Abstract
Background
There is growing concern about the human resources needed to care for increasing numbers of patients receiving antiretroviral therapy in resource-limited settings. We evaluated an alternative model, community-based, comprehensive antiretroviral program staffed primarily by peer health workers and nurses.
Methods
We conducted a retrospective cohort study of patients receiving antiretroviral therapy during the first 10 months of program enrollment beginning in late 2003. Virologic, immunologic, clinical, and adherence data were collected.
Results
Of 360 patients started on treatment, 258 (72%) were active and on therapy approximately two years later. Viral load testing demonstrated that 86% of active patients (211 of 246 tested) had a viral load <400 copies/mL. The median CD4 increase for active patients was 197 cells/mm3 (IQR, 108–346). Patients with either a history of antiretroviral use or lack of CD4 response were more likely to experience virologic failure. Survival was 84% at one year and 82% at two years. WHO stage 4 was predictive of both not sustaining therapy and increased mortality.
Conclusions
A community-based antiretroviral treatment program in a resource-limited setting can provide excellent AIDS care over at least a two year period. A comprehensive program based upon peer health workers and nurses provides an effective alternative model for AIDS care.
Keywords: adherence, Africa, antiretroviral treatment, community health services, nurses, program evaluation
Introduction
The global health workforce crisis is a significant barrier to the continued expansion of antiretroviral therapy (ART) programs in resource-limited settings (RLS).1, 2 Alternative models of care that rely on nurses and lay health workers rather than physicians may provide one solution. However, these programs must be carefully evaluated by appropriate criteria to determine their effectiveness and long-term sustainability.
The Reach Out Mbuya Parish HIV/AIDS Initiative is a community and faith-based AIDS care program in the urban slums of Kampala, Uganda. The Reach Out program is staffed predominantly by nurses and peer health workers who are persons living with HIV (PLHIV) themselves and receiving care from the program. In October 2003, Reach Out began proving free ART purchased through private donations, along with a continued, comprehensive package of support services and programs directed at patients, their families, and the larger community. In early 2004, this program became the first in the world to provide antiretrovirals with funding from the US-based President’s Emergency Plan for AIDS Relief (PEPFAR). Evaluation of the Reach Out program therefore provides an opportunity to assess the longer-term outcomes and sustainability of ART by a program using an alternative model of care.
Methods
We conducted a retrospective cohort evaluation of all patients who had been started on ART through the Reach Out program from October 1, 2003 to July 31, 2004. Patients were classified as active, died, transferred, lost to follow-up, or stopped therapy based upon their status on April 1, 2006. Patients were defined as active if they had a clinic visit within the last 90 days and were on ART. Patients were defined as lost to follow-up if they were last known to be on ART but did not visit the Reach Out clinic in the past 90 days. Patients were defined as transferred if they had discontinued care at Reach Out but follow-up care with another provider had been clearly arranged. Patients were defined as stopped therapy if they were off of ART for a period of greater than one week, continued to be followed by Reach Out, and were never subsequently restarted on therapy. Finally, patients were defined as died if they were on a list of deceased clients maintained by Reach Out staff through active follow-up and verbal autopsies. Clinical, virologic, immunologic, and adherence outcomes were collected from clinical and programmatic databases and records. History of alcohol abuse was based on patient self-report of significant alcohol use during baseline evaluation for ART. Viral load testing results were collected on active patients from April 2006 to January 2007. The end time point for data collection and analyses was either the date the patient died, the date of viral load testing if active, or the date of the last clinic visit if lost, transferred, or stopped therapy. If a patient was classified as active but no viral load testing was available, then April 1, 2006 was designated as their study end date. This study was approved by the institutional review boards of Johns Hopkins University, the Uganda Virus Research Institute Science and Ethics Committee, and the Uganda National Council for Science and Technology.
