Abstract
Disorders of the central nervous system (CNS) are complex disease states that represent a major challenge for modern medicine. Although etiology is often unknown, it is established that multiple factors such as defects in genetics and/or epigenetics, the environment as well as imbalance in neurotransmitter receptor systems are all at play in determining an individual’s susceptibility to disease. Gene therapy is currently not available and therefore, most conditions are treated with pharmacological agents that modify neurotransmitter receptor signaling. Here, I provide a review of ionotropic glutamate receptors (iGluRs) and the roles they fulfill in numerous CNS disorders. Specifically, I argue that our understanding of iGluRs has reached a critical turning point to permit, for the first time, a comprehensive re-evaluation of their role in the cause of disease. I illustrate this by highlighting how defects in AMPA receptor trafficking are important to Fragile X mental retardation and ectopic expression of kainate (KA) receptor synapses contributes to the pathology of temporal lobe epilepsy. Finally, I discuss how parallel advances in studies of other neurotransmitter systems may allow pharmacologists to work towards a cure for many CNS disorders rather than developing drugs to treat their symptoms.
Keywords: Kainate receptors, AMPA receptors, epilepsy, Fragile-X Syndrome, mental retardation, neurodegeneration, glioblastoma, neurogenesis
CNS disorders were first recorded in cultures as far back as antiquity and thus have long troubled society [1,2]. Amongst the many notable historical personages afflicted with various nervous ailments, both Aristotle and Julius Caesar were said to have suffered from epilepsy (see Traicté de l'Epilepsie by Jean Taxol, 1602) whereas Mozart may have been a manic depressive [3]. By any modern medical definition, such individuals would have been incapacitated significantly during their life; however, societies were often ambivalent to this. Instead, most were eager to attribute special qualities or even greatness to them. The 15th century French heroine, Joan of Arc, has been revered for her religious visions and revelations. However, more recent evaluations has suggested, at least to some scholars, that her behaviour was consistent with schizophrenia and/or epilepsy [4,5]. Although this and other examples may always remain a matter of conjecture and debate, CNS disorders are complex illnesses that continue to represent a major challenge to neurology and psychiatry.
In this review, I discuss the contribution of ionotropic glutamate receptors to disorders of the CNS. iGluRs represent the major excitatory neurotransmitter pathway in the brain and consequently, they are essential for normal CNS function but are also implicated in numerous disease states. I provide an assessment of their role in disease with special reference to AMPA- and KA-type iGluRs. Research in neuroscience over the last two decades has brought important advances in our understanding of the brain. I argue that this progress has reached a turning point for future work on iGluRs and disease.
AETIOLOGY OF CNS DISORDERS IS MULTIFACTORIAL
With the advent of reliable diagnostic criteria, it is now clear that CNS disorders can affect any member of any society at any point in life. Postnatal neurodevelopmental disorders, such as Autism, strike in the first years of an infant’s life [6], mood or anxiety disorders first appear in early adulthood [7] whereas neurodegenerative conditions, such as parkinsonism or dementias like Alzheimer’s disease, typically develop later in life [8]. Although most CNS disorders have unknown aetiology, dysfunction is commonly attributed to not one but a combination of defects that often include excitatory or inhibitory neurotransmission, its modulation by other neurotransmitter pathways (e.g. dopaminergic, cholinergic, serotonergic), environmental or epigenetic factors as well as genetics (Fig. 1). For example, although the genetic origin of some CNS disorders, such as Rett’s syndrome and Fragile X mental retardation [9,10], is well established, the environment and quality of care [11] as well as epigenetic factors [12] all contribute to affect long-term prognosis. Despite this level of understanding, treatment in most cases relies on targeting a patient’s symptoms rather than tackling the underlying cause(s). As explained in the following section, the development of present day therapeutic compounds often occurred by chance observations rather than exploiting an approach focused on a knowledge-base of the disease or rationale-drug design.
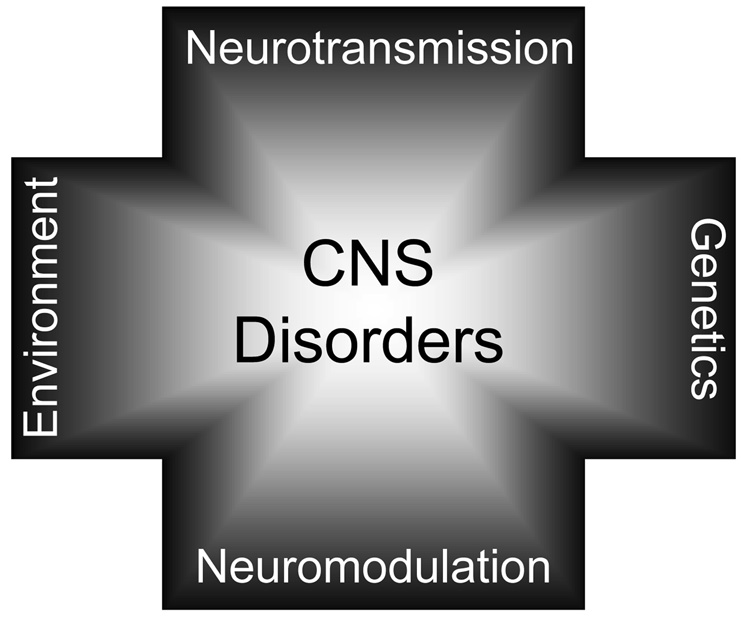
Figure 1. CNS DISORDERS ARE COMPLEX PSYCHIATRIC CONDITIONS.
Multiple factors predispose the developing and adult CNS to disease. This axis includes defects in glutamatergic and GABAergic neurotransmission, neuromodulation by other transmitter systems such as dopaminergic and cholinergic, genetic and epigenetic factors. Although the contribution of each predisposing factor may vary depending on the disease state, in most cases, all can negatively impact the prognosis.
CNS DISORDERS ARE TREATED BY TARGETING SYMPTOMS
The origin of current drug therapy used to alleviate the symptoms of CNS disorders was developed in the 1950s, in many cases, quite serendipitously (Fig. 2). For example, the early typical antipsychotic, chlorpromazine, was synthesized in 1949 as an agent to potentiate anaesthesia before being recognised in 1951 for its value in the treatment of schizophrenia [13]. The hypothesis that schizophrenia may represent hyperfunction of dopaminergic signaling was proposed, in part, after chlorpromazine and other phenothiazines were shown to act as D2 receptor antagonists [14,15]. From then on, the possibility that neurochemical disturbances may underlie the cause of many CNS disorders began in earnest [16,17]. In Alzheimer’s disease, a striking loss in neurotransmitter content, particularly for acetylcholine, was shown to parallel neuronal loss associated with the onset of dementia [18]. With the observation that central cholinergic antagonists precipitate confusion reminiscent of dementia [18] attention was focused on agents that would mimic or enhance the actions of acetylcholine. Although newer compounds have been developed to treat CNS disorders in the last five decades, in almost all cases, the overall strategy has been to treat the symptoms whilst offering the patient a drug regime with as few side-effects as possible. Many drugs however are completely ineffective or exhibit appreciable side-effects accounting for poor drug compliance amongst many patients [19–22].
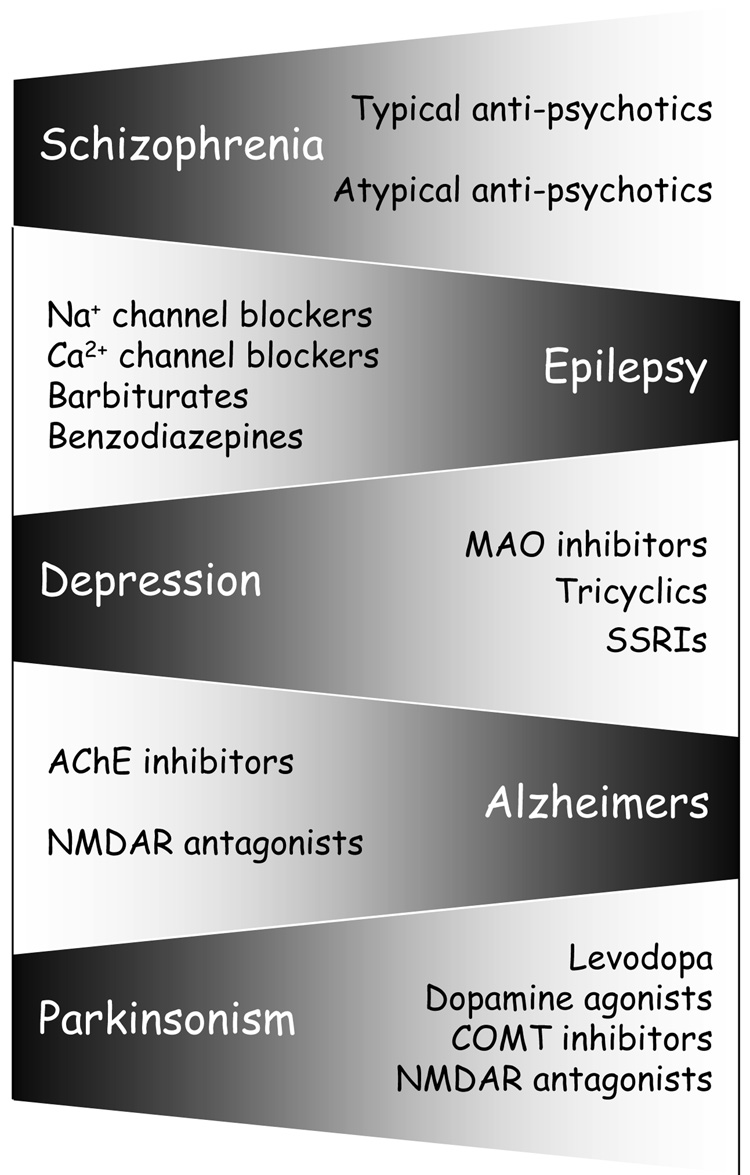
Figure 2. CNS DISORDERS ARE TREATED BY TARGETING THE SYMPTOMS.
A list of most of the major CNS disorders that afflict society and the drugs commonly used to treat them. Despite advances in our understanding of the CNS, most conditions are treated by attempting to alleviate symptoms rather than tackling the root cause of the disease.
If substantial improvement in the treatment of CNS illness is to be achieved, two factors need to be considered; (i) identify the underlying causes of the CNS disorder and (ii) employ rationale drug design to limit potential side-effects. As explained below, advances in our understanding of ionotropic glutamate receptors (iGluRs) over the last 30 years have now made it possible to re-examine CNS disorders associated with this important neurotransmitter receptor family. Before doing so, it is first necessary to provide an overview of the some of the roles played by iGluRs in the CNS.
IONOTROPIC GLUTAMATE RECEPTORS ARE PREDOMINANT NEUROTRANSMITTER RECEPTORS
The pivotal position that we now associate with iGluRs in the CNS was not immediately realised by early neuroscientists. In fact, much doubt on the suitability of L-glutamate (L-Glu) as a putative neurotransmitter candidate was passionately debated (K. Krnjevič, personal communication). The principal argument was that L-Glu was already known to play key roles in the animal and plant kingdoms. Specifically, it linked the metabolism of carbon and nitrogen and, depending on requirement, served either as a source of energy or source of ammonia [23]. Therefore, although L-Glu was originally identified in 1866 by H. Ritthausen [24], it took almost another 100 years before its role in the CNS was considered (Fig. 3). Early on, L-Glu was known to be an important constituent of nervous tissues [25], however, it was not until Hayashi in 1954 demonstrated convulsant effects of L-Glu in the cerebral cortex that a specific and direct excitatory effect on gray matter was noted [26,27] (Fig. 3). The action of L-Glu was subsequently investigated by Curtis and colleagues [28,29] by intracellular recordings which showed that L-Glu depolarised individual neurons in the cat spinal cord. At this time, L-Glu was still being viewed more as a pharmacological agent rather than a neurotransmitter candidate. However in 1963, Krnjevič & Phillis formally argued in favour of L-Glu being a major excitatory neurotransmitter by studying iontophoretic Glu-responses in the cerebral cortex [30,31] (Fig. 3).
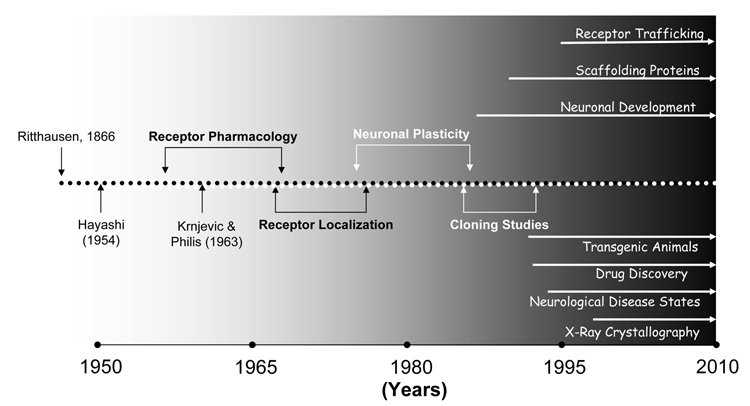
Figure 3. DISCOVERY TIMELINE HIGHLIGHTS MAJOR ADVANCES AND TRENDS IN UNDERSTANDING IONOTROPIC GLUTAMATE RECEPTORS.
Although the amino acid, L-Glu, was identified by Ritthausen almost 150 years ago, neurophysiologists in the 1950s ardently debated whether it played any role at all as a neurotransmitter in the CNS. However, early work by Hayashi (1953) and Krnjevič & Philis (1963) lead the way as other groups, particularly Jeffrey Watkins and colleagues, identified selective agonists and antagonists. This work, in turn, permitted the distribution of different iGluR families to be mapped out in the CNS. During the 1980s–90s, cloning studies and advances in understanding the prominent role of iGluRs in synaptic plasticity precipitated an influx of biomedical researchers into the iGluR field which has sustained a comprehesive and ongoing analysis of their function in the CNS.
Over the next two decades, work on iGluRs intensified due to two main reasons. First, medicinal chemist, Jeff Watkins and his colleagues, headed advances in receptor pharmacology by identifying a number of naturally occurring or synthetic agonists and antagonists that distinguished between iGluR subtypes [32–34] (Fig. 3). N-methyl-D-aspartate (NMDA)-type iGluRs were identified by their sensitivity to the agonist, NMDA, a synthetic analogue of L-Asp [35], and block by Mg2+ ions [36,37]. Non-NMDA receptors responded to L-α-kainic acid (KA), isolated from seaweed [38] (see below), and quisqualic acid (QA), extracted from a Cambodian nut of the plant genus Quisqualis [39], and were insensitive to Mg2+ [32]. Later, non-NMDA receptors were further subdivided into QA/AMPA- and KA-preferring classes as selective antagonists such as L-glutamic acid diethyl ester (GDEE) [40] and γ-D-glutamylglycine (γ-GG) [41] were tested [32–34]. The second reason for rapid growth in interest of iGluRs was the emergence of radioligand binding and autoradiographic techniques. Both approaches permitted large-scale screening of receptor ligands and the mapping of the various iGluR subtypes to distinct CNS regions [42,43] (Fig. 3). Interestingly, this work lagged behind advances using electrophysiology primarily due to the difficulty of identifying optimal conditions that would permit specific binding. Eventually it was realised that [3H]-Glu binding in the presence of Cl− ions primarily reflected transport of amino acids into resealed membrane vesicles [44]. Determination of iGluR subtypes and their localization was then attained by simply eliminating Cl− from the assay medium [42,45] often substituted with the chaotropic anion, thiocynanate [46–48].
