Abstract
Objectives: Chronic subacute inflammation is implicated in the pathogenesis of insulin resistance and type 2 diabetes. Salicylates were shown years ago to lower glucose and more recently to inhibit NF‐κB activity. Salsalate, a prodrug form of salicylate, has seen extensive clinical use and has a favorable safety profile. We studied the efficacy of salsalate in reducing glycemia and insulin resistance and potential mechanisms of action to validate NF‐κB as a potential pharmacologic target in diabetes.
Methods and Results: In open label studies, both high (4.5 g/d) and standard (3.0 g/d) doses of salsalate reduced fasting and postchallenge glucose levels after 2 weeks of treatment. Salsalate increased glucose utilization during euglycemic hyperinsulinemic clamps, by approximately 50% and 15% at the high and standard doses, respectively, and insulin clearance was decreased. Dose‐limiting tinnitus occurred only at the higher dose. In a third, double‐masked, placebo‐controlled trial, 1 month of salsalate at maximum tolerable dose (no tinnitus) improved fasting and postchallenge glucose levels. Circulating free fatty acids were reduced and adiponectin increased in all treated subjects.
Conclusions: These data demonstrate that salsalate improves in vivo glucose and lipid homeostasis, and support targeting of inflammation and NF‐κB as a therapeutic approach in type 2 diabetes.
Keywords: salicylate, salsalate, inflammation, type 2 diabetes, insulin resistance, glucose, adiponectin
Introduction
Inflammation participates in the pathogenesis of insulin resistance, type 2 diabetes (T2D), 1 , 2 , 3 and cardiovascular disease (CVD). 4 , 5 Weight gain and obesity are accompanied by activation of at least two inflammatory pathways in adipose tissue and liver, the stress kinase JNK 6 , 7 and the transcription factor NF‐kB, 8 , 9 which increases the production of proinflammatory cytokines and chemokines (e.g., TNF‐α, IL‐6, IL‐1b, resistin, and MCP‐1) and promotes the recruitment of macrophages to adipose tissue. 10 , 11 Inflammatory mediators induce insulin resistance locally in fat and liver, and systemically in skeletal muscle. The subacute chronic inflammation of obesity may therefore provide pharmacological targets for intervention.
Salicylates were shown over a century ago to lower glucoses in patients with diabetes 12 , 13 , 14 ; recent studies confirm earlier observations. 15 , 16 , 17 , 18 While nonacetylated (e.g., sodium salicylate, salsalate, and trilisate) and acetylated (aspirin) forms of salicylate are widely used to treat rheumatic pain, it is important to understand distinctions in mechanism of action. Even at low doses, aspirin effectively inhibits cyclooxygenase enzymes, Cox 1 and Cox 2, through covalent transacetylation of active site serine residues; this irreversibly inactivates the enzymes and prevents catalysis of the committed step in prostaglandin synthesis. 19 Lacking an acetyl group, nonacetylated salicylates do not modify cyclooxygenase enzymes. At significantly higher concentrations than those required for Cox inhibition, both nonacetylated salicylates and aspirin inhibit the IKKb/NF‐kB axis, 20 , 21 , 22 , 23 a pharmacologically distinct drug target.
While a 2‐week course of high‐dose (approximately 7 g/d) aspirin reduces glucose and lipid levels and improves insulin sensitivity in patients with diabetes, 24 prolonged exposure to such high doses of aspirin would have unacceptable side effects, especially potentially serious gastrointestinal bleeding. Nonacetylated salicylates do not modify Cox enzymes, inhibit platelets, or prolong bleeding time, 25 and are therefore not associated with increased bleeding risk. 26 , 27 , 28 Salsalate (Disalsid™), a dimeric prodrug comprising two esterified salicylate moieties, is used to treat patients with rheumatologic conditions. Salsalate is advantageous over sodium salicylate because it is insoluble at the acid pH of the stomach and passes suspended but undissolved into the small intestine, sparing the gastric mucosa direct contact. 26 , 27 , 28 Blood salicylate levels are nonetheless comparable to those following administration of sodium salicylate. Furthermore, salsalate is generic and inexpensive, so established safety and efficacy in diabetes would have potential health–economic benefit worldwide. In proof‐of‐concept studies, we assessed the effects of targeting inflammation with salsalate to lower glycemia in patients with type 2 diabetes.
Experimental Methods
Study subjects and design
Protocols were approved by the Institutional Review Boards; informed consent was obtained from all participants. Subjects without clear documentation of diabetes underwent a 75‐g oral glucose tolerance test. 29 Three distinct studies were conducted sequentially utilizing three distinct patient cohorts; subjects were not randomized by study. The first two studies used an open‐label trial design of 2‐week duration, one dosed at 4.5 g/d salsalate (1.5 g thrice daily) to match the dosage and duration used historically to improve glycosuria 12 , 17 , 24 and the second at 3 g/d (1.5 g twice daily) as recommended to minimize side effects (Caraco Pharmaceuticals). The third study utilized a randomized double‐masked, placebo‐controlled trial design of 4‐week duration to evaluate efficacy at maximum tolerable dose.
Trials 1 and 2
Antihyperglycemic medications were discontinued 1 month prior to baseline. Subjects were instructed to monitor fasting blood glucose levels and with symptoms of hyperglycemia or hypoglycemia, and to avoid changing dietary or exercise habits. Subjects received misoprostol (200 μg orally four times daily), beginning 3 days prior and throughout the 2‐week treatment period, to compare results to those from the previously conducted high‐dose aspirin study. 24
IVGTTs and hyperinsulinemic‐euglycemic clamps
Frequently sampled intravenous glucose tolerance tests (FSIGTT) and hyperinsulinemic‐euglycemic clamp studies were performed following an overnight fast, prior to and after 14 days of salsalate. In brief, blood samples were collected at baseline and 2, 3, 4, 5, 6, 8, 10, 12, 14, 18, 20, 30, 40, 50, 60, 70, 80, 90, 100, 120, 140, 160, and 180 minutes following intravenous administration of dextrose solution (0.3 g/kg). Acute insulin response to glucose (AIRg) was calculated as area under the curve (AUC) for insulin and C‐peptide during the first 10 minutes following glucose administration (corrected for baseline levels). Second phase insulin or C‐peptide production was determined as AUC for insulin or C‐peptide from 10 to 180 minutes.
The following day, after an overnight fast subjects underwent hyperinsulinemic‐euglycemic clamps. Intravenous lines were placed for infusion of test substances and collection of blood samples. The hand bearing the blood‐sampling catheter was placed into a heated box at 65°C to “arterialize” venous blood. 30 After collection of baseline samples for hormones and substrates, hyperinsulinemia was induced by primed (480 mU/m2/min × 5 min and 240 mU/m2/min × 5 min) followed by continuous (120 mU/m2/min × 240 min) infusion of insulin. 24 Euglycemia was maintained at 80 mg/dL by monitoring plasma glucose at 5‐minute intervals and adjusting rates of glucose (20%) infusion. 31
6,6‐[2H2]‐Glucose was infused during hyperinsulinemic‐euglycemic clamps to evaluate endogenous glucose production. A priming dose (181 mg/m2) was administered 4 hours before the first infusion of insulin and a continuous infusion (1.81 mg/m2/min) was maintained throughout the study (480 minutes). The variable rate glucose infusion solutions were also enriched with 2.5% of 6,6‐[2H2]‐glucose. 32 Hepatic glucose production (i.e., rate of glucose appearance) was calculated by dividing the 6,6‐[2H2]‐glucose infusion rate by the measured steady‐state level of deuterated glucose achieved during the last 20 minutes of the basal period and during hyperinsulinemia, using the formula: rate of endogenous glucose production (EGP, mg/kg/min) = isotope infusion rate × [enrichmentinfusate/enrichmentplasma) – 1]. Insulin suppression of endogenous glucose production (clampEGP) cEGP = GIR × [enrichmentinfusate/enrichmentplasma) – 1], with GIR being the mean rate of infusion of exogenous glucose during minutes 210 to 240 of the clamp. Insulin sensitivity was expressed as the metabolic rate (M) of glucose uptake at steady‐state hyperinsulinemia, normalized for mass (kg) of the subject. Insulin clearance rate (mL/min) = insulin infusion rate (pmol/min)/plasma insulin concentration (pmol/mL).
