Abstract
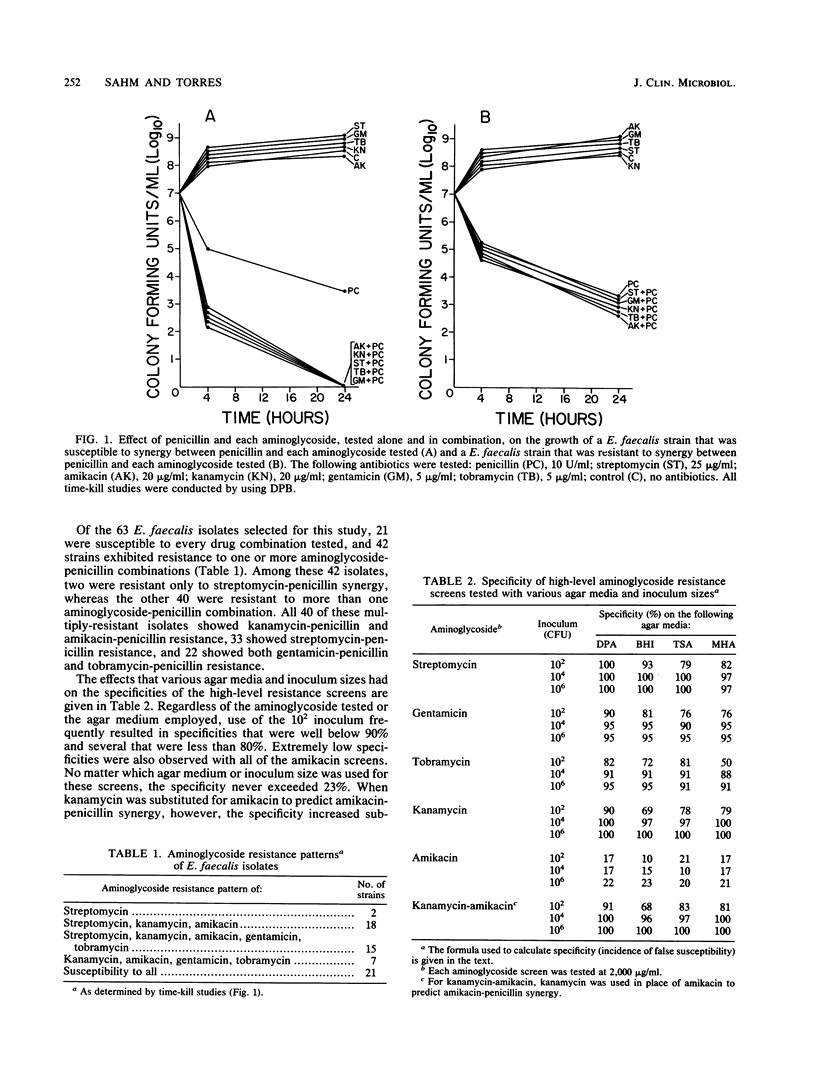
Enterococcus faecalis isolates that are refractory to aminoglycoside-penicillin synergy can be detected by their ability to grow in the presence of high concentrations of aminoglycoside (2,000 micrograms/ml). In past studies investigators have used a variety of media and inoculum sizes to perform high-level aminoglycoside resistance screens, but little is known about how these variations affect test accuracy. We screened 63 E. faecalis strains on different media by using various inoculum sizes and correlated the results with synergy test results obtained by time-kill studies. Screens were done with dextrose-phosphate agar, brain heart infusion agar, Trypticase soy agar with 5% sheep blood, Mueller-Hinton agar with 5% sheep blood, dextrose-phosphate broth, and Mueller-Hinton broth. Agar screens were inoculated with 10(2), 10(4), and 10(6) CFU; and broth screens contained a final inoculum of 10(5) CFU/ml. The E. faecalis isolates were tested for high-level resistance to streptomycin, kanamycin, amikacin, gentamicin, and tobramycin. Of the 63 isolates tested, 21 did not show high-level resistance to any of the aminoglycosides tested, and 42 demonstrated high-level resistance to one or more drugs. The sensitivity of most screens was greater than or equal to 90%. Regardless of the inoculum size or medium used, false-resistance results were seldom encountered. Screen specificity, which was used as the indicator of false susceptibility, was markedly influenced by both the inoculum size and the drug being tested. Specificity was low whenever a 10(2)-CFU inoculum was used, when amikacin was tested with any inoculum, and when tobramycin was tested in broth media. Data for kanamycin could be used to predict amikacin-penicillin synergy, and the highly accurate gentamicin screen obviated the need for the testing of tobramycin. We recommend a 10(6) -CFU inoculum for agar screens and a 10(5) -CFU/ml inoculum for broth screens. The type of medium used did not substantially influence screen accuracy. Among the aminoglycosides, only streptomycin, gentamicin, and occasionally, kanamycin need to be used to screen E. faecalis isolates for aminoglycoside-penicillin synergy.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basker M. J., Slocombe B., Sutherland R. Aminoglycoside-resistant enterococci. J Clin Pathol. 1977 Apr;30(4):375–380. doi: 10.1136/jcp.30.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood S. A., Wennersten C., Moellering R. C., Jr, Kunz L. J., Krogstad D. J. Resistance to six aminoglycosidic aminocyclitol antibiotics among enterococci: prevalence, evolution, and relationship to synergism with penicillin. Antimicrob Agents Chemother. 1977 Sep;12(3):401–405. doi: 10.1128/aac.12.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P., Carlier C., Collatz E. Plasmid-mediated resistance to aminocyclitol antibiotics in group D streptococci. J Bacteriol. 1980 Aug;143(2):541–551. doi: 10.1128/jb.143.2.541-551.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos G. M., Farber B. F., Murray B. E., Wennersten C., Moellering R. C., Jr Ribosomal resistance of clinical enterococcal to streptomycin isolates. Antimicrob Agents Chemother. 1984 Mar;25(3):398–399. doi: 10.1128/aac.25.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horodniceanu T., Bougueleret L., El-Solh N., Bieth G., Delbos F. High-level, plasmid-borne resistance to gentamicin in Streptococcus faecalis subsp. zymogenes. Antimicrob Agents Chemother. 