Abstract
Objective
The urinary proteome is a potential easily accessible source of biomarkers for inflammatory bladder diseases including interstitial cystitis. In the present study, we subjected rat urine to multiplex cytokine analysis in an attempt to identify an inflammatory signature of the temporal course of cyclophosphamide (CYP) - induced cystitis.
Methods
Rat urine was collected for 12h following CYP injection (150mg/kg) for multiplex analysis of 14 cytokines by a multiple antigen bead assay (Luminex™ 100 IS). Urine from each void was collected and voiding frequency was determined. Bladder tissue was analyzed for cytokines levels and histological evidence of inflammation.
Results
Significant fold changes were noticed in urine levels of all cytokines with respect to baseline at 2, 4, 6 and 10h after CYP injection. Elevation was noticed at all times for most cytokines except for MCP-1 that showed a five fold decrease at 2h time point. Urine and tissue levels of IL-1β, IL-4 and GRO/KC were significantly correlated, with a positive spearman correlation also noticed for GM-CSF, MCP-1, IL-18 and IFN-γ. Tissue levels for most cytokines except IL-2 and urinary frequency were significantly elevated in CYP treated rats over control vehicle treated rats. The hints of severe inflammation in bladder indicated by urinary cytokines were confirmed by bladder histology and tissue cytokine levels on animal sacrifice.
Conclusions
The progression of CYP-induced cystitis is clearly reflected in the urine matrix by temporal and quantitative changes in cytokine levels. Further delineation of urine and bladder tissue cytokine expression may yield biomarkers for cystitis.
Keywords: cystitis, multiplex analysis, rat, cyclophosphamide, cytokine, interstitial cystitis
Introduction
Interstitial cystitis (IC) is a chronic inflammatory disease of unknown etiology characterized by urinary frequency, urgency, and suprapubic pain1. The National Institutes of Health (NIH) have established a diagnostic criteria for IC based on the presence of irritative voiding symptoms in the absence of other identifiable pathology2. In the present study, we hypothesized that analysis of urinary cytokines may allow for the discovery of disease-specific targets and/or biomarkers that may be of use as diagnostic and prognostic markers for inflammatory bladder disease3.
The urine is one of the ideal biological samples for the discovery of noninvasive biomarkers for human diseases, because it is available in almost all patients and its collection is simple and does not require any invasive procedures. Recent studies have suggested that cytokines or chemokines contribute to lower urinary tract dysfunction. Therefore, cytokines may serve as direct therapeutic targets or as potential biomarkers for the development of targeted therapy designed to prevent the long term sequelae of chronic bladder inflammatory conditions such as interstitial cystitis4.
Systemic or intraperitoneal injection with Cyclophosphamide5 induces a reproducible dose-dependant chemical cystitis in both mice and rats and for this reason has been utilized as an experimental model of IC6. Increased voiding frequency, decrease urine volume per void, and increased permeability of the bladder wall is seen in this model5,7. Similar studies reported previously only examined cytokine expression in rat bladder tissue with ELISA and not in the urine8. Saban et al. reported up-regulation of cytokines in pooled urine samples from inflammatory mouse model utilizing a multiplex suspension array9. The multiplex analysis (xMAP®) of urinary proteins following CYP-induced cystitis in rat using Luminex™ technology has not been reported.
The present study was designed to examine acute changes in urinary levels of 14 cytokines (interleukin 1α [IL-1α], IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-18, granulocyte macrophage colony-stimulating factor [GM-CSF], monocyte chemotactic protein-1 [MCP-1], interferon γ [IFNγ], growth related oncogene/keratinocyte-derived chemokine [GRO/KC], and tumor necrosis factor α [TNFα] after CYP-induced cystitis. Bladder inflammation revealed by urinary cytokines was verified by tissue analysis and histology.
Materials and Methods
Animals
All animal experimentation described was performed in accordance with institutional guidelines and the University of Pittsburgh Institutional Animal Care and Use Committee (IACUC) approval. Intraperitoneal10 CYP injections (150 mg/kg) were performed in female Sprague-Dawley rats (276–292 g). Urine specimens obtained from rats kept in metabolic cages were frozen immediately in liquid nitrogen and stored at −80°C prior to analysis. Baseline urine samples were obtained 24 h prior to CYP injection as well as from vehicle treated rats. Bladder tissue was harvested from CYP treated and vehicle treated rats.