Reach Out Mbuya Parish HIV/AIDS Initiative
The Reach Out Mbuya Parish HIV/AIDS Initiative began in an urban slum of Kampala, Uganda in May 2001 and has grown to currently serve over 2500 PLHIV, approximately 1200 of whom are on ART. The population is of relatively low socio-economic status in an already poor country and a significant proportion consists of displaced persons from the civil war in northern Uganda. About two-thirds of Reach Out’s overall staff are PLHIV who are patients themselves. Many of these peer health workers receive training in home-based care, are typically assigned 10–15 patients to follow, and regularly visit these patients in their homes to assess clinical, social, and adherence issues and provide social support. The Reach Out ART clinic is staffed predominantly by nurses rather than clinical and medical officers (at approximately a 7:1 ratio) and continuing medical education and regular advanced training sessions are strongly emphasized. The patient to provider ratio typically varies between approximately 50 to 100:1. The Reach Out care model emphasizes a patient-led, holistic approach to AIDS care with the provision of comprehensive health and social services, including clinic, laboratory, and pharmacy services, voluntary counselling and testing, microfinance and income generation programs, a school fees program, food aid via the World Food Program, an adult literacy program, and extensive community education and prevention activities. All Reach Out services, including ART, are free, with funding provided through church funds, private and institutional donations, the Uganda Ministry of Health, and the United States government (Centers for Disease Control and Prevention-Uganda and PEPFAR). Reach Out is a faith-based organization which began as a project within the Catholic church of Mbuya Parish and continues to be supported by the church. However, Reach Out has staff, volunteers, and patients from all the major religions and does not discriminate based on religion, ethnicity, sexual orientation, or gender in the care of its patients or in the hiring and placement of staff and volunteers. In April 2006, Reach Out was granted non-governmental organization (NGO) status.
Patients
Reach Out serves patients from the Mbuya parish catchment area (population ~60,000); patients must have residency confirmed prior to initiating therapy. Reach Out first began providing ART in October 2003. Initially, ART was purchased with private funding, and priority was given to the sickest patients. Starting in March 2004, PEPFAR-funded ART became available and all patients meeting more generalized criteria were eligible for initiating therapy. These eligibility criteria for starting ART included a CD4 count <250 cells/mm3 or WHO Stage 3 or 4 illness. Available antiretrovirals included stavudine (D4T), zidovudine (AZT), lamivudine (3TC), Combivir (AZT/3TC), efavirenz (EFV) and nevirapine (NVP). Tuberculosis medications were freely available from the Ministry of Health. Only patients ≥13 years of age were included in this study.
Adherence
Clinic-based pill counts were performed at every patient clinic visit and also during intermittent home visits. A summary pill count adherence percent was calculated by dividing the number of pills taken over the study period by the sum of pills expected to be taken over the study period.
Viral load and CD4 testing
Limited viral load testing first became available at Reach Out in April 2006. Testing was performed on all available active patients who had started on antiretroviral therapy from October 2003 to July 2004, and test results were used by clinicians to assist with patient management. HIV viral loads were measured using the Amplicor Monitor Assay, version 1.5 (Roche Diagnostics, Branchburg, NJ, USA) with a lower limit of detection of <400 copies/mL. CD4 counts have been generally available at Reach Out since they began providing antiretroviral therapy and were usually obtained upon initial registration of patients and repeated every 6 months if the initial CD4 was <=400 cells/mm3 or every 12 months if >400 cells/mm3. CD4 cell counts were measured using FACScan (Becton Dickinson, San Jose, CA, USA). For this study, baseline CD4 cell count was designated as the CD4 count within two months of starting ART or, if this was not available, the most recent CD4 prior to ART. End of study CD4 count was designated as the CD4 count closest to the study end date.
Statistical analysis
The characteristics of those who sustained treatment were compared to those who did not sustain treatment by using the chi-square test or Fischer’s exact test for categorical variables and two-tailed t tests for continuous variables. Bivariate analyses of predictors of virologic failure were performed in a similar manner. For multivariate analyses of variables associated with virologic failure (defined as a viral load >400 copies/mL), we constructed a descriptive multivariate logistic model. Given our small sample size and number of events, our multivariate model contained a limited number of pre-selected predictor variables previously reported to have been associated with virologic failure. For analysis of sustainability of therapy, a Kaplan-Meier probability estimate was constructed from time of initiation of ART to either death, lost to follow-up or stopping therapy. A separate Kaplan-Meier estimate was also constructed for survival. For analyses of both sustainability of therapy and survival, we used the log rank test to compare different strata of interest. Finally, a descriptive Cox’s proportional hazard model was constructed for multivariate analyses of predictors of sustainability of therapy and survival using a descriptive modelling strategy with a limited number of known predictor variables, again due to small sample size and events. Analyses were conducted using SAS, version 9.0, SAS Institute Inc., Cary, NC, USA.