By the beginning of the early 1990s, several important breakthroughs were made that have subsequently shaped much of the current research into iGluRs. First, the molecular identity of AMPA-,KA- and NMDA-type iGluRs was elucidated in a series of landmark cloning studies primarily from the labs of Steve Heinemann, Peter Seeburg and Shigetada Nakanishi [49–51]. Orphan-class iGluR subunits, δ1 and δ2, were also identified at this time [52,53]. By identifying the molecular identity of all iGluR subunits, these studies have been pivotal for the generation of genetically modified animals which, as described below, have given significant insight into the role of iGluRs in several CNS disorders. Additionally, iGluR clones provided the foundation for recent advances in structure-function analysis of iGluRs [54–57] which is motivating efforts towards improved drug design (Fig. 3). The second key observation that has shaped present work on iGluRs was proof of their central role in synaptic plasticity such as long-term potentiation (LTP) and long-term depression (LTD) [58–60] (Fig. 3). Importantly, this body of work underlined the importance of iGluRs in learning and memory [61,62], neuronal development [63–66] as well as neurodegenerative disorders such as stroke [67]. More recently, attempts to understand the molecular basis of synaptic plasticity has led to the identification of trafficking pathways for AMPARs, NMDARs, KARs and orphan-class delta-2 receptors into and out of central synapses [68–75] and their reliance on numerous trafficking proteins such as the PDZ-domain family [76,77] and membrane associate guanylate kinases (MAGUKs) [78], auxiliary proteins such as transmembrane AMPAR regulatory proteins (TARPs) [79,80] and the pentraxins which cluster iGluRs through extracellular protein interactions [81] (Fig. 3).
IONOTROPIC GLUTAMATE RECEPTOR SUBTYPES FULFILL DISTINCT ROLES IN THE CNS
It is now possible to assign distinct functional roles to the different iGluR subfamilies (Fig. 4). NMDARs primarily operate as coincidence detectors at central synapses [82,83] and/or as entry points for Ca2+ ions into the cell to drive neuronal development [84,85] (Fig. 4). Coincidence detection is established because tonic Mg2+ block of the channel pore [86,87] permits signalling through the NMDAR only when postsynaptic depolarization is paired with presynaptic release of neurotransmitter [88]. Depolarization can occur either by activation of AMPARs [89], which are often co-localized with NMDARs [90], or by backpropagating action potentials [91–93]. In terms of neuronal development, high Ca2+-permeability of NMDARs [94,95] coupled with the tardiness of their gating kinetics [96] makes them ideal for triggering Ca2+-dependent signalling events in the cytoplasm.
Figure 4. IONOTROPIC GLUTAMATE RECEPTORS FULFILL DISTINCT ROLES IN THE CNS.
Summary table identifying each iGluR subfamily (left column) with the individual subunits that assemble as mature receptors (middle column) and their respective roles within the CNS (right column).
In contrast to NMDARs, native AMPARs have rapid gating kinetics [97–100] and, at most synapses, are weakly permeable to external Ca2+ ions [101–104]. These functional properties of AMPARs and their close association with NMDARs established early on their fundamental role in the hardwiring of neuronal circuits (Fig. 4). However, this original function assigned to AMPARs is more complicated on two counts. First, studies over the last decade or so have identified synapses which are devoid of AMPARs but contain NMDARs [105,106]. From a functional standpoint, synapses of this nature are rendered “silent” to glutamate release since NMDARs are tonically blocked by Mg2+ at resting membrane potentials. The proportion of AMPAR-deficient synapses is greater in the neonatal CNS than in the adult [107–109] suggesting that re-organization of different synaptic iGluRs is important for neuronal development. The second complication is that not all AMPARs are Ca2+-impermeable. In fact, many inhibitory interneurons and glial cells primarily express AMPARs with an appreciable Ca2+-permeability (CP-AMPARs) [110,111] which, as explained below, often implicates this iGluR subtype in CNS disorders. Interestingly, recent findings in the developing retina suggest that CP-AMPARs are probably the only iGluR expressed in inhibitory cells [112,113] suggesting that they may replace the traditional role of NMDARs in providing Ca2+ entry for neuronal maturation. An added complication in the retina is that not all CP-AMPARs can be blocked by external polyamines, such as philanthotoxin (PhTX), as once thought [110,111,114], but acquire PhTX-insensitive CP-AMPARs during development [112]. Whether CP-AMPARs expressed in other CNS regions also exhibit this unconventional pharmacology remains to be established.
Unlike AMPARs, KARs are present at fewer synapses and therefore are thought to fulfill more of a neuromodulatory role in the CNS [115–117]. Although recombinant KARs exhibit similar fast activation and desensitization similar to AMPARs [54,118], synaptic events mediated by native KARs curiously have slow kinetics [119–121]. The molecular basis for this difference is presently unresolved though a number of possibilities have been considered. It was initially thought that slow events reflected the extrasynaptic location of KARs. However, ultrastructural staining for KAR subunits [122] and experiments interfering with glutamate clearance [123] place KARs in the synapse directly apposed to neurotransmitter release sites. It was also proposed that the association of recombinant KARs with the scaffolding protein, PSD-95, was sufficient to convert their phenotype to that of synaptic receptors [124]. However, recent work has demonstrated a much more modest effect of PSD-95 on KAR properties [125]. Bowie & Lange (2002) have suggested that slow recovery from desensitization could trap synaptic KARs into a conducting state with slow activation kinetics [118]. Although this possibility remains to be formally tested, several studies have now recorded KAR-mediated synaptic events with kinetic properties comparable to recombinant receptors [126–128]. How these more recent observations relate to earlier findings clearly requires further investigation.
Although orphan iGluR subunits, δ1 and δ2, are found in the CNS, their functional properties remain elusive. This is due primarily to the fact that neither δ1 or δ2 apparently form functional channels alone or in combination with other iGluR subunits [52,53]. δ2 is largely expressed in Purkinje cells of the cerebellum where it has been shown to fulfill several critical roles. In knockout or hotfoot mice, where δ2 fails to exit the endoplasmic reticulum [129], the absence of surface expressed δ2 causes ataxia and disrupts fear conditioning [130–133]. At the synaptic level, deletion of δ2 interferes with LTD at the parallel-fiber-Purkinje cell synapse [131] which may account for some of these behavioral correlates [134].
Taken together, this work has established the prevalence of iGluRs in the vertebrate brain. In terms of CNS disorders, it has been recognized for some time that excessive activation of NMDARs and subsequent Ca2+-load triggers cell death in cerebral insult, such as in stroke [67], or in neurodegenerative conditions, such as Alzheimer’s [135] or Huntington’s disease [136]. Indeed, the NMDAR channel blocker, memantine [137], is currently used in Europe, the US and Canada in the treatment of moderate-to-severe Alzheimer’s disease [138,139] representing the only therapeutic compound targeted to iGluRs currently in clinical use. The role of AMPARs and KARs has not been given as much attention and, therefore, will be the subject of the remainder of this review. As outlined below, both iGluR subtypes have been implicated in a number of CNS disorders. However, in some cases, improper signaling through KARs and AMPARs may be at the heart of the disease state. I will illustrate this perspective by reviewing how our understanding of AMPAR trafficking is providing insight into the postnatal neurodevelopmental disorder, Fragile-X mental retardation and how ectopic expression of KARs contributes significantly to the pathology of temporal lobe epilepsy.
AMPA Receptors & CNS Disorders
NEURODEGENERATION, CANCER & MENTAL RETARDATION
Given the wide distribution of AMPA-selective ionotropic glutamate receptors (iGluRs) in the vertebrate CNS [54], it is perhaps not wholly surprising that this class of neurotransmitter receptor is involved in a large number of CNS disorders (Fig. 5). To date, AMPARs have been implicated in several neurodevelopmental disorders, such as Fragile-X mental retardation [140–145] and schizophrenia [145–147], neurodegenerative conditions, such as Alzheimer’s disease [145,147], motor neuron disease or Amyotrophic Lateral Sclerosis (ALS)[148–153], stroke [146,149,154–159] and parkinsonism [146,147,160–164], as well as contributing to the proliferation of glioblastoma tumors [165–168]. Furthermore, AMPARs have also been suggested to fulfill roles in depression [146,147,160] as well as the seizure spread and the neuronal damage associated with epilepsy [169] [158,170–172] and Rasmussen's syndrome [173,174].
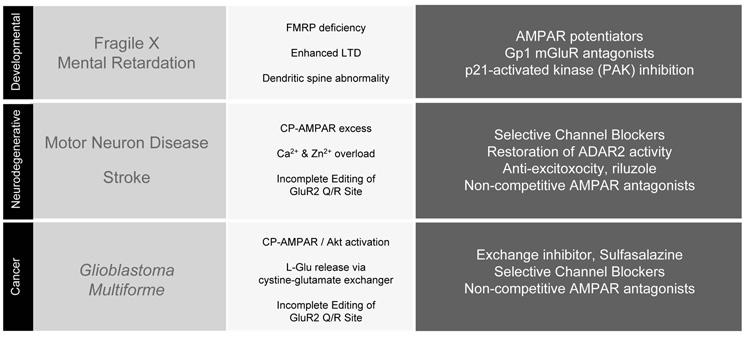
Figure 5. AMPA RECEPTORS AND CNS DISORDERS.
Table listing a number of disease states (left column) where defective signaling through AMPARs has been established. The middle column summarizes key characteristics associated with each disorder and the right column refers to the drug classes whose actions may have therapeutic value.
In acute or chronic neurodegenerative conditions, such as stroke and ALS respectively, a number of studies point to a common mechanism whereby excessive activation of Ca2+-permeable AMPARs (CP-AMPARs) leads to cell death [110,149] (Fig. 5). NMDARs and transient receptor potential (TRP) channels have significant roles in stroke [175] and many factors contribute to ALS [176]. Therefore, the detrimental effects mediated by CP-AMPARs can account for only some of the features in each disease. With this in mind, the selective expression of CP-AMPARs in the adult CNS [102,103,110,111,114] may explain why only certain neuronal populations are vulnerable to excitotoxicity. More specific factors may be important to consider too. For example, in ALS it has been proposed that the selective loss of motor neurons in the spinal cord reflects the excessive number of CP-AMPARs coupled with low Ca2+-buffering capacity [148]. How overload then leads to neuronal injury may be dependent on the magnitude of Ca2+ accumulation. In circumstances where it is excessive, Ca2+ sequestered into the mitochondria may trigger neuronal death through the release of apoptotic mediators such as cytochrome c which trigger caspase-dependent cell death [149]. With more intermediate changes, caspaseindependent pathways may come into play triggered, for example, by activation of Poly(ADP-ribose) polymerase 1 (PARP-1) [177]. With these factors in mind, particular attention has focused on which features of the AMPAR regulate Ca2+-permeability.
Early studies highlighted the role of GluR2 which is the only AMPAR subunit to form Ca2+-impermeable homomers [178,179]. Expression of all other AMPAR subunits, GluR1, 3 and 4, leads to appreciable divalent permeability. The reason for this distinction is due to a single amino acid residue, the so called Q/R site [180,181], which is located at the apex of the ion-channel pore region [182,183], and therefore ideally positioned to influence divalent ion-flow. The Q/R site of GluR1, 3 and 4 subunits contain the neutral amino acid, glutamine (i.e. Q) whereas GluR2 subunits possess a positively charged arginine (i.e. R) which most likely restricts divalent ion transport through electrostatic repulsion [114]. With this understanding, it was then shown in several studies that inclusion of the GluR2 subunit into heteromeric AMPARs reduces Ca2+-permeability [103,184,185]. In models of ischemia, as in stroke, it has been postulated that insult leads to downregulation of GluR2 with the result that AMPARs exhibit much greater Ca2+-permeability [186,187]. In support of this, CP-AMPARs have been identified in the principal neurons of the CA1 region of the hippocampus which is particularly vulnerable to ischemic insult [188–190]. Other factors may also be at play however. Contrary to earlier reports [103], the Q/R site of GluR2 subunit is not fully edited. Indeed, several studies suggest that in ALS the efficiency of the editing process is reduced rendering AMPARs more permeable to Ca2+ [191–193] (Fig. 5). Finally, Ca2+ is not the only divalent ion, however, that can initiate cell death by permeation through CP-AMPARs. Zn2+ transport is proposed to also lead to cytoplasmic overload and, much like Ca2+, mitochondrial damage occurs with caspase-induction and PARP-1 activation [194] (Fig. 5).
Several therapeutic strategies aimed at affecting AMPAR signaling have been proposed for the treatment of ALS and stroke (Fig. 5). Selective channel blockers of CP-AMPARs, such as 1-naphthyl acetyl spermine, are being considered given their effectiveness in models of forebrain ischemia [195]. With incomplete editing of GluR2 subunit Q/R site in both ALS and stroke, several studies have proposed restoring the activity of ADAR2, a mammalian adenosine deaminase [196], by using constitutively-active CREB [154,159]. In ALS, the anti-excitoxic drug, riluzole, is currently being used to delay the onset of ventilator-dependence in patients [148]. Finally, non-competitive AMPAR antagonists, such as the 2,3 benzodiazepines, exhibit valuable properties that may be useful in the treatment of stroke [155].
CP-AMPARs also play significant roles in glioblastoma multiforme; the most common and aggressive CNS tumor thought to originate from glial progenitor cells [197]. Treatment usually involves palliative measures such as chemotherapy, radiotherapy and surgery, all of which slow disease progression without providing a cure. The survival rate of the disease has remained in most cases at 3 percent over the last 30 years [198], in part, due to the ineffectiveness of surgery in stemming infiltration and invasion [199]. Clearly, additional therapeutic approaches are needed to compliment ongoing strategies. The first indication that CP-AMPARs may be important to consider in the pathology of gliomas came from analysis of the editing state of the GluR2 Q/R site [165]. Using postmortem tissue from malignant brain tumors, Maas and colleagues (2001) were able to show that the Q/R site was only 69 % to 88 % edited. Ishiuchi and colleagues extended this observation by showing that the tumor migration could be supressed with the induction of apoptosis if CP-AMPARs were rendered impermeable to Ca2+ [166]. Importantly, this finding suggests that selective antagonism of CP-AMPARs, perhaps with ion-channel blockers [114,166], may represent a novel therapeutic strategy in the treatment of glioblastoma multiforme. More recent evidence suggests that the activation of CP-AMPARs leads to proliferation and migration through a pathway mediated by the serine-threonine kinase Akt [167]. Interestingly, malignant gliomas have another feature that supports their ability to invade and proliferate. Unlike non-malignant glia [200,201], glioblastomas lack excitatory amino-acid transporters and thus release L-Glu via a cystine-glutamate exchanger [168,202]. This, in turn, makes way for tumor expansion by killing surrounding tissue through excitotoxicity [203]. Thus, glioblastomas exploit normal homeostatic mechanisms of nervous tissue through a regenerative loop that starts with release of L-Glu which leads to proliferation and migration by activation of CP-AMPARs and Akt. This finding has suggested that a combination of AMPAR antagonists [204] with growth factor inhibitors [167,203] may be useful in the treatment of malignant gliomas. An additional strategy that has been proposed is to prevent L-Glu release with sulfasalazine by inhibiting the cystine-glutamate exchanger [168].
AMPA Receptors & Fragile-X Syndrome
WHAT IS FRAGILE-X SYNDROME?
