Abstract
Background
Epidemiological studies of prostate cancer (PCA) which are based on case control comparisons may be effected by verification bias. Verification bias exists when the experimental group has verified PCA, while the control group is presumed to be cancer free, but this is not histologically verified. Materials and
Methods
Review of the literature and our recent experience with case control studies of PCA in an autopsy model. Results: When autopsied prostates were evaluated for cancer based on prostatic specific antigen <4 ng/ml, negative biopsy or both criteria, the contamination rate was 22%, 15% or 12%, respectively. The effect of contamination by occult PCA alters the odds ratio and p-value of the results.
Conclusion
It is important to recognize that contamination of the control population by occult carcinomas reduces the reliability of the results. Rigorous characterization of the experimental and control groups is needed in order to preserve the integrity of the conclusions.
Keywords: Prostate cancer, polymorphisms, autopsy, occult cancer, prostate specific antigen
Verification bias is the result of identifying experimental groups by histological confirmation of a disease or condition (such as cancer) while the control group is presumed to be free of this condition, but not histologically verified (1, 2). Prostate cancer (PCA) case control studies suffer from this bias because all of the men in the study population have been confirmed to have PCA, but the control group is presumed to be free of cancer, but lacks histological confirmation. It is well known that the prevalence of occult PCA is high and dependent on age, race, national origin, family history and prostate specific antigen (PSA) levels (3–5).
The carcinogenesis of PCA is not known at this time. Many environmental, dietary and chemical causes have been studied (6), however most efforts to find positive associations have been unsuccessful. A family history of PCA may be involved in about 10% of PCA cases (7). Evidence has been presented that environmental factors may also be involved in these carcinomas (6). DNA adducts derived from the exposure to carcinogens present in cooked meat as well as the exposure of prostatic glands to urine suggest the involvement of urine-borne aromatic and heterocyclic amines in the carcinogenesis of the prostate (8). Recently several epidemiological studies of PCA have reported that obese men and smokers are more likely to develop aggressive and fatal PCA (9–11). Many nutritional factors have also been associated with aggressive and fatal PCA (9). We have recently identified evidence that statistical flaws effecting the design of case control studies may inadvertently mask existing associations (12). The confounding factor appears to be the presence of occult, unsuspected PCA in the control population.
Manganese superoxide dismutase (MnSOD) is an enzyme responsible for the detoxification of reactive oxygen species by converting the super oxide radical to hydrogen peroxide. However, genetic polymorphism of the enzyme has been identified in the malignant transformation of PCA. Unfortunately, many case control studies have reported no (13), or only weak association (14–16). When we tested the association in an autopsy study, variable association was found depending upon the extent of our effort to exclude occult PCA (12). Only when complete step sectioning and histological confirmation of the absence of PCA in the control group was carried out, was the association of MnSOD polymorphism and PCA identified. Therefore, verification bias and the failure to account for occult PCA in the control group of case control studies could have an adverse effect on the accuracy of identifying associations (12).
PCA is the most prevalent non-skin malignancy in the US male and it is estimated that 1 in 6 males probably will develop invasive PCA in his lifetime (17). Unlike other malignancies, occult cancer of the prostate is present in men as young as 30 years and its prevalence is above 30% in men older than 50 years and 60% to 70% in men older than 80 years (18). The prevalence increases with advancing age. When men with normal digital rectal examination and PSA <4 ng/ml were sextant biopsied as part of the Prostate Cancer Prevention Trial, 15.2% were found to have unsuspected cancer (3). A 12-core biopsy regimen could have identified even more carcinomas (4). Clinical data suggest that more than 30% of men without a history of PCA have occult PCA (4, 18), however most case control studies are not large enough to be able to overcome a 30 % occult cancer inclusion rate in the control population Traditional epidemiology studies do not take into account the possibility of occult cancer in the control group and therefore may result in false negative results.
High grade prostatic intraepithelial neoplasia has been associated with the presence and development of PCA (19). Clinical data suggest that occult cancer can develop into significant cancer because nearly half of localized low-grade PCA cases may progress to clinically significant cancer over the next 20 years (20, 21). Although there are large variations in the incidence and mortality of PCA among various geographical and ethnic groups, the prevalence of PCA appears similar among the groups. When Japanese men immigrated to the United States, their incidence and mortality started to approximate that of the local population (22). It is possible that exposure to environmental factors promotes occult cancer to significant cancer and PCA etiology must encompass the steps leading to both the initiation of histological cancer and the progression to clinically significant cancer.