Results
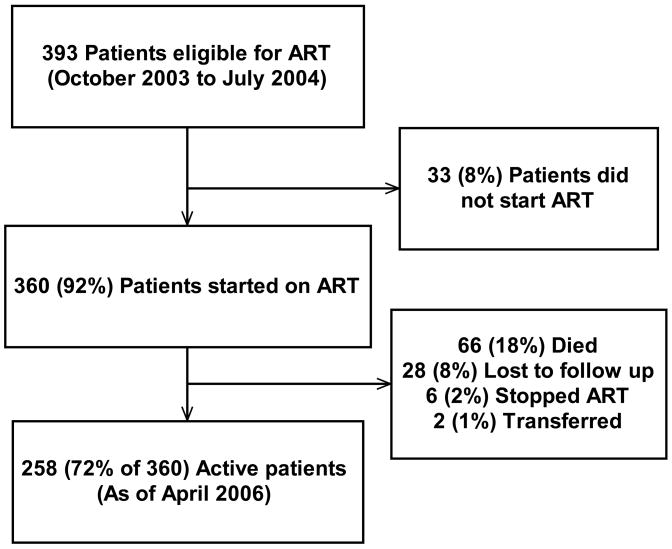
Between October 1, 2003 to July 31, 2004, 393 patients were evaluated at Reach Out and eligible for starting ART; 360 (92%) of these patients eventually started ART during this period. By April 1, 2006, 258 (72%) of these 360 patients were still active and on therapy through Reach Out (Figure 1). Baseline characteristics comparing patients who were active at study end date versus those who had not sustained therapy, i.e. died, lost to follow-up, transferred, or stopped therapy, are shown in Table 1.
Figure 1.
Profile of Reach Out cohort.
Table 1.
Comparison of characteristics of 360 patients by therapy status on April 2006.*
| Active on therapy | Not on therapy (Died, Lost, Transferred, Stopped) | ||||
|---|---|---|---|---|---|
| Characteristic | N | Value (%) | n | Value (%) | p value |
| Female sex | 258 | 175 (68) | 102 | 63 (62) | 0.27† |
| Age, median, [range] | 258 | 38, [15–66] | 102 | 37, [13–61] | 0.07† |
| ≤30 | 39 (15) | 25 (25) | |||
| 31–39 | 111 (43) | 34 (33) | |||
| ≥40 | 108 (42) | 43 (42) | |||
| WHO stage at start of therapy | 247 | 100 | |||
| 1 | 0 | 1 (1) | 0.14† | ||
| 2 | 71 (29) | 22 (22) | |||
| 3 | 121 (49) | 47 (47) | |||
| 4 | 55 (22) | 30 (30) | |||
| Any prior history of tuberculosis | 249 | 59 (24) | 79 | 16 (20) | 0.53† |
| Any tuberculosis treatment during ART | 249 | 64 (26) | 80 | 22 (28) | 0.75† |
| Any pregnancy during ART | 169 | 22 (13) | 49 | 0 | 0.003§ |
| Any prior history of PMTCT¶ | 169 | 4 (2) | 50 | 0 | 0.58§ |
| Any prior history of ART | 249 | 17 (7) | 80 | 9 (11) | 0.20† |
| Any history of alcohol abuse | 249 | 48 (19) | 79 | 9 (11) | 0.11† |
| Education Level > Primary School | 244 | 107 (44) | 69 | 29 (42) | 0.79† |
| Body Weight (kg) | |||||
| Men | 77 | 30 | |||
| Median | 59 | 57 | 0.31‡ | ||
| IQR | 53–65 | 53–67 | |||
| Women | 166 | 50 | |||
| Median | 54 | 51 | 0.27‡ | ||
| IQR | 49–61 | 45–60 | |||
| Baseline CD4 Count (cells/mm3) | 239 | 92 | |||
| Median | 107 | 81 | 0.94‡ | ||
| IQR | 50–179 | 18–178 | |||
| Initial ART Regimen | 249 | 77 | |||
| CBV/EFV | 102 (41) | 28 (36) | <0.0001† | ||
| CBV/NVP | 69 (28) | 13 (17) | |||
| D4T/3TC/EFV | 4 (2) | 8 (10) | |||
| D4T/3TC/NVP | 74 (30) | 28 (36) | |||
IQR, Interquartile Range; kg, kilogram.
