Abstract
We examined changes in the efficiency of visual selection over the first postnatal year with an adapted version of a spatial negative priming paradigm. In this task, when a previously ignored location becomes the target to be selected, responses to it are impaired, providing a measure of visual selection. Oculomotor latencies to target selection were the dependent measure. Each trial consisted of a prime and a probe presentation, separated by a 67-, 200-, or 550-msec interstimulus interval (ISI), to test the efficiency of selection as a function of processing time. In the prime, the target was accompanied by a distractor item. In the probe, the target appeared either in the location formerly occupied by the distractor (repeated distractor trials) or in one of the other two locations (control trials). We tested 41 infants in each of 3 age groups (3, 6, and 9 months) on the three different ISIs. Nine-month-old infants’ saccade latencies were slowed on repeated distractors relative to control trials, given sufficiently long ISIs. Saccade latencies in the youngest two age groups showed only facilitation on repeated distractor trials at short ISIs. These results suggest that visual selection efficiency is a function of the interaction of the processing limitations of a system with environmental conditions, in this case the time allotted for the selection process.
The development of visual selection mechanisms supports efficient information gathering and exploration, and in turn efficient perception and learning (Amso & Johnson, 2006). Eye movements are quickly directed at targets, even as they compete with distractors in cluttered visual scenes. The goal of this study is to examine the dynamics of visual selection under conditions of competition between simultaneously presented elements.
Spatial cuing paradigms have yielded important information about the development of visual selection. In the covert spatial cuing paradigm, infants’ attention is engaged with a central visual stimulus, followed by a brief cue flashed to the right or left. After a delay, the peripheral target is presented either in the cued location (valid trial) or in the opposite location (e.g., Harman, Posner, Rothbart, & Thomas-Thrapp, 1994; Hood, 1993, 1995; Richards 2000, 2001). Infants produce faster saccade latencies to valid trials than to invalid trials when the delay between presentations is short, termed facilitation. However, inhibition of return (IOR) will obtain if the delay is longer (see Figure 1). The facilitation effect has been observed in 3- and 4- month-olds (Johnson & Tucker, 1996; Richards 2000, 2001). IOR following covert shifts of attention has not been observed consistently until 6 months. IOR effects presumably reflect a cost to reselecting an item, and theoretically guide effective visual search away from previously attended locations (Klein, 2000).
Figure 1.
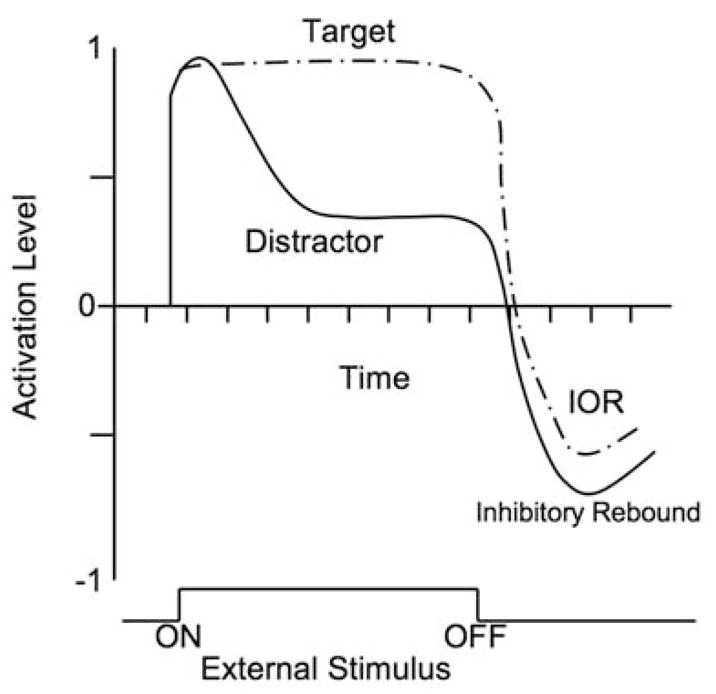
This schematic model depicts the theoretical time course of excitation and inhibition, beginning at display onset and proceeding postoffset, for both the target and distractor items. Adapted from Houghton and Tipper (1996), with permission from the publisher.