Materials and Methods
This study utilized 194 consecutive prostate glands from deceased men over the age of 45, collected as part of a larger study of the prevalence of PCA (4). The deceased had no known history of PCA. Total PSA levels were measured in the sera collected within 24 hours from the time of death (23). All the prostate glands were subjected to 12-core biopsy regimens performed as previously described (4). Briefly, biopsies were taken ex-vivo by an experienced urologist (GPH) in a manner similar to how clinical transrectal ultrasound guided biopsies are performed. The biopsies were taken from both sides of the glands in a symmetrical fashion from the mid-peripheral, lateral-peripheral and central zones at the level of the apex, mid-gland and base. The entire prostate was fixed, step sectioned at 5-mm intervals and each section was embedded in paraffin, cut into 5 μm sections, and stained with hematoxylin-eosin. Histological evaluation was performed and the presence or absence of PCA on the biopsies and final pathology was compared. The specimens were then studied for MnSOD polymorphism (V16A) as previously described (12). Briefly, DNA was extracted from the prostate tissues using QIAamp DNA Blood Mini Kits (Qiagen, Valencia, CA, USA). The MnSOD gene was PCR-amplified and then digested with BsaWI (60°C, 1 hour; New England BioLabs, Beverly, MA, USA). The fragment patterns specific for the three MnSOD genotypes were VV (GTT; 176 bp, 37 bp), VA (GTT/GCT; 213 bp, 176 bp, 37 bp), and AA (GCT; 213 bp). The frequency of the various MnSOD genotypes were compared between the experimental group of histologically confirmed carcinomas and the control groups set by PSA cut-off values of 4 ng/ml, negative biopsy results or both.
Results
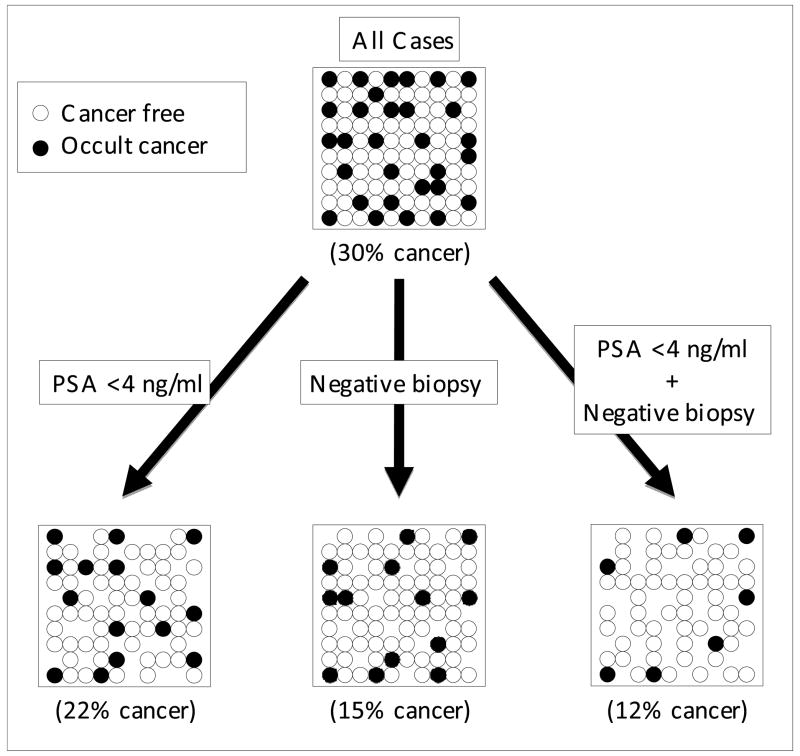
Out of the 194 cases of autopsied prostates, 57 carcinomas (29.4%) were found. Out of the 135 cases with PSA <4 ng/ml, 30 carcinomas (22.2%) were found. Despite 161 cases with negative biopsies, 25 carcinomas were found (15.5%). Finally, there were 118 cases with PSA <4 ng/ml and a negative biopsy, and out of these 14 carcinomas were found (11.9%). Thus, the percentage of cancer varied and was inversely proportional to the intensity of the effort to exclude it (Figure 1).
Figure 1.
Reduction of the contamination of occult cancer in control group by various selection criteria. This is a schematic illustration; the circles do not represent the actual number of cases in each group.