Pearson chi-square test statistic.
2 tailed t test statistic.
Fischer’s exact test statistic.
PMTCT=Prevention of Mother To Child Transmission
Virologic and immunologic response
At least one HIV viral load test was performed from April 2006 to January 2007 on 246 (95%) of the 258 active patients. The median time from ART initiation to viral load testing was 24.8 months (IQR, 24.4–25.9). Of those tested, 211 patients (86%) had an undetectable (<400 copies/mL) viral load. Multivariate analyses found lack of CD4 response (defined as no change or decline of CD4 count from baseline to endpoint CD4 measurement) and any history of previous antiretroviral use to be associated with virologic failure (Table 2). Pill count adherence less than 95% was predictive of virologic failure in univariate analyses (p=0.02), but was not included in the multivariate model as it produced an unstable model due to small numbers. CD4 counts were increased in 222 (95%) of 233 active patients in whom both baseline and endpoint CD4 measurements were available. The median CD4 increase from baseline for active patients was 197 cells/mm3 (IQR, 108–346).
Table 2.
Bivariate and multivariate analyses of predictors of virologic failure for 246 patients.*
| Characteristic | OR (95% CI) | aOR (95% CI) | p value | |
|---|---|---|---|---|
| Female gender | 1.04 (0.48–2.24) | 1.17 (0.43–3.20) | 0.75 | |
| Age (per year) | 0.95 (0.91–1.00) | 0.95 (0.89–1.00) | 0.09 | |
| Baseline CD4<100 | 1.08 (0.53–2.21) | 1.21 (0.51–2.86) | 0.66 | |
| WHO Stage 4 (versus Stage 2 or 3) | 0.59 (0.22–1.61) | 0.37 (0.11–1.21) | 0.10 | |
| Initial ART Regimen† | ||||
| CBV/EFV | 1.0 | 1.0 | ||
| CBV/NVP | 1.08 (0.43–2.69) | 0.78 (0.24–2.55) | 0.68 | |
| D4T/3TC/NVP | 1.46 (0.63–3.36) | 0.60 (0.20–1.81) | 0.37 | |
| Any prior history of ART | 6.52 (2.32–18.3) | 7.88 (2.03–30.5) | 0.003 | |
| No CD4 response‡ | 6.28 (1.79–22.0) | 7.12 (1.63–31.1) | 0.009 | |
OR, Odds Ratio; aOR, adjusted Odds Ratio; CI, Confidence Interval; p values are for aOR.
Patients on D4T/3TC/EFV not included in this analysis secondary to small numbers.
Defined as no change or decline in CD4 response from baseline.
Sustainability of therapy
As shown in the Kaplan-Meier curve of sustainability of therapy (death, lost to follow-up, and stopped being designated as failures), 85% (95% CI, 81% to 89%) of patients remained active on ART at six months, 77% (73% to 82%) were active at one year, and 73% (69% to 78%) were still on therapy at two years (Figure 2). Using log rank testing, we found patients with baseline CD4 counts less than 100 cells/mm3 to be significantly more likely to not remain on therapy (p=0.02). A baseline antiretroviral regimen of D4T/3TC/EFV was also associated with not remaining on therapy (p<0.0004). Patients who were WHO Stage 4 when starting ART trended toward more likely to not remain on therapy (p=0.06). Using a Cox proportional hazards model, we found baseline D4T/3TC/EFV regimen and WHO Stage 4 were significant predictors of not sustaining therapy (Table 3).