Fragile X syndrome (FXS) is the most common cause of inherited mental retardation affecting 1 in 1250 – 4000 males and 1 in 2500 – 8000 females [144,205,206]. The impairment ranges from learning difficulties to more severe cognitive or intellectual disabilities [141,144,207]. Afflicted individuals have problems in retaining information over short periods to poor linguistic processing. Characteristics that distinguish FXS children include impulsivity, hyper-arousal and over-anxiety. Many affected boys display symptoms of attention deficit hyperexcitability disorder (ADHD) and autistic-like features, such as gaze avoidance and stereotyped repetitive movements, which can often lead to a misdiagnosis of autism. Wide variability in the clinical presentation is one reason the diagnosis may be missed or incorrectly identified. Although certain physical and behavioral features are often associated with FXS, they are not always present. In at least 10% of males, intellectual impairment is the only presenting feature. The classic triad of long face, prominent ears and macroorchidism (i.e. testicular enlargement) is present in just 60% of cases. Nor is mental retardation always present. Approximately 15% of males with FXS have an IQ above 70 [208]. In such cases, it is possible that diagnosis of FXS may not be considered. Similarly, females with FXS may be incorrectly diagnosed due to the subtlety of the symptoms with about one third exhibiting intellectual disability [207].
FRAGILE-X SYNDROME IS A GENETIC DISORDER
Fragile X mental retardation is a genetic disorder caused by mutation of the FMR1 gene on the X chromosome [141,144,207]. Today it is known that, in most cases, the mutational mechanism results from expansion of an unstable non-coding CGG repeat of the untranslated UTR [209]. Normally, the FMR1 gene contains between 6 and 54 CGG repeats whereas individuals with FXS have over 230 repeats [209]. Expansion of the CGG repeat permits aberrant methylation of that portion of the DNA [210] and decreased histone acetylation [211] which effectively silences expression of the FMR1 protein. FMRP is a selective RNA-binding protein that is predominately expressed in the cytoplasm but shuttles back and forth from the nucleus [212,213]. In this role, FMRP associates with polyribosomes and acts as a negative regulator of translation [207,213] and, as described below, regulates synaptic plasticity [214,215] by regulating the synthesis of proteins encoded by certain dendritic mRNAs [216–219].
IMPAIRED AMPAR PLASTICITY PLAYS A CRUCIAL ROLE IN THE FRAGILE-X SYNDROME
The identification of the FMR1 gene as being responsible for FXS [141,210] led to the development of a knockout (KO) mouse model [220]. KO mice lack functional FMRP in any tissues and, like the human condition the phenotype is mild with no major pathological deficits in the CNS and elsewhere including an unimpaired reproductive capability. KO mice show significant macroorchidism, audiogenic seizures and exhibit characteristic behavioral abnormalities [220] suggesting that the FMR1 KO mouse is useful for studying the human disease state. With this in mind and guided by earlier evidence showing that dendritic synthesis of FMRP is controlled by metabotropic glutamate receptor (mGluR) activation [221], long-term depression (LTD) of AMPARs was shown to be accentuated in FMR1 KO mice [214]. The finding led the way to the mGluR hypothesis of FXS which argues that exaggerated mGluR signaling causes a greater internalization of synaptic AMPARs.
To date, exaggerated LTD in FMR1 KO mice has been reported in principal cells of the CA1 region of the hippocampus [214] and inhibitory Purkinje cells of the cerebellum [215]. The disruption appears to be specific to this plasticity mechanism since NMDAR-dependent LTD, which is also found at CA1 synapses, is unaffected in null mice [214]. A key molecular difference is that mGluR-triggered LTD requires protein synthesis [222–224] which is not required for NMDAR-dependent LTD [223]. In support of this, the rate of protein synthesis and levels of synaptic proteins are elevated in FMRP KO mice [225] which is consistent with earlier reports identifying FMRP is a suppressor of protein synthesis [226–228]. By functioning primarily as a RNA-binding protein, FMRP associates with brain mRNA transcripts thus forming a mRNA-protein complex to regulate local dendritic protein synthesis [225,229,230]. This interaction seems quite specific for only a subset of mRNAs since protein synthesis-dependent late-phase hippocampal LTP is unaffected in FXS mice [231,232]. Despite this established role of FMRP in synaptic plasticity, correlating learning deficits in KO mice to clinical symptoms seen in patients has been challenging [220,232,233]. Indeed, how the various types of hippocampal plasticity, including LTD, relate to animal behavior is still the subject of debate [234]. Fortunately, in the cerebellum, the case is different. Cerebellar LTD has an important role in eye-blink conditioning which is a simple form of associative learning [235]. Using this experimental paradigm, both Fmr1 KO mice and human patients with FXS have been shown to have impairment in the conditioned eye-blink [215]. The importance of this observation is that it permits deficits in AMPAR trafficking to be tied to cognitive deficits. With this in mind, it may prove very useful in assessing the response of a patient to drug therapy.
DEVELOPMENT OF THERAPEUTIC COMPOUNDS TO TREAT FRAGILE-X SYNDROME
Several therapeutic approaches have been proposed to alleviate FXS which include the use of 5-azadeoxycytidine to reduce hypermethylation of the FMR1 promoter [236] and the inhibition of p21-activated kinase to reverse dendritic spine abnormalities [237]. Given the proposed hypofunction associated with AMPARs, it has been suggested that AMPAkines, which are positive allosteric modulators, may be of clinical valuable. To date, the AMPAkine, CX-516, has made it into Phase II clinical trails to assess its value in treating FXS [238,239]. Although, the study did not report any adverse side-effects associated with CX-516, the AMPAkine provided little improvement in behaviorial tests when compared to the placebo [239]. Given this, the most compelling evidence so far supports the view that correction of FXS may be attained by downregulating mGluR5 signaling [140,142,240,241]. In support of this, blockade of mGluR5 signaling with the inverse agonist, 2-methyl-6-(phenylethynyl)-pyridine (MPEP) in KO mice abolishes hyperactivity [242], audiogenic seizures and epileptiform activity [242,243] as well as the characteristic pattern of abnormal protein synthesis seen in FXS [244]. Furthermore, recent analysis of a mouse model of FXS revealed that 50 % reduction in mGluR5 also corrects many of the cognitive, behavioral, and neurological correlates of the disease state [241]. Taken together, this work suggest that the debilitating symptoms suffered by individuals with FXS may be reversed or at least improved by targeting group 1 mGluR signaling. If AMPAkines are to be considered further, drug potency may need to be looked at more carefully given the suggestion that dosing in previous clinical trials may have been insufficient to elicit a therapeutic effect [239].
Kainate Receptors & CNS Disorders
NEUROPATHIC PAIN, FEAR MEMORY &EPILEPSY
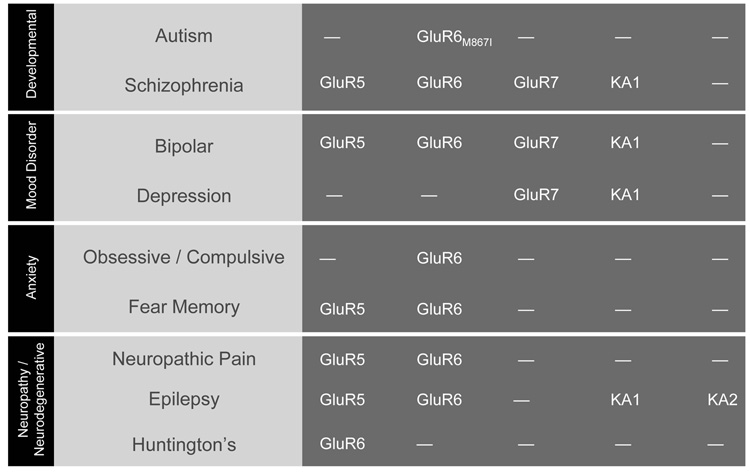
Compared to AMPA receptors, our understanding of the potential roles fulfilled by KARs in CNS disorders is just beginning to emerge. Several studies have attempted to link KARs to specific illnesses by comparing the relative amounts of KAR subunits in control and diseased postmortem tissue (Fig. 6). In this way, a few studies have implicated the human GluR6 KAR subunit, GRIK2, in obsessive-compulsive disorder [245], schizophrenia [246] and autism ([247,248,248–250] though see [251]). Similarly, recent work has added that genes of the human GluR7, GRIK3, and KA1 subunit, GRIK4, may be susceptibility factors in major depressive disorders [252,253]. It is unlikely however that these disease states reflect a selective dysfunction only in KARs. Indeed, more extensive studies of mood disorders and schizophrenia find that an array of proteins involved in glutamatergic transmission are altered [254–257] suggesting, perhaps not too surprisingly, that these psychiatric illnesses involve aberrations in multiple aspects of glutamate signaling as well as others.
Figure 6. KAINATE RECEPTORS AND CNS DISORDERS.
Summary table identifying which KAR subunits are implicated in distinct disorders of the CNS. As work progress, it is likely that this information will be more complete. For example, it is commonly assumed that native KARs are heteromers assembled from GluR5–7 subunits with KA1 and/or KA2. In view of this, in disease states where GluR5–7 have been implicated, it is possible that future work will also implicate KA1 and/or KA2 subunits.
More compelling evidence supports a role for KARs in Huntington’s disease, neuropathic pain and fear memory. The role of KARs in Huntington’s disease stems from early observations showing that kainic acid lesioning produces a similar pattern of neurodegeneration in the striatum [258] the CNS region most affected in this disease state [136]. Consistent with this observation, selective loss of [3H] kainic acid binding sites has been reported in another study examining postmortem brains with Huntington’s disease [259]. More recent genetic studies [260,261] and analysis of knockout animals [262] provides the most prevalent view that the GluR6 subunit is responsible for much of the KAR-mediated neurodegeneration in early-onset Huntington’s disease (Fig. 6).
Pain transmission from the periphery to the CNS is mediated by a combination of GluR5- and GluR6-containing KARs [116]. In support of this, a number of studies have reported the benefit of GluR5- and GluR6-selective antagonists in alleviating nociception in different models of pain [263–266]. Interestingly in knockout studies, responsiveness to persistent pain is reduced only in GluR5- and not GluR6-lacking mice [267]. In the periphery, small diameter dorsal root ganglion neurons, which carry nociceptive and thermoreceptive information to the spinal cord, express KARs [268–271] that contain GluR5 subunits [271]. Here, one of their primary roles is to act as presynaptic autoreceptors to reduce transmitter release at glutamatergic synapses in the dorsal horn [272–274]. In the dorsal horn of the spinal cord, there is a combination of GluR5- and GluR6-containing KARs [272,275–277].
KARs containing GluR5 and GluR6 receptor subunits also particpate in important functional aspects of the amygdala [267,278–281]; the CNS region predominately associated with learned fear [282,283]. Although GluR5-containing KARs fulfill important roles in plasticity mechanisms of the amygdala [278–280], a recent study using knockout animals suggests that it is only GluR6-containing KARs that are involved in fear memory [267].
Kainate Receptors & Epilepsy
TEMPORAL LOBE EPILEPSY: DEFINITION, AETIOLOGY & TREATMENT
Epilepsy is the second most common neurological condition in North America afflicting as many as 0.5 to 1 % of the population with the highest incidence occurring in young children and the elderly [284]. Current therapy is directed towards alleviating the patients’ symptoms since neither effective prophylaxis nor a cure have been identified. Many individuals with epilepsy experience transient loss of consciousness that can result in bodily harm as well as having long-term detrimental effects on education and employment. Although available medications are initially effective, over the long-term, patient compliance is problematic as well as the development of drug resistant seizures in about 30 – 40 % of those treated [285]. Consequently, much emphasis has been placed on understanding the underlying cause of epilepsy as well as identifying novel therapeutic approaches [286].
Although epilepsy exists in various forms [287,288], the role of KARs in the initiation and maintenance of epileptic seizures primarily comes from work on temporal lobe epilepsy (TLE) [289–292]. Two main types are internationally recognized (i) mesial TLE which occurs in the hippocampus, parahippocampal gyrus and amygdala regions of the CNS whereas (ii) lateral TLE which is less common and associated with the neocortex [293].
The onset of TLE is thought to be triggered by an initial insult or brain injury that occurs early in childhood. The nature of the insult varies but is often related to febrile seizures which account for about 2 out of 3 cases of TLE [294–296]. This finding has provided an explanation for the susceptibility and higher incidence of TLE in newly born babies. That is, insult arises due to the immature nature of the neonatal thermoregulatory system which fails to safely regulate elevations in core body temperatures that accompany infection [297]. In almost all cases, patients experience a seizure-free interval between the presumed cerebral insult and the development of habitual seizures [295]. Less commonly, TLE develops after birth due to head traumas, spinal meningitis or brain tumours [295,298]. When seizure onset occurs in childhood, atrophy of the hippocampus can often be observed with magnetic resonance imaging whereas no abnormalities are usually visible when symptoms develop in adulthood [296].
The International League Against Epilepsy has identified three types of seizure associated with TLE; simple partial seizures (SPS), complex partial seizures (CPS) and secondarily generalized tonic-clonic seizures (SGTCS) [293]. SPS manifest as auras in individuals with TLE [295] which often serve as an early warning before the onset of more severe symptoms. Patients have described feelings of nausea, epigastric rising, fear, déjà vu and/or altered states of olfaction [295,298] reflecting seizure activity in restricted areas of the temporal lobe [292]. Often, SPS develop into complex partial seizures (CPS) as neuronal activity spreads into greater areas of the temporal lobe. The onset of CPS can be identified by the inability of the patient to interact with others. This impairment in consciousness is often associated with repetitive motor movements of the hand or mouth, motionless staring and/or unusual speech and behavior that may last a few seconds up to several minutes [293]. Finally in some cases, seizure activity restricted to the temporal lobe spreads to the entire brain resulting in secondarily generalized tonic-clonic seizures (SGTCS) [293]. SGTCS are characterized by both tonic and clonic phases. Patients in the tonic phase lose consciousness and develop muscle rigidity that can often lead to imbalance when in a standing position. The clonic phase is characterized by convulsions that occur as violent, involuntary muscular contractions of the extremities, trunk and head. The eventual cessation of SGTCS is usually accompanied by confusion and amnesia which is referred to as the postictal state or sleep.
In principle, TLE can be treated with any number of anticonvulsant or antiseizure drugs though the most commony used fall into two main categories based on their mechanism of action. Phenytoin, carbamazepine, valproate and topiramate all act by limiting neuronal excitability by prolonging the time voltage-gated Na+-channels reside in the inactivated state [299]. Topiramate may also act by antagonising clonic seizures elicited by the selective GluR5 KAR agonist, (RS)-2-amino-3-(3-hydroxy-5-tert-butylisoxazol-4-yl)propanoic acid (ATPA) [300]. Other compounds such as phenobarbital and the more recent gabapentin enhance GABA-ergic synaptic inhibition either by positive allosteric regulation of the GABAA receptor or by affecting release of the neurotransmitter by acting on presynaptic Ca2+-channels [301–303]. In patients where seizure control through medication is inadequate [285,304], the only option is removal of part of the hippocampus or amygdala through resective surgery.
HISTORICAL EMERGENCE OF KAINATE RECEPTORS IN EPILEPSY
The view that KARs may be implicated in epileptic disorders emerged more than three decades ago with the observation that systematic or intracerebral injections of the neurotoxin, L-α-kainic acid (KA), elicits epileptiform seizures originating in the hippocampal formation and amygdala [305,306].