Indirect calorimetry
Oxidative versus nonoxidative glucose disposal was assessed with continuous indirect calorimetry (Sensormedic, DeltaTrac) at baseline and during the last 60 minutes of the hyperinsulinemic clamp. Whole body O2 consumption and CO2 production were measured and the respiratory quotient calculated. A timed urine collection was performed to measure the nitrogen excretion rate as an index of protein oxidation. Carbohydrate, lipid, and protein oxidation rates and energy expenditure were calculated from O2 consumption, CO2production, and urinary nitrogen excretion data. Respiratory quotient (RQ) and resting energy expenditure (REE) were calculated using the equations RQ = VCO2/VO2, with VO2= oxygen uptake (mL/min) and VCO2= carbon dioxide output (mL/min) and REE = 1.44 × (3.9 [VO2] + 1.1 [VCO2]) according to the modified Weir equation. 33
Trial 3
The third group of diabetic subjects participated in a double‐mask, placebo‐controlled parallel study design of 1‐month duration. At entry, participants were treated by lifestyle modification alone or in conjunction with either sulfonylurea or biguanide therapy (stably dosed for >4 weeks and continued for the trial duration). After an overnight fast, subjects received a mixed meal tolerance test with blood samples collected at baseline and every 30 minutes for 2 hours (8 oz. Boost™, Novartis, Freemont, MI, USA; 240 calories; 41 g carbohydrate, 10 g protein, 4 g fat). Subjects were then randomized to receive placebo or salsalate (4.0 g/d), orally, divided twice daily. Misoprostol was not administered to subjects in trial 3. Fasting laboratory profiles were assessed after 2 and 4 weeks, and mixed meal tolerance tests were repeated after 4 weeks. In the event of tinnitus, drug or placebo was held for one dose and resumed with dose reduced by 500 mg/d.
Assays
Plasma glucose was measured with a Beckman Instrument Glucose Analyzer‐2 (Beckman Coulter, Fullerton, CA, USA). Immunoassays were performed in duplicate by commercial assay including RIA for insulin and C‐peptide (Diagnostic Systems, Webster, TX, USA), adiponectin (LINCO, a division of Millipore, Billerica, MA, USA), and ELISA for free fatty acids (WAKO, Richmond, VA, USA), IL‐6 (R&D, Minneapolis, MN, USA) and sCD40L (Bender Medsystems, Burlingame, CA, USA) and nitrite (Cayman Chemicals, Ann Arbor, MI, USA). hsCRP was analyzed by immunoturbidometry (WAKO). Glycated albumin was evaluated by Hitachi 911 lipid and protein analyzer and kits from AsahiKasei (Tokyo, Japan). Deuterated glucose (6,6‐[2H2]‐glucose) enrichment was measured by gas chromatography‐mass spectrometry analysis. 32 NF‐kB DNA binding activity was assessed by ELISA using neutra‐avidin plates (Pierce Biotechnology, Rockford, IL, USA) preimmobilized with 5’‐biotin‐labeled, double‐stranded NF‐kB binding oligonucleotides. Isolated peripheral blood mononuclear cells (PBMC) were lysed with Passive Lysis Buffer (Promega, Madison, WI, USA). DNA‐bound NF‐κB was detected using anti‐p65 antibody (Santa Cruz sc‐372, Santa Cruz Biotechnology, Santa Cruz, CA, USA), and quantified using HRP‐conjugated antirabbit IgG.
Statistical analyses
Subject characteristics are presented as mean ± standard deviation, data as mean ± standard error. Statistical analyses included paired (pre to post) and unpaired (salsalate to placebo) Student's t‐tests and analysis of variance (ANOVA, StatView); results are considered significant with two‐tailed p‐values <0.05. Bonferroni corrections for multiple endpoint testing were not performed in these proof‐of‐principle studies.
Results
Trials 1 and 2
Since the trials were conducted sequentially, subjects were not randomized and baseline characteristics for trials 1 and 2 differed (Table 1). Of note, the seven subjects dosed at 4.5 g/d (trial 1) differed from the nine subjects dosed at 3.0 g/d (trial 2) by having lower fasting glucose and HbA1c and by the inclusion in trial 1 of two persons with IGT.
Table 1.
Subject characteristics for the open label 4.5 g/d and 3.0 g/d trials (mean ± SD).
| Salsalate dose | 4.5 (g/d) | 3.0 (g/d) |
|---|---|---|
| Glucose Tolerance | 2 IGT, 5 T2D | 9 T2D |
| Gender | 5F, 2M | 6F, 3M |
| Age (year) | 49 ± 9 | 51 ± 3 |
| BMI (kg/m2) | 32 ± 6 | 34 ± 3 |
| FBS (mmol/L) | 6.2 ± 2.4 | 11.1 ± 1.3 |
| HbA1c (%) | 6.3 ± 1.7 | 8.1 ± 0.5 |
F = female; M = male; BMI = body mass index; FBG = fasting blood glucose (mg/dL × 0.0555 = mmol/L).
Fasting glucose levels fell by approximately 1.1 mmol/L (20 mg/dL) in both 4.5 and 3.0 g/d cohorts (Figure 1A). For the 4.5 g/d group, this represents a substantial reduction of 19% (6.2 ± 0.9 vs. 5.1 ± 0.4 mmol/L [112 ± 16 vs. 91 ± 7 mg/dL], p < 0.03). Reductions for the 3.0 g/d trial were more modest at 9% (11.2 ± 1.3 vs. 10.2 ± 1.1 mmol/L [201 ± 23 vs. 183 ± 20 mg/dL], p < 0.05). Similarly, there was a 19% reduction in glycated albumin at 4.5 g/d (13.3 ± 2.2 vs. 10.7 ± 1.6 %, p= 0.005) and a trend at 3.0 g/d (21.4 ± 1.6 vs. 20.3 ± 1.7%, p= 0.07) (Figure 1J). While one cannot directly compare results between the two trials because patient characteristics and their overall glycemic control differ, it is important to note it is more difficult to improve glycemia within the near‐normal range (e.g., trial 1) than when glucoses are less well controlled (trial 2). There was no clinical hypoglycemia, or change in body weight (Table 2).
Figure 1.
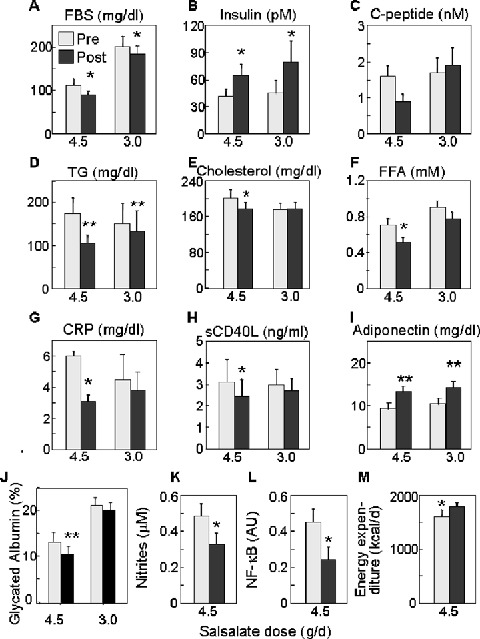
Metabolic parameters in subjects prior to treatment (pre, grey boxes) and after 2‐week treatment with 4.5 g/d (n= 7) or 3.0 g/d (n= 9) salsalate (post, black boxes). Fasting data are displayed for (A) blood glucose (mg/dL × 0.0555 = mmol/L), (B) insulin (pM/6.945 =μU/mL), (C) C‐peptide (nM/0.333 = ng/mL), (D) triglyceride (TG, mg/mL × 0.0113 = mmol/L), (E) total cholesterol (mg/dL × 0.0259 = mmol/L), (F) free fatty acids (FFA, mM), (G) C‐reactive protein (CRP, mg/dL), (H) sCD40L (ng/mL), (I) adiponectin (mg/dL), (J) glycated albumin (%), (K) nitrites (μM), (L) NF‐κB activity (arbitrary units), and (M) energy expenditure (kcal/d). Data represent mean ± SEM; *p < 0.05, **p < 0.005.