Various models of target selection stress the concept of inhibition of simultaneously competing distractors (e.g., Godijn & Theeuwes, 2002; Houghton & Tipper, 1994). For example, in the spatial negative priming (SNP) paradigm, a distractor location that was previously ignored on one trial becomes the target to be selected on the next trial, resulting in impairment of its selection (Neill, 1977; Tipper, 1985). A model proposed by Houghton and Tipper (1994, 1996) suggests that the distractor initially receives a high level of internal activation that soon becomes inhibited or suppressed as the selected target “wins” competition for attention over the distractor. At stimulus offset, inhibition remains and the item is suppressed below its baseline, known as inhibitory rebound (Figure 1), resulting in a delay in the reactivation of the previously ignored representation compared to the activation generated by a novel input. This is taken as an indication of functional selection via suppression of competing information. Variations in the interstimulus interval (ISI) should result in differences in the magnitude of the observed cost (Gibbons & Rammsayer, 2004; Ortells, Noguera, Abad, & Lupianez, 2001), as they allow more or less time for the initial activation and subsequent distractor suppression.
Amso and Johnson (2005) examined visual selection, under conditions of simultaneous target–distractor competition using an adapted SNP method in 9-month-olds and adults. In the prime, the target was accompanied by a distractor. In the probe, a new target appeared in the same location as the distractor (repeated distractor [RD] trials), or in one of the other two locations (control trials). We varied the ISI between prime and probe displays to examine both the presence of excitation, or suppression, and also the ability of the system to maintain that information over a delay. Saccade latencies to the probe in the 67-msec ISI condition were taken as an indication of processing during the 200-msec prime display with virtually no maintenance requirement. The 200-msec and 550-msec ISI conditions allowed more time for the inhibition to continue, if initiated during the prime, below baseline. Successful utilization of these longer intervals for suppression requires maintaining the spatial location “excite or suppress” code over the delay period. Saccade latencies to probe target locations were the dependent measure. Amso and Johnson (2005) found that adults’ saccade latencies were reliably slower in RD versus control trials at all three ISIs tested, corroborating findings from a similar paradigm (Crawford, Hill, & Higham, 2005). Likewise, infants’ saccade latencies were reliably slower in RD trials at the 550- and 200-msec ISIs, although no reliable inhibition or facilitation effect was found at the 67-msec interval. These data provide evidence for functional visual selection in 9-month-olds. Because previous work has shown the first 6 postnatal months to be a time of considerable change in the efficiency of visual selection, here we consider the functionality and efficiency of the dynamics of visual selection in the presence of competition under increasingly demanding temporal conditions at 3, 6, and 9 months of age.
METHOD
Participants
Forty-one infants were included in the final sample. Seventeen 9-month-old infants contributed a full data set (M age = 275 days, SD = 8.35; 10 girls and 7 boys). Eighteen 9-month-old infants were observed but excluded from the sample due to excessive head or body motion yielding insufficient data or poor calibration of the point of gaze (POG; n = 3), fussiness or failure to complete all three ISI conditions (n = 10), or program or experimenter error (n = 5). Twelve 6-month-old infants contributed a full data set (M age = 184.9 days, SD = 13.7; 7 girls and 5 boys). Thirteen 6-month-old infants were observed but excluded from the sample due to excessive movement (n = 2), fussiness or failure to complete all three ISI conditions (n = 6), or program or experimenter error (n = 5). Twelve 3-month-old infants contributed a full data set (M age = 92.5 days, SD = 8.3 days; 6 girls and 6 boys). Twenty 3-month-old infants were observed but excluded from the sample due to excessive movement (n = 6), fussiness or failure to complete all three ISI conditions (n = 13), or program error (n = 1). All infants were full term with no known, developmental disabilities. Infants were recruited via a letter sent to parents listed, in a commercially available database and follow-up phone call. Parents were compensated for travel expenses, and the infant received a toy or t-shirt as a thank-you gift.