KA was originally isolated from the marine red algae Digenea simplex in 1955 [38] due to its anthelmintic value in traditional Japanese medicine for expelling parasitic worms [307]. An important turning point came with a series of observations revealing that KA was a key pharmacological tool for distinguishing between NMDA and non-NMDA ionotropic glutamate receptor families [32]. From this work, the consensus view emerged that KA was selective for non-NMDARs [308,309] where its effects were insensitive to NMDAR antagonists such as external Mg2+ ions [36,37], D-α-aminoadipate [310] and 2-amino-5-phosphovaleric acid (APV) [311,312] but sensitive to block by L-glutamic acid diethyl ester (GDEE) [313], γ-D-glutamylglycine (γ-GG) [314]and kynurenic acid [315].
Evidence was also accumulating for the subdivision of non-NMDARs into KA- and quisqualate (QA/AMPA)-preferring receptors [32]. Here again the use of KA was pivotal in providing much of the support for the existence of distinct KARs. For example, early radioligand binding studies with [3H]KA helped identify the distinct expression pattern of high-affinity KAR binding sites in the CNS [316]. Moreover, KA was observed to exert a selective depolarization of afferent dorsal root C-fibres [317] further establishing the existence of a distinct population of KARs insensitive to agonists acting on NMDA and/or QA/AMPA receptor classes. Although cloning studies during the 1990s finally confirmed the existence of 3 major iGluR families [49,50], ongoing voltage-clamp experiments of native iGluRs expressed in neuronal culture revealed a more complicated pharmacology for KA than initially anticipated [33].
Specifically, the emergence of rapid drug application techniques [318,319] revealed that KA elicits a non-desensitizing response at AMPARs [320–322](though see [323]) but a rapidly inactivating current at KARs [269,324]. The fact that KA repetitively activates AMPARs, and not KARs, initially suggested that AMPARs may fulfill more important roles in epilepsy especially in terms of neuronal damage [325]. Indeed, to date, AMPARs have established roles in neurodegeneration [326–328], however, it was long recognised that AMPAR activation cannot account for the long-lasting effects of nanomolar concentrations of KA on hippocampal excitability [329]. The problem was that native AMPARs are activated at much higher concentrations of KA (i.e. µM – mM [321,322]), consequently, the long-lasting effects on excitability had to reflect activation of some other membrane conductance, possibly KARs [330,331]; a possibility supported by early findings of high-affinity [3H]KA binding sites in the hippocampus [332]. Indeed, this turned out to be the case but it was only conclusively shown following the generation of genetically-modified mice lacking specific KAR subunits.
To date, mice either entirely lacking or possessing modified GluR5 [333], GluR6 [334], GluR7 [335] and KA2 [336] subunits have been studied. Mice deficient in the KA1 subunit, though available, have yet to be studied in any detail (though see (Catches et al (2007) Soc Neurosci Abstr 877.2)). Although the development of selective receptor antagonists helped delineate between AMPAR- and KAR-mediated responses [337], the availability of KAR knockout mice has been instrumental in providing insight into the composition of native KARs, their synaptic location and as described below, the roles they fulfill in regulating seizure activity in the hippocampus.
KAINATE RECEPTORS ARE KEY PLAYERS IN THE PATHOLOGY OF EPILEPSY
GluR5 [338–342], GluR6 [338,339,343,344], KA1 [345,346] and KA2 [346,347] have all been implicated in the initiation or maintenance in various animal models of epileptic seizures. Comparative studies on wildtype and knockout mice suggest that GluR5 and GluR6 subunits contribute similarly to establishment of hippocampal gamma oscillations and epileptiform activity [339,343]. The involvement of GluR5 is further supported by studies showing that GluR5-selective antagonists inhibit seizure activity [340–342]. In this respect, the hippocampal area CA3 has been argued to be most vulnerable in generating epileptic seizures from gamma oscillations when excitation outweighs inhibition [339,348]. However, the mechanism by which the hippocampus is rendered more vulnerable to seizures remains to be identified. In this respect, several studies have proposed that changes in the expression levels of KAR subunits and/or the edited state of their respective Q/R site may be important to consider [338,344–347]. Although the global expression pattern of KARs is different in hippocampal tissue from epileptic patients [338,345,346], an elegant study has revealed the sprouting of aberrant KAR-containing synapses in granule cells of the hippocampal dentate gyrus is an additional factor [128].
Seizure-triggered reactive plasticity in the dentate gyrus has been well documented accounting for its presumed key role in the pathogenesis of TLE [349,350]. In the normal brain, the dentate gyrus operates as a filter that inhibits excitatory information from the entorhinal cortex propagating to the hippocampus. In addition to loss of hilar interneurons in TLE, granule cells of the dentate gyrus establish a recurrent excitation pathway following seizure-related brain damage. This sprouting of glutamatergic mossy fibers establishes aberrant synapses back onto granule cells reducing the threshold for granule cell synchronization which, in turn, is thought to increase the propensity for seizures [350]. In this context, KARs are particularly important to consider since the axonal rewiring is associated with a change in the nature of glutamatergic transmission onto granule cells. Specifically, Epsztein and colleagues have observed de novo expression of KARs by granule cells in chronic epileptic tissue that is absent in the control hippocampus [128]. KARs provide half of the non-NMDA receptor-mediated excitatory drive in granule cells further underlining their importance to the physiopathology of TLE. Taken together, this work establishes the value of working towards more selective KAR antagonists and the need to identify the signaling factors responsible for hippocampal re-modeling in TLE.
Future Perspectives
CONSIDERING THE WAY FORWARD FOR IGLURS AND CNS DISORDERS
Can a future be imagined where drugs are given to cure CNS disorders and not to simply alleviate their symptoms? Several studies focusing on other neurotransmitter systems may fuel new ideas for work on iGluRs. The first example relates to observations that have given insight into the treatment of posttraumatic stress disorder (PTSD). Individuals develop PTSD after a traumatic life event where grave physical harm has occurred or has been threatened. PTSD was first referred to as shell shock or traumatic war necrosis to describe the confounding distress observed amongst soldiers of the 1st and 2nd World Wars [351,352]. Today, the condition has a much broader definition so that PTSD is used to describe an individual’s reaction to, for example, sexual assault, incarceration, torture or medical complications [353]. Not everyone develops PTSD; therefore in those who are susceptible, it represents a failure to recover from the normal effects of trauma. Diagnosis is based on the persistent re-experience and avoidance of situations associated with the trauma. Importantly, persistent symptoms of increased arousal, including sleep disturbances, for example, negatively impact the afflicted person’s social interactions as well as their ability to remain in gainful employment.
Insight into potential treatments of this condition first developed from animal studies which showed that emotional trauma triggered the β-adrenergic hormonal signalling system in the amygdala during and after the event [354,355]. It was shown subsequently in human subjects that administration of β-blockers, such as propranolol, selectively impaired memory of an emotionally-charged narrative and not of a closely-matched neutral story [356]. This finding led the way for more recent work showing that β-blockers act prophylactically if given within hours [357] or even after the retrieval of memories of past traumatic events [23].
The principle that acute administration of pharmacological agents can elicit long-lasting beneficial effects on the mentally ill may have broad applications especially given the plasticity mechanisms associated with iGluRs. In another study, analysis of antidepressants (ADs) also highlights the beneficial effects of drugs acting through an unconventional mechanism. Traditionally, it has been assumed that the clinical value of ADs is due to their ability to elevate extracellular serotonin or noradrenaline [358]. However recently, Santarelli and colleagues (2003) show that ADs alleviate the symptoms of depression and anxiety by increasing adult neurogenesis in the hippocampus. Specifically, ADs appear to be able to stimulate the dentate gyrus to generate new neurons from stem cells. Their findings explain why there is a delay of 3–4 weeks before relief of clinical symptoms is observed with ADs [359]. In contrast, serotonin and/or noradrenaline levels rise almost immediately after acute administration of ADs [359]. As our understanding of stem cells and how they can be manipulated is still emerging [360], it would be tempting in future work to establish if iGluRs also play a role in neurogenesis of the adult CNS.
And finally, a recent study using an animal model of schizophrenia suggests a generalized approach for treating neurodevelopmental disorders [361]. A major defect in schizophrenia is due to hyperfunction of D2 dopaminergic neurotransmission [14]. Despite this, it has remained unclear why typical and atypical antipsychotics, which block D2 receptors, improve only the positive symptoms, such as hallucinations and delusions, whilst having little effect on the negative symptoms, such as deficits in working memory and attention [362]. The issue has been all the more perplexing since an upregulation of D2 receptors has been shown in the schizophrenic brain such as in the striatum (e.g. [363,364]) which has been linked to the pathophysiology of schizophrenia [365]. To address this conundrum, Kellendonk and colleagues (2006) reversibly increased D2 receptors in the striatum of transgenic mice and noted the behaviorial and physiological consequences. As predicted, the modified mice exhibited defects in working memory consistent with deficits in schizophrenia. However, the authors were surprised in two ways. Firstly, the memory deficit resulted from excess D2 receptor signaling during development and not in the adult brain. Secondly, overexpression of D2 receptors in the striatum disrupts other aspects of dopaminergic signaling in the prefrontal cortex which are insensitive to D2 receptor antagonists [361]. Since the prefrontal cortex is thought to perform the task of working memory [366], this latter observation explains why deficits in the working memory of schizophrenics are insensitive to antipsychotics. It remains to be established whether other neurodevelopmental disorders which involve iGluRs, such as autism or Rett’s syndrome, can also be treated before the onset of clinical symptoms. A turning point in our understanding of iGluRs has been reached, however, so that correction of even the most intractable CNS disorders might be imagined in the future.
Acknowledgements
I am indebted to Drs. Johannes Krupp and Brian Collier for providing critical comments on the content of this review. In addition, I am grateful to Drs. John Phillis and especially Kris Krnjevič for sharing their many memories of early work on ionotropic glutamate receptors. Finally, I would like to thank past and present members of my lab and numerous colleagues who create an intellectually stimulating environment at McGill. This work was supported by operating grants from the Canadian Institutes of Health Research, National Institutes of Health and the Fragile X Research Foundation of Canada as well as a personal award from the Canada Research Chair program.
REFERENCES
- 1.Mills J. Psychoanal. Rev. 2003;90:269. doi: 10.1521/prev.90.3.269.23621. [DOI] [PubMed] [Google Scholar]
- 2.King LJ. Ann. Clin. Psychiatry. 1999;11:47. doi: 10.1023/a:1022379729697. [DOI] [PubMed] [Google Scholar]
- 3.Huguelet P, Perroud N. Psychiatry. 2005;68:130. doi: 10.1521/psyc.2005.68.2.130. [DOI] [PubMed] [Google Scholar]
- 4.Allen C. Hist Med. 1975;6:4. [PubMed] [Google Scholar]
- 5.d'Orsi G, Tinuper P. Epilepsy Behav. 2006;9:152. doi: 10.1016/j.yebeh.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 6.Zoghbi HY. Science. 2003;302:826. doi: 10.1126/science.1089071. [DOI] [PubMed] [Google Scholar]
