343–351. doi: 10.1016/j.schres.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Trzeciak HI, Kalaciński W, Małecki A, Kokot D. Effect of neuroleptics on phospholipase A2 activity in the brain of rats. Eur Arch Psychiatry Clin Neurosci. 1995;245(3):179–182. doi: 10.1007/BF02193092. [DOI] [PubMed] [Google Scholar]
- Vik-Mo AO, Birkenaes AB, Fernø J, Jonsdottir H, Andreassen OA, Steen VM. Increased expression of lipid biosynthesis genes in peripheral blood cells of olanzapine-treated patients. Int J Neuropsychopharmacol. 2008;11:679–684. doi: 10.1017/S1461145708008468. [DOI] [PubMed] [Google Scholar]
- Williard DE, Nwankwo JO, Kaduce TL, Harmon SD, Irons M, Moser HW, Raymond GV, Spector AA. Identification of a fatty acid delta6-desaturase deficiency in human skin fibroblasts. J Lipid Res. 2001;42(4):501–508. [PubMed] [Google Scholar]
- Xiao Y, Huang Y, Chen ZY. Distribution, depletion and recovery of docosahexaenoic acid are region-specific in rat brain. Br J Nutr. 2005;94:544–550. doi: 10.1079/bjn20051539. [DOI] [PubMed] [Google Scholar]
- Yao JK, Leonard S, Reddy RD. Membrane phospholipid abnormalities in postmortem brains from schizophrenic patients. Schizophr Res. 2000;42:7–17. doi: 10.1016/s0920-9964(99)00095-x. [DOI] [PubMed] [Google Scholar]