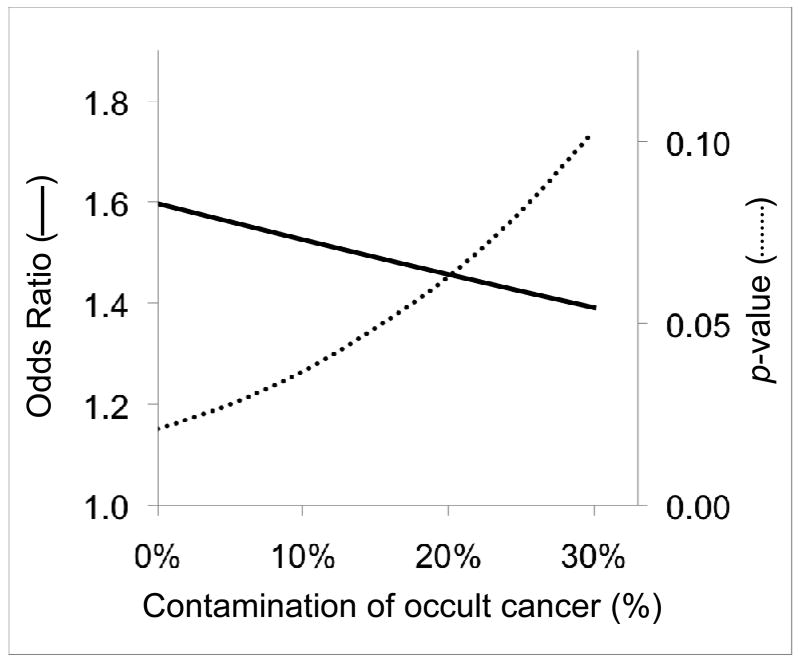
The association between MnSOD polymorphism and PCA was then investigated. The frequency of MnSOD AA genotype was 30% and 42% in the cancer-free and the PCA group, respectively. When the MnSOD AA genotype prevalence was compared between the histologically verified cancer cases (n=57) versus those glands with no cancer (n=137), the results demonstrated an association of MnSOD AA genotype and PCA (p=0.049). However if the control group was selected based on negative biopsy (occult cancer rate: 15.5%), there was no association (p=0.115). When the control group was selected based on PSA <4 ng/ml and negative biopsy (occult cancer rate: 11.9%), there was still no association (p=0.131). The increase of occult cancer miscategorized in the control group decreased the odds ratio and the power of analysis as shown in a hypothetical case control study with 200 subjects in each group (Figure 2).
Figure 2.
The influence on odds ratio and p-value by contamination of occult cancer in control group. In a hypothetical study consisting of 200 each of controls and PCA, the odds ratio for MnSOD AA genotpe is 1.6 with a p-value of 0.02 when the control group is free from occult cancer. As the contamination of occult cancer in the control group is increased, the odds ratio is decreased and p-value is increased.
Discussion
In most PCA case control studies, control subjects are selected on the basis of an absent history of PCA and/or a low PSA level (<4 ng/ml). Biopsies are not usually done to verify the absence of cancer and the inclusion of 15 to 30% of occult carcinomas in the control group would reduce the power of analysis and might result in false negative results. How can control subjects be selected in a case control study of PCA in order to exclude occult PCA? If the control population is based on an absent history of PCA, the practicality is high, but the accuracy of being cancer-free is low. Normal digital rectal examination does not improve the accuracy. If the control is selected based on low PSA, it improves the accuracy. Biopsy would greatly improve the accuracy, but the practicality is low because fewer volunteers would be willing to participate in the clinical trial. However the influence of the contamination of occult cancer may be minimized in a study consisting of a larger number of samples. The only way to confirm that the control population does not contain occult PCA is to examine the entire prostate gland histologically by thin step sections, but this is possible only for autopsy studies and not for case control studies.
PSA cut-off values of <4 ng/ml can reduce the number of occult carcinomas by approximately 20% and biopsy can detect approximately 50% of occult cancers in the control group. These data are consistent with reports that prostate biopsy has high specificity, but low sensitivity and PSA screening with cut-off values of 4 ng/ml has high specificity (94%), but low sensitivity (21%) for detecting PCA (24). Because it has been reported that PSA cut-off values of 1, 2 and 3 ng/ml yielded sensitivities of 83%, 53% and 32%, and specificities of 39%, 73% and 87%, respectively (24), it may be possible to exclude 50% of occult carcinomas from the control group by using the PSA cut-off values of 2 ng/ml. However, many cancer free subjects would be rejected. Therefore, the control groups should be selected by as low PSA as possible and/or negative biopsy. Increased restrictions in the selection of control subjects, such as requiring a negative biopsy to participate, may diminish participation, thereby leading to a selection bias.