Urine and Bladder Tissue Cytokine Expression
Frozen urine samples from each time point and bladder tissue protein isolates from end of the study were thawed, and 50 μL from each sample was analyzed in duplicate on the Luminex™ 100 IS (MiraiBio, South San Francisco, CA) using a LINCOplex Cytokine/Chemokine Luminex® Bead immunoassay Kit 14-Plex beadset (LINCO Research, St. Charles, MO). Cytokine concentrations provided by Luminex for each time point were normalized to the creatinine concentration in urine for that time point and expressed as amount of cytokine in picograms (pg) excreted per mg of creatinine. Creatinine in urine was measured using the previously published HPLC method11. Cytokine concentrations in the tissue at the end of the study were normalized to respective bladder weight of each animal.
Histological Analyses
Bladders were fixed in 4% buffered formaldehyde, followed by cryo-preservation and serial sectioning into 20 μm sections. Sections were stained with hematoxylin and eosin (H&E)12,13. Stained sections were visualized for leukocyte infiltration, destruction of urothelium, and edema. Toluidine blue staining was performed for mast cells.
Statistical Analysis
Data comparison was done between mean cytokine levels at baseline, 2, 4, 6 and 10 h after CYP treatment using one way ANOVA followed by Dunnett’s multiple comparison test for statistical significance. Significance was considered at p<0.05. In addition, cytokine values in last voided urine were compared to bladder tissue expression. Values are expressed as mean ± SEM (standard error of mean).
Results
Temporal Quantitative Changes in Urinary Cytokine Levels after CYP-induced Cystitis
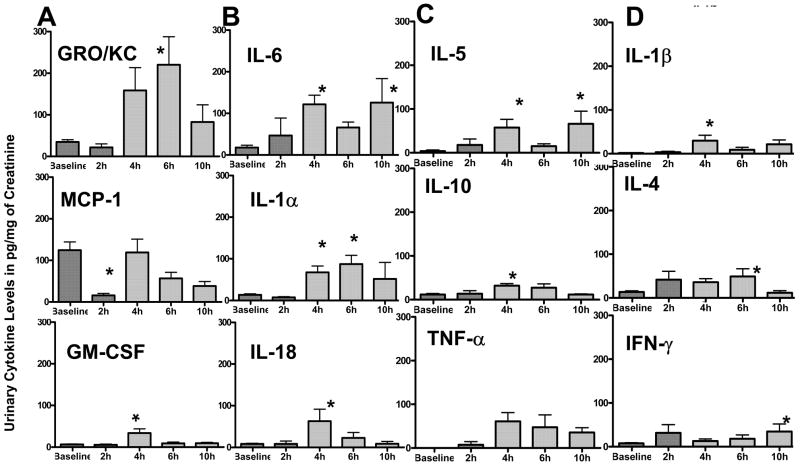
Acute disease progression was profiled by the CYP-induced time-dependent changes in urinary cytokine levels relative to baseline values. All the 14 cytokines assayed, were detected in the urine of CYP-treated rats (Fig 1–2). Levels of urinary cytokines at baseline and after CYP treatment were normally distributed to justify the use of parametric tests for statistical significance. Significant changes compared to baseline values were noted within 4 h of CYP-injection for IL-1α, IL-1β, IL-5, IL-6, IL-10, IL-18, and GM-CSF (p<0.05; Fig. 1A–D). Most robust changes were seen at 4h with as 5–6 fold increase relative to baseline in the levels of GRO/KC, IL-6, IL-1α and GM-CSF was seen compared to a 10 fold increase in the levels of IL-18 at the same time point (p<0.05; Fig. 1A–B). Seven fold increase in levels of GRO/KC at 6h time point was statistically significant (top panel of Fig. 1A). Levels of IL-6 doubled by 2h and were measured 6-fold higher at 4 and 10h time point (p<0.05; top panel of Fig. 1B). Interestingly, urinary levels of MCP-1 showed a significant decrease of eight fold at 2h relative to baseline (middle panel of Fig. 1A). The levels of IL-5 and IL-1β rose sharply to nearly 15 fold relative to baseline at 4h time point (p<0.05; top panels of Fig. 1C & Fig. 1D). Comparatively as modest three fold elevation relative to baseline was seen in the levels of IL-10 and IL-4 at 4 and 6h, respectively (p<0.05; middle panels of Fig. 1C & Fig. 1D). Levels of IFN-γ showed a significant 4- fold increase by 10h (p<0.05), whereas a 60 fold rise of TNF-α at 4h did not reach statistical significance(bottom panels of Fig. 1C & Fig. 1D).