1979 Nov;16(5):686–689. doi: 10.1128/aac.16.5.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannini P. B., Ehret J., Eickhoff T. C. Effects of ampicillin-amikacin and ampicillin-rifampin on enterococci. Antimicrob Agents Chemother. 1976 Mar;9(3):448–451. doi: 10.1128/aac.9.3.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda D. P., Barry A. L., Andersen S. G. Emergence of Streptococcus faecalis isolates with high-level resistance to multiple aminocyclitol aminoglycosides. Diagn Microbiol Infect Dis. 1984 Jun;2(3):171–177. doi: 10.1016/0732-8893(84)90027-0. [DOI] [PubMed] [Google Scholar]
- Jacob A. E., Hobbs S. J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974 Feb;117(2):360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawetz E., Gunnison J. B., Coleman V. R. The Combined Action of Penicillin with Streptomycin or Chloromycetin on Enterococci in Vitro. Science. 1950 Mar 10;111(2880):254–256. doi: 10.1126/science.111.2880.254. [DOI] [PubMed] [Google Scholar]
- Korzeniowski O. M., Wennersten C., Moellering R. C., Jr, Sande M. A. Penicillin-netilmicin synergism against Streptococcus faecalis. Antimicrob Agents Chemother. 1978 Mar;13(3):430–434. doi: 10.1128/aac.13.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogstad D. J., Korfhagen T. R., Moellering R. C., Jr, Wennersten C., Swartz M. N. Aminoglycoside-inactivating enzymes in clinical isolates of Streptococcus faecalis. An explanation for resistance to antibiotic synergism. J Clin Invest. 1978 Aug;62(2):480–486. doi: 10.1172/JCI109149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell G. L., Kaye D., Levison M. E., Hook E. W. Enterococcal endocarditis. An analysis of 38 patients observed at the New York Hospital-Cornell Medical Center. Arch Intern Med. 1970 Feb;125(2):258–264. doi: 10.1001/archinte.125.2.258. [DOI] [PubMed] [Google Scholar]
- Mederski-Samoraj B. D., Murray B. E. High-level resistance to gentamicin in clinical isolates of enterococci. J Infect Dis. 1983 Apr;147(4):751–757. doi: 10.1093/infdis/147.4.751. [DOI] [PubMed] [Google Scholar]
- Moellering R. C., Jr, Wennersten C., Weinberg A. N. Studies on antibiotic synergism against enterococci. I. Bacteriologic studies. J Lab Clin Med. 1971 May;77(5):821–828. [PubMed] [Google Scholar]
- Moellering R. C., Jr, Wennersten C., Weinberg A. N. Synergy of penicillin and gentamicin against Enterococci. J Infect Dis. 1971 Dec;124 (Suppl):S207–S209. doi: 10.1093/infdis/124.supplement_1.s207. [DOI] [PubMed] [Google Scholar]
- Moellering R. C., Jr, Wennersten C., Weinstein A. J. Penicillin-tobramycin synergism against enterococci: a comparison with penicillin and gentamicin. Antimicrob Agents Chemother. 1973 Apr;3(4):526–529. doi: 10.1128/aac.3.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Tsao J., Panida J. Enterococci from Bangkok, Thailand, with high-level resistance to currently available aminoglycosides. Antimicrob Agents Chemother. 1983 Jun;23(6):799–802. doi: 10.1128/aac.23.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zervos M. J., Kauffman C. A., Therasse P. M., Bergman A. G., Mikesell T. S., Schaberg D. R. Nosocomial infection by gentamicin-resistant Streptococcus faecalis. An epidemiologic study. Ann Intern Med. 1987 May;106(5):687–691. doi: 10.7326/0003-4819-106-5-687. [DOI] [PubMed] [Google Scholar]
- Zimmermann R. A., Moellering R. C., Jr, Weinberg A. N. Mechanism of resistance to antibiotic synergism in enterococci. J Bacteriol. 1971 Mar;105(3):873–879. doi: 10.1128/jb.105.3.873-879.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]