- 7.Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, Ustun TB. Curr. Opin. Psychiatry. 2007;20:359. doi: 10.1097/YCO.0b013e32816ebc8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maccioni RB, Munoz JP, Barbeito L. Arch. Med. Res. 2001;32:367. doi: 10.1016/s0188-4409(01)00316-2. [DOI] [PubMed] [Google Scholar]
- 9.O'Donnell WT, Warren ST. Annu. Rev. Neurosci. 2002;25:315. doi: 10.1146/annurev.neuro.25.112701.142909. [DOI] [PubMed] [Google Scholar]
- 10.Van dVI, Zoghbi HY. Ment. Retard. Dev. Disabil. Res. Rev. 2002;8:82. doi: 10.1002/mrdd.10025. [DOI] [PubMed] [Google Scholar]
- 11.Dagnan D. Curr. Opin. Psychiatry. 2007;20:456. doi: 10.1097/YCO.0b013e3282ab9963. [DOI] [PubMed] [Google Scholar]
- 12.Tsankova N, Renthal W, Kumar A, Nestler EJ. Nat. Rev. Neurosci. 2007;8:355. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- 13.Swazey JP. Chlorpromazine In psychiatry: A study in therapeutic innovation. Vol. 74. Cambridge: M.I.T. Press; [Google Scholar]
- 14.Carlsson A. Science. 2001;294:1021. doi: 10.1126/science.1066969. [DOI] [PubMed] [Google Scholar]
- 15.Snyder SH. Neuron. 2006;49:484. doi: 10.1016/j.neuron.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Miller NE. Science. 1965;148:328. doi: 10.1126/science.148.3668.328. [DOI] [PubMed] [Google Scholar]
- 17.Fisher AE. Sci. Am. 1964;210:60. doi: 10.1038/scientificamerican0664-60. [DOI] [PubMed] [Google Scholar]
- 18.Perry EK. Br. Med. Bull. 1986;42:63. doi: 10.1093/oxfordjournals.bmb.a072100. [DOI] [PubMed] [Google Scholar]
- 19.Arana GW. J Clin. Psychiatry. 2000;61 Suppl 8:5. [PubMed] [Google Scholar]
- 20.Leppik IE. Adv. Exp. Med. Biol. 2002;497:1. doi: 10.1007/978-1-4615-1335-3_1. [DOI] [PubMed] [Google Scholar]
- 21.Tierney JG. J Manag. Care Pharm. 2007;13:S2. doi: 10.18553/jmcp.2007.13.s6-a.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen HV, Kessing LV. Expert. Rev. Neurother. 2007;7:57. doi: 10.1586/14737175.7.1.57. [DOI] [PubMed] [Google Scholar]
- 23.Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, Pitman RK. J Psychiatr. Res. 2007 doi: 10.1016/j.jpsychires.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Ritthausen H. J. Prakt. Chem. 1866;99:454. [Google Scholar]
- 25.Abderhalden E, Weil A. Z. Physiol. Chem. 1913;83:425. [Google Scholar]
- 26.Hayashi T. Keio J. Med. 1954;3:183. [Google Scholar]
- 27.Krnjevic K. Phys. Rev. 1974;54:418. [Google Scholar]
- 28.Curtis DR, Phillis JW, Watkins JC. Nature Lond. 1959;183:611. doi: 10.1038/183611a0. [DOI] [PubMed] [Google Scholar]
- 29.Curtis DR, Phillis JW, Watkins JC. J Physiol. 1960;150:656. doi: 10.1113/jphysiol.1960.sp006410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krnjevic K, Phillis JW. Br. J Pharmacol. Chemother. 1963;20:471. doi: 10.1111/j.1476-5381.1963.tb01484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krnjevic K, Phillis JW. J Physiol. 1963;165:274. doi: 10.1113/jphysiol.1963.sp007057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watkins JC, Evans RH. Ann. Rev. Pharmacol. Toxicol. 1981;21:165. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- 33.Mayer ML, Westbrook GL. Prog. Neurobiol. 1987;28:197. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- 34.Collingridge GL, Lester RA. Pharmacol. Rev. 1989;41:143. [PubMed] [Google Scholar]
- 35.Watkins JC. J. Med. Pharm. Chem. 1962;5:1187. doi: 10.1021/jm01241a010. [DOI] [PubMed] [Google Scholar]
- 36.Davies J, Watkins JC. Brain Res. 1977;130:364. doi: 10.1016/0006-8993(77)90284-0. [DOI] [PubMed] [Google Scholar]
- 37.Evans RH, Francis AA, Watkins JC. Experientia. 1977;33:489. doi: 10.1007/BF01922227. [DOI] [PubMed] [Google Scholar]
- 38.Ueno Y, Nama H, Ueganagi J, Morimoto H, Nakamori R, Matsuoka T. J Pharmac. Soc. Japan. 1955;75:807. [Google Scholar]
- 39.Takemoto T, Koike K, Nakajima T, Arihara S. Yakugaku Zasshi. 1975;95:448. doi: 10.1248/yakushi1947.95.4_448. [DOI] [PubMed] [Google Scholar]
- 40.McLennan H, Lodge D. Brain Res. 1979;169:83. doi: 10.1016/0006-8993(79)90375-5. [DOI] [PubMed] [Google Scholar]
- 41.Davies J, Watkins JC. Brain Res. 1981;206:172. doi: 10.1016/0006-8993(81)90111-6. [DOI] [PubMed] [Google Scholar]
- 42.Foster AC, Fagg GE. Brain Res. 1984;319:103. doi: 10.1016/0165-0173(84)90020-1. [DOI] [PubMed] [Google Scholar]
- 43.Fagg GE, Foster AC. Neurosci. 1983;9:701. doi: 10.1016/0306-4522(83)90263-4. [DOI] [PubMed] [Google Scholar]
- 44.Monaghan DT, Bridges RJ, Cotman CW. Annu. Rev. Pharmacol. Toxicol. 1989;29:365. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- 45.Fagg GE, Matus A. Proc. Natl. Acad. Sci. U. S. A. 1984;81:6876. doi: 10.1073/pnas.81.21.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olsen RW, Szamraj O, Houser CR. Brain Res. 1987;402:243. doi: 10.1016/0006-8993(87)90030-8. [DOI] [PubMed] [Google Scholar]
- 47.Nielsen EO, Cha JH, Honore T, Penney JB, Young AB. Eur. J Pharmacol. 1988;157:197. doi: 10.1016/0014-2999(88)90383-4. [DOI] [PubMed] [Google Scholar]
- 48.Bowie D, Smart TG. Br. J Pharmacol. 1993;109:779. doi: 10.1111/j.1476-5381.1993.tb13642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seeburg PH. Trends Neurosci. 1993;16:359. doi: 10.1016/0166-2236(93)90093-2. [DOI] [PubMed] [Google Scholar]
- 50.Hollmann M, Heinemann S. Ann. Rev. Neurosci. 1994;17:31. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 51.Nakanishi S, Masu M. Annu. Rev. Biophys. Biomol. Struct. 1994;23:319. doi: 10.1146/annurev.bb.23.060194.001535. [DOI] [PubMed] [Google Scholar]
- 52.Lomeli H, Sprengel R, Laurie DJ, Köhr G, Herb A, Seeburg PH, Wisden W. FEBS Lett. 1993;315:318. doi: 10.1016/0014-5793(93)81186-4. [DOI] [PubMed] [Google Scholar]
- 53.Araki K, Meguro H, Kushiya E, Takayama C, Inoue Y, Mishina M. Biochem. Biophys. Res. Commun. 1993;197:1267. doi: 10.1006/bbrc.1993.2614. [DOI] [PubMed] [Google Scholar]
- 54.Dingledine R, Borges K, Bowie D, Traynelis SF. Pharmacol. Rev. 1999;51:7. [PubMed] [Google Scholar]
- 55.Mayer ML, Armstrong N. Annu. Rev. Physiol. 2004;66:161. doi: 10.1146/annurev.physiol.66.050802.084104. [DOI] [PubMed] [Google Scholar]
- 56.Wollmuth LP, Sobolevsky AI. Trends Neurosci. 2004;27:321. doi: 10.1016/j.tins.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 57.Erreger K, Chen PE, Wyllie DJ, Traynelis SF. Crit Rev. Neurobiol. 2004;16:187. doi: 10.1615/critrevneurobiol.v16.i3.10. [DOI] [PubMed] [Google Scholar]
- 58.Cotman CW, Monaghan DT, Ganong AH. Annu. Rev. Neurosci. 1988;11:61. doi: 10.1146/annurev.ne.11.030188.000425. [DOI] [PubMed] [Google Scholar]
- 59.Nicoll RA, Kauer JA, Malenka RC. Neuron. 1988;1:97. doi: 10.1016/0896-6273(88)90193-6. [DOI] [PubMed] [Google Scholar]
- 60.Ito M. Annu. Rev. Neurosci. 1989;12:85. doi: 10.1146/annurev.ne.12.030189.000505. [DOI] [PubMed] [Google Scholar]
- 61.Bear MF. Proc. Natl. Acad. Sci. U. S. A. 1996;93:13453. doi: 10.1073/pnas.93.24.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Collingridge GL, Singer W. Trends Pharmacol. Sci. 1990;11:290. doi: 10.1016/0165-6147(90)90011-v. [DOI] [PubMed] [Google Scholar]
- 63.Constantine-Paton M, Cline HT, Debski E. Annu. Rev. Neurosci. 1990;13:129. doi: 10.1146/annurev.ne.13.030190.001021. [DOI] [PubMed] [Google Scholar]
- 64.Singer W. J Exp. Biol. 1990;153:177. doi: 10.1242/jeb.153.1.177. [DOI] [PubMed] [Google Scholar]
- 65.Shatz CJ. Neuron. 1990;5:745. doi: 10.1016/0896-6273(90)90333-b. [DOI] [PubMed] [Google Scholar]
- 66.Crepel F, Audinat E. Prog. Biophys. Mol. Biol. 1991;55:31. doi: 10.1016/0079-6107(91)90010-p. [DOI] [PubMed] [Google Scholar]
- 67.Choi DW. Stroke. 1990;21:III20. [PubMed] [Google Scholar]
- 68.Sheng M. Proc. Natl. Acad. Sci. U. S. A. 2001;98:7058. doi: 10.1073/pnas.111146298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barry MF, Ziff EB. Curr. Opin. Neurobiol. 2002;12:279. doi: 10.1016/s0959-4388(02)00329-x. [DOI] [PubMed] [Google Scholar]
- 70.Malinow R, Malenka RC. Annu. Rev. Neurosci. 2002;25:103. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 71.Bredt DS, Nicoll RA. Neuron. 2003;40:361. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- 72.Collingridge GL, Isaac JT, Wang YT. Nat. Rev. Neurosci. 2004;5:952. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- 73.Jaskolski F, Coussen F, Mulle C. Trends Pharmacol. Sci. 2005;26:20. doi: 10.1016/j.tips.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 74.Perez-Otano I, Ehlers MD. Trends Neurosci. 2005;28:229. doi: 10.1016/j.tins.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 75.Yuzaki M. Cerebellum. 2004;3:89. doi: 10.1080/14734220410028921. [DOI] [PubMed] [Google Scholar]
- 76.Kim E, Sheng M. Nat. Rev. Neurosci. 2004;5:771. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 77.Uemura T, Mori H, Mishina M. Mol. Cell Neurosci. 2004;26:330. doi: 10.1016/j.mcn.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 78.Elias GM, Nicoll RA. Trends Cell Biol. 2007;17:343. doi: 10.1016/j.tcb.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 79.Nicoll RA, Tomita S, Bredt DS. Science. 2006;311:1253. doi: 10.1126/science.1123339. [DOI] [PubMed] [Google Scholar]
- 80.Ziff EB. Neuron. 2007;53:627. doi: 10.1016/j.neuron.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 81.Gerrow K, El Husseini A. Sci. STKE. 2007;2007:e56. doi: 10.1126/stke.4082007pe56. [DOI] [PubMed] [Google Scholar]
- 82.Seeburg PH, Burnashev N, Kohr G, Kuner T, Sprengel R, Monyer H. Recent Prog. Horm. Res. 1995;50:19. doi: 10.1016/b978-0-12-571150-0.50006-8. [DOI] [PubMed] [Google Scholar]
- 83.Freitas dR, Pereira A, Jr, Bezerra Coutinho FA. Prog. Neurobiol. 2001;64:555. doi: 10.1016/s0301-0082(00)00069-1. [DOI] [PubMed] [Google Scholar]
- 84.Constantine-Paton M. Cold Spring Harb. Symp. Quant. Biol. 1990;55:431. doi: 10.1101/sqb.1990.055.01.043. [DOI] [PubMed] [Google Scholar]
- 85.Debski EA, Cline HT, Constantine-Paton M. J Neurobiol. 1990;21:18. doi: 10.1002/neu.480210103. [DOI] [PubMed] [Google Scholar]
- 86.Nowak LM, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Nature Lond. 1984;307:462. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- 87.Mayer ML, Westbrook GL, Guthrie PB. Nature Lond. 1984;309:261. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- 88.McBain CJ, Mayer ML. Physiol Rev. 1994;74:723. doi: 10.1152/physrev.1994.74.3.723. [DOI] [PubMed] [Google Scholar]
- 89.Herron CE, Lester RA, Coan EJ, Collingridge GL. Neurosci. Lett. 1985;60:19. doi: 10.1016/0304-3940(85)90375-1. [DOI] [PubMed] [Google Scholar]
- 90.Bekkers JM, Stevens CF. Nature Lond. 1989;341:230. doi: 10.1038/341230a0. [DOI] [PubMed] [Google Scholar]
- 91.Markram H, Lubke J, Frotscher M, Sakmann B. Science. 1997;275:213. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- 92.Stuart G, Spruston N, Sakmann B, Hausser M. Trends Neurosci. 1997;20:125. doi: 10.1016/s0166-2236(96)10075-8. [DOI] [PubMed] [Google Scholar]
- 93.Kampa BM, Clements J, Jonas P, Stuart GJ. J Physiol. 2004;556:337. doi: 10.1113/jphysiol.2003.058842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.MacDermott AB, Mayer ML, Westbrook GL, Smith SJ, Barker JL. Nature Lond. 1986;321:519. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- 95.Wollmuth LP, Sakmann B. J. Gen. Physiol. 1998;112:623. doi: 10.1085/jgp.112.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lester RAJ, Clements JD, Westbrook GL, Jahr CE. Nature Lond. 1990;346:565. doi: 10.1038/346565a0. [DOI] [PubMed] [Google Scholar]
- 97.Hestrin S. Neuron. 1992;9:991. doi: 10.1016/0896-6273(92)90250-h. [DOI] [PubMed] [Google Scholar]
- 98.Livsey CT, Costa E, Vicini S. J. Neurosci. 1993;13:5324. doi: 10.1523/JNEUROSCI.13-12-05324.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jonas P, Sakmann B. J Physiol. 1992;455:143. doi: 10.1113/jphysiol.1992.sp019294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Colquhoun D, Jonas P, Sakmann B. J Physiol. 1992;458:261. doi: 10.1113/jphysiol.1992.sp019417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lambolez B, Audinat E, Bochet P, Crepel F, Rossier J. Neuron. 1992;9:247. doi: 10.1016/0896-6273(92)90164-9. [DOI] [PubMed] [Google Scholar]
- 102.Jonas P, Racca C, Sakmann B, Seeburg PH, Monyer H. Neuron. 1994;12:1281. doi: 10.1016/0896-6273(94)90444-8. [DOI] [PubMed] [Google Scholar]
- 103.Geiger JRP, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, Monyer H. Neuron. 1995;15:193. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 104.Tempia F, Kano M, Schneggenburger R, Schirra C, Garaschuk O, Plant T, Konnerth A. J. Neurosci. 1996;16:456. doi: 10.1523/JNEUROSCI.16-02-00456.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liao D, Hessler NA, Malinow R. Nature Lond. 1995;375:400. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- 106.Malenka RC, Nicoll RA. Neuron. 1997;19:473. doi: 10.1016/s0896-6273(00)80362-1. [DOI] [PubMed] [Google Scholar]
- 107.Wu G, Malinow R, Cline HT. Science. 1996;274:972. doi: 10.1126/science.274.5289.972. [DOI] [PubMed] [Google Scholar]
- 108.Petralia RS, Esteban JA, Wang YX, Partridge JG, Zhao HM, Wenthold RJ, Malinow R. Nat. Neurosci. 1999;2:31. doi: 10.1038/4532. [DOI] [PubMed] [Google Scholar]