Figure 1. A–D. Temporal profile of urinary cytokines following CYP-injection.
1A) Urine levels of cytokines normalized to creatinine excretion in urine at baseline and at 2, 4, 6 and 10h after CYP injection. A 5–6 fold increase relative to baseline in the levels of GRO/KC, IL-6, IL-1α and GM-CSF was seen at 4h compared to a 10 fold increase in the levels of IL-18 at the same time point (*p<0.05; Fig 1A&B). Levels of MCP-1 showed a significant decrease of eight fold at 2h relative to baseline (*p<0.05; middle panel of Fig. 1A). A 15 fold significant rise relative to baseline was observed in levels of IL-5 and IL-1β at 4h time point (*p<0.05; top panels of Fig 1C&D). A three fold increase relative to baseline was seen in the levels of IL-10 and IL-4 at 4 and 6h, respectively (*p<0.05; middle panels of Fig 1 C&D, respectively). Levels of IFN-γ showed a significant 4- fold by 10h, whereas a 60 fold rise of TNF-α at 4h did not reach statistical significance (bottom panels of Fig 1C&D). Values are expressed as mean ± SEM and considered significant relative to baseline at p <0.05*; n=8.
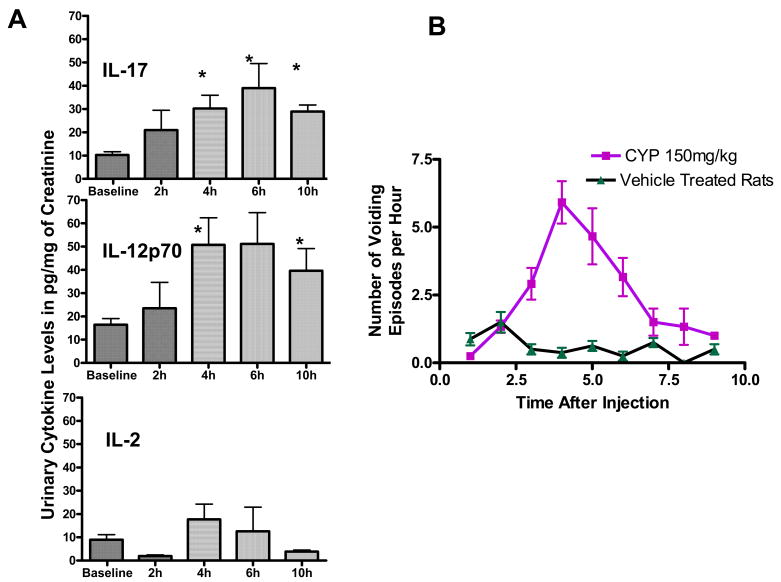
Figure 2.
Figure 2A. Temporal profile of urinary cytokines following CYP-injection Urine levels of IL-17, IL-12p70, and IL-2 normalized to creatinine excretion in urine at baseline and at 2, 4, 6 and 10h after CYP injection. Levels of IL-17 and IL-12p70 tripled significantly by 4h after CYP injection (*p<0.05; n=4; top and middle panel, respectively). In contrast, levels only doubled for IL-2 (bottom panel) without statistical significance (n=8) at the same time point.
Figure 2B. Time dependent changes in urinary frequency following CYP injection. Peak urinary frequency following CYP injection was concurrent with peak urinary cytokine expression between 4 and 6h and preceded the peak IL-6 elevation. Vehicle treated rats showed a relatively constant voiding frequency of one void every hour or every second hour.
Levels of IL-17 and IL-12p70 tripled significantly by 4h after CYP injection (p<0.05;; top & middle panel of Fig. 2A), whereas levels of IL-2 only doubled without statistical significance at the same time point (bottom panel of Fig. 2A). Urinary frequency was defined as the number of voiding episodes per hour for each individual rat following CYP-injection (Fig 2B). A mean peak urinary frequency 6.1±2.8 voids/hr was observed between 4 and 6h following CYP-injection (n=8). Vehicle treated rats (n=8) voided with relatively constant frequency.