Contamination by occult cancer in a control group can yield a false negative result. For example, MnSOD polymorphism (V16A) has been reported as a risk factor for PCA (14–16), but some studies have reported negative results (13). Certainly, MnSOD polymorphism as a risk for PCA was borderline significant in some case control studies, which used control groups contaminated by occult cancer. In the present study, it was possible to demonstrate their association when the presence of cancer in the study group and the absence of cancer in the control group were histologically verified.
Autopsy studies, which can eliminate occult cancer from the control group, are important for studying the association between genetic polymorphism and PCA. It is important to recognize that contamination of the control population by PCA reduces the reliability of the results. Rigorous characterization of the experimental and control groups is urged in order to preserve the integrity of the conclusions.
Acknowledgments
This study was supported by the National Cancer Institute (CA097751; G.P. Haas) and the National Institute on Aging (AG021389; G. P. Haas).
Abbreviations
- PCA
prostate cancer
- MnSOD
manganese superoxide dismutase
- PSA
prostate specific antigen
References
- 1.Bates AS, Margolis PA, Evans AT. Verification bias in pediatric studies evaluating diagnostic tests. J Pediatr. 1993;122:585–590. doi: 10.1016/s0022-3476(05)83540-1. [DOI] [PubMed] [Google Scholar]
- 2.Punglia RS, D’Amico AV, Catalona WJ, Roehl KA, Kuntz KM. Effect of verification bias on screening for prostate cancer by measurement of prostate-specific antigen. N Engl J Med. 2003;349:335–342. doi: 10.1056/NEJMoa021659. [DOI] [PubMed] [Google Scholar]
- 3.Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, Minasian LM, Ford LG, Lippman SM, Crawford ED, Crowley JJ, Coltman CA., Jr Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004;350:2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 4.Haas GP, Delongchamps NB, Jones RF, Chandan V, Serio AM, Vickers AJ, Jumbelic M, Threatte G, Korets R, Lilja H, de la Roza G. Needle biopsies on autopsy prostates: sensitivity of cancer detection based on true prevalence. J Natl Cancer Inst. 2007;99:1484–1489. doi: 10.1093/jnci/djm153. [DOI] [PubMed] [Google Scholar]
- 5.Haas GP, Delongchamps N, Brawley OW, Wang CY, de la Roza G. The worldwide epidemiology of prostate cancer: perspectives from autopsy studies. Can J Urol. 2008;15:3866–3871. [PMC free article] [PubMed] [Google Scholar]
- 6.Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, Pukkala E, Skytthe A, Hemminki K. Environmental and heritable factors in the causation of cancer-analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 7.Carter BS, Beaty TH, Steinberg GD, Childs B, Walsh PC. Mendelian inheritance of familial prostate cancer. Proc Natl Acad Sci U S A. 1992;89:3367–3371. doi: 10.1073/pnas.89.8.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Paolo OA, Teitel CH, Nowell S, Coles BF, Kadlubar FF. Expression of cytochromes P450 and glutathione S-transferases in human prostate, and the potential for activation of heterocyclic amine carcinogens via acetyl-coA-, PAPS-and ATP-dependent pathways. Int J Cancer. 2005;117:8–13. doi: 10.1002/ijc.21152. [DOI] [PubMed] [Google Scholar]
- 9.Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int J Cancer. 2007;121:1571–1578. doi: 10.1002/ijc.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong Z, Agalliu I, Lin DW, Stanford JL, Kristal AR. Cigarette smoking and prostate cancer-specific mortality following diagnosis in middle-aged men. Cancer Causes Control. 2008;19:25–31. doi: 10.1007/s10552-007-9066-9. [DOI] [PubMed] [Google Scholar]
- 11.Wright ME, Chang SC, Schatzkin A, Albanes D, Kipnis V, Mouw T, Hurwitz P, Hollenbeck A, Leitzmann MF. Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer. 2007;109:675–684. doi: 10.1002/cncr.22443. [DOI] [PubMed] [Google Scholar]