- 109.Xiao MY, Wasling P, Hanse E, Gustafsson B. Nat. Neurosci. 2004;7:236. doi: 10.1038/nn1196. [DOI] [PubMed] [Google Scholar]
- 110.Cull-Candy S, Kelly L, Farrant M. Curr. Opin. Neurobiol. 2006;16:288. doi: 10.1016/j.conb.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 111.Isaac JT, Ashby M, McBain CJ. Neuron. 2007;54:859. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 112.Osswald IK, Galan A, Bowie D. J Physiol. 2007;582:95. doi: 10.1113/jphysiol.2007.127894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Diamond JS. J Physiol. 2007;582:3. doi: 10.1113/jphysiol.2007.135145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bowie D, Bähring R, Mayer ML. Handbook of Experimental Pharmacology. In: Jonas P, Monyer H, editors. Ionotropic Glutamate Receptors in the CNS. Berlin: Springer-Verlag; 1999. pp. 251–373. [Google Scholar]
- 115.Lerma J, Paternain AV, Rodriguez-Moreno A, López-García JC. Phys. Rev. 2001;81:971. doi: 10.1152/physrev.2001.81.3.971. [DOI] [PubMed] [Google Scholar]
- 116.Huettner JE. Prog. Neurobiol. 2003;70:387. doi: 10.1016/s0301-0082(03)00122-9. [DOI] [PubMed] [Google Scholar]
- 117.Pinheiro P, Mulle C. Cell Tissue Res. 2006;326:457. doi: 10.1007/s00441-006-0265-6. [DOI] [PubMed] [Google Scholar]
- 118.Bowie D, Lange GD. J. Neurosci. 2002;22:3392. doi: 10.1523/JNEUROSCI.22-09-03392.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Castillo PE, Malenka RC, Nicoll RA. Nature Lond. 1997;388:182. doi: 10.1038/40645. [DOI] [PubMed] [Google Scholar]
- 120.Vignes M, Collingridge GL. Nature Lond. 1997;388:179. doi: 10.1038/40639. [DOI] [PubMed] [Google Scholar]
- 121.Kidd FL, Isaac JTR. Nature Lond. 1999;400:569. doi: 10.1038/23040. [DOI] [PubMed] [Google Scholar]
- 122.Petralia RS, Wang YX, Wenthold RJ. J. Comp. Neurol. 1994;349:85. doi: 10.1002/cne.903490107. [DOI] [PubMed] [Google Scholar]
- 123.Kidd FL, Isaac JTR. J. Neurophysiol. 2001;86:1139. doi: 10.1152/jn.2001.86.3.1139. [DOI] [PubMed] [Google Scholar]
- 124.Garcia EP, Mehta S, Blair LAC, Wells DG, Shang J, Fukushima T, Fallon JR, Garner CC, Marshall J. Neuron. 1998;21:727. doi: 10.1016/s0896-6273(00)80590-5. [DOI] [PubMed] [Google Scholar]
- 125.Bowie D, Garcia EP, Marshall J, Traynelis SF, Lange GD. J. Physiol. (Lond.) 2003;547:373. doi: 10.1113/jphysiol.2002.033076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.DeVries SH, Schwartz EA. Nature Lond. 1999;397:157. doi: 10.1038/16462. [DOI] [PubMed] [Google Scholar]
- 127.DeVries SH. Neuron. 2000;28:847. doi: 10.1016/s0896-6273(00)00158-6. [DOI] [PubMed] [Google Scholar]
- 128.Epsztein J, Represa A, Jorquera I, Ben Ari Y, Crepel V. J. Neurosci. 2005;25:8229. doi: 10.1523/JNEUROSCI.1469-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Matsuda S, Yuzaki M. Eur. J Neurosci. 2002;16:1507. doi: 10.1046/j.1460-9568.2002.02219.x. [DOI] [PubMed] [Google Scholar]
- 130.Lalouette A, Lohof A, Sotelo C, Guenet J, Mariani J. Neurosci. 2001;105:443. doi: 10.1016/s0306-4522(01)00193-2. [DOI] [PubMed] [Google Scholar]
- 131.Kashiwabuchi N, Ikeda K, Araki K, Hirano T, Shibuki K, Takayama C, Inoue Y, Kutsuwada T, Yagi T, Kang Y. Cell. 1995;81:245. doi: 10.1016/0092-8674(95)90334-8. [DOI] [PubMed] [Google Scholar]
- 132.Sacchetti B, Scelfo B, Tempia F, Strata P. Neuron. 2004;42:973. doi: 10.1016/j.neuron.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 133.Sacchetti B, Scelfo B, Strata P. Neuroscientist. 2005;11:217. doi: 10.1177/1073858405276428. [DOI] [PubMed] [Google Scholar]
- 134.Yuzaki M. Neurosci. Res. 2003;46:11. doi: 10.1016/s0168-0102(03)00036-1. [DOI] [PubMed] [Google Scholar]
- 135.Chohan MO, Iqbal K. J Alzheimers. Dis. 2006;10:81. doi: 10.3233/jad-2006-10112. [DOI] [PubMed] [Google Scholar]
- 136.Fan MM, Raymond LA. Prog. Neurobiol. 2007;81:272. doi: 10.1016/j.pneurobio.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 137.Johnson JW, Kotermanski SE. Curr. Opin. Pharmacol. 2006;6:61. doi: 10.1016/j.coph.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 138.Cosman KM, Boyle LL, Porsteinsson AP. Expert. Opin. Pharmacother. 2007;8:203. doi: 10.1517/14656566.8.2.203. [DOI] [PubMed] [Google Scholar]
- 139.Scarpini E, Scheltens P, Feldman H. Lancet Neurol. 2003;2:539. doi: 10.1016/s1474-4422(03)00502-7. [DOI] [PubMed] [Google Scholar]
- 140.Bear MF, Huber KM, Warren ST. Trends Neurosci. 2004;27:370. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 141.O'Donnell WT, Warren ST. Annu. Rev. Neurosci. 2002;25:315. doi: 10.1146/annurev.neuro.25.112701.142909. [DOI] [PubMed] [Google Scholar]
- 142.Bear MF. Genes Brain Behav. 2005;4:393. doi: 10.1111/j.1601-183X.2005.00135.x. [DOI] [PubMed] [Google Scholar]
- 143.Dolen G, Bear MF. Neuron. 2005;45:642. doi: 10.1016/j.neuron.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 144.Koukoui SD, Chaudhuri A. Brain Research Reviews. 2007;53:27. doi: 10.1016/j.brainresrev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 145.Marenco S, Weinberger DR. CNS. Drugs. 2006;20:173. doi: 10.2165/00023210-200620030-00001. [DOI] [PubMed] [Google Scholar]
- 146.Black MD. Psychopharmacology (Berl) 2005;179:154. doi: 10.1007/s00213-004-2065-6. [DOI] [PubMed] [Google Scholar]
- 1477.O'Neill MJ, Bleakman D, Zimmerman DM, Nisenbaum ES. Curr. Drug Targets. CNS. Neurol. Disord. 2004;3:181. doi: 10.2174/1568007043337508. [DOI] [PubMed] [Google Scholar]
- 148.Van Den BL, Van Damme P, Bogaert E, Robberecht W. Biochim. Biophys. Acta. 2006;1762:1068. doi: 10.1016/j.bbadis.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 149.Kwak S, Weiss JH. Curr. Opin. Neurobiol. 2006;16:281. doi: 10.1016/j.conb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 150.Kawahara Y, Kwak S. Amyotroph. Lateral. Scler. Other Motor Neuron Disord. 2005;6:131. doi: 10.1080/14660820510037872. [DOI] [PubMed] [Google Scholar]
- 151.Kwak S, Kawahara Y. J. Mol. Med. 2005;83:110. doi: 10.1007/s00109-004-0599-z. [DOI] [PubMed] [Google Scholar]
- 152.Heath PR, Shaw PJ. Muscle Nerve. 2002;26:438. doi: 10.1002/mus.10186. [DOI] [PubMed] [Google Scholar]
- 153.Shaw PJ, Eggett CJ. J. Neurol. 2000;247 Suppl 1:I17. doi: 10.1007/BF03161151. [DOI] [PubMed] [Google Scholar]
- 154.Soundarapandian MM, Tu WH, Peng PL, Zervos AS, Lu Y. Mol. Neurobiol. 2005;32:145. doi: 10.1385/MN:32:2:145. [DOI] [PubMed] [Google Scholar]
- 155.Weiser T. Curr. Drug Targets. CNS. Neurol. Disord. 2005;4:153. doi: 10.2174/1568007053544129. [DOI] [PubMed] [Google Scholar]
- 156.Weiser T. Curr. Pharm. Des. 2002;8:941. doi: 10.2174/1381612024607135. [DOI] [PubMed] [Google Scholar]
- 157.Tanaka H, Grooms SY, Bennett MV, Zukin RS. Brain Res. 2000;886:190. doi: 10.1016/s0006-8993(00)02951-6. [DOI] [PubMed] [Google Scholar]
- 158.Kullmann DM, Asztely F, Walker MC. Cell Mol. Life Sci. 2000;57:1551. doi: 10.1007/PL00000640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Peng PL, Zhong X, Tu W, Soundarapandian MM, Molner P, Zhu D, Lau L, Liu S, Liu F, Lu Y. Neuron. 2006;49:719. doi: 10.1016/j.neuron.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 160.O'Neill MJ, Witkin JM. Curr. Drug Targets. 2007;8:603. doi: 10.2174/138945007780618517. [DOI] [PubMed] [Google Scholar]
- 161.Wu SS, Frucht SJ. CNS. Drugs. 2005;19:723. doi: 10.2165/00023210-200519090-00001. [DOI] [PubMed] [Google Scholar]
- 162.Brotchie JM. Mov Disord. 2005;20:919. doi: 10.1002/mds.20612. [DOI] [PubMed] [Google Scholar]
- 163.O'Neill MJ, Murray TK, Clay MP, Lindstrom T, Yang CR, Nisenbaum ES. CNS. Drug Rev. 2005;11:77. doi: 10.1111/j.1527-3458.2005.tb00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Johnston TH, Brotchie JM. Curr. Opin. Investig. Drugs. 2004;5:720. [PubMed] [Google Scholar]
- 165.Maas S, Patt S, Schrey M, Rich A. Proc. Natl. Acad. Sci. U. S. A. 2001;98:14687. doi: 10.1073/pnas.251531398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ishiuchi S, Tsuzuki K, Yoshida Y, Yamada N, Hagimura N, Okado H, Miwa A, Kurihara H, Nakazato Y, Tamura M, Sasaki T, Ozawa S. Nat. Med. 2002;8:971. doi: 10.1038/nm746. [DOI] [PubMed] [Google Scholar]
- 167.Ishiuchi S, Yoshida Y, Sugawara K, Aihara M, Ohtani T, Watanabe T, Saito N, Tsuzuki K, Okado H, Miwa A, Nakazato Y, Ozawa S. J. Neurosci. 2007;27:7987. doi: 10.1523/JNEUROSCI.2180-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lyons SA, Chung WJ, Weaver AK, Ogunrinu T, Sontheimer H. Cancer Res. 2007;67:9463. doi: 10.1158/0008-5472.CAN-07-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Howes JF, Bell C. Neurotherapeutics. 2007;4:126. doi: 10.1016/j.nurt.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rogawski MA. Epilepsy Res. 2006;69:273. doi: 10.1016/j.eplepsyres.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.De Sarro G, Gitto R, Russo E, Ibbadu GF, Barreca ML, De Luca L, Chimirri A. Curr. Top. Med. Chem. 2005;5:31. doi: 10.2174/1568026053386999. [DOI] [PubMed] [Google Scholar]
- 172.Madsen U, Stensbol TB, Krogsgaard-Larsen P. Curr. Med. Chem. 2001;8:1291. doi: 10.2174/0929867013372210. [DOI] [PubMed] [Google Scholar]
- 173.Granata T. Neurol. Sci. 2003;24 Suppl 4:S239. doi: 10.1007/s10072-003-0086-2. [DOI] [PubMed] [Google Scholar]
- 174.Lang B, Dale RC, Vincent A. Curr. Opin. Neurol. 2003;16:351. doi: 10.1097/01.wco.0000073937.19076.d5. [DOI] [PubMed] [Google Scholar]
- 175.MacDonald JF, Xiong ZG, Jackson MF. Trends Neurosci. 2006;29:75. doi: 10.1016/j.tins.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 176.Boillee S, Vande VC, Cleveland DW. Neuron. 2006;52:39. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 177.Hong SJ, Dawson TM, Dawson VL. Trends Pharmacol. Sci. 2004;25:259. doi: 10.1016/j.tips.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 178.Verdoorn TA, Burnashev N, Monyer H, Seeburg PH, Sakmann B. Science. 1991;252:1715. doi: 10.1126/science.1710829. [DOI] [PubMed] [Google Scholar]
- 179.Hollmann M, Hartley M, Heinemann S. Science. 1991;252:851. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- 180.Hume RI, Dingledine R, Heinemann SF. Science. 1991;253:1028. doi: 10.1126/science.1653450. [DOI] [PubMed] [Google Scholar]
- 181.Burnashev N, Monyer H, Seeburg PH, Sakmann B. Neuron. 1992;8:189. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- 182.Kuner T, Beck C, Sakmann B, Seeburg PH. J. Neurosci. 2001;21:4162. doi: 10.1523/JNEUROSCI.21-12-04162.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sprengel R, Aronoff R, Volkner M, Schmitt B, Mosbach R, Kuner T. Trends Pharmacol. Sci. 2001;22:7. doi: 10.1016/s0165-6147(00)01588-1. [DOI] [PubMed] [Google Scholar]
- 184.Schmieden V, Kuhse J, Betz H. Science. 1993;262:256. doi: 10.1126/science.8211147. [DOI] [PubMed] [Google Scholar]
- 185.Washburn MS, Numberger M, Zhang S, Dingledine R. J. Neurosci. 1997;17:9393. doi: 10.1523/JNEUROSCI.17-24-09393.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gorter JA, Petrozzino JJ, Aronica EM, Rosenbaum DM, Opitz T, Bennett MV, Connor JA, Zukin RS. J. Neurosci. 1997;17:6179. doi: 10.1523/JNEUROSCI.17-16-06179.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pellegrini-Giampietro DE, Gorter JA, Bennett MV, Zukin RS. Trends Neurosci. 1997;20:464. doi: 10.1016/s0166-2236(97)01100-4. [DOI] [PubMed] [Google Scholar]
- 188.Yin HZ, Sensi SL, Carriedo SG, Weiss JH. J Comp Neurol. 1999;409:250. [PubMed] [Google Scholar]
- 189.Ogoshi F, Weiss JH. J. Neurosci. 2003;23:10521. doi: 10.1523/JNEUROSCI.23-33-10521.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Plant K, Pelkey KA, Bortolotto ZA, Morita D, Terashima A, McBain CJ, Collingridge GL, Isaac JT. Nat. Neurosci. 2006;9:602. doi: 10.1038/nn1678. [DOI] [PubMed] [Google Scholar]
- 191.Kawahara Y, Ito K, Sun H, Aizawa H, Kanazawa I, Kwak S. Nature Lond. 2004;427:801. doi: 10.1038/427801a. [DOI] [PubMed] [Google Scholar]
- 192.Kwak S, Kawahara Y. J Mol. Med. 2005;83:110. doi: 10.1007/s00109-004-0599-z. [DOI] [PubMed] [Google Scholar]
- 193.Takuma H, Kwak S, Yoshizawa T, Kanazawa I. Ann. Neurol. 1999;46:806. doi: 10.1002/1531-8249(199912)46:6<806::aid-ana2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 194.Kim YH, Koh JY. Exp. Neurol. 2002;177:407. doi: 10.1006/exnr.2002.7990. [DOI] [PubMed] [Google Scholar]
- 195.Noh KM, Yokota H, Mashiko T, Castillo PE, Zukin RS, Bennett MV. Proc. Natl. Acad. Sci. U. S. A. 2005;102:12230. doi: 10.1073/pnas.0505408102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Seeburg PH. Neuron. 2002;35:17. doi: 10.1016/s0896-6273(02)00760-2. [DOI] [PubMed] [Google Scholar]
- 197.Noble M, Gutowski N, Bevan K, Engel U, Linskey M, Urenjak J, Bhakoo K, Williams S. Glia. 1995;15:222. doi: 10.1002/glia.440150304. [DOI] [PubMed] [Google Scholar]
- 198.Senger D, Cairncross JG, Forsyth PA. Cancer J. 2003;9:214. doi: 10.1097/00130404-200305000-00009. [DOI] [PubMed] [Google Scholar]