Figure 2.
Kaplan-Meier estimate with 95% confidence intervals of the proportion of adults remaining in care and on antiretroviral therapy.
Table 3.
Cox’s proportional hazards analyses for predictors of not sustaining therapy and mortality for 360 patients.*
| Not sustaining therapy | Mortality | |||
|---|---|---|---|---|
| Characteristic | Hazard Ratio (95% CI) | p value | Hazard Ratio (95% CI) | p value |
| Baseline CD4 <100 | 1.33 (0.82–2.17) | 0.25 | 2.44 (1.24–4.81) | 0.01 |
| WHO Stage 4 (versus Stage 1, 2 or 3) | 1.87 (1.14–3.07) | 0.01 | 3.49 (1.89–6.46) | <0.0001 |
| D4T/3TC/EFV baseline regimen | 3.51 (1.49–8.29) | 0.004 | 3.75 (1.19–11.8) | 0.02 |
Model contains all variables in Table 2 except no CD4 response; D4T/3TC/EFV baseline regimen was included in this model; CI, Confidence Interval.
Survival
As shown in the Kaplan-Meier survival curve, survival was 87% (95% CI, 84% to 91%) at six months, 84% (80% to 89%) at one year, and 82% (78% to 86%) at two years (Figure 3). Using log rank testing, we found similar results to sustainability of therapy analyses with baseline antiretroviral regimen of D4T/3TC/EFV (p<0.01), CD4 count <100 cells/mm3 (p=0.001), and WHO stage 4 (p=0.0003) associated with worse survival. Using a Cox proportional hazards model, we found baseline D4T/3TC/EFV regimen, WHO Stage 4 and baseline CD4 count <100 cells/mm3 were significant predictors of worse survival (Table 3). Patients started on ART prior to the availability of PEPFAR-funded antiretrovirals (n=89, 25%) accounted for a disproportionate number of the total deaths, 27 of 66 (40%); pre-PEPFAR patients had lower CD4 counts than those started afterward (mean of 99 cells/mm3 versus 120 cells/mm3, t-test p value <0.0001).
Figure 3.
Kaplan-Meier estimate with 95% confidence intervals of the proportion of adults surviving after initiation of antiretroviral therapy.
Drug toxicities and regimen changes
In the active patients, a total of 57 adverse drug events were noted in 36 (14%) patients. Common toxicities included 17 (30%) cases of peripheral neuropathy, 10 (18%) cases of anemia, 6 (11%) cases of rash, 5 (9%) cases of nausea/vomiting, 4 (7%) cases of CNS-related side effects, and 3 (5%) cases of lipodystrophy. Active patients were changed from their baseline antiretroviral regimen 39 times (15%). Changes were due to drug toxicities and/or side effects in 21 (54%) of these patients and 6 changes (15%) were due to concerns about tuberculosis drug interactions.
Adherence and clinical outcomes
Pill count adherence information was available for 237 active patients and the median adherence was 99%. Only 2 patients had adherence <95%, and both of these patients had detectable viral loads. For the 228 active patients in whom baseline and end study weights were available, the median weight increase was 4 kilograms (IQR, 1–9). A total of 178 patients (78%) had an increase in weight. 49 active patients (19%) were diagnosed with at least one WHO Stage 3 or 4 illness during the course of therapy. The majority (61%) of these new illnesses developed more than six months after ART initiation. The most common new illnesses (n=62 total) included extrapulmonary cryptococcosis (n=12, 19%), pulmonary tuberculosis (n=11, 17%), chronic diarrhea >1 month (n=9, 15%), oral candidiasis (n=7, 11%), esophageal candidiasis (n=7, 11%), chronic herpes simplex virus infection >1 month (n=5, 8%), and extrapulmonary tuberculosis (n=3, 5%).
Discussion
This study demonstrates that a peer health worker and nurse-staffed ART program in a RLS can provide excellent AIDS care over at least a two year period. Viral load and CD4 responses were comparable and in many cases better than reports from the developing and developed world.3–13 Previous ART program evaluations were mostly in settings of relatively well-resourced research, government, and academic programs and concerns have been raised about barriers to future scale-up, the long term sustainability of ART in RLSs, and an end to the “honeymoon” period of ART roll-out.1, 2, 14–16 This study provides evidence that a community-based program in a RLS can sustain ART for years with good virologic outcomes.