Changes in Tissue Cytokine Protein Levels after CYP-Induced Cystitis
At 24 h following CYP-injection, tissue levels for majority of cytokines in CYP treated rats were elevated at least two fold over vehicle treated control bladder tissue levels. Levels of IL-6 showed most drastic elevations of nearly 24 fold over sham treated rats and a 10-fold elevation was noticed in the tissue levels of GRO/KC and TNF-α over controls (Table 1). Nearly 3–4 fold increase was seen in the levels of IL-1β and MCP-1. In contrast, tissue levels of IL-2 decreased by half following CYP treatment. A positive correlation between urine and tissue levels was noticed for GM-CSF, MCP-1, IL-18 and IFN-γ with significant spearman correlation for IL-1β, IL-4 and GRO/KC (p<0.05).
Table 1.
Correlation between cytokine levels in bladder at time of animal sacrifice and in last voided urine specimen before sacrifice.
| Cytokine | Last Voided Urine (pg/mg creatinine) | Tissue cytokine levels expressed per bladder (ng) | Spearman’s correlation coefficients | Fold change In CYP treated bladder over Vehicle treated bladder |
|---|---|---|---|---|
| GM-CSF | 5.2±1.07 | 27.8±2.1 | 0.87 | 1 |
| IL-1α | 26.3± 11.5 | 47.1±13.1 | 0.5 | 2.5 |
| MCP-1 | 121.6± 47.1 | 213.2±58.8 | 0.8 | 3.5 |
| IL-4 | 64.9±48.2 | 35±3.28 | 0.97* | 2 |
| IL-1β | 13.9±8.7 | 76.4±18.4 | 0.97* | 4 |
| IL-2 | 2.4±1.7 | 33.6±10.4 | −0.05 | 0.5 |
| IL-6 | 130.2±62.9 | 438.7±184.3 | 0.5 | 24 |
| IL-10 | 13.7±4.3 | 28.3±15.3 | −0.6 | 2.5 |
| IL-12p70 | 16.7±5.2 | 25.7±11.3 | −0.5 | 2 |
| IL-5 | 0 | 34.8±7.5 | 0 | 2 |
| IFN-γ | 44.9±37.9 | 16.4±0.6 | 0.66 | 2 |
| IL-18 | 10.3±3.7 | 1285.2±297.0 | 0.80 | 2 |
| GRO/KC | 184.3±100.2 | 522.7±265.1 | 0.9* | 10 |
| TNF-α | 0 | 23.9±7.7 | 0 | 10 |
| IL-17 | 16.6±2.4 | 17.5±1.5 | −0.5 | 2 |
Cytokine concentrations in the tissue were normalized to respective bladder weight of each animal. A positive correlation between urine and tissue levels was noticed for GM-CSF, MCP-1, IL-18 and IFN-γ with significant spearman correlation for IL-1β, IL-4 and GRO/KC. The measured Spearman correlation coefficient between levels in tissue and in last voided urine sample for each cytokine was tested for significant difference from 0 by setting a two tailed
p≤0.05; n=4–8.
Histological Analysis
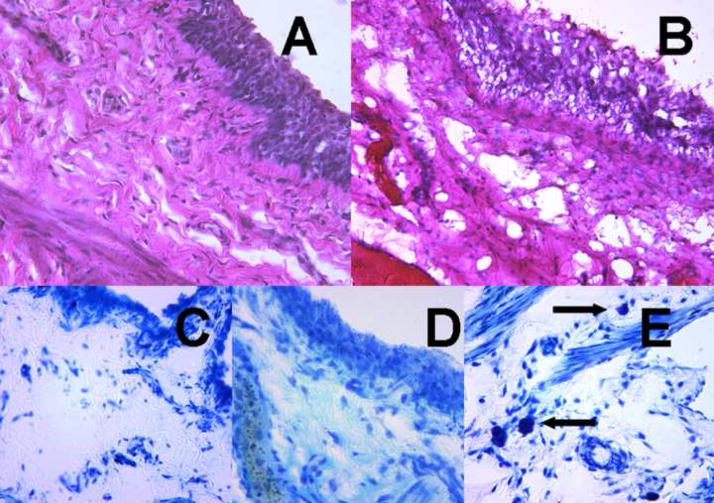
Histological evaluation of bladder with H&E staining 24h following CYP-treatment revealed signs of CYP- induced hemorrhagic cystitis of varying severity marked by severe edema and inflammation, erosion and ulceration of the urothelium, and hemorrhage when compared to untreated specimens (Figure 3a, b). Toluidine blue staining failed to reveal the presence of mast cells in control bladders or CYP-treated bladders at 24 hours (Figure 3c, d), but did demonstrate the presence of mast cells in positive control bladders 9 days following CYP treatment (Figure 3e).