- 199.Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK, DePinho RA. Genes Dev. 2001;15:1311. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- 200.Bar-Peled O, Ben Hur H, Biegon A, Groner Y, Dewhurst S, Furuta A, Rothstein JD. J. Neurochem. 1997;69:2571. doi: 10.1046/j.1471-4159.1997.69062571.x. [DOI] [PubMed] [Google Scholar]
- 201.Danbolt NC. Prog. Neurobiol. 2001;65:1. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 202.Ye ZC, Rothstein JD, Sontheimer H. J. Neurosci. 1999;19:10767. doi: 10.1523/JNEUROSCI.19-24-10767.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sontheimer H. Trends Neurosci. 2003;26:543. doi: 10.1016/j.tins.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 204.Rzeski W, Turski L, Ikonomidou C. Proc. Natl. Acad. Sci. U. S. A. 2001;98:6372. doi: 10.1073/pnas.091113598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Turner G, Webb T, Wake S, Robinson H. Am. J Med. Genet. 1996;64:196. doi: 10.1002/(SICI)1096-8628(19960712)64:1<196::AID-AJMG35>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 206.Warren ST, Nelson DL. JAMA. 1994;271:536. [PubMed] [Google Scholar]
- 207.Orr HT, Zoghbi HY. Annu. Rev. Neurosci. 2007;30:575. doi: 10.1146/annurev.neuro.29.051605.113042. [DOI] [PubMed] [Google Scholar]
- 208.Hagerman RJ, Hull CE, Safanda JF, Carpenter I, Staley LW, O'Connor RA, Seydel C, Mazzocco MM, Snow K, Thibodeau SN. Am. J. Med. Genet. 1994;51:298. doi: 10.1002/ajmg.1320510404. [DOI] [PubMed] [Google Scholar]
- 209.Fu YH, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, Verkerk AJ, Holden JJ, Fenwick RG, Jr, Warren ST. Cell. 1991;67:1047. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- 210.Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP. Cell. 1991;65:905. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- 211.Coffee B, Zhang F, Warren ST, Reines D. Nat. Genet. 1999;22:98. doi: 10.1038/8807. [DOI] [PubMed] [Google Scholar]
- 212.Siomi H, Siomi MC, Nussbaum RL, Dreyfuss G. Cell. 1993;74:291. doi: 10.1016/0092-8674(93)90420-u. [DOI] [PubMed] [Google Scholar]
- 213.Penagarikano O, Mulle JG, Warren ST. Annu. Rev. Genomics Hum. Genet. 2007;8:109. doi: 10.1146/annurev.genom.8.080706.092249. [DOI] [PubMed] [Google Scholar]
- 214.Huber KM, Gallagher SM, Warren ST, Bear MF. Proc. Natl. Acad. Sci. U. S. A. 2002;99:7746. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Koekkoek SK, Yamaguchi K, Milojkovic BA, Dortland BR, Ruigrok TJ, Maex R, De Graaf W, Smit AE, VanderWerf F, Bakker CE, Willemsen R, Ikeda T, Kakizawa S, Onodera K, Nelson DL, Mientjes E, Joosten M, De Schutter E, Oostra BA, Ito M, De Zeeuw CI. Neuron. 2005;47:339. doi: 10.1016/j.neuron.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 216.Brown V, Jin P, Ceman S, Darnell JC, O'Donnell WT, Tenenbaum SA, Jin X, Feng Y, Wilkinson KD, Keene JD, Darnell RB, Warren S. T. Cell. 2001;107:477. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- 217.Zhang YQ, Bailey AM, Matthies HJ, Renden RB, Smith MA, Speese SD, Rubin GM, Broadie K. Cell. 2001;107:591. doi: 10.1016/s0092-8674(01)00589-x. [DOI] [PubMed] [Google Scholar]
- 218.Lu R, Wang H, Liang Z, Ku L, O'Donnell WT, Li W, Warren ST, Feng Y. Proc. Natl. Acad. Sci. U. S. A. 2004;101:15201. doi: 10.1073/pnas.0404995101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Muddashetty RS, Kelic S, Gross C, Xu M, Bassell GJ. J. Neurosci. 2007;27:5338. doi: 10.1523/JNEUROSCI.0937-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.The Dutch-Belgian Fragile X Consortium. Cell. 1994;78:23. [PubMed] [Google Scholar]
- 221.Weiler IJ, Irwin SA, Klintsova AY, Spencer CM, Brazelton AD, Miyashiro K, Comery TA, Patel B, Eberwine J, Greenough WT. Proc. Natl. Acad. Sci. U. S. A. 1997;94:5395. doi: 10.1073/pnas.94.10.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Huber KM, Kayser MS, Bear MF. Science. 2000;288:1254. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- 223.Huber KM, Roder JC, Bear MF. J. Neurophysiol. 2001;86:321. doi: 10.1152/jn.2001.86.1.321. [DOI] [PubMed] [Google Scholar]
- 224.Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. Nat. Neurosci. 2001;4:1079. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- 225.Antar LN, Bassell GJ. Neuron. 2003;37:555. doi: 10.1016/s0896-6273(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 226.Feng Y, Absher D, Eberhart DE, Brown V, Malter HE, Warren ST. Mol. Cell. 1997;1:109. doi: 10.1016/s1097-2765(00)80012-x. [DOI] [PubMed] [Google Scholar]
- 227.Brown V, Jin P, Ceman S, Darnell JC, O'Donnell WT, Tenenbaum SA, Jin X, Feng Y, Wilkinson KD, Keene JD, Darnell RB, Warren ST. Cell. 2001;107:477. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- 228.Darnell JC, Jensen KB, Jin P, Brown V, Warren ST, Darnell RB. Cell. 2001;107:489. doi: 10.1016/s0092-8674(01)00566-9. [DOI] [PubMed] [Google Scholar]
- 229.Jin P, Alisch RS, Warren ST. Nat. Cell Biol. 2004;6:1048. doi: 10.1038/ncb1104-1048. [DOI] [PubMed] [Google Scholar]
- 230.Pfeiffer BE, Huber KM. J. Neurosci. 2006;26:7147. doi: 10.1523/JNEUROSCI.1797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Godfraind JM, Reyniers E, De Boulle K, D'Hooge R, De Deyn PP, Bakker CE, Oostra BA, Kooy RF, Willems PJ. Am. J Med. Genet. 1996;64:246. doi: 10.1002/(SICI)1096-8628(19960809)64:2<246::AID-AJMG2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 232.Paradee W, Melikian HE, Rasmussen DL, Kenneson A, Conn PJ, Warren ST. Neurosci. 1999;94:185. doi: 10.1016/s0306-4522(99)00285-7. [DOI] [PubMed] [Google Scholar]
- 233.Dobkin C, Rabe A, Dumas R, El Idrissi A, Haubenstock H, Brown WT. Neurosci. 2000;100:423. doi: 10.1016/s0306-4522(00)00292-x. [DOI] [PubMed] [Google Scholar]
- 234.Neves G, Cooke SF, Bliss TV. Nat. Rev. Neurosci. 2008;9:65. doi: 10.1038/nrn2303. [DOI] [PubMed] [Google Scholar]
- 235.Medina JF, Nores WL, Ohyama T, Mauk MD. Curr. Opin. Neurobiol. 2000;10:717. doi: 10.1016/s0959-4388(00)00154-9. [DOI] [PubMed] [Google Scholar]
- 236.Chiurazzi P, Pomponi MG, Willemsen R, Oostra BA, Neri G. Hum. Mol. Genet. 1998;7:109. doi: 10.1093/hmg/7.1.109. [DOI] [PubMed] [Google Scholar]
- 237.Hayashi ML, Rao BS, Seo JS, Choi HS, Dolan BM, Choi SY, Chattarji S, Tonegawa S. Proc. Natl. Acad. Sci. U. S. A. 2007;104:11489. doi: 10.1073/pnas.0705003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Danysz W. Curr. Opin. Investig. Drugs. 2002;3:1081. [PubMed] [Google Scholar]
- 239.Berry-Kravis E, Krause SE, Block SS, Guter S, Wuu J, Leurgans S, Decle P, Potanos K, Cook E, Salt J, Maino D, Weinberg D, Lara R, Jardini T, Cogswell J, Johnson SA, Hagerman R. J Child Adolesc. Psychopharmacol. 2006;16:525. doi: 10.1089/cap.2006.16.525. [DOI] [PubMed] [Google Scholar]
- 240.Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Neuron. 2007;56:955. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Bear MF, Dolen G, Osterweil E, Nagarajan N. Neuropsychopharmacology. 2008;33:84. doi: 10.1038/sj.npp.1301610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Neuropharm. 2005;49:1053. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 243.Chuang SC, Zhao W, Bauchwitz R, Yan Q, Bianchi R, Wong RK. J. Neurosci. 2005;25:8048. doi: 10.1523/JNEUROSCI.1777-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Aschrafi A, Cunningham BA, Edelman GM, Vanderklish PW. Proc. Natl. Acad. Sci. U. S. A. 2005;102:2180. doi: 10.1073/pnas.0409803102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Delorme R, Krebs MO, Chabane N, Roy I, Millet B, Mouren-Simeoni MC, Maier W, Bourgeron T, Leboyer M. Neuroreport. 2004;15:699. doi: 10.1097/00001756-200403220-00025. [DOI] [PubMed] [Google Scholar]
- 246.Bah J, Quach H, Ebstein RP, Segman RH, Melke J, Jamain S, Rietschel M, Modai I, Kanas K, Karni O, Lerer B, Gourion D, Krebs MO, Etain B, Schurhoff F, Szoke A, Leboyer M, Bourgeron T. Neuroreport. 2004;15:1987. doi: 10.1097/00001756-200408260-00031. [DOI] [PubMed] [Google Scholar]
- 247.Strutz-Seebohm N, Korniychuk G, Schwarz R, Baltaev R, Ureche ON, Mack AF, Ma ZL, Hollmann M, Lang F, Seebohm G. Cell Physiol Biochem. 2006;18:287. doi: 10.1159/000097675. [DOI] [PubMed] [Google Scholar]
- 248.Kim SA, Kim JH, Park M, Cho IH, Yoo HJ. Neurosci. Res. 2007;58:332. doi: 10.1016/j.neures.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 249.Jamain S, Betancur C, Quach H, Philippe A, Fellous M, Giros B, Gillberg C, Leboyer M, Bourgeron T. Mol. Psychiatry. 2002;7:302. doi: 10.1038/sj.mp.4000979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Shuang M, Liu J, Jia MX, Yang JZ, Wu SP, Gong XH, Ling YS, Ruan Y, Yang XL, Zhang D. Am. J Med. Genet. B Neuropsychiatr. Genet. 2004;131:48. doi: 10.1002/ajmg.b.30025. [DOI] [PubMed] [Google Scholar]
- 251.Dutta S, Das S, Guhathakurta S, Sen B, Sinha S, Chatterjee A, Ghosh S, Ahmed S, Ghosh S, Usha R. Cell Mol. Neurobiol. 2007 doi: 10.1007/s10571-007-9193-6. [DOI] [PubMed] [Google Scholar]
- 252.Schiffer HH, Heinemann SF. Am. J Med. Genet. B Neuropsychiatr. Genet. 2007;144:20. doi: 10.1002/ajmg.b.30374. [DOI] [PubMed] [Google Scholar]
- 253.Paddock S, Laje G, Charney D, Rush AJ, Wilson AF, Sorant AJ, Lipsky R, Wisniewski SR, Manji H, McMahon FJ. Am. J Psychiatry. 2007;164:1181. doi: 10.1176/appi.ajp.2007.06111790. [DOI] [PubMed] [Google Scholar]
- 254.Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH. Neuropsychopharmacology. 2007;32:1888. doi: 10.1038/sj.npp.1301312. [DOI] [PubMed] [Google Scholar]
- 255.Wilson GM, Flibotte S, Chopra V, Melnyk BL, Honer WG, Holt RA. Hum. Mol. Genet. 2006;15:743. doi: 10.1093/hmg/ddi489. [DOI] [PubMed] [Google Scholar]
- 256.Pickard BS, Malloy MP, Christoforou A, Thomson PA, Evans KL, Morris SW, Hampson M, Porteous DJ, Blackwood DH, Muir WJ. Mol. Psychiatry. 2006;11:847. doi: 10.1038/sj.mp.4001867. [DOI] [PubMed] [Google Scholar]
- 257.Blackwood DH, Pickard BJ, Thomson PA, Evans KL, Porteous DJ, Muir WJ. Neurotox. Res. 2007;11:73. doi: 10.1007/BF03033484. [DOI] [PubMed] [Google Scholar]
- 258.Coyle JT, Ferkany JW, Zaczek R. Neurobehav. Toxicol. Teratol. 1983;5:617. [PubMed] [Google Scholar]
- 259.Wagster MV, Hedreen JC, Peyser CE, Folstein SE, Ross CA. Exp. Neurol. 1994;127:70. doi: 10.1006/exnr.1994.1081. [DOI] [PubMed] [Google Scholar]
- 260.MacDonald ME, Vonsattel JP, Shrinidhi J, Couropmitree NN, Cupples LA, Bird ED, Gusella JF, Myers RH. Neurology. 1999;53:1330. doi: 10.1212/wnl.53.6.1330. [DOI] [PubMed] [Google Scholar]
- 261.Rubinsztein DC, Leggo J, Chiano M, Dodge A, Norbury G, Rosser E, Craufurd D. Proc. Natl. Acad. Sci. U. S. A. 1997;94:3872. doi: 10.1073/pnas.94.8.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Diguet E, Fernagut PO, Normand E, Centelles L, Mulle C, Tison F. Neurobiol. Dis. 2004;15:667. doi: 10.1016/j.nbd.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 263.Sutton JL, Maccecchini ML, Kajander KC. Neurosci. 1999;91:283. doi: 10.1016/s0306-4522(98)00621-6. [DOI] [PubMed] [Google Scholar]
- 264.Ta LE, Dionne RA, Fricton JR, Hodges JS, Kajander KC. Brain Res. 2000;858:106. doi: 10.1016/s0006-8993(99)02437-3. [DOI] [PubMed] [Google Scholar]
- 265.Blackburn-Munro G, Bomholt SF, Erichsen HK. Neuropharm. 2004;47:351. doi: 10.1016/j.neuropharm.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 266.Palecek J, Neugebauer V, Carlton SM, Iyengar S, Willis WD. Pain. 2004;111:151. doi: 10.1016/j.pain.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 267.Ko S, Zhao MG, Toyoda H, Qiu CS, Zhuo M. J. Neurosci. 2005;25:977. doi: 10.1523/JNEUROSCI.4059-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Agrawal SG, Evans RH. Br. J. Pharmacol. 1986;87:345. doi: 10.1111/j.1476-5381.1986.tb10823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Huettner JE. Neuron. 1990;5:255. doi: 10.1016/0896-6273(90)90163-a. [DOI] [PubMed] [Google Scholar]
- 270.Wong LA, Mayer ML. Mol. Pharmacol. 1993;44:504. [PubMed] [Google Scholar]
- 271.Partin KM, Patneau DK, Winters CA, Mayer ML, Buonanno A. Neuron. 1993;11:1069. doi: 10.1016/0896-6273(93)90220-l. [DOI] [PubMed] [Google Scholar]
- 272.Kerchner GA, Wilding TJ, Li P, Zhuo M, Huettner JE. J. Neurosci. 2001;21:59. doi: 10.1523/JNEUROSCI.21-01-00059.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Kerchner GA, Wilding TJ, Huettner JE, Zhuo M. J. Neurosci. 2002;22:8010. doi: 10.1523/JNEUROSCI.22-18-08010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Hwang SJ, Pagliardini S, Rustioni A, Valtschanoff JG. J Comp Neurol. 2001;436:275. [PubMed] [Google Scholar]
- 275.Li P, Wilding TJ, Kim SJ, Calejesan AA, Huettner JE, Zhuo M. Nature Lond. 1999;397:161. doi: 10.1038/16469. [DOI] [PubMed] [Google Scholar]
- 276.Wilding TJ, Huettner JE. J Physiol. 2001;532:411. doi: 10.1111/j.1469-7793.2001.0411f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Kerchner GA, Wang GD, Qiu CS, Huettner JE, Zhuo M. Neuron. 2001;32:477. doi: 10.1016/s0896-6273(01)00479-2. [DOI] [PubMed] [Google Scholar]
- 278.Li H, Rogawski MA. Neuropharm. 1998;37:1279. doi: 10.1016/s0028-3908(98)00109-9. [DOI] [PubMed] [Google Scholar]
- 279.Li H, Chen A, Xing G, Wei ML, Rogawski MA. Nat. Neurosci. 2001;4:612. doi: 10.1038/88432. [DOI] [PubMed] [Google Scholar]