Apparatus and Stimuli
Infants were seated approximately 100 cm from a 76-cm monitor while eye movements were recorded using a remote-optics corneal reflection, eye tracker (Applied Science Laboratories Model 504). All trials consisted of a prime and a probe display, each lasting 2,000-msec and separated by an ISI of 550-, 200-, or 67-msec. Each display was made up of a white cross-shaped grid against a black background with four possible locations in which a stimulus could appear (see Figure 2). Infants saw two types of trials, RD and. control. Targets were selected randomly from a series of colorful, animated images that moved in synchrony with various sounds. Targets varied within and between prime and probe presentations to maintain infant interest. In all prime trials, the target and an accompanying distractor (static silent gray diamond) appeared in separate locations. In RD probe trials, a target appeared in the location that the distractor had previously occupied. Evidence of inhibitory distractor effects have been found whether a distractor is present or absent during the probe (Amso & Johnson, 2005; Buckholz, Van Damme, & O’Donnell, 1998; Crawford et al., 2005; Milliken, Tipper, Houghton, & Lupianez, 2000; Neill, Terry, & Valdes, 1994; Tipper, Brehaut, & Driver, 1990). We elected to omit the distractor during the probe to prevent additional data loss due to looking at the distractor during the probe. Control probe trials were identical except the probe target appeared in a different location. We generated 36 possible prime– probe combinations, encompassing both horizontal and vertical grid locations. The program randomly selected from these trials for presentation, with the provision that half of the trials presented were control and half were RD. Trial order was shuffled for each participant. The interval between trials (prime–probe pairs) was 1,500-msec.
Figure 2.
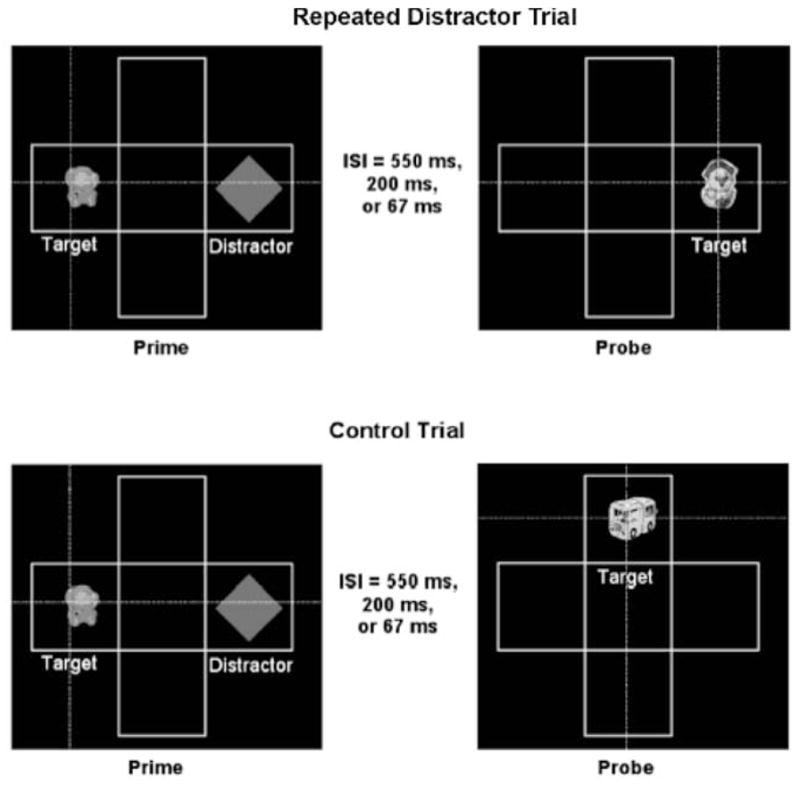
The two types of trials presented to infants. Each trial consisted of two presentations, a prime and a probe. In the repeated distractor probe display, the target appeared in a location occupied by the distractor in the prime. In the control probe display, the target appeared in one of the other possible locations outlined by the grid. The dashed lines represent the observer’s point of gaze. Adapted with permission from Amso and Johnson (2005).
Data from individual trials were considered invalid if the infant (a) fixated the distractor during the prime presentation, (b) did not fixate the target during the prime presentation, (c) produced a preprogrammed eye movement toward the target location in probe trials (saccade latency less than 133-msec for 6- and 9-month-olds and 167-msec for 3-month-olds; see Canfield, Smith, Brezsnyak, & Snow, 1997), or (d) if the POG was not recorded or was directed elsewhere on the stimulus.