Figure 3. Histological examination of untreated and CYP injected rat bladders.
Bladders were fixed in 4% buffered formaldehyde, cryopreserved, cut serially into 20μm sections, and subsequently stained with hematoxylin and eosin (A,B) Representative photomicrographs demonstrated CYP induced histological changes at 24 h (40X). A. Normal bladder. B. CYP treated bladder demonstrated urothelial sloughing, submucosal inflammatory infiltrate, scattered areas of hemorrhage, and intramural edema. Sections stained with toluidine blue (C–E). C. Normal bladder. D. CYP treated bladder showing no mast cells. E. Positive control bladder (9 days after CYP treatment) with mast cells (arrows).
Discussion
The present report describes the application of multiplex analysis of inflammatory cytokines using Luminex™ technology in order to analyze microliter quantities of urine specimens collected at various time points following chemically induced cystitis in rats. Our results suggest that urine multiplex analysis may be a promising approach for the identification of IC biomarkers, aiming at the detection of IC from a single voided urine specimen. In comparison, most current urine proteome testing requires urine collection over a twenty four hour period14.
Clinical studies have reported elevated urinary levels of IL-614–16 in patients with IC, and demonstrated an association between IL-6 expression and clinical symptom severity14. CYP is well known to induce frank inflammation in the bladder17 and results from these study demonstrate that inflammation at tissue level is reflected by variable urinary cytokines levels. Similar studies in the literature on urine measurement of cytokines in animal models have not reported the inter-individual variability of cytokines between animals9.
Significant increases in urinary cytokines specifically produced by T helper 1 cells (IL-2, IL-18, IL-12 IFN-γ) as well as by T helper 2 cells (IL-4, MCP-1, IL-5, IL-6 and IL-10) were observed. GM-CSF and TNF-α produced by both Th1 and Th2 cells were also elevated by CYP and is not surprising that effect of CYP on Th1/Th2 immune system has been previously exploited for immune suppression in the clinic18. Moreover, our study is first to report the involvement of a distinct subset of IL-17-secreting T-Helper cells19 in the acute cystitis induced by CYP. Levels of IL-17 were doubled from baseline within 2h after CYP injection and levels at 6h were twice the levels measured at 2h (Fig. 2A). Another unique observation of this study is the dramatic increase in levels of chemokine GRO/KC following CYP injection. Increased levels of GRO/KC have been associated with the release of calcitonin gene-related peptide20 and sympathetic remodeling21. Production of GRO/KC in bladder may be involved in neuro-immune modulation induced by CYP.
We observed that the temporal elevation of most cytokines occurred concurrent to increase in urinary frequency of CYP treated rats. The increased vascular permeability and urothelial damage caused by CYP led to the observation of macroscopic gross hematuria in few urine specimens. The signs of severely inflamed bladder orchestrated by temporal elevation of urinary cytokines were verified by histological analysis of bladder. Bladder histology 24 hours following CYP-injection revealed demonstrable acute inflammatory infiltrate and edema, with minimal mast cell infiltration. While mastocytosis has been implicated in the mediation of the severity of several experimental models of inflammatory cystitis22 as well as IC in human subjects 23, this phenomenon has been mostly observed in chronic disease models.
Tissue cytokine levels were normalized to respective bladder weights and urinary levels of all cytokines were thousand folds lower than the tissue levels. Elevation of urinary cytokines after systemic injection of CYP can arise from activation of immune cells in response to bladder injury within and outside the bladder18. Multi-site action of CYP can probably explain the lack of correlation between urine and tissue levels of cytokines listed in Table I. The tissue levels of few cytokines reported here corroborate those reported by Malley et al8. The 4 fold and 2 fold increase of IL-1β and IL-10 at 24h reported here, agree with similar increase in tissue levels measured at 4h after CYP injection to suggest that elevation seen at 4h is sustained for next 20 hours8. In contrast, the progressive decrease in levels of IL-6 from the peak of 80 fold increase at 4h to only a 4 fold increase at 48h8 was confirmed by a 24 fold increase at 24h reported here after CYP injection.