- 280.Braga MF, Aroniadou-Anderjaska V, Xie J, Li H. J. Neurosci. 2003;23:442. doi: 10.1523/JNEUROSCI.23-02-00442.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Braga MF, Aroniadou-Anderjaska V, Li H. Mol. Neurobiol. 2004;30:127. doi: 10.1385/MN:30:2:127. [DOI] [PubMed] [Google Scholar]
- 282.Phelps EA, LeDoux JE. Neuron. 2005;48:175. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 283.Lang PJ, Davis M. Prog. Brain Res. 2006;156:3. doi: 10.1016/S0079-6123(06)56001-7. [DOI] [PubMed] [Google Scholar]
- 284.Theodore WH, Spencer SS, Wiebe S, Langfitt JT, Ali A, Shafer PO, Berg AT, Vickrey BG. Epilepsia. 2006;47:1700. doi: 10.1111/j.1528-1167.2006.00633.x. [DOI] [PubMed] [Google Scholar]
- 285.Kwan P, Brodie MJ. Seizure. 2000;9:464. doi: 10.1053/seiz.2000.0442. [DOI] [PubMed] [Google Scholar]
- 286.Jacobs MP, Fischbach GD, Davis MR, Dichter MA, Dingledine R, Lowenstein DH, Morrell MJ, Noebels JL, Rogawski MA, Spencer SS, Theodore WH. Neurology. 2001;57:1536. doi: 10.1212/wnl.57.9.1536. [DOI] [PubMed] [Google Scholar]
- 287.Westbrook GL. In: Principles of Neural Science. Kandel ER, Schwartz JH, Jessell TM, editors. New York: McGraw-Hill Companies; 2000. pp. 910–935. [Google Scholar]
- 288.McNamara JO. In: Goodman & Gilman's the pharmacological basis of therapeutics. Hardman JG, Limbard LE, Goodman AG, editors. New York: McGraw-Hill Companies; 2001. pp. 521–547. [Google Scholar]
- 289.Scharfman HE. Curr. Neurol. Neurosci. Rep. 2007;7:348. doi: 10.1007/s11910-007-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Blume WT. Epilepsia. 2006;47 Suppl 1:71. doi: 10.1111/j.1528-1167.2006.00665.x. [DOI] [PubMed] [Google Scholar]
- 291.Devinsky O. Rev. Neurol. Dis. 2004;1:2. [PubMed] [Google Scholar]
- 292.Avoli M, Louvel J, Pumain R, Kohling R. Prog. Neurobiol. 2005;77:166. doi: 10.1016/j.pneurobio.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 293.Epilepsia. 1981;22:489. doi: 10.1111/j.1528-1157.1981.tb06159.x. [DOI] [PubMed] [Google Scholar]
- 294.Falconer MA. Epilepsia. 1971;12:13. doi: 10.1111/j.1528-1157.1971.tb03912.x. [DOI] [PubMed] [Google Scholar]
- 295.French JA, Williamson PD, Thadani VM, Darcey TM, Mattson RH, Spencer SS, Spencer DD. Ann. Neurol. 1993;34:774. doi: 10.1002/ana.410340604. [DOI] [PubMed] [Google Scholar]
- 296.VanLandingham KE, Heinz ER, Cavazos JE, Lewis DV. Ann. Neurol. 1998;43:413. doi: 10.1002/ana.410430403. [DOI] [PubMed] [Google Scholar]
- 297.Armstrong LE, Stoppani J. Rev. Neurosci. 2002;13:271. doi: 10.1515/revneuro.2002.13.3.271. [DOI] [PubMed] [Google Scholar]
- 298.Mathern GW, Babb TL, Vickrey BG, Melendez M, Pretorius JK. Brain. 1995;118(Pt 1):105. doi: 10.1093/brain/118.1.105. [DOI] [PubMed] [Google Scholar]
- 299.Hille B. Ionic channels of excitable membranes. 01. Sunderland: Sinauer Associates; [Google Scholar]
- 300.Kaminski RM, Banerjee M, Rogawski MA. Neuropharm. 2004;46:1097. doi: 10.1016/j.neuropharm.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 301.Dooley DJ, Taylor CP, Donevan S, Feltner D. Trends Pharmacol. Sci. 2007;28:75. doi: 10.1016/j.tips.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 302.Davies A, Hendrich J, Van Minh AT, Wratten J, Douglas L, Dolphin AC. Trends Pharmacol. Sci. 2007;28:220. doi: 10.1016/j.tips.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 303.Cascio M. AAPS. J. 2006;8:E353. doi: 10.1007/BF02854906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Regesta G, Tanganelli P. Epilepsy Res. 1999;34:109. doi: 10.1016/s0920-1211(98)00106-5. [DOI] [PubMed] [Google Scholar]
- 305.Nadler JV. Life Sci. 1981;29:2031. doi: 10.1016/0024-3205(81)90659-7. [DOI] [PubMed] [Google Scholar]
- 306.Ben Ari Y. Neurosci. 1985;14:375. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- 307.Kobayashi Y, Bando T, Ishizaki T. Jpn. J Pharmacol. 1952;1:130. doi: 10.1254/jjp.1.2_130. [DOI] [PubMed] [Google Scholar]
- 308.McCulloch RM, Johnston GA, Game CJ, Curtis DR. Exp. Brain Res. 1974;21:515. doi: 10.1007/BF00237169. [DOI] [PubMed] [Google Scholar]
- 309.Johnston GA, Curtis DR, Davies J, McCulloch RM. Nature Lond. 1974;248:804. doi: 10.1038/248804a0. [DOI] [PubMed] [Google Scholar]
- 310.Biscoe TJ, Evans RH, Francis AA, Martin MR, Watkins JC, Davies J, Dray A. Nature Lond. 1977;270:743. doi: 10.1038/270743a0. [DOI] [PubMed] [Google Scholar]
- 311.Davies J, Francis AA, Jones AW, Watkins JC. Neurosci. Lett. 1981;21:77. doi: 10.1016/0304-3940(81)90061-6. [DOI] [PubMed] [Google Scholar]
- 312.Evans RH, Francis AA, Jones AW, Smith DA, Watkins JC. Br. J Pharmacol. 1982;75:65. doi: 10.1111/j.1476-5381.1982.tb08758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.McLennan H, Lodge D. Brain Res. 1979;169:83. doi: 10.1016/0006-8993(79)90375-5. [DOI] [PubMed] [Google Scholar]
- 314.Davies J, Watkins JC. Brain Res. 1981;206:172. doi: 10.1016/0006-8993(81)90111-6. [DOI] [PubMed] [Google Scholar]
- 315.Coleman PA, Massey SC, Miller RF. Brain Res. 1986;381:172. doi: 10.1016/0006-8993(86)90708-0. [DOI] [PubMed] [Google Scholar]
- 316.London ED, Coyle JT. Mol. Pharmacol. 1979;15:492. [PubMed] [Google Scholar]
- 317.Davies J, Evans RH, Francis AA, Watkins JC. J Physiol (Paris) 1979;75:641. [PubMed] [Google Scholar]
- 318.Sugihara H, Moriyoshi K, Ishii T, Masu M, Nakanishi S. Biochem. Biophys. Res. Commun. 1992;185:826. doi: 10.1016/0006-291x(92)91701-q. [DOI] [PubMed] [Google Scholar]
- 319.Mayer ML, Vyklicky L, Patneau DK. Adv. Exp. Med. Biol. 1990;268:3. doi: 10.1007/978-1-4684-5769-8_1. [DOI] [PubMed] [Google Scholar]
- 320.Kutsuwada T, Kashiwabuchi N, Mori H, Sakimura K, Kushiya E, Araki K, Meguro H, Masaki H, Kumanishi T, Arakawa M, Mishina M. Nature Lond. 1992;358:36. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- 321.Patneau DK, Mayer ML. J. Neurosci. 1990;10:2385. doi: 10.1523/JNEUROSCI.10-07-02385.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Patneau DK, Mayer ML. Neuron. 1991;6:785. doi: 10.1016/0896-6273(91)90175-y. [DOI] [PubMed] [Google Scholar]
- 323.Patneau DK, Vyklicky L, Mayer ML. J. Neurosci. 1993;13:3496. doi: 10.1523/JNEUROSCI.13-08-03496.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Lerma J, Paternain AV, Naranjo JR, Mellstrom B. Proc. Natl. Acad. Sci. USA. 1993;90:11688. doi: 10.1073/pnas.90.24.11688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Dingledine R, McBain CJ, McNamara JO. Trends Pharmacol. Sci. 1990;11:334. doi: 10.1016/0165-6147(90)90238-4. [DOI] [PubMed] [Google Scholar]
- 326.Weiss JH, Sensi SL. Trends Neurosci. 2000;23:365. doi: 10.1016/s0166-2236(00)01610-6. [DOI] [PubMed] [Google Scholar]
- 327.Kwak S, Weiss JH. Curr. Opin. Neurobiol. 2006;16:281. doi: 10.1016/j.conb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 328.Liu SJ, Zukin RS. Trends Neurosci. 2007;30:126. doi: 10.1016/j.tins.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 329.Ben Ari Y, Gho M. J Physiol. 1988;404:365. doi: 10.1113/jphysiol.1988.sp017294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Ben Ari Y, Cossart R. Trends Neurosci. 2000;23:580. doi: 10.1016/s0166-2236(00)01659-3. [DOI] [PubMed] [Google Scholar]
- 331.Pinheiro P, Mulle C. Cell Tissue Res. 2006;326:457. doi: 10.1007/s00441-006-0265-6. [DOI] [PubMed] [Google Scholar]
- 332.Monaghan DT, Cotman CW. Brain Res. 1982;252:91. doi: 10.1016/0006-8993(82)90981-7. [DOI] [PubMed] [Google Scholar]
- 333.Sailer A, Swanson GT, Perez-Otano I, O'Leary L, Malkmus SA, Dyck RH, Dickinson-Anson H, Schiffer HH, Maron C, Yaksh TL, Gage FH, O'Gorman S, Heinemann SF. J. Neurosci. 1999;19:8757. doi: 10.1523/JNEUROSCI.19-20-08757.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Darstein M, Petralia RS, Swanson GT, Wenthold RJ, Heinemann SF. J. Neurosci. 2003;23:8013. doi: 10.1523/JNEUROSCI.23-22-08013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Pinheiro PS, Perrais D, Coussen F, Barhanin J, Bettler B, Mann JR, Malva JO, Heinemann SF, Mulle C. Proc. Natl. Acad. Sci. U. S. A. 2007;104:12181. doi: 10.1073/pnas.0608891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Contractor A, Sailer AW, Darstein M, Maron C, Xu J, Swanson GT, Heinemann SF. J. Neurosci. 2003;23:422. doi: 10.1523/JNEUROSCI.23-02-00422.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Bleakman D, Lodge D. Neuropharm. 1998;37:1187. doi: 10.1016/s0028-3908(98)00139-7. [DOI] [PubMed] [Google Scholar]
- 338.Kortenbruck G, Berger E, Speckmann EJ, Musshoff U. Neurobiol. Dis. 2001;8:459. doi: 10.1006/nbdi.2001.0394. [DOI] [PubMed] [Google Scholar]
- 339.Fisahn A. J Physiol. 2005;562:65. doi: 10.1113/jphysiol.2004.077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Smolders I, Bortolotto ZA, Clarke VR, Warre R, Khan GM, O'Neill MJ, Ornstein PL, Bleakman D, Ogden A, Weiss B, Stables JP, Ho KH, Ebinger G, Collingridge GL, Lodge D, Michotte Y. Nat. Neurosci. 2002;5:796. doi: 10.1038/nn880. [DOI] [PubMed] [Google Scholar]
- 341.Rogawski MA, Gryder D, Castaneda D, Yonekawa W, Banks MK, Lia H. Ann. N. Y. Acad. Sci. 2003;985:150. doi: 10.1111/j.1749-6632.2003.tb07079.x. [DOI] [PubMed] [Google Scholar]
- 342.Kaminski RM, Banerjee M, Rogawski MA. Neuropharm. 2004;46:1097. doi: 10.1016/j.neuropharm.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 343.Mulle C, Sailer A, Perez-Otano I, Dickinson-Anson H, Castillo PE, Bureau I, Maron C, Gage FH, Mann JR, Bettler B, Heinemann SF. Nature Lond. 1998;392:601. doi: 10.1038/33408. [DOI] [PubMed] [Google Scholar]
- 344.Vissel B, Royle GA, Christie BR, Schiffer HH, Ghetti A, Tritto T, Perez-Otano I, Radcliffe RA, Seamans J, Sejnowski T, Wehner JM, Collins AC, O'Gorman S, Heinemann SF. Neuron. 2001;29:217. doi: 10.1016/s0896-6273(01)00192-1. [DOI] [PubMed] [Google Scholar]
- 345.Grigorenko E, Glazier S, Bell W, Tytell M, Nosel E, Pons T, Deadwyler SA. J Neurol. Sci. 1997;153:35. doi: 10.1016/s0022-510x(97)00180-9. [DOI] [PubMed] [Google Scholar]
- 346.Mathern GW, Pretorius JK, Kornblum HI, Mendoza D, Lozada A, Leite JP, Chimelli L, Born DE, Fried I, Sakamoto AC, Assirati JA, Peacock WJ, Ojemann GA, Adelson PD. Neurobiol. Dis. 1998;5:151. doi: 10.1006/nbdi.1998.0200. [DOI] [PubMed] [Google Scholar]
- 347.Porter BE, Cui XN, Brooks-Kayal AR. Eur. J Neurosci. 2006;23:2857. doi: 10.1111/j.1460-9568.2006.04839.x. [DOI] [PubMed] [Google Scholar]
- 348.Epsztein J, Milh M, Bihi RI, Jorquera I, Ben Ari Y, Represa A, Crepel V. J. Neurosci. 2006;26:7082. doi: 10.1523/JNEUROSCI.1666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Ben Ari Y, Cossart R. Trends Neurosci. 2000;23:580. doi: 10.1016/s0166-2236(00)01659-3. [DOI] [PubMed] [Google Scholar]
- 350.Nadler JV. Neurochem. Res. 2003;28:1649. doi: 10.1023/a:1026004904199. [DOI] [PubMed] [Google Scholar]
- 351.Jones E, Fear NT, Wessely S. Am. J Psychiatry. 2007;164:1641. doi: 10.1176/appi.ajp.2007.07071180. [DOI] [PubMed] [Google Scholar]
- 352.Lamprecht F, Sack M. Psychosom. Med. 2002;64:222. doi: 10.1097/00006842-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 353.Yehuda R, LeDoux J. Neuron. 2007;56:19. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 354.McGaugh JL. Annu. Rev. Neurosci. 1989;12:255. doi: 10.1146/annurev.ne.12.030189.001351. [DOI] [PubMed] [Google Scholar]
- 355.McGaugh JL, Introini-Collison IB, Nagahara AH, Cahill L, Brioni JD, Castellano C. Neurosci. Biobehav. Rev. 1990;14:425. doi: 10.1016/s0149-7634(05)80065-x. [DOI] [PubMed] [Google Scholar]
- 356.Cahill L, Prins B, Weber M, McGaugh JL. Nature Lond. 1994;371:702. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- 357.Pitman RK, Sanders KM, Zusman RM, Healy AR, Cheema F, Lasko NB, Cahill L, Orr SP. Biol. Psychiatry. 2002;51:189. doi: 10.1016/s0006-3223(01)01279-3. [DOI] [PubMed] [Google Scholar]
- 358.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Science. 2003;301:805. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 359.Wong ML, Licinio J. Nat. Rev. Neurosci. 2001;2:343. doi: 10.1038/35072566. [DOI] [PubMed] [Google Scholar]
- 360.Gould E. Nat. Rev. Neurosci. 2007;8:481. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- 361.Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, Moore H, Kandel ER. Neuron. 2006;49:603. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 362.Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Mol. Psychiatry. 2005;10:79. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- 363.Seeman P, Kapur S. Proc. Natl. Acad. Sci. U. S. A. 2000;97:7673. doi: 10.1073/pnas.97.14.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Hirvonen J, van Erp TG, Huttunen J, Aalto S, Nagren K, Huttunen M, Lonnqvist J, Kaprio J, Hietala J, Cannon TD. Arch. Gen. Psychiatry. 2005;62:371. doi: 10.1001/archpsyc.62.4.371. [DOI] [PubMed] [Google Scholar]
- 365.Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT. Neuron. 2006;52:139. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 366.Levy R, Goldman-Rakic PS. Exp. Brain Res. 2000;133:23. doi: 10.1007/s002210000397. [DOI] [PubMed] [Google Scholar]