Procedure
Each infant’s POG was calibrated by showing an attention-attracting stimulus that, contracted and expanded in synchrony with a rhythmic sound at the top left and bottom, right corners of an imaginary rectangle that contained the possible stimulus locations. The infant then viewed the calibration stimulus at several random locations on the screen. If the POG was not within 0.5° of the center of the attention-getter at all locations (minimum of six), the calibration procedure was repeated. Infants were presented with 48 trials of each ISI condition presented in separate blocks. The order of ISI blocks was counterbalanced. Data (the POG superimposed on the stimuli) were recorded onto digital videotape and coded offline. Interrater reliability (two coders) was high (Pearson r = .95).
RESULTS
The dependent variable was saccade latency to the probe target, defined as the duration between probe target onset and initiation of an eye movement that ended in a fixation, on. the target stimulus. Fixations were defined as portions of eye movement records in which the POG remained within a 1° radius for at least 100-msec. We reasoned that distractor suppression during the prime would be revealed by a difference in saccade latency between RD and control conditions. No sex differences in performance were revealed, nor were there order effects that informed the principal hypotheses; therefore analyses in the three experiments were collapsed across these two variables.
There were differences in overall saccade latency among the three age groups, F(2, 38) = 32.55, p < .001, η2 = .63. Linear contrasts showed that 3-month-old infants were slower (M= 391.30-msec, SD = 63.36) than 6-month-olds (M = 300.70-msec, SD = 59.17), and both were slower than 9-month-old infants (M = 240.89-msec, SD = 25.31), all ps < .01, Bonferroni adjusted alpha level of .017 (.05/3). Hence, we treated average saccade latency as a covariate in the subsequent general linear model (GLM) repeated measures analysis of variance (ANOVA) for two reasons: (a) our principal hypotheses were concerned with indexing target excitation or distractor suppression during the prime display, before oculomotor output saccades were executed to the probe target, and (b) we aimed to reduce effects of within-group variability in saccade latencies, especially in the youngest group, on the RD versus control manipulation of interest.
Average saccade latency values per participant were treated as a covariate in a 3 (ISI: 550, 200, or 67-msec) × 2 (condition: RD vs. control) × 3 (age group: 3-, 6-, 9-month-old infants) GLM repeated measures ANOVA. The analysis yielded a significant main effect of ISI, F(2, 74) = 3.31, p < .05, η2p = .08. Pairwise comparisons showed that latencies were longer overall (all ps < .001, Bonferroni adjusted alpha level of .017) in the 550-msec ISI condition (M = 336.82-msec, SD = 94.82) relative to both the 200-msec and 67-msec ISI conditions (M = 283.57-msec, SD = 93.65, η2 = .37 and M = 286.85-msec, SD = 80.77, η2 = .32, respectively).
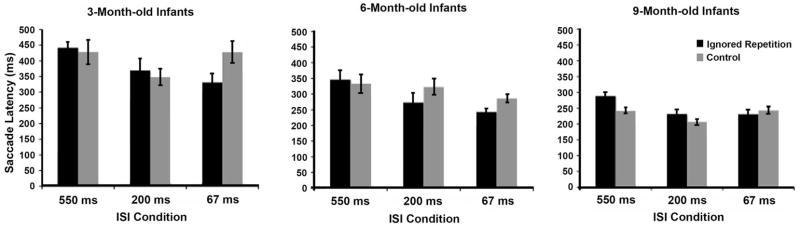
Figure 3 illustrates average RD and control saccade latency values by age group. The analysis indicated no main effect of condition but yielded a significant Condition × ISI interaction, F(2, 74) = 8.35, p = .001, η2p = .18. Planned comparisons (simple effects tests) revealed no reliable differences between RD and control trials in either the 550-msec or 200-msec ISI conditions, but saccade latencies were faster to RD relative to control trials at the shortest ISI, F(1, 40) = 9.56, p < .005, η2 = .19. That is, there was facilitation at 67-msec. We also found a significant Condition × ISI × Age interaction, F(4, 74) = 2.69, p < .05, η2p = .13. Planned comparisons examining performance as a function of age group showed that 9-month-olds were slower on RD relative to control trials at the longest ISI, F(1, 16) = 2.84, p < .05, η2 = .34, and marginally significant at the 200-msec ISI, F(1, 16) = 1.66, p = .117, η2 = .15, and nothing reliable at the 67-msec ISI condition, F(1, 16) = −1.62, ns. Given sufficient time, therefore, 9-month-old infants appear to have suppressed the distractor stimulus during prime target selection. In contrast, 6-month-old infants provided no evidence of inhibition but did show facilitation in both the 200-msec, F(1, 11) = −2.37, p < .05, η2 = .34, and 67-msec ISI conditions, F(1, 11) = −3.81, p < .005, η2 = .57. Finally, 3-month-olds showed some evidence of facilitation only in the 67-msec ISI condition, F(1, 11) = −2.11 p < .06, η2 = .29. These data suggest that the time course of inhibition in 3- and 6-month-old infants is slower than in 9-month-olds.