- 12.Iguchi T, Wang CY, Delongchamps NB, Sunheimer R, Nakatani T, de la Roza G, Haas GP. Association of prostate cancer and manganese superoxide dismutase AA genotype is influenced by the presence of occult cancer in the control group. Urol. doi: 10.1016/j.urology.2008.03.064. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi JY, Neuhouser ML, Barnett M, Hudson M, Kristal AR, Thornquist M, King IB, Goodman GE, Ambrosone CB. Polymorphisms in oxidative stress-related genes are not associated with prostate cancer risk in heavy smokers. Cancer Epidemiol Biomarkers Prev. 2007;16:1115–1120. doi: 10.1158/1055-9965.EPI-07-0040. [DOI] [PubMed] [Google Scholar]
- 14.Woodson K, Tangrea JA, Lehman TA, Modali R, Taylor KM, Snyder K, Taylor PR, Virtamo J, Albanes D. Manganese superoxide dismutase (MnSOD) polymorphism, alpha-tocopherol supplementation and prostate cancer risk in the alpha-tocopherol, beta-carotene cancer prevention study (Finland) Cancer Causes Control. 2003;14:513–518. doi: 10.1023/a:1024840823328. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Kantoff PW, Giovannucci E, Leitzmann MF, Gaziano JM, Stampfer MJ, Ma J. Manganese superoxide dismutase polymorphism, prediagnostic antioxidant status, and risk of clinical significant prostate cancer. Cancer Res. 2005;65:2498–2504. doi: 10.1158/0008-5472.CAN-04-3535. [DOI] [PubMed] [Google Scholar]
- 16.Kang D, Lee KM, Park SK, Berndt SI, Peters U, Reding D, Chatterjee N, Welch R, Chanock S, Huang WY, Hayes RB. Functional variant of manganese superoxide dismutase (SOD2 V16A) polymorphism is associated with prostate cancer risk in the prostate, lung, colorectal, and ovarian cancer study. Cancer Epidemiol Biomarkers Prev. 2007;16:1581–1586. doi: 10.1158/1055-9965.EPI-07-0160. [DOI] [PubMed] [Google Scholar]
- 17.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 18.Reiter RE, de Kernion JB. Epidemiology, etiology, and prevention of prostate cancer. In: Walsh PC, Retik AB, Vaughan ED, Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell’s Urology IV. Readfield, ME: Saunders; 2002. pp. 3003–3024. [Google Scholar]
- 19.Bostwick DG, Pacelli A, Lopez-Beltran A. Molecular biology of prostatic intraepithelial neoplasia. Prostate. 1996;29:117–134. doi: 10.1002/(SICI)1097-0045(199608)29:2<117::AID-PROS7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 20.Holmberg L, Bill-Axelson A, Helgesen F, Salo JO, Folmerz P, Haggman M, Andersson SO, Spangberg A, Busch C, Nordling S, Palmgren J, Adami HO, Johansson JE, Norlen BJ. A randomized trial comparing radical prostatectomy with watchful waiting in early prostate cancer. N Engl J Med. 2002;347:781–789. doi: 10.1056/NEJMoa012794. [DOI] [PubMed] [Google Scholar]
- 21.Adolfsson J, Tribukait B, Levitt S. The 20-yr outcome in patients with well- or moderately differentiated clinically localized prostate cancer diagnosed in the pre-PSA era: the prognostic value of tumour ploidy and comorbidity. Eur Urol. 2007;52:1028–1035. doi: 10.1016/j.eururo.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Dunn JE. Cancer epidemiology in populations of the United States-with emphasis on Hawaii and California-and Japan. Cancer Res. 1975;35:3240–3245. [PubMed] [Google Scholar]
- 23.Jones RF, Sunheimer R, Friedman H, Miller D, Ginsburg R, Jumbelic M, Threatte G, Haas GP. Comparison of ante- and post-mortem PSA levels for epidemiological studies. Anticancer Res. 2005;25:1263–1267. [PubMed] [Google Scholar]
- 24.Thompson IM, Ankerst DP, Chi C, Lucia MS, Goodman PJ, Crowley JJ, Parnes HL, Coltman CA., Jr Operating characteristics of prostate-specific antigen in men with an initial PSA level of 3.0 ng/ml or lower. JAMA. 2005;294:66–70. doi: 10.1001/jama.294.1.66. [DOI] [PubMed] [Google Scholar]