Table 2.
Indices for fasting subjects studied in the open label trials before salsalate therapy (pre) and after 2‐week treatment (post) (mean ± SEM).
| Salsalate 4.5 (g/d) | Salsalate 3.0 (g/d) | |||||
|---|---|---|---|---|---|---|
| Pre | Post | p‐value | Pre | Post | p‐value | |
| Weight (kg) | 88.6 ± 8.3 | 88.4 ± 8.4 | 0.8 | 93.7 ± 7.2 | 94.2 ± 7.1 | 0.2 |
| HDL (mmol/L) | ‐‐1.2 ± 0.1 | 1.1 ± 0.1 | 0.01 | 1.1 ± 0.1 | 1.1 ± 0.1 | 0.6 |
| LDL (mmol/L) | ‐‐3.2 ± 0.5 | 3.0 ± 0.4 | 0.2 | 2.7 ± 0.3 | 2.9 ± 0.3 | 0.2 |
| SBP (mm Hg) | 135 ± 4 | 128 ± 5 | 0.2 | 135 ± 6 | 125 ± 7 | 0.1 |
| DBP (mm Hg) | ‐75 ± 2 | 71 ± 4 | 0.3 | 77 ± 4 | 71 ± 5 | 0.02 |
| AST (U/L) | ‐25 ± 3 | 24 ± 4 | 0.6 | 20 ± 2 | 20 ± 2 | 0.7 |
| ALT (U/L) | ‐21 ± 1 | 24 ± 2 | 0.2 | 26 ± 4 | 22 ± 3 | 0.01 |
| Cr (μmol/L) | ‐67 ± 5 | 77 ± 5 | 0.005 | 68 ± 6 | 72 ± 6 | 0.04 |
HDL and LDL mg/dL × 0.0259 = mmol/L; Cr = creatinine mg/dL × 88.4 =μmol/L.
Improved glycemia was accompanied by increases in fasting insulin levels in both cohorts (44 ± 8 vs. 67 ± 14 pM [6.3 ± 1.1 vs. 9.6 ± 2.0 μU/mL], p= 0.03 and 47 ± 15 vs. 83 ± 24 pM [6.7 ± 2.2 vs. 11.9 ± 3.5 μU/mL], p= 0.05, at 4.5 g/d and 3.0 g/d respectively) (Figure 1B). This effect could be due to augmented insulin secretion, decreased clearance, or both. However, fasting C‐peptide (0.52 ± 0.13 vs. 0.30 ± 0.06 nM [1.6 ± 0.4 vs. 0.9 ± 0.2 ng/mL], p= 0.06) tended to be lower in subjects receiving 4.5 g/d salsalate (Figure 1C), consistent with diminished insulin clearance demonstrated previously following high‐dose aspirin 24 and during insulin clamp studies described below. Fasting C‐peptide concentrations were unchanged in the cohort treated with 3.0 g/d salsalate.
Salsalate therapy was also accompanied by decreases in fasting triglycerides (Figure 1D), 40% (2.0 ± 0.4 vs. 1.2 ± 0.2 mmol/L [174 ± 37 vs. 105 ± 20 mg/dL], p= 0.007) at 4.5 g/d and 11% (1.7 ± 0.5 vs. 1.5 ± 0.5 mmol/L [150 ± 47 vs. 133 ± 46 mg/dL], p= 0.007) at 3.0 g/d. Other changes in fasting lipids were seen only at the higher dose, including reductions in total cholesterol by 12% (5.2 ± 0.5 vs. 4.6 ± 0.4 mmol/L [201 ± 19 vs. 177 ± 14 mg/dL], p= 0.04) (Figure 1E) and nonesterified free fatty acids (FFA, Figure 1F) by 28% (0.71 ± 0.05 vs. 0.51 ± 0.05 mM, p= 0.02) with major fatty acid subtypes reduced equivalently (data not shown). While there was a reduction in HDL cholesterol (1.2 ± 0.1 vs. 1.1 ± 0.1 mmol/L [47 ± 3 vs. 43 ± 3 mg/dL], p= 0.01), parallel but nonsignificant reductions in LDL cholesterol (3.2 ± 0.5 vs. 3.0 ± 0.3 mmol/L [124 ± 18 vs. 114 ± 13 mg/dL], p= 0.2) resulted in no overall change in HDL/LDL cholesterol ratio.
Although blood pressure was already well controlled in these patients, there was an 8% drop in diastolic pressure in the group receiving 3.0 g/d (77 ± 4 vs. 71 ± 5 mm Hg, p= 0.02); the downward trends in systolic blood pressure in both groups and diastolic blood pressure at 4.5 g/d did not reach statistical significance (Table 2). Alanine aminotransferase (ALT) was reduced by 16% (26 ± 4 vs. 22 ± 3 mg/dL, p= 0.01) with 3.0 g/d, whereas other liver enzyme measures were unchanged in these small trials (Table 2).
Euglycemic‐hyperinsulinemic clamps
In the 4.5 g/d salsalate treatment cohort, exogenous glucose infusion rates (GIR) during euglycemic‐hyperinsulinemic clamps increased by 44% (p= 0.002), with corresponding 43% improvement in glucose disposal (4.2 ± 0.7 vs. 6.0 ± 0.9 mg/kg/min, p= 0.007) (Figure 2A and 2B). Indirect calorimetry indicates that enhanced glucose utilization is due primarily to improvements in nonoxidative glucose disposal (i.e., glycogen synthesis) (1.9 ± 0.5 vs. 3.2 ± 0.5 mg/kg/min, p= 0.006) without change in oxidative glucose disposal (2.2 ± 0.2 vs. 2.6 ± 0.4 mg/kg/min, p= 0.2) (Figure 2C). Lower fasting glucose levels could not be ascribed to altered endogenous glucose production rates, as this was unchanged at baseline and during hyperinsulinemia. Interestingly, at the higher salsalate dose resting energy expenditure measured by indirect calorimetry increased by 11% (Figure 1M; 1605 ± 114 vs. 1785 ± 81 kcal/day, p= 0.007), which could contribute to lower fasting glucose levels.
Figure 2.
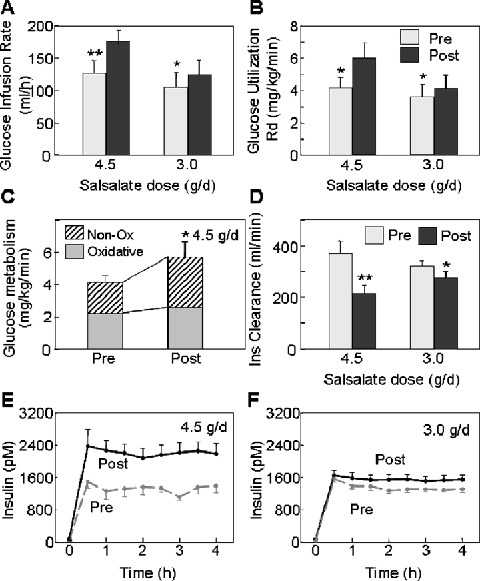
Euglycemic‐hyperinsulinemic clamp data are shown for subjects treated for 2 weeks with 4.5 g/d or 3.0 g/d salsalate. (A) Exogenous glucose infusion rates (GIR) and (B) rates of total body glucose utilization (Rd) were determined during clamps before (grey boxes) and after (black boxes) 2‐week treatment with 4.5 g/d or 3.0 g/d salsalate. (C) Rates of oxidative (grey boxes) and nonoxidative glucose metabolism (non‐ox, hatched boxes) are shown before and after 2‐week treatment with 4.5 g/d salsalsate. (D) Insulin clearance rates are shown at baseline (pre, grey boxes) and after 2‐week treatment (post, black boxes) with 4.5 g/d or 3.0 g/d salsalate. (E, F) Insulin levels achieved during the clamps (pM/6.945 =μU/mL) are before (dashed grey lines) or after (solid black lines) 4.5 g/d and 3.0 g/d salsalate, respectively. Data represent mean ± SEM; A, *p= 0.02, **p= 0.002. B, *p < 0.009. C, *p= 0.006. D, **p= 0.01, *p= 0.05. E, ANOVA, p < 0.005. F, ANOVA, p < 0.02.