Figure 3.
Repeated distractor and control saccade latency data for infants in each of the three ISI conditions. Only 9-month-olds provided reliable evidence of spatial negative priming. Three- and 6-month-old infants showed only facilitation.
Inefficiency in distractor suppression during the prime might lead to interference from the distractor. One possible consequence is an increased tendency to look at the distractor, a tendency that might be greater in infants who are the most susceptible to interference. Figure 4 shows looks to the distractor during the prime, calculated as a proportion of total number of trials provided per participant. An ANOVA examining differences in distractibility as a function of age revealed significant differences in the proportion of looks to the distractor during the prime display, F(2, 38) = 33.88, p < .001, η2 = .64. Three-month-olds were reliably more distractible than both 6- and 9-month-old infants (all ps < .001, Bonferroni adjusted alpha level of .017).
Figure 4.
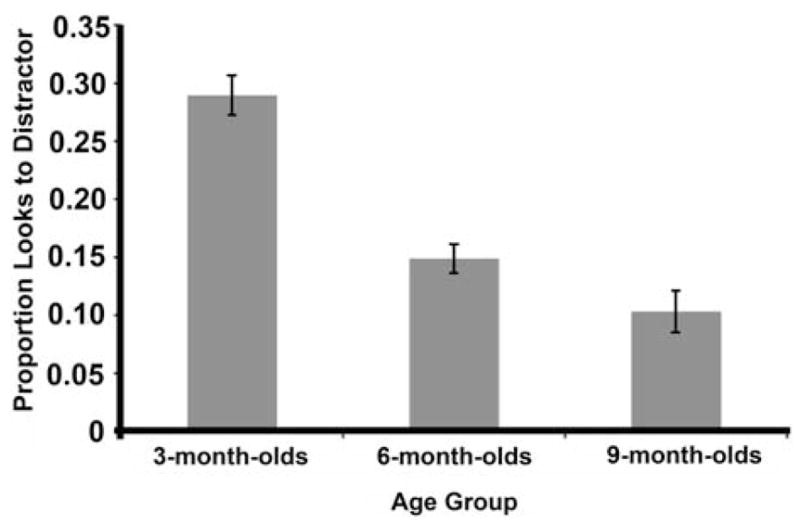
Proportion of looks to the distractor during the prime display. Three-month-olds looked reliably more often at the distractor than did either 6- or 9-month-old infants, suggesting greater susceptibility to interference.
GENERAL DISCUSSION
We tested the functionality and efficiency of visual selection via suppression of distractor interference across the first postnatal year. Three-, 6-, and 9-month-old infants were tested in an adapted SNP paradigm, each participant completing three task conditions that differed in the length of the interval between prime and probe presentations (550-, 200-, and 67-msec ISIs). Slower saccade latencies in RD relative to control probe trials were observed in 9-month-olds after the 550-msec ISI, and marginally after the 200-msec ISI conditions. This suggests that the process of target excitation or distractor suppression during the prime induced a cost of reselecting the previously ignored location. As predicted, longer ISI intervals allowed for the maintenance of the target excitation or distractor suppression process in the absence of visual input, and the distractor was suppressed below baseline levels. As in the Amso and Johnson (2005) study, the prime duration coupled with the shortest 67-msec ISI was insufficient for completion of this process in 9-month-old infants. Note that the 200-msec ISI condition yielded an only marginally significant result in this study, whereas this effect was significant in Amso and Johnson (2005). Given the same general pattern of results as a function of ISI in this age group, and a lack of an ISI presentation order effect, it may be that the within-subjects design (necessitating a greater number of trials) resulted in greater fatigue, and as a consequence more variability in this data set.