This study has several limitations including its retrospective nature and reliance on clinical and programmatic records, some of which were incomplete and all of which was originally collected and utilized for intentions other than research activities. The number of patients lost to follow-up likely resulted in an underestimation of survival. Outcome measurements were not measured at exacting time intervals. This study would also have benefited from resistance data to provide additional insight into our virologic outcomes.
Virologic failure was associated with past history of antiretroviral use, raising concerns about virologic resistance. Lack of CD4 response was also associated with virologic failure though the use of immunologic responses to predict virologic failure should be approached cautiously based upon other reports.17 Beginning ART at WHO Stage 4 rather than at an earlier stage was clearly predictive of both not sustaining therapy and earlier mortality which is supported by previous studies; lower baseline CD4 counts were also predictive of early mortality.10 Significant differences between other WHO Stages were not demonstrated, possibly due to inadequate power. This study also found that patients started on the baseline antiretroviral regimen of D4T/3TC/EFV were less likely to sustain therapy and had increased mortality. A possible explanation for this surprising finding is that this ART regimen was predominantly initiated in patients on concurrent tuberculosis therapy during the beginning of the Reach Out program before Combivir became widely available. Indeed, 75% of patients started on D4T/3TC/EFV had a history of tuberculosis treatment during ART compared to 23% of all other patients (OR=9.35; 95% CI, 2.47–35.4; p value<0.0001). Therefore these patients likely represented a group of sicker patients at higher risk of both not sustaining therapy and dying. Additionally, as only a small number of patients (12 total) were started on this regimen, these findings should be interpreted cautiously.
The initial drop off of patients from care over the first six months can largely be explained by high early mortality. While the 16% mortality at 12 months is greater than some reports, it is largely consistent with local findings.18, 19 This high early mortality reflects the known disparity in mortality outcomes between low-income and high-income countries and suggests the limitations of quality primary care if provided too late to patients presenting with late-stage disease.19 In addition, high early mortality likely reflects the provision of ART from a limited, pre-PEPFAR-funded supply to only the sickest patients during the early months of Reach Out’s program enrolment. The significant number of patients lost to follow-up demonstrates the challenges of caring for a dynamic urban slum population that often has ongoing rural to urban to rural patterns of migration. These issues will require continued vigilance as well as interventions to encourage early care-seeking and retention.
While there are clear areas for improvement, the overall success of the Reach Out program offers insight into successful components of an ART program in RLSs. Reach Out uses a large number of patients trained in home-based care to serve the simultaneous roles of peer educators, patient social supporters, and adherence and clinical monitors. The use of these types of peer health workers deserves closer investigation to understand their myriad effects on the delivery and sustainability of ART.20 In addition, Reach Out’s nurse-based model of AIDS care responds to the healthcare workforce crisis in RLSs by task shifting.21 This study is consistent with a growing body of evidence that nurses are capable of delivering quality HIV care in RLSs.1, 10
The Reach Out program also provides a comprehensive set of services to patients and the community in an approach to AIDS care that attempts to address many of the known structural barriers to antiretroviral uptake and adherence.22 It is impossible to separate out the individual effects of programs such as microfinance, paying school fees, food aid, and community education to reduce stigma, but these combined efforts are likely beneficial in improving AIDS care outcomes in this population and reflect the strong community-based nature of Reach Out.23 Adherence, as measured by pill counts, was remarkably high in our study and reflects intensive efforts by Reach Out to address adherence when any pill counts demonstrate possible non-compliance though there may also be a selection bias towards adherence as our study population focused on patients who have remained on therapy for two years. However, while worse adherence was associated with virologic failure, most patients with virologic failure had pill count adherence percentages >95%. This finding may indicate the lack of sensitivity of this isolated adherence measure as well as raises concerns for pre-existing resistance as a cause of virologic failure. Finally, it is also important that ART and all services at Reach Out are provided at no cost; the provision of free medications has been associated with lower mortality and improved virologic outcomes.13, 19
Finally, Reach Out’s religious roots are fundamental to its origin, growth, and vision of taking comprehensive care of the whole patient. Reach Out, as with many faith-based organizations thoughout RLSs, had extensive existing community relationships which helped promote “buy-in”, follow-up, adherence, and dissemination of HIV care and prevention knowledge within the community. The role of faith-based institutions in HIV care and prevention has stimulated considerable discussion in the lay media; however, there has been minimal scientific evaluations of faith-based treatment programs prior to this study.12, 13, 24 Assessments of faith-based interventions and programs will continue to benefit from peer-reviewed, scientific evaluations and reports.
A comprehensive community-based AIDS care program in a RLS using peer health workers and nurses and integrating the delivery of ART with personal, family, and community services demonstrated excellent outcomes over a two year period. The success of this model lends support to similar strategies to address the global health workforce crisis.
Acknowledgments
We thank the volunteers, staff, and patients of the Reach Out program for their dedication, support, and compassion. We thank Caitlin Kennedy for comments on an initial draft of this paper, Matthew Burkey for assistance with data collection, and Kevin Newell and Blake Charvat for statistical assistance. This study was supported by The Division of Intramural Research, The National Institute for Allergy and Infectious Diseases, National Institutes of Health.
Supported by The Division of Intramural Research, The National Institute for Allergy and Infectious Diseases, National Institutes of Health.
References
- 1.Van Damme W, Kober K, Laga M. The real challenges for scaling up ART in sub-Saharan Africa. Aids. 2006 Mar 21;20(5):653–656. doi: 10.1097/01.aids.0000216364.44409.b1. [DOI] [PubMed] [Google Scholar]
- 2.Colebunders R, Ronald A, Katabira E, Sande M. Rolling out antiretrovirals in Africa: there are still challenges ahead. Clin Infect Dis. 2005 Aug 1;41(3):386–389. doi: 10.1086/431490. [DOI] [PubMed] [Google Scholar]
- 3.Staszewski S, Miller V, Sabin C, et al. Virological response to protease inhibitor therapy in an HIV clinic cohort. Aids. 1999 Feb 25;13(3):367–373. doi: 10.1097/00002030-199902250-00009. [DOI] [PubMed] [Google Scholar]
- 4.Lucas GM, Chaisson RE, Moore RD. Highly active antiretroviral therapy in a large urban clinic: risk factors for virologic failure and adverse drug reactions. Ann Intern Med. 1999 Jul 20;131(2):81–87. doi: 10.7326/0003-4819-131-2-199907200-00002. [DOI] [PubMed] [Google Scholar]
- 5.Deeks SG, Hecht FM, Swanson M, et al. HIV RNA and CD4 cell count response to protease inhibitor therapy in an urban AIDS clinic: response to both initial and salvage therapy. Aids. 1999 Apr 16;13(6):F35–43. doi: 10.1097/00002030-199904160-00001. [DOI] [PubMed] [Google Scholar]
- 6.Bartlett JA, DeMasi R, Quinn J, Moxham C, Rousseau F. Overview of the effectiveness of triple combination therapy in antiretroviral-naive HIV-1 infected adults. Aids. 2001 Jul 27;15(11):1369–1377. doi: 10.1097/00002030-200107270-00006. [DOI] [PubMed] [Google Scholar]
- 7.Severe P, Leger P, Charles M, et al. Antiretroviral therapy in a thousand patients with AIDS in Haiti. N Engl J Med. 2005 Dec 1;353(22):2325–2334. doi: 10.1056/NEJMoa051908. [DOI] [PubMed] [Google Scholar]
- 8.Spacek LA, Shihab HM, Kamya MR, et al. Response to antiretroviral therapy in HIV-infected patients attending a public, urban clinic in Kampala, Uganda. Clin Infect Dis. 2006 Jan 15;42(2):252–259. doi: 10.1086/499044. [DOI] [PubMed] [Google Scholar]
- 9.Bekker LG, Myer L, Orrell C, Lawn S, Wood R. Rapid scale-up of a community-based HIV treatment service: programme performance over 3 consecutive years in Guguletu, South Africa. S Afr Med J. 2006 Apr;96(4):315–320. [PubMed] [Google Scholar]
- 10.Stringer JS, Zulu I, Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. Jama. 2006 Aug 16;296(7):782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 11.Weidle PJ, Wamai N, Solberg P, et al. Adherence to antiretroviral therapy in a home-based AIDS care programme in rural Uganda. Lancet. 2006 Nov 4;368(9547):1587–1594. doi: 10.1016/S0140-6736(06)69118-6. [DOI] [PubMed] [Google Scholar]
- 12.Akileswaran C, Lurie MN, Flanigan TP, Mayer KH. Lessons learned from use of highly active antiretroviral therapy in Africa. Clin Infect Dis. 2005 Aug 1;41(3):376–385. doi: 10.1086/431482. [DOI] [PubMed] [Google Scholar]
- 13.Ivers LC, Kendrick D, Doucette K. Efficacy of antiretroviral therapy programs in resource-poor settings: a meta-analysis of the published literature. Clin Infect Dis. 2005 Jul 15;41(2):217–224. doi: 10.1086/431199. [DOI] [PubMed] [Google Scholar]
- 14.Reynolds SJ, Bartlett JG, Quinn TC, Beyrer C, Bollinger RC. Antiretroviral therapy where resources are limited. N Engl J Med. 2003 May 1;348(18):1806–1809. doi: 10.1056/NEJMsb035366. [DOI] [PubMed] [Google Scholar]
- 15.Gill CJ, Hamer DH, Simon JL, Thea DM, Sabin LL. No room for complacency about adherence to antiretroviral therapy in sub-Saharan Africa. Aids. 2005 Aug 12;19(12):1243–1249. doi: 10.1097/01.aids.0000180094.04652.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bangsberg DR, Ware N, Simoni JM. Adherence without access to antiretroviral therapy in sub-Saharan Africa? Aids. 2006 Jan 2;20(1):140–141. doi: 10.1097/01.aids.0000196168.50303.31. author reply 141–142. [DOI] [PubMed] [Google Scholar]
- 17.Moore DM, Mermin J, Awor A, Yip B, Hogg RS, Montaner JS. Performance of immunologic responses in predicting viral load suppression: implications for monitoring patients in resource-limited settings. J Acquir Immune Defic Syndr. 2006 Dec 1;43(4):436–439. doi: 10.1097/01.qai.0000243105.80393.42. [DOI] [PubMed] [Google Scholar]
- 18.Weidle PJ, Malamba S, Mwebaze R, et al. Assessment of a pilot antiretroviral drug therapy programme in Uganda: patients’ response, survival, and drug resistance. Lancet. 2002 Jul 6;360(9326):34–40. doi: 10.1016/S0140-6736(02)09330-3. [DOI] [PubMed] [Google Scholar]
- 19.Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006 Mar 11;367(9513):817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. Scaling up HIV/AIDS care: service delivery and human resource perspectives. 2004 [Google Scholar]
- 21.World Health Organization. The world health report 2006: working together for health. 2006 [Google Scholar]
- 22.Weiser S, Wolfe W, Bangsberg D, et al. Barriers to antiretroviral adherence for patients living with HIV infection and AIDS in Botswana. J Acquir Immune Defic Syndr. 2003 Nov 1;34(3):281–288. doi: 10.1097/00126334-200311010-00004. [DOI] [PubMed] [Google Scholar]
- 23.Walton DA, Farmer PE, Lambert W, Leandre F, Koenig SP, Mukherjee JS. Integrated HIV prevention and care strengthens primary health care: lessons from rural Haiti. J Public Health Policy. 2004;25(2):137–158. doi: 10.1057/palgrave.jphp.3190013. [DOI] [PubMed] [Google Scholar]
- 24.Green EC, Halperin DT, Nantulya V, Hogle JA. Uganda’s HIV prevention success: the role of sexual behavior change and the national response. AIDS Behav. 2006 Jul;10(4):335–346. doi: 10.1007/s10461-006-9073-y. discussion 347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]