Salicylates have previously been shown to reduce insulin clearance. 24 Similarly, insulin levels achieved during the clamp following salsalate administration were higher than those at baseline (1267 ± 109 vs. 2209 ± 230 pM [182 ± 16 vs. 318 ± 33 μU/mL], p= 0.01) (Figure 2E), despite identical insulin infusion rates. This was due to a 40% reduction (p= 0.007) in insulin clearance after salsalate (Figure 2D). Higher circulating insulin concentrations are expected to participate in the antihyperglycemic effects of salsalate and other salicylates.
Subjects receiving 3.0 g/d salsalate also had significantly improved glucose utilization during euglycemic‐hyperinsulinemic clamp analyses, albeit a more modest increment of 15% (3.6 ± 0.8 vs. 4.2 ± 0.8 mg/kg/min, p= 0.003) (Figure 2B). Nonoxidative glucose disposal tended to increase, although this did not reach significance at the lower dose (p= 0.06, data not shown). Again, insulin levels achieved during steady state in the clamps post‐salsalate were 18% higher (1317 ± 72 vs. 1555 ± 84 pM [190 ± 10 vs. 224 ± 12 μU/mL], p= 0.05) (Figure 2F), which could largely be attributed to a comparable 14% reduction in insulin clearance (p= 0.04) (Figure 2D).
Intravenous glucose tolerance testing
To evaluate effects of salsalate on β‐cell function, the acute insulin secretory response was assessed during IVGTT. Glucose concentrations were significantly lower in subjects treated with 4.5 g/d salsalate compared to baseline (Figure 3A) (p < 0.01, ANOVA). During the first 10 minutes (Figure 3C), insulin concentrations increased by 72% (ΔAUC, 865 ± 521 vs. 2196 ± 1008 pM·min, p= 0.005). During the same period, there was a 50% increase in C‐peptide levels (Figure 3E; ΔAUC, 5.7 ± 3.6 vs. 10.6 ± 4.8 ng/mL·min, p= 0.04), consistent with this being a true improvement in the first phase insulin release. Although insulin levels were also higher over the second phase of insulin secretion (10–180 minutes), C‐peptide levels were not increased during this later phase (Figure 3F), suggesting an important contribution of altered insulin clearance, as opposed to enhanced second phase secretion at the later times. While glycemia was marginally lower (ANOVA, p= 0.09) following salsalate at 3.0 g/d dosing (Figure 3B), and insulin levels were higher (ANOVA, p < 0.001), neither acute insulin secretory response expressed as change from baseline (ΔAUC) nor C‐peptide response from 0 to 10 minutes (Figure 3E) or 10 to 180 minutes (Figure 3F) was significantly altered at the lower dose.
Figure 3.
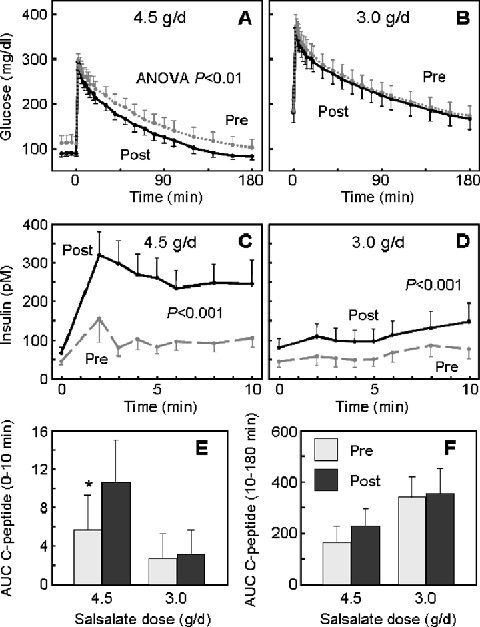
Intravenous glucose tolerance tests (IVGTT). (A, B) Glucose excursions (mg/dL × 0.0555 = mmol/L) and (C, D) insulin responses (pM/6.945 =μU/mL) during IVGTTs were before (pre, dashed grey lines) or after (post, solid black lines) 2‐week treatment with (A, C) 4.5 g/d or (B, D) 3.0 g/d salsalate. (E) The acute (0–10 minutes) and (F) delayed (10–180 minutes) insulin secretory responses to glucose, calculated as the area under the curve (AUC) C‐peptide, at baseline (pre, grey boxes) and after 2‐week treatment (post, black boxes) with 4.5 g/d or 3.0 g/d salsalate. Data represent mean ± SEM; *p= 0.04.
Inflammation
Since salsalate's primary mechanism of action is antiinflammatory, we also looked at more conventional parameters of inflammation. CRP was reduced approximately 50% (6.0 ± 2.7 vs. 3.1 ± 1.7 mg/dL, p < 0.05) in subjects treated with salsalate at 4.5 g/d, but was not significantly lowered in the 3.0 g/d cohort (4.5 ± 1.6 vs. 3.3 ± 1.2 mg/dL, p= 0.4) (Figure 1G). Soluble CD40L, another circulating marker of inflammation, was decreased by 22% (3.1 ± 1.0 vs. 2.4 ± 0.8 ng/dL, p < 0.05) at 4.5 g/d, but was not significantly lowered in the 3.0 g/d group (3.0 ± 0.7 vs. 2.7 ± 0.6 mg/dL, p= 0.4) (Figure 1H). Adiponectin also increased by 40% in the 4.5 g/d group (9.3 ± 2.1 vs. 13.0 ± 2.5 mg/mL, p= 0.008) and 35% in the 3.0 g/d group (10.7 ± 1.8 vs. 14.4 ± 2.2 mg/mL, p= 0.002) (Figure 1I). Additional markers and mediators of inflammation were assessed only at the higher dose where the clinical response was greater. Both interleukin‐6 (IL‐6) (2.7 ± 0.2 vs. 1.8 ± 0.2 pg/mL, p= 0.1) and white blood cell counts (6.2 ± 0.8 vs. 5.3 ± 0.4%, p= 0.06) tended to decrease, although these changes did not reach statistical significance in this small trial. Fasting serum nitrites decreased by 33% during salsalate therapy (0.49 ± 0.05 vs. 0.33 ± 0.04 μM, p= 0.02) (Figure 1K).
To evaluate salsalate effects on NF‐κB more directly, we looked at its DNA binding capacity in homogenates isolated from circulating PBMCs. NF‐κB activity decreased after drug treatment by about 65% (p= 0.04) (Figure 1L). The effect was reversible and began to return toward baseline levels 1 week after discontinuing the drug (data not shown). While it is unknown whether PBMCs actively participate in the pathogenesis of insulin resistance, some studies suggest that they might do. 34 , 35 At a minimum, circulating leukocytes provide a readily accessible source of cells for assessing in vivo NF‐κB activity.
Safety and tolerability
Six of the seven subjects treated with 4.5 g/d salsalate experienced tinnitus, and two required dose reductions due to tinnitus or headache. These are well known and expected side effects of high‐dose salicylate. Symptoms improved upon dose reduction and all subjects completed the study. Serum salicylate levels were 0.21 ± 0.01 and 0.15 ± 0.01 mmol/L [28.4 ± 2.0 and 19.0 ± 2.0 mg/dL], at 1 and 2 weeks of treatment at 4.5 g/d, respectively. Lower levels at the second week are due, at least in part, to reduced doses. Salicylate levels seen in the 4.5 g/d trial were comparable to those achieved in the previous study using high‐dose (approximately 7 g/d) aspirin (27 mg/dL). 24 At the standard salsalate dose of 3.0 g/d, no subject experienced tinnitus or required dose reduction, but clinical responses were modest. Serum salicylate levels were lower as well, 0.4 ± 0.01 mmol/L [5.4 ± 1.3 mg/dL], which would be considered subtherapeutic by conventional rheumatologic standards.