However, a two fold increase in IL-4 tissue levels at 24h is not in agreement with previously reported lack of change in tissue levels of IL-4 at any time point after CYP injection8. Similarly, the two fold decrease in tissue levels of IL-2 in CYP treated rats at 24h is in disagreement with 4.5 fold increase in mRNA for IL-2 at 4 and 48h8 found in CYP treated tissue. Our results suggest that multiplex analysis of urine cytokines accurately and non-invasively discriminates CYP-treated rats from controls. Future studies on urine of IC patients will be undertaken to validate these findings.
Conclusions
Our findings report a profile of inflammation-associated cytokines in urine and bladder tissue of CYP treated rats. These results demonstrate the utility of the multiplex analysis for urinary proteomics and its potential in the non-invasive assessment of inflammatory bladder disease.
Acknowledgments
This work was supported in part by NIDDK grant DK 066138 (PT) NIDRR grant H133E070024 (YV), NIGMS grant P50-GM-53789-09
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parsons CL. The role of the urinary epithelium in the pathogenesis of interstitial cystitis/prostatitis/urethritis. Urology. 2007;69:9–16. doi: 10.1016/j.urology.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 2.Hanno PM, Landis JR, Matthews-Cook Y, Kusek J, Nyberg L., Jr The diagnosis of interstitial cystitis revisited: lessons learned from the National Institutes of Health Interstitial Cystitis Database study. J Urol. 1999;161:553–7. doi: 10.1016/s0022-5347(01)61948-7. [DOI] [PubMed] [Google Scholar]
- 3.Keay S, Zhang CO, Chai T, Warren J, Koch K, Grkovic D, Colville H, Alexander R. Antiproliferative factor, heparin-binding epidermal growth factor-like growth factor, and epidermal growth factor in men with interstitial cystitis versus chronic pelvic pain syndrome. Urology. 2004;63:22–6. doi: 10.1016/j.urology.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 4.Erickson DR, Tomaszewski JE, Kunselman AR, Bentley CM, Peters KM, Rovner ES, Demers LM, Wheeler MA, Keay SK. Do the National Institute of Diabetes and Digestive and Kidney Diseases cystoscopic criteria associate with other clinical and objective features of interstitial cystitis? J Urol. 2005;173:93–7. doi: 10.1097/01.ju.0000146466.71311.ab. [DOI] [PubMed] [Google Scholar]
- 5.Eichel L, Scheidweiler K, Kost J, Shojaie J, Schwarz E, Messing E, Wood R. Assessment of murine bladder permeability with fluorescein: validation with cyclophosphamide and protamine. Urology. 2001;58:113–8. doi: 10.1016/s0090-4295(01)01007-x. [DOI] [PubMed] [Google Scholar]
- 6.Lanteri-Minet M, Bon K, de Pommery J, Michiels JF, Menetrey D. Cyclophosphamide cystitis as a model of visceral pain in rats: model elaboration and spinal structures involved as revealed by the expression of c-Fos and Krox-24 proteins. Exp Brain Res. 1995;105:220–32. doi: 10.1007/BF00240958. [DOI] [PubMed] [Google Scholar]
- 7.Wood R, Eichel L, Messing EM, Schwarz E. Automated noninvasive measurement of cyclophosphamide-induced changes in murine voiding frequency and volume. J Urol. 2001;165:653–9. doi: 10.1097/00005392-200102000-00089. [DOI] [PubMed] [Google Scholar]
- 8.Malley SE, Vizzard MA. Changes in urinary bladder cytokine mRNA and protein after cyclophosphamide-induced cystitis. Physiol Genomics. 2002;9:5–13. doi: 10.1152/physiolgenomics.00117.2001. [DOI] [PubMed] [Google Scholar]
- 9.Saban MR, Simpson C, Davis C, Wallis G, Knowlton N, Frank MB, Centola M, Gallucci RM, Saban R. Discriminators of mouse bladder response to intravesical Bacillus Calmette-Guerin (BCG) BMC Immunol. 2007;8:6. doi: 10.1186/1471-2172-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batler RA, Sengupta S, Forrestal SG, Schaeffer AJ, Klumpp DJ. Mast cell activation triggers a urothelial inflammatory response mediated by tumor necrosis factor-alpha. J Urol. 2002;168:819–25. [PubMed] [Google Scholar]
- 11.George SK, Dipu MT, Mehra UR, Singh P, Verma AK, Ramgaokar JS. Improved HPLC method for the simultaneous determination of allantoin, uric acid and creatinine in cattle urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;832:134–7. doi: 10.1016/j.jchromb.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 12.Davidson RA, McCloskey KD. Morphology and localization of interstitial cells in the guinea pig bladder: structural relationships with smooth muscle and neurons. J Urol. 2005;173:1385–90. doi: 10.1097/01.ju.0000146272.80848.37. [DOI] [PubMed] [Google Scholar]
- 13.Tyagi P, Chancellor MB, Li Z, De Groat WC, Yoshimura N, Fraser MO, Huang L. Urodynamic and immunohistochemical evaluation of intravesical capsaicin delivery using thermosensitive hydrogel and liposomes. J Urol. 2004;171:483–9. doi: 10.1097/01.ju.0000102360.11785.d7. [DOI] [PubMed] [Google Scholar]
- 14.Erickson DR, Xie SX, Bhavanandan VP, Wheeler MA, Hurst RE, Demers LM, Kushner L, Keay SK. A comparison of multiple urine markers for interstitial cystitis. J Urol. 2002;167:2461–9. [PubMed] [Google Scholar]
- 15.Erickson DR, Belchis DA, Dabbs DJ. Inflammatory cell types and clinical features of interstitial cystitis. J Urol. 1997;158:790–3. doi: 10.1097/00005392-199709000-00025. [DOI] [PubMed] [Google Scholar]
- 16.Lotz M, Villiger P, Hugli T, Koziol J, Zuraw BL. Interleukin-6 and interstitial cystitis. J Urol. 1994;152:869–73. doi: 10.1016/s0022-5347(17)32594-6. [DOI] [PubMed] [Google Scholar]
- 17.Westropp JL, Buffington CA. In vivo models of interstitial cystitis. J Urol. 2002;167:694–702. doi: 10.1016/S0022-5347(01)69129-8. [DOI] [PubMed] [Google Scholar]
- 18.Perini P, Calabrese M, Rinaldi L, Gallo P. The safety profile of cyclophosphamide in multiple sclerosis therapy. Expert Opin Drug Saf. 2007;6:183–90. doi: 10.1517/14740338.6.2.183. [DOI] [PubMed] [Google Scholar]
- 19.Chang H, Hanawa H, Yoshida T, Hayashi M, Liu H, Ding L, Otaki K, Hao K, Yoshida K, Kato K, et al. Alteration of IL-17 Related Protein Expressions in Experimental Autoimmune Myocarditis and Inhibition of IL-17 by IL-10-Ig Fusion Gene Transfer. Circ J. 2008;72:813–9. doi: 10.1253/circj.72.813. [DOI] [PubMed] [Google Scholar]
- 20.Qin X, Wan Y, Wang X. CCL2 and CXCL1 trigger calcitonin gene-related peptide release by exciting primary nociceptive neurons. J Neurosci Res. 2005;82:51–62. doi: 10.1002/jnr.20612. [DOI] [PubMed] [Google Scholar]
- 21.Xie WR, Deng H, Li H, Bowen TL, Strong JA, Zhang JM. Robust increase of cutaneous sensitivity, cytokine production and sympathetic sprouting in rats with localized inflammatory irritation of the spinal ganglia. Neuroscience. 2006;142:809–22. doi: 10.1016/j.neuroscience.2006.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bjorling DE, Jerde TJ, Zine MJ, Busser BW, Saban MR, Saban R. Mast cells mediate the severity of experimental cystitis in mice. J Urol. 1999;162:231–6. doi: 10.1097/00005392-199907000-00073. [DOI] [PubMed] [Google Scholar]
- 23.Theoharides TC, Kempuraj D, Sant GR. Mast cell involvement in interstitial cystitis: a review of human and experimental evidence. Urology. 2001;57:47–55. doi: 10.1016/s0090-4295(01)01129-3. [DOI] [PubMed] [Google Scholar]