We found no evidence of reliable selection via inhibition in 3- and 6-month-olds. Instead, both younger age groups were faster to the probe target on RD relative to control trials in the 67-msec ISI task condition, providing evidence of facilitation and indicating that the prime and ISI interval was not sufficient for distractor suppression. Rather, responses to the distractor were still benefiting from activation in the prime. Note that the ISI manipulation impacted response latencies in the RD and not the control condition; observed facilitation is not a function of a slowing of responses to the control condition across ISIs (see Figure 2). The 550-msec ISI may have placed excessive demands on maintenance, resulting in decay and lack of subsequent facilitation. Three-month-olds showed facilitation only in the 67-msec ISI interval, whereas 6-month-olds showed facilitation to the location previously occupied by the distractor in both the 67-msec and 200-msec ISI. One interpretation of these findings is that 3-month-olds were still experiencing concurrent target or distractor excitation at prime trial end, but that this representation was either too fragile to maintain over the 200-msec interval or that the 200-msec interval placed excessive demands on maintenance in general. Ineffective maintenance should yield poor attenuation of distractor interference during prime selection. Consistent with this interpretation, we found developmental differences in susceptibility to this distractor interference, as measured by the proportion of looks to the distractor during the prime presentation (see Figure 4). Future work can further test this interpretation with the addition of a condition in which the target location is repeated in the probe. Facilitation to the repeated target location should not obtain in 3- relative to 6-month-olds at longer delays. Another question for future work is whether a longer prime duration will allow sufficient time for the youngest infants to provide evidence of selection via inhibition when no delay is imposed.
We have shown that the efficiency of selection changes over the first postnatal year and suggest that it bears on information gathering and visual exploration in infancy. Target elements are in continuous competition with other elements in a visual scene. The robustness of the selection process is determined by the capacity of the system to suppress distractor interference. Susceptibility to interference may result in weak representations of target features during active exploration, and the immature (distractible) system may require more exposure to an item to fully extract its characteristic information.
This work makes an important contribution to the developmental selection literature by measuring the cost of recent distractor effects. One difference between this and standard SNP tasks is the omission of a distractor on probe trials. Amso and Johnson (2005) found the expected cost to selecting a previously ignored item using this adapted approach. Indeed, although some work found the effect intact with this omission, other work has found the effect weakened by it. This limitation may have impacted selection state and contributed to the weakened inhibitory effect found in the 3- and 6-month-olds. Regardless, our focus was on prime trial selection in the presence of a competing distractor rather than on the dynamics of probe trial selection. Selection efficiency improved over the first postnatal year and is provisional on the time allotted for effective suppression to take place. However, there is likely no one set of circumstances that support processing efficiency, but rather numerous conditions under which the environment is more or less optimally interacting with the capabilities of the system.
Contributor Information
Dima Amso, Department of Psychiatry, Sackler Institute for Developmental Psychobiology, Weill Medical College of Cornell University.