Only the high dose of salsalate was associated with a tendency toward increased anion gap. Both doses were associated with small but significant increases in serum creatinine (Table 2), although this remained in the normal range and returned to baseline 1 week after discontinuation of salsalate.
Trial 3
While our trials demonstrated improved glucose metabolism at both 4.5 and 3.0 g/d doses, side effect of tinnitus would likely limit clinical applicability at the higher dose and efficacy was modest at the lower, standard dose. Thus, we sought to examine evidence of clinical efficacy in type 2 diabetes at the maximum tolerable dose. As participation in any clinical trial could alter glycemia by subtle changes in lifestyle, we employed a placebo‐controlled, double‐masked design in this third clinical trial, conducted for 4 weeks in contrast to the previous 2‐week trials, to begin to assess durability, as well. Subjects randomized to active and treated groups were generally similar at baseline, although cholesterol levels were modestly higher in the placebo‐treated group (Table 3).
Table 3.
Subject characteristics for the placebo‐controlled trial of salsalate at maximum tolerated dose (mean ± SD).
| Salsalate | Placebo | p‐value | |
|---|---|---|---|
| Age (year) | 51 ± 12 | 54 ± 8 | 0.6 |
| Gender | 3 M, 5 F | 5 M, 4 F | 0.5 |
| BMI (kg/m2) | 32.5 ± 6.4 | 31.5 ± 4.9 | 0.7 |
| FBS (mmol/L) | 7.5 ± 1.0 | 7.0 ± 1.4 | 0.4 |
| HbA1c (%) | 7.1 ± 1.2 | 6.7 ± 0.5 | 0.3 |
| SBP (mm Hg) | 125 ± 12 | 132 ± 15 | 0.3 |
| DBP (mm Hg) | 71 ± 4 | 78 ± 10 | 0.1 |
| Chol (mmol/L) | 3.9 ± 0.8 | 4.7 ± 0.7 | 0.04 |
| Treatment | 5 Met, 1 SFU, 2 LS | 6 Met, 1 SFU, 2 LS | NS |
F = female; M = male; BMI = body mass index; FBG = fasting blood glucose; Met = metformin; SFU = sulfonylurea; LS = lifestyle; Chol = cholesterol (mg/dL × 0.0259 = mmol/L); NS = not significant.
Five of the eight subjects randomized to salsalate tolerated the initial 4.0 g/d dose; three developed mild tinnitus that resolved with reduction to 3.5 g/d. Blood salicylate levels were in the range considered to be therapeutic, 0.15 ± 0.03 mmol/L [21 ± 4 mg/dL] at 2 weeks and 0.09 ± 0.02 mmol/L [13 ± 3 mg/dL] at 4‐week treatment. Neither serum creatinine nor anion gap changed in the 4‐week trial, although systolic blood pressure was 8% higher in the treatment group (125 ± 4 vs. 135 ± 6, p= 0.01) and unchanged with placebo.
The lowering of fasting glucose (1.0 mmol/L [18 mg/dL]; 13%) that was present following 2 weeks was sustained for 4‐week salsalate (Figure 4A: 7.5 ± 0.3 vs. 6.4 ± 0.3 mmol/L [136 ± 6 vs. 116 ± 5 mg/dL], p= 0.002); placebo was ineffective (Figure 4B: 7.0 ± 0.4 vs. 7.1 ± 0.3 mmol/L [127 ± 8 vs. 128 ± 6 mg/dL], p= 0.8). The change from baseline in a between‐group comparison was highly significant (p= 0.003) (Figure 4C). Fasting insulin increased by 77% following 4‐week salsalate (108 ± 22 vs. 193 ± 22 pM [15.6 ± 3.2 vs. 27.8 ± 3.2 μU/mL], p= 0.007), whereas fasting C‐peptide was reduced by 28% (1.6 ± 0.25 vs. 1.1 ± 0.21 nM [4.7 ± 0.7 vs. 3.4 ± 0.6 ng/mL], p= 0.002). Neither insulin (132 ± 20 vs. 131 ± 14 pM [19 ± 3 vs. 19 ± 2 μU/mL], p= 0.4) nor C‐peptide (1.9 ± 0.4 vs. 1.5 ± 0.2 nM [5.6 ± 1.3 vs. 4.4 ± 0.5 ng/mL], p= 0.4) was changed with placebo.
Figure 4.
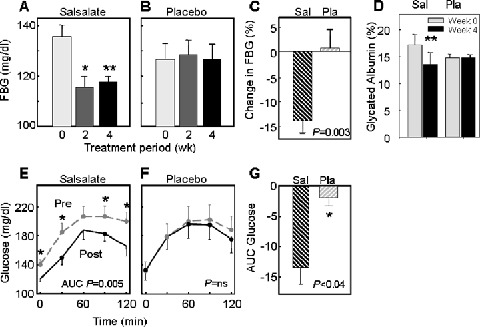
Metabolic parameters of subjects treated with salsalate at highest tolerated dose. (A, B) Fasting glucose levels (mg/dL × 0.0555 = mmol/L) are at baseline and after 2 or 4 weeks of treatment or placebo; (C) change in fasting glucose values are for the salsalate (Sal) and placebo (Pla) groups at 4 weeks. (D) Glycated hemoglobin (%) is shown at baseline and after 4‐week salsalate (Sal) or placebo (Pla). (E, F) Glucose excursions following a liquid meal are before (pre, dashed grey lines) or after (post, solid black lines) 4‐week salsalate or placebo. (G) Changes in glucose (area under the curve, AUC) following liquid meal for salsalate (Sal) and placebo (Pla). Data represent mean ± SEM. *p < 0.04, **p= 0.002
Salsalate also reduced the glycemic response to a mixed meal, with differences in the fasting measurements sustained throughout the postprandial period (Figure 4D). Since placebo did not alter fasting or postprandial blood glucose concentrations (Figure 4E), there was a significant difference in ΔAUC glucose values (p= 0.006) (Figure 4F). Similarly, glycated albumin was reduced by 21% following salsalate (17.2 ± 1.9 vs. 13.5 ± 2.2%, p= 0.0001) but unchanged following placebo (14.8 ± 0.6 vs. 14.8 ± 0.5%, p= 0.8). While 1 month is a short time to demonstrate changes in glycohemoglobin, a significant reduction was observed in the 4‐week salsalate treatment group (HbA1c: 7.1 ± 0.4% vs. 6.8 ± 0.4%, p= 0.03) that was not present in the placebo group (6.7 ± 0.2% vs. 6.5 ± 0.2%, p= 0.2).
In the placebo‐controlled trial, salsalate was also associated with a 33% reduction in fasting free fatty acid concentrations (0.57 ± 0.07 vs. 0.38 ± 0.06 mM, p= 0.0009), which was sustained following a mixed meal challenge (Figure 5A). These metabolic changes were associated with a 66% increase in plasma insulin levels (Figure 5C; ΔAUC, p= 0.004) and a 19% reduction in C‐peptide concentration (Figure 5E; ΔAUC, p= 0.04), consistent with reduced insulin clearance and an improvement in insulin sensitivity leading to a reduction in insulin secretion as assessed by C‐peptide concentrations. Plasma glucose, free fatty acids, insulin, and C‐peptide concentrations were all unchanged from baseline during mixed meal tolerance testing of the placebo group (4, 5).
Figure 5.
