Abstract
Androgens are critical steroid hormones that determine the expression of the male phenotype, including the outward development of secondary sex characteristics as well as the initiation and maintenance of spermatogenesis. Their actions are mediated by the androgen receptor (AR), a member of the nuclear receptor superfamily. AR functions as a ligand-dependent transcription factor, regulating expression of an array of androgen-responsive genes. Androgen and the AR play important roles in male spermatogenesis and fertility. The recent generation and characterization of male total and conditional AR knockout mice from different laboratories demonstrated the necessity of AR signaling for both external and internal male phenotype development. As expected, the male total AR knockout mice exhibited female-typical external appearance (including a vagina with a blind end and a clitoris-like phallus), the testis was located abdominally, and germ cell development was severely disrupted, which was similar to a human complete androgen insensitivity syndrome or testicular feminization mouse. However, the process of spermatogenesis is highly dependent on autocrine and paracrine communication among testicular cell types, and the disruption of AR throughout an experimental animal cannot answer the question about how AR in each type of testicular cell can play roles in the process of spermatogenesis. In this review, we provide new insights by comparing the results of cell-specific AR knockout in germ cells, peritubular myoid cells, Leydig cells, and Sertoli cells mouse models that were generated by different laboratories to see the consequent defects in spermatogenesis due to AR loss in different testicular cell types in spermatogenesis. Briefly, this review summarizes these results as follows: 1) the impact of lacking AR in Sertoli cells mainly affects Sertoli cell functions to support and nurture germ cells, leading to spermatogenesis arrest at the diplotene primary spermatocyte stage prior to the accomplishment of first meiotic division; 2) the impact of lacking AR in Leydig cells mainly affects steroidogenic functions leading to arrest of spermatogenesis at the round spermatid stage; 3) the impact of lacking AR in the smooth muscle cells and peritubular myoid cells in mice results in similar fertility despite decreased sperm output as compared to wild-type controls; and 4) the deletion of AR gene in mouse germ cells does not affect spermatogenesis and male fertility. This review tries to clarify the useful information regarding how androgen/AR functions in individual cells of the testis. The future studies of detailed molecular mechanisms in these in vivo animals with cell-specific AR knockout could possibly lead to useful insights for improvements in the treatment of male infertility, hypogonadism, and testicular dysgenesis syndrome, and in attempts to create safe as well as effective male contraceptive methods.
I. Introduction
II. Generation of Various Testicular Cell-Specific Androgen Receptor (AR) Knockout Mice
- III. Serum Testosterone Levels in Various Testicular Cell-Specific AR Knockout Mice
- A. Testosterone biosynthesis in the Leydig cells
- B. Leydig cell development and maturation
- C. Tfm mice and humans with AIS
- D. T-AR−/y mice
- E. S-AR−/y mice
- F. L-AR−/y mice
- G. PM-AR−/y mice and G-AR−/y mice
IV. Phenotypes of External Genitalia and Internal Male Accessory Genital Organ Size in Various Testicular Cell-Specific AR Knockout Mice
V. Testis Position in Various Testicular Cell-Specific AR Knockout Mice
- VI. Testis Size in Various Testicular Cell-Specific AR Knockout Mice
- A. S-AR−/y mice
- B. Tfm mice and T-AR−/y mice
- C. L-AR−/y mice, PM-AR−/y mice, and G-AR−/y mice
- VII. Testis Morphology, Epididymal Sperm Count, and Fertility Test in Various Testicular Cell-Specific AR Knockout Mice
- A. Humans with AIS, Tfm mice, and T-AR−/y mice
- B. S-AR−/y mice
- C. L-AR−/y mice
- D. PM-AR−/y mice
- E. G-AR−/y mice
VIII. Concluding Remarks and Future Directions
I. Introduction
SPERMATOGENESIS (EXOCRINE) and androgen biosynthesis (endocrine) are the major functions of mammalian testis. Both functions are complicated and highly regulated. Spermatogenesis is a process of generating mature sperm with half the number of chromosomes (haploid) produced from germ cell precursors (diploid). Androgens, by signaling through the androgen receptor (AR), mediate a wide range of physiological responses and developmental processes, involving both reproductive and nonreproductive systems in the male (1,2,3). The appropriate regulation of androgen activity via the hypothalamic-pituitary-testis axis is necessary for development of the male phenotype, as well as for initiation and maintenance of spermatogenesis (2,4).