Scott P. Johnson, Department of Psychology, University of California, Los Angeles
References
- Amso D, Johnson SP. Selection and inhibition in infancy: Evidence from the spatial negative priming paradigm. Cognition. 2005;95:B27–B36. doi: 10.1016/j.cognition.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Amso D, Johnson SP. Learning by selection: Visual search and object perception in young infants. Developmental Psychology. 2006;42:1236–1245. doi: 10.1037/0012-1649.42.6.1236. [DOI] [PubMed] [Google Scholar]
- Buckholz E, Van Damme J, O’Donnell C. Positive priming: Locations containing relevant information are labeled accordingly. Human Movement Science. 1998;17:781–799. [Google Scholar]
- Canfield RL, Smith EG, Brezsnyak MP, Snow KL. Information processing through the first year of life: A longitudinal study using the visual expectation paradigm. Monographs of the Society for Research in Child Development. 1997;62 (2, Serial No. 250) [PubMed] [Google Scholar]
- Crawford TJ, Hill S, Higham S. The inhibitory effect of a recent distractor. Vision Research. 2005;45:3365–3378. doi: 10.1016/j.visres.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Gibbons H, Rammsayer TH. Differential effects of prime-probe duration on positive and negative location priming: Evidence for opponent facilitatory and inhibitory influences in priming tasks. The Quarterly Journal of Experimental Psychology. 2004;57:61–86. doi: 10.1080/02724980343000125. [DOI] [PubMed] [Google Scholar]
- Godijn R, Theeuwes J. Programming of endogenous and exogenous saccades: Evidence for a competitive integration model. Journal of Experimental Psychology: Human Perception and Performance. 2002;28:1039–1054. doi: 10.1037//0096-1523.28.5.1039. [DOI] [PubMed] [Google Scholar]
- Harman C, Posner MI, Rothbart MK, Thomas-Thrapp L. Development of orienting to locations and objects in human infants. Canadian Journal of Psychology. 1994;43:301–318. doi: 10.1037/1196-1961.48.2.301. [DOI] [PubMed] [Google Scholar]
- Hood BM. Inhibition of return produced by covert shifts of visual attention in 6-month-old infants. Infant Behavior and Development. 1993;16:245–254. [Google Scholar]
- Hood BM. Shifts of visual attention in the human infant: A neuroscientific approach. Advances in Infancy Research. 1995;10:163–216. [Google Scholar]
- Houghton G, Tipper SP. A model of inhibitory mechanisms in selective attention. In: Dagenbach D, Carr T, editors. Inhibitory mechanisms of attention, memory, and language. New York: Academic; 1994. pp. 53–112. [Google Scholar]
- Houghton G, Tipper SP. Inhibitory mechanisms of neural and cognitive control: Applications to selective attention and sequential action. Brain & Cognition. 1996;1:20–43. doi: 10.1006/brcg.1996.0003. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Tucker LA. The development and temporal dynamics of spatial orienting in infants. Journal of Experimental Child Psychology. 1996;63:171–188. doi: 10.1006/jecp.1996.0046. [DOI] [PubMed] [Google Scholar]
- Klein RM. Inhibition of return. Trends in Cognitive Sciences. 2000;4(4):138–147. doi: 10.1016/s1364-6613(00)01452-2. [DOI] [PubMed] [Google Scholar]
- Milliken B, Tipper SP, Houghton G, Lupianez J. Attending, ignoring, and repetition: On the relation between negative priming and inhibition of return. Perception and Psychophysics. 2000;62:1280–1296. doi: 10.3758/bf03212130. [DOI] [PubMed] [Google Scholar]
- Neill WT. Inhibitory and facilitatory processes in selective attention. Journal of Experimental Psychology: Human Perception and Performance. 1977;3:444–450. [Google Scholar]
- Neill WT, Terry KM, Valdes L. Negative priming without probe selection. Psychonomic Bulletin and Review. 1994;1:119–121. doi: 10.3758/BF03200767. [DOI] [PubMed] [Google Scholar]
- Ortells JJ, Noguera C, Abad MJF, Lupianez J. Influence of prime-probe stimulus onset asynchrony and prime cueing manipulations on semantic priming effects with words in a lexical-decision task. Journal of Experimental Psychology: Human Perception and Performance. 2001;27:75–91. doi: 10.1037//0096-1523.27.1.75. [DOI] [PubMed] [Google Scholar]
- Richards JE. Localizing the development of covert attention in infants using scalp event-related-potentials. Developmental Psychology. 2000;36:91–108. [PubMed] [Google Scholar]
- Richards JE. Cortical indices of saccade planning following covert orienting in 20-week-old infants. Infancy. 2001;2:135–157. [Google Scholar]
- Tipper SP. The negative priming effect: Inhibitory effects of ignored primes. Quarterly Journal of Experimental Psychology. 1985;37A:571–590. doi: 10.1080/14640748508400920. [DOI] [PubMed] [Google Scholar]
- Tipper SP, Brehaut JC, Driver J. Selection of moving and static objects for the control of spatially directed action. Journal of Experimental Psychology: Human Perception and Performance. 1990;16:492–504. doi: 10.1037//0096-1523.16.3.492. [DOI] [PubMed] [Google Scholar]