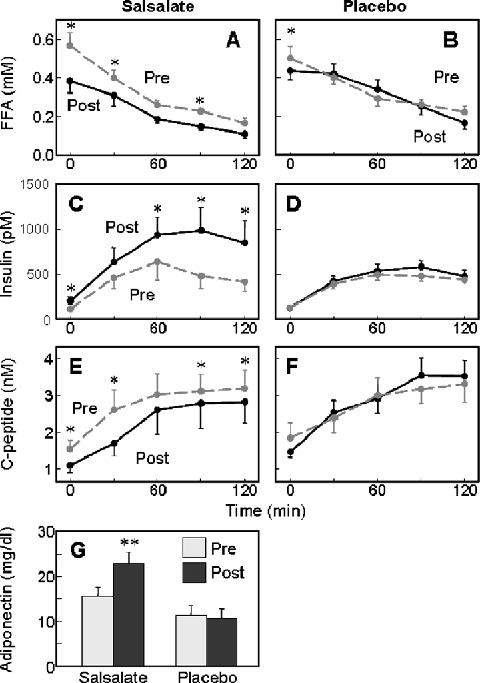
Metabolic parameters. Responses to the liquid meal for subjects treated with (A, C, E) highest tolerated salsalate dose or (B, D, F) placebo are before (pre, dashed grey lines) or after (post, solid black lines) 4‐week treatment. (G) Adiponectin (mg/dL) levels were determined before and after 4‐week maximum tolerated dose salsalate or placebo, **p= 0.001. Insulin, pm/6.945 =μU/mL; C‐peptide nM/0.333 = ng/mL. Data represent mean ± SEM.
CRP levels did not change significantly during this third small trial in either active or placebo treatment groups. However, adiponectin levels increased by 46% in the salsalate group (15.5 ± 1.9 vs. 22.7 ± 2.5 mg/mL, p= 0.001) with no change following placebo (11.2 ± 2.2 vs. 10.6 ± 2.0, p= 0.3), associated with a highly significant change from baseline in the between group comparison (Figure 5G, p < 0.0003).
Discussion
The purpose of the current set of clinical trials was two‐fold, first to translate previous preclinical findings suggesting the IKKb/NF‐κB pathway is a new pharmacological target for treatment and prevention in insulin resistance and type 2 diabetes, and second to provide preliminary assessments of efficacy, tolerability, and durability of salsalate as a new treatment for insulin resistance and type 2 diabetes.
The three small‐scale trials consistently showed clinical efficacy for the antiinflammatory drug salsalate in the treatment of type 2 diabetes. The benefits include about 10% to 20% reductions in fasting and postchallenge hyperglycemia, albeit with different magnitudes at different doses in patients with differing severity of dysglycemia. Two‐week durations are too short to expect significant changes in HbA1c, but consistent with the shorter half‐life of albumin, glycated albumin improved within 2 weeks in the 4 and 4.5 g/d dose studies and tended to improve with 3.0 g/d. Even a 4‐week trial duration is too short to expect a change in HbA1c, but a decrease of 0.3% in HbA1c and a highly significant drop in glycated albumin also supported sustained improvements in glycemic control in the 4‐week, highest tolerated dose trial. Impressive reductions were also seen in circulating levels of triglyceride and free fatty acids, particularly at the higher salsalate doses, consistent with previous findings in rodents treated with high‐dose sodium salicylate or aspirin 8 , 9 and humans treated with high‐dose aspirin. 24 This is an important result as patients with diabetes often have elevated circulating lipids that potentially contribute to both the pathophysiology of type 2 diabetes and its associated complications.
Glucose utilization improved following both 4.5 and 3.0 g/d doses of salsalate, although the 50% improvement at the higher dose was more dramatic. Nevertheless, the 15% increase in glucose utilization seen with the lower dose could have meaningful clinical benefit with prolonged use. Two mechanisms may contribute to improved glucose utilization, increased insulin concentration, as demonstrated, and insulin sensitization. Increased insulin concentration likely contributes to lower glycemia and improved metabolism. Improvements in insulin sensitivity were more specifically addressed in the previous study using high‐dose aspirin in which insulin levels during euglycemic‐hyperglycemic clamps were equalized. 24 Both the previous study and our current study show that high‐dose salicylates reduce insulin clearance, explaining higher insulin levels. Pharmacokinetics of salicylates differ in rodents and increased insulin levels are not seen, thus the mechanism of reduced insulin clearance in humans cannot be easily explored in rodent models. In the aspirin study, insulin sensitization persisted when insulin levels were matched. 24 Other potential physiologic mechanisms for improving glycemia with salsalate include improvements in resting energy expenditure and nonoxidative glucose metabolism, although these mechanisms were significant only at the higher dose in these small trials. Improved nonoxidative glucose metabolism is often seen with other insulin‐sensitizing agents, including metformin and thiazolidendiones. However, increased resting energy expenditure is not generally seen with antidiabetic drugs and has potential clinical importance in diabetes and obesity.
Insulin and C‐peptide concentrations increased during the first 10 minutes following intravenous glucose challenge with 4.5 g salsalate, consistent with improvement in the acute insulin secretory response to glucose and with previous studies suggesting favorable effects of salicylates on beta cell function. 36 , 37 However, in the 2 hours following mixed meal stimulation there were simultaneous increases in insulin and decreases in C‐peptide concentration, suggesting altered insulin clearance and/or insulin sensitization confounds interpretation at these later times. Reduced free fatty acid levels could contribute to benefit in insulin secretion and/or glucose utilization.
Given that salsalate is a nonsteroidal antiinflammatory drug, albeit an unusual one that inhibits NF‐κB and not the cyclooxygenases, it is encouraging to have found changes in circulating levels of inflammatory mediators and markers of cardiovascular and diabetes risk. There may be both direct and indirect antiinflammatory mechanisms as insulin itself may have antiinflammatory properties and insulin levels increase following salicylate administration. 38 , 39 Circulating CRP concentrations decreased by 50% following 2 weeks of salsalate at 4.5 g/d, although not significantly at lower doses. Like CRP, the expression of sCD40L, IL‐6, and inducible nitric oxide synthase (iNOS) are NF‐κB regulated; the targeted disruption of iNOS, a major enzyme involved in nitric oxide (NO) synthesis, protects against obesity‐linked insulin resistance. 40 While NO is sufficiently volatile, with a plasma half‐life of under 15 seconds, that its in vivo measurement is difficult, it is metabolized to more stable products 41 , 42 ; nitrites can be used as a measure of NO production. Similarly, circulating levels of sCD40L and nitrites were reduced at the high dose of salsalate. Adiponectin increased by 35% to 45% in all salsalate treated groups, consistent with improved insulin sensitivity and which may have independent cardioprotective value. 43 , 44 , 45 The decreases in levels of circulating proteins and enzymatic products are consistent with inhibition of NF‐κB and support it as a molecular target of high‐dose salicylate. We hypothesize even small decreases in NF‐κB activity, as with salicylate therapy, would induce small but relevant decreases in expression of multiple, potentially pathogenic, inflammatory mediators and improve metabolism in patients with type 2 diabetes.
There is substantial evidence for activation of NF‐κB in multiple tissues in obesity, type 2 diabetes, and cardiovascular disease, including adipose tissue, liver, and the vasculature. Parenchymal cells and associated immune cells participate in these inflammatory processes. 1 , 4 , 5 We did not obtain biopsies and therefore did not measure NF‐κB activity directly in these tissues. However, NF‐κB activity is also increased in circulating blood mononuclear cells in obesity and the metabolic syndrome, 34 , 35 and was accordingly reduced following high‐dose salsalate. As effects of salicylate are systemic, reduction in NF‐κB seen in circulating mononuclear cells likely reflects changes in other tissues.
Additional drugs in current clinical practice may reduce NF‐κB activity in various cell types. While the primary effect of PPAR‐γ agonists is to promote lipid storage into adipocytes, thiazolidinediones have also been shown to lower NF‐κB activity in circulating leukocytes from obese patients with type 2 diabetes, 46 , 47 perhaps through the transrepression of NF‐κB by ligated PPAR‐γ. 48 Statins reduce circulating cholesterol levels. These drugs have similarly been shown to modestly reduce NF‐κB activity, although statins are not known to improve dysglycemia in diabetic patients. The antiinflammatory “side” effects of these drugs are thought to have potential beneficial roles in reducing risk for atherosclerosis in predisposed subjects.
Salsalate has been used for decades for management of rheumatic pain. In these conditions, salsalate is administered to maximum tolerable doses based on tinnitus, typically ranging from 3.0 to over 4.0 g/d. 49 While many people tolerate higher doses, for regulatory reasons the lower, uniformly tolerated 3.0 g/d dose is now recommended on the package insert, despite being marginally effective. Therapeutic salicylate levels of 10 to 25 mg/dL are established for rheumatologic conditions. Levels achieved at the higher doses in our studies with diabetic patients in trials 1 and 3 were within this range. However, the mean serum level achieved at 3.0 g/d in trial 2 was subtherapeutic at 5.4 mg/dL. Despite the vastly different clinical endpoints of pain in one case and metabolic measures including blood glucose in the other, the pharmacology appears similar. These findings suggest that the molecular target for high‐dose salicylate is the same in rheumatologic and metabolic disorders, and likely to be NF‐κB.
No serious adverse events were associated with administration of salsalate. The most noteworthy side effect in these studies was dose‐dependent and dose‐limiting tinnitus. Salicylate‐associated tinnitus was established long ago to be dose limiting in patients with rheumatologic conditions. The frequency, severity, and reversibility of this side effect appear to be identical in patients with diabetes. Although clinical efficacy was greatest at 4.5 g/d dosing, tinnitus will limit this dose in most patients. None experienced tinnitus at 3.0 g/d, but clinical efficacy at this lower dose was marginal. Tinnitus could be largely avoided at the intermediate but effective doses of 3.5 to 4.0 g/d.
Patients treated with metformin or thiazolidinediones are susceptible to hypoglycemia when used concomitantly with insulin or secretagogues. Similar precautions with salicylates are likely warranted. Serum creatinine rose modestly, albeit within normal limits, in the two open‐label cohorts but not in the double‐masked treatment group. It is possible that a mild prerenal condition secondary to phlebotomy during IVGTT and clamp procedures contributed to transiently alter renal function, as subjects in the double‐masked trial had substantially less phlebotomy. Doses of 3.0 g/d and higher have been well tolerated in terms of renal function for extended periods in large numbers of patients treated for rheumatologic conditions. 50 Despite being widely used for decades there are no reports in medical literature of salsalate causing a decline in renal function in patients who did not already have baseline renal insufficiency. This should be contrasted with other NSAIDs, which may decrease renal function through alterations in prostaglandin production. While misoprostol was administered in studies 1 and 2, so changes could be ascribed to this agent, it was administered prior to baseline evaluation and not used in trial 3 and thus is unlikely to underlie metabolic changes. Salsalate has less gastrointestinal side effects than aspirin or other NSAIDs and concomitant use of misoprostol is unlikely to be required for the chronic treatment of diabetes in most patients.
Conclusions
Data from these three small trials demonstrate that salsalate may benefit patients with diabetes. These are the first studies showing that potentially safe and tolerable doses of salsalate positively impact multiple endpoints in type 2 diabetes, although dosing of salsalate may need to be close to that producing tinnitus. Salsalate is generic, and inexpensive to manufacture. Given its long‐term safety profile in humans, salsalate thus provides the potential for large healthcare economic benefits. However, enthusiasm must be tempered by limitations of the current studies, including that the 4.5 and 3.0 g/d cohorts were not masked, small numbers of subjects were used in each treatment group, durations of drug treatment were short, and studies were conducted at a single site and sequentially so subjects were not randomized to the protocols. Notwithstanding these limitations and based on the encouraging findings of these small trials, larger randomized trials are warranted and underway, in the NIDDK‐sponsored Targeting Inflammation with Salsalate in T2D (TINSAL‐T2D) trial.
Acknowledgments
We thank the staff of the Brigham and Women's Hospital General Clinical Research Center for their assistance, M. Freeman and J. Manning for analyses of fatty acid composition, Drs. Ernie Schaefer and Bela Asztalos for special assays, and G. I. Shulman and G. Cline for isotope analyses. These studies were supported by NIH grants R01 DK51729 and R01 DK45943 (SES), K23 DK02795 (ABG), P30 DK36836 and M01 RR02635 (Joslin Diabetes Center), and M01 RR01032 (Brigham and Women's Hospital), fellowships from the William Randolph Hearst Foundation (RJS) and American Diabetes Association 7‐04‐MN‐47 (SES), and the Helen and Morton Adler Chair (SES).
This trial was registered at http://clinicaltrials.gov; access #NCT00258128.
References
- 1. Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006; 116: 1793–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tataranni PA, Ortega E. A burning question: does an adipokine‐induced activation of the immune system mediate the effect of overnutrition on type 2 diabetes? Diabetes. 2005; 54(4): 917–927. [DOI] [PubMed] [Google Scholar]
- 3. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006; 444(7121): 860–867. [DOI] [PubMed] [Google Scholar]
- 4. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005; 352(16): 1685–1695. [DOI] [PubMed] [Google Scholar]
- 5. Hansson GK, Libby P. The immune response in atherosclerosis: a double‐edged sword. Nat Rev Immunol. 2006; 6(7): 508–519. [DOI] [PubMed] [Google Scholar]
- 6. Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c‐Jun N‐terminal kinase promotes insulin resistance during association with insulin receptor substrate‐1 and phosphorylation of Ser(307). J Biol Chem. 2000; 275(12): 9047–9054. [DOI] [PubMed] [Google Scholar]
- 7. Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002; 420(6913): 333–336. [DOI] [PubMed] [Google Scholar]
- 8. Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity‐ and diet‐induced insulin resistance with salicylates or targeted disruption of Ikkβ. Science. 2001; 293(5535): 1673–1677. [DOI] [PubMed] [Google Scholar]
- 9. Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKKβ and NF‐κB. Nat Med. 2005; 11(2): 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW, Jr . Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003; 112(12): 1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity‐related insulin resistance. J Clin Invest. 2003; 112(12): 1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ebstein W. Zur therapie des diabetes mellitus, insbesondere uber die anwendeng der salicylauren natron bei demselben. Berliner Klinische Wochenschrift. 1876; 13: 337–340. [Google Scholar]
- 13. Bartels‐Kiel. Ueber die therapeutiche Verwerthung der Salicylsãure und ihres Natronsalzes in der inneren Medicin. Deutsche Medicinische Wochenschrift. 1878; 4: 423–425. [Google Scholar]
- 14. Williamson RT. On the treatment of glycosuria and diabetes mellitus with sodium salicylate. Br Med J. 1901; 1: 760–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reid J, Macdougall AI, Andrews MM. On the efficacy of salicylate in treating diabetes mellitus. Br Med J. 1957; 2: 1071–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hecht A, Goldner MG. Reappraisal of the hypoglycemic action of acetylsalicylate. Metabolism. 1959; 8: 418–428. [PubMed] [Google Scholar]
- 17. Gilgore SG. The influence of salicylate on hyperglycemia. Diabetes. 1960; 9(5): 392–393. [Google Scholar]
- 18. Baron SH. Salicylates as hypoglycemic agents. Diabetes Care. 1982; 5(1): 64–71. [DOI] [PubMed] [Google Scholar]
- 19. Loll PJ, Picot D, Garavito RM. The structural basis of aspirin activity inferred from the crystal structure of inactivated prostaglandin H2 synthase. Nat Struct Biol. 1995; 2(8): 637–643. [DOI] [PubMed] [Google Scholar]
- 20. Kopp E, Ghosh S. Inhibition of NF‐κB by sodium salicylate and aspirin. Science. 1994; 265(5174): 956–959. [DOI] [PubMed] [Google Scholar]
- 21. Pierce JW, Read MA, Ding H, Luscinskas FW, Collins T. Salicylates inhibit I kappa B‐alpha phosphorylation, endothelial‐leukocyte adhesion molecule expression, and neutrophil transmigration. J Immunol. 1996; 156(10): 3961–3969. [PubMed] [Google Scholar]
- 22. Cai D, Frantz JD, Tawa NE, Jr. , Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKβ/NF‐κB activation causes severe muscle wasting in mice. Cell. 2004; 119(2): 285–298. [DOI] [PubMed] [Google Scholar]
- 23. Yin MJ, Yamamoto Y, Gaynor RB. The anti‐inflammatory agents aspirin and salicylate inhibit the activity of IκB kinase‐β. Nature. 1998; 396(6706): 77–80. [DOI] [PubMed] [Google Scholar]
- 24. Hundal RS, Petersen KF, Mayerson AB, Randhawa PS, Inzucchi S, Shoelson SE, Shulman GI. Mechanism by which high‐dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest. 2002; 109(10): 1321–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sweeney JD, Hoernig LA. Hemostatic effects of salsalate in normal subjects and patients with hemophilia A. Thromb Res. 1991; 61(1): 23–27. [DOI] [PubMed] [Google Scholar]
- 26. Edmar D. Effects of salicylates on the gastric mucosa as revealed by roentgen examination and the gastrocamera. Acta Radiol Diagn (Stockh). 1971; 11(1): 57–64. [DOI] [PubMed] [Google Scholar]
- 27. Lanza F, Rack MF, Doucette M, Ekholm B, Goldlust B, Wilson R. An endoscopic comparison of the gastroduodenal injury seen with salsalate and naproxen. J Rheumatol. 1989; 16(12): 1570–1574. [PubMed] [Google Scholar]
- 28. Roth S, Bennett R, Caldron P, Hartman R, Mitchell C, Doucette M, Ekholm B, Goldlust B, Lee E, Wilson R. Reduced risk of NSAID gastropathy (GI mucosal toxicity) with nonacetylated salicylate (salsalate): an endoscopic study. Semin Arthritis Rheum. 1990; 19(4 Suppl 2): 11–19. [PubMed] [Google Scholar]
- 29. National Diabetes Data Group . Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979; 28(12): 1039–1057. [DOI] [PubMed] [Google Scholar]
- 30. McGuire EA, Helderman JH, Tobin JD, Andres R, Berman M. Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J Appl Physiol. 1976; 41(4): 565–573. [DOI] [PubMed] [Google Scholar]
- 31. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979; 237(3): E214–E223. [DOI] [PubMed] [Google Scholar]
- 32. DeFronzo RA, Ferrannini E, Simonson DC. Fasting hyperglycemia in non‐insulin‐dependent diabetes mellitus: contributions of excessive hepatic glucose production and impaired tissue glucose uptake. Metabolism. 1989; 38(4): 387–395. [DOI] [PubMed] [Google Scholar]
- 33. Ferrannini E. The theoretical bases of indirect calorimetry: a review. Metabolism. 1988; 37(3): 287–301. [DOI] [PubMed] [Google Scholar]
- 34. Aljada A, Ghanim H, Dandona P. Activation of nuclear factor‐kappa B (NF‐kappa B) in mononuclear cells (MNC). Methods Mol Biol. 2002; 196: 105–110. [DOI] [PubMed] [Google Scholar]
- 35. Ghanim H, Aljada A, Hofmeyer D, Syed T, Mohanty P, Dandona P. Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation. 2004; 110(12): 1564–1571. [DOI] [PubMed] [Google Scholar]
- 36. Chen M, Robertson RP. Restoration of the acute insulin response by sodium salicylate. A glucose dose‐related phenomenon. Diabetes. 1978; 27(7): 750–756. [DOI] [PubMed] [Google Scholar]
- 37. Fujimoto WY, Metz SA. Phasic glucose‐stimulated insulin secretion by neonatal rat pancreatic islet cells. Enhancement by sodium salicylate. Diabetes. 1984; 33(9): 872–878. [DOI] [PubMed] [Google Scholar]
- 38. Dandona P, Aljada A, Mohanty P, Ghanim H, Hamouda W, Assian E, Ahmad S. Insulin inhibits intranuclear nuclear factor kappaB and stimulates IkappaB in mononuclear cells in obese subjects: evidence for an anti‐inflammatory effect? J Clin Endocrinol Metab. 2001; 86(7): 3257–3265. [DOI] [PubMed] [Google Scholar]
- 39. Aljada A, Ghanim H, Saadeh R, Dandona P. Insulin inhibits NFkappaB and MCP‐1 expression in human aortic endothelial cells. J Clin Endocrinol Metab. 2001; 86(1): 450–453. [DOI] [PubMed] [Google Scholar]
- 40. Perreault M, Marette A. Targeted disruption of inducible nitric oxide synthase protects against obesity‐linked insulin resistance in muscle. Nat Med. 2001; 7(10): 1138–1143. [DOI] [PubMed] [Google Scholar]
- 41. Marletta MA. Nitric oxide synthase: aspects concerning structure and catalysis. Cell. 1994; 78(6): 927–930. [DOI] [PubMed] [Google Scholar]
- 42. Stamler JS, Singel DJ, Loscalzo J. Biochemistry of nitric oxide and its redox‐activated forms. Science. 1992; 258(5090): 1898–1902. [DOI] [PubMed] [Google Scholar]
- 43. Matsuda M, Shimomura I, Sata M, Arita Y, Nishida M, Maeda N, Kumada M, Okamoto Y, Nagaretani H, Nishizawa H, Kishida K, Komuro R, Ouchi N, Kihara S, Nagai R, Funahashi T, Matsuzawa Y. Role of adiponectin in preventing vascular stenosis. The missing link of adipo‐vascular axis. J Biol Chem. 2002; 277(40): 37487–37491. [DOI] [PubMed] [Google Scholar]
- 44. Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003; 278(45): 45021–45026. [DOI] [PubMed] [Google Scholar]
- 45. Ouchi N, Ohishi M, Kihara S, Funahashi T, Nakamura T, Nagaretani H, Kumada M, Ohashi K, Okamoto Y, Nishizawa H, Kishida K, Maeda N, Nagasawa A, Kobayashi H, Hiraoka H, Komai N, Kaibe M, Rakugi H, Ogihara T, Matsuzawa Y. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension. 2003; 42(3): 231–234. [DOI] [PubMed] [Google Scholar]
- 46. Ghanim H, Garg R, Aljada A, Mohanty P, Kumbkarni Y, Assian E, Hamouda W, Dandona P. Suppression of nuclear factor‐kappaB and stimulation of inhibitor kappaB by troglitazone: evidence for an anti‐inflammatory effect and a potential antiatherosclerotic effect in the obese. J Clin Endocrinol Metab. 2001; 86(3): 1306–1312. [DOI] [PubMed] [Google Scholar]
- 47. Mohanty P, Aljada A, Ghanim H, Hofmeyer D, Tripathy D, Syed T, Al‐Haddad W, Dhindsa S, Dandona P. Evidence for a potent antiinflammatory effect of rosiglitazone. J Clin Endocrinol Metab. 2004; 89(6): 2728–2735. [DOI] [PubMed] [Google Scholar]
- 48. Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, Rose DW, Willson TM, Rosenfeld MG, Glass CK. A SUMOylation‐dependent pathway mediates transrepression of inflammatory response genes by PPAR‐gamma. Nature. 2005; 437(7059): 759–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. The Multicenter Salsalate/Aspirin Comparison Study Group . Does the acetyl group of aspirin contribute to the antiinflammatory efficacy of salicylic acid in the treatment of rheumatoid arthritis? J Rheumatol. 1989; 16(3): 321–327. [PubMed] [Google Scholar]
- 50. April P, Abeles M, Baraf H, Cohen S, Curran N, Doucette M, Ekholm B, Goldlust B, Knee CM, Lee E, Marcus R, Parrino GR, Reese R, Riskin W, Samuels B, Utsinger P, Virshup A, Wenger M, Whelton J, Wilson R. Does the acetyl group of aspirin contribute to the antiinflammatory efficacy of salicylic acid in the treatment of rheumatoid arthritis? Semin Arthritis Rheum. 1990; 19(4 Suppl 2): 20–28. [PubMed] [Google Scholar]