AR, which has been localized to the long arm of the X chromosome (at Xq11-12), is a member of the nuclear receptor superfamily and acts as a ligand-inducible transcription factor to modulate expression of target genes (5,6,7). The binding of testosterone or its metabolite 5α-dihydroxytestosterone (DHT) to AR induces receptor dimerization, facilitating the ability of AR to bind to its cognate response elements and recruit coregulators to promote the expression of target genes (4,8). Failure of the mutated receptor to activate its target genes causes a spectrum of hereditary disorders of androgen insensitivity syndrome (AIS) or testicular feminization (Tfm) mutation (9,10,11,12). The humans with the most severe type of AIS, presumably complete AIS (CAIS), display feminized external genitalia and intraabdominal testis and do not undergo development of secondary sex characteristics at puberty. In less severe cases, where some level of androgen responsiveness is maintained, there are a wide range of phenotypes. In addition to men, the inherited syndrome of Tfm has been described in several species, including the dog (13), the rat (14), the mouse (15,16), and the cat (17). In those species, the affected male pseudohermaphrodites manifest several common features, such as feminized external genitalia, an absence of internal genitalia other than testis (13,14,15,16), and marked resistance to endogenous and exogenous androgens (14,16).
The mammalian testis has two distinct functional compartments known as the seminiferous tubules and the interstitium, with spermatogenesis arising in the seminiferous tubules and androgen biosynthesis in the interstitial Leydig cells. Both testicular compartments contain a variety of different cell types, and the spermatogenesis is highly dependent on autocrine and paracrine communication among all cell types (18). The interstitial space, or space between the seminiferous tubules, consists of the testosterone-producing Leydig cells (19), macrophages, perivascular smooth muscle cells, and vascular endothelial cells. The Sertoli cells comprise the main structural component of the seminiferous tubules. They are responsible for the structural support for germ cell development (20,21), facilitating germ cell movement and mature germ cell release (18,22), and secretion of diverse functional glycoproteins and peptides to nourish germ cells (23,24), as well as maintenance of the blood-testis barrier, and secretion of seminiferous tubular fluid (25).
The blood-testis barrier is located in the basal third of the seminiferous tubules and segregates the seminiferous tubules into the basal compartment (containing spermatogonia, preleptotene, and leptotene spermatocytes) and the adluminal compartment (containing different stages of meiotic spermatocytes, round spermatids, elongated spermatids, and spermatozoa). The blood-testis barrier is known to function as a natural barrier to regulate the passage of various molecules into and out of the adluminal compartment of seminiferous tubules, and an immunological barrier to create a specialized environment for the differentiation and movement of developing germ cells (22).
Peritubular myoid (PM) cells are mesenchymal cells that form the outer border of the seminiferous tubules and, in conjunction with Sertoli cells, produce the basement membrane required to maintain normal tubule morphology. Several important functions of PM cells have been proposed: 1) they can synthesize several secretory products, such as P-Mod-S (peritubular factors that modulate Sertoli cell function), to modulate a number of Sertoli cell functions (26,27); and 2) they have the ability to contract, thus inducing peristalsis-like waves and impulses in the seminiferous tubule, which will help the transport of spermatozoa through the tubular lumen and into the epididymis to control testicular output of both fluid and sperm (28). PM cells have also been indicated as one of the principal target cells in testis that respond to androgens and participate in the androgenic control of spermatogenesis (29).
There is a general agreement that AR can be detected in Sertoli cells, PM cells, and cells in the interstitial spaces including Leydig cells and perivascular smooth muscle cells in testis (30,31,32,33,34,35). However, using antibody staining, the localization of the AR in male germ cells remains controversial. Several studies indicated that AR is present in germ cells in different species (32,34,35,36,37,38,39,40,41), but other reports show that there is no AR staining in the germ cells (30,33,42,43,44,45,46). Interestingly, some previous studies have revealed that the AR expression in male germ cells is stage specific, only expressing in elongated spermatid at spermatogenic stage XI in rat testis (34). Furthermore, the presence of testosterone binding sites has been detected on monkey sperm and correlated with motility (41). The presence of the AR in human sperm has been demonstrated by Western blot and by immunofluorescence assay (38). These studies suggest that AR might play a direct role in germ cells.
The recent generation and characterization of male total AR knockout (T-AR−/y) mice, which confirmed the similar phenotype to a human with CAIS or Tfm mouse, exhibited female-typical external appearance (including a vagina with a blind end and a clitoris-like phallus). Testes were located abdominally, and germ cell development was severely disrupted. These observations further emphasized the necessity of AR signaling for both external and internal male phenotype development (47,48). However, a gene alteration in the whole body might cause a complex phenotype in which it is hard to differentiate direct effects in a particular tissue or particular cell type from those secondary effects arising from a gene change in other cell types. In view of the fact that the AR has developmental roles in establishing the male phenotype and the process of spermatogenesis is highly dependent on autocrine and paracrine communication among testicular cell types, the disruption of AR throughout an experimental animal cannot answer the question about how AR in each cell type within the testis can play roles in the spermatogenesis. Therefore, it is necessary to use a cre-lox strategy in transgenic mice with cell-specific expression of the cre recombinase (Cre) to generate a mouse model in which disruption of AR function is exclusively in a particular cell type in the testis. Here, we summarize the results of cell-specific AR knockout (AR−/y) in germ cells (G-AR−/y), peritubular myoid cells (PM-AR−/y), Leydig cells (L-AR−/y), and Sertoli cells (S-AR−/y) mouse models to understand the spermatogenetic consequences of AR loss in different testicular cell types.
II. Generation of Various Testicular Cell-Specific Androgen Receptor (AR) Knockout Mice
To generate tissue-specific AR−/y mice, a Cre-loxP strategy for conditional knockout is necessary. The Cre-loxP system utilizes the expression of P1 phage Cre to catalyze the excision of DNA located between flanking loxP sites (49). This strategy differs from the standard targeted disruption procedure in that embryonic stem cells are generated in which the target segment is not disrupted but is flanked by loxP sites (floxed). The target gene thus functions normally, and mice can be bred to homozygosity for the targeted locus. The C57-B6/129/SvEv loxP-floxed AR mice were first generated (47,48) and then mated with different testicular cell-specific Cre mice for the study of total or cell-specific AR−/y mice (Fig. 1). T-AR−/y mice were generated by mating the floxed AR mice with β-actin (ACTB)-Cre (ACTB-Cre is under the control of the β-actin promoter) transgenic mice (47); S-AR−/y mice were generated by mating the floxed AR mice with anti-Müllerian hormone (AMH)-Cre (AMH-Cre is under the control of the anti-Müllerian hormone promoter) transgenic mice (50,51,52,53); L-AR−/y mice were generated by mating the floxed AR mice with AMHRII-Cre (AMHRII-Cre is under the control of the anti-Müllerian hormone receptor II promoter) transgenic mice (54); PM-AR−/y mice were generated by mating the floxed AR mice with Transgelin-Cre (Transgelin-Cre is under the control of the transgelin promoter) transgenic mice (55); and G-AR−/y mice were generated by mating the floxed AR mice with Sycp1-Cre [Sycp1-Cre is under the control of the synaptonemal complex protein 1 promoter, expressed at an early stage of the male meiosis (leptotene to zygotene)] transgenic mice (56). Male wild-type AR (AR+/y) or floxed AR littermate mice without Cre transgene were used as control AR+/y to study the AR roles in individual cells within the testis.
Figure 1.
The mating strategy to generate T-AR−/y, S-AR−/y, L-AR−/y, PM-AR−/y, and G-AR−/y mice.
III. Serum Testosterone Levels in Various Testicular Cell-Specific AR Knockout Mice
Serum testosterone levels are influenced by two key factors: testosterone biosynthesis rate in the Leydig cells, and number of functional Leydig cells available in the testis.
A. Testosterone biosynthesis in the Leydig cells
The conversion of cholesterol to testosterone involves a number of steps that are catalyzed by enzymes, including hydroxylase [17α-hydroxylase (P450c17)], two cleavage enzymes [P450 side-chain cleavage (P450scc) and 17,20 lyase(P450c17)], and one isomerization Δ5-Δ4 enzyme [3β-hydroxysteroid dehydrogenase (3β-HSD), conversion of Δ5-3β-hydroxysteroids to Δ4-3-ketosteroids], predominantly belonging to the cytochrome P450 family. LH, through the LH receptor (LHR) on the surface of Leydig cells, directly stimulates the synthesis of a steroidogenic acute regulatory (StAR) protein and the outer mitochondrial membrane translocator protein. StAR and outer mitochondrial membrane translocator protein are key regulators of cholesterol transport from the outer to the inner mitochondrial membrane (57,58). Once delivered to the inner mitochondrial membrane, cholesterol is enzymatically converted to pregnenolone by the enzyme cytochrome P450scc (59). Pregnenolone diffuses across the mitochondrial membranes and might progress to testosterone production through either the Δ4 or Δ5 pathway (60) by enzymes associated with the smooth endoplasmic reticulum. Pregnenolone can be converted to progesterone through the enzyme 3β-HSD (the Δ4 pathway) or can be hydroxylated at the 17α position by the enzyme 17α-hydroxylase to form 17α hydroxypregnenolone (the Δ5 pathway). The Δ5 pathway predominates in human Leydig cells (61), whereas in the rodent, both pathways are catalyzed, although the Δ4 pathway is preferred (62). The further conversion of 17α hydroxypregnenolone (the Δ5 pathway) involves the formation of the 19C steroid dehydroepiandrosterone catalyzed by the enzyme 17,20 lyase. Both 17α-hydroxylation and cleavage of the 17,20 carbon-carbon bond are catalyzed by a single microsomal enzyme cytochrome P450c17, which is encoded by a single gene on chromosome 10 (63,64). The final step in the production of testosterone in the testis is via conversion of androstenedione (a weak androgen) to testosterone in the Δ4 pathway or dehydroepiandrosterone to androstenediol in the Δ5 pathway by the enzyme 17β-hydroxysteroid dehydrogenase (17β-HSD)/17-ketosteroid reductase (65). Biosynthesis of all biologically active steroid hormones requires conversion of Δ5-3β-hydroxysteroids to Δ4-3-ketosteroids, and this reaction is catalyzed by the enzyme 3β-HSD (66). Notably, the 3β-HSD type I isoform is expressed at significant levels in fetal-type Leydig cells, whereas the expression of 3β-HSD type VI isoform is specific to the adult population of Leydig cells in the mouse testis (67). Testosterone is the principal steroid produced and secreted by the testis, although numerous other steroids (C18, C19, and C21) are also produced (68,69).
B. Leydig cell development and maturation
In mammals, there are two distinct populations of Leydig cells arising in a sequential manner during normal testicular development (70,71). In the mouse testis, the first population, fetal Leydig cells, develops at around 12.5 d after coitum after normal testicular differentiation (71,72) and starts to produce testosterone at around 13 d after coitum for masculinization of the male urogenital system (71,73). The fetal Leydig cell population persists after birth but becomes ancillary to the newly developed secondary population, adult Leydig cells, which originate from undifferentiated mesenchymal spindle-shaped precursors in the testicular interstitium (71,74). The intermediates of adult Leydig cells first become apparent by around postnatal d 7–11 (67,75,76). Subsequently, the total number of Leydig cell precursors increases rapidly in a LH-dependent manner between postnatal d 10 and d 20, finally reaching a maximal number at about postnatal d 35 (77,78,79). Earlier studies reviewing the morphological change of adult Leydig cell population showed that the mesenchymal spindle-shaped precursor cells are mainly found in the peritubular region in the interstitium and are transformed to the progenitor Leydig cells in the same region. Subsequently, the progenitor Leydig cells are transformed from spindle-shaped to round immature adult Leydig cells, most commonly seen in the testis during postnatal d 28 to d 56 (76). The immature adult Leydig cells contain numerous lipid droplets and abundant smooth endoplasmic reticulum and are moved to the central interstitial region. An increase in cell size, the volume of smooth endoplasmic reticulum, and the decline in cytoplasmic lipid droplets characterize the transition between immature and mature adult Leydig cells by postnatal d 56 (76,79). The factors regulating the proliferation and differentiation of the two populations of Leydig cells are still largely unknown, but gonadotropins such as LH (80,81) and FSH (82,83), thyroid hormone (84,85), adrenocorticotrophic hormone (81), estrogens (86), and androgens (87) are suggested to play an important role. In addition, a body of evidence either from in vitro studies using testicular-derived mesenchymal cells in culture or from in vivo studies using the Tfm mice have demonstrated that the development of adult Leydig cell function definitely requires the action of androgens (88,89).
C. Tfm mice and humans with AIS
The serum testosterone levels in Tfm mice are reduced (1.8 ± 0.3 vs. 9.3 ± 2.0 nmol/liter; P < 0.05) (90), and serum LH levels are increased as compared with AR+/y controls (89,91). In addition, examination of Leydig cell-specific steroidogenic enzyme and functional gene expressions revealed that in adult Tfm mice, the 17β-HSD III, 3β-HSD VI, and prostaglandin synthetase gene expressions are barely detectable, and the P450c17 and relaxin-like factor (RLF) gene expressions are largely reduced compared with AR+/y controls. However, the gene expression levels of P450scc, StAR, LHR, 3β-HSD I, and thrombospondin type 2 (TSP-2) are normal or increased in adult Tfm mice compared with AR+/y controls. The highly abnormal patterns of Leydig cell-specific gene expression in Tfm mice shows that the initial differentiation of adult Leydig cells occurs, but there is a developmental failure of adult Leydig cell maturation, which leads to the adult Leydig cells in Tfm mice only acquiring partial maturational characteristics of the adult Leydig cell (92). These findings are consistent with the earlier studies in which the Tfm mice showed elevated plasma LH levels, increased Leydig cell oxidative enzyme activities indicating that Leydig cells were hyperstimulated, and reduced testosterone production and secretion (93). Earlier studies showed that the Leydig cell number increased 26-fold from postnatal d 12 to d 140, wherein most increases in Leydig cell number occurred during the period between postnatal d 12 and d 50 (94). The evidence from Tfm mice showed that the Leydig cell number and function are normal up to postnatal d 5, indicating that fetal Leydig cell proliferation, development, and function are not dependent on androgens before postnatal d 5 (92). The normal prepubertal rise in Leydig cell number is significantly reduced and only reaches 30% of AR+/y control values on postnatal d 20. Between postnatal d 20 and adulthood, Leydig cell number shows a similar trend of increase in both AR+/y control and Tfm mice, indicating that only the early part of the developmental phase is associated with AR function (92). In contrast to rodents, wherein the AR might play a role in the differentiation of Leydig cell precursors into mature adult Leydig cells, the humans with CAIS have relatively normal or slightly increased levels of plasma testosterone, elevated LH levels, and normal FSH levels (12,95,96), and Leydig cells are shown to have hyperplastic changes (97). In consideration of the wide variation of clinical characteristics in AIS and the wide variation of serum testosterone levels between individuals, there are still debates existing in regard to this issue in human patients.
D. T-AR−/y mice
The serum testosterone levels in T-AR−/y mice are decreased (7.9 ± 0.8 vs. 192 ± 13.1 ng/dl; P < 0.05), and serum LH levels are increased compared with AR+/y controls in our study (Fig. 2F) (50,56). These results are similar to Tfm mouse (90). Another study also showed the similar trend of serum testosterone level changes in T-AR−/y mice compared with AR+/y controls, although this was not statistically significant due to a wide variation of serum testosterone levels between animals within the same genotype groups (94). The results of quantitative RT-PCR for Leydig cell-specific steroidogenic enzymes and functional gene expressions revealed that adult T-AR−/y mice have reduced P450c17 gene expression and increased 3β-HSD I and P450scc gene expressions compared with AR+/y controls (94). Moreover, the result from T-AR−/y mice showed that the Leydig cell number reached 37% of AR+/y values on postnatal d 12 and reached only 22% of AR+/y values on postnatal d 140. Similarly, the Leydig cell cytoplasmic volume in T-AR−/y mice achieved only 60% of AR+/y values on postnatal d 140 (94). Overall, the result from the above studies clearly demonstrated that loss of AR in whole testis, including Leydig cells, PM cells, and Sertoli cells, will cause a profound functional defect in Leydig cells. There are reduced Leydig cell numbers, size, and steroidogenic enzyme gene expressions in T-AR−/y mice compared with AR+/y controls, but these findings in T-AR−/y mice are not explained by the coincidental cryptorchidism (92).
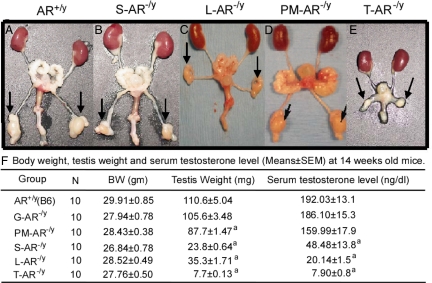
Figure 2.
The internal genitalia of 14-wk-old male AR+/y (A), S-AR−/y (B), L-AR−/y (C), PM-AR−/y (D), and T-AR−/y (E). Arrows indicate the testis. [Sections of figures were reproduced from Refs. 50 and 55. Copyright 2004 and 2006, respectively, Proceedings of the National Academy of Sciences.] F, Body weight, testis weight, and serum testosterone levels (mean ± sem) of 14-wk-old male AR+/y, G-AR−/y, PM-AR−/y, S-AR−/y, L-AR−/y, and T-AR−/y mice. a, Significant difference (P < 0.05; t test) compared with AR+/y. All of these results originated from our previous publications. The other studies also showed the similar trend of serum testosterone level changes in T-AR−/y mice and S-AR−/y mice compared with AR+/y controls, although this was not statistically significant due to a wide variation of serum testosterone levels between animals within the same genotype groups.
E. S-AR−/y mice
The serum testosterone levels in S-AR−/y mice are decreased by 74.7% of AR+/y values (48.48 ± 13.8 vs. 192 ± 13.1 ng/dl; P < 0.05) (Fig. 2F), and the serum LH levels are increased compared with AR+/y controls in our study (50,53). The other studies also showed the similar trend of serum testosterone level changes in S-AR−/y mice compared with AR+/y controls, although this was not statistically significant (51,94). But one independent study has shown almost a 40-fold increase in serum testosterone levels in both loxP-floxed AR mice and S-AR−/y mice compared with AR+/y controls (52). In view of the wide variation of serum testosterone levels between animals within genotype groups, this issue is still debatable. The results of quantitative RT-PCR for Leydig cell-specific steroidogenic enzyme and functional gene expressions also revealed that adult S-AR−/y mice have significant increases of P450scc, P450c17, and 3β-HSD I gene expressions compared with AR+/y controls (94). Our studies also found that adult S-AR−/y mice have significant increases of P450c17, 3β-HSD I, 3β-HSD VI, 17β-HSDIII, and LHR compared with AR+/y controls (data not shown). The evidence from S-AR−/y mice showed that the Leydig cell numbers are comparable with AR+/y controls up to postnatal d 12, and progressive decreases in Leydig cell numbers are evident at all later ages; for example, the Leydig cell number is reduced by 42% of AR+/y values on postnatal d 20 and by 54% of AR+/y values on postnatal d 140. In contrast, the Leydig cell cytoplasmic volume in S-AR−/y mice is 23% greater than AR+/y controls on postnatal d 140 (94). Based on above T-AR−/y and S-AR−/y mice data, it is suggested that the androgen/AR signals in Sertoli cells contribute to the establishment of approximately 50% of adult Leydig cell number in testes, whereas the establishment of normal Leydig cell size is not dependent on such action. In addition, the evidence indicated that androgen/AR signals on testicular cell types other than Sertoli cells are essential for the establishment of approximately 25% of the final adult Leydig cell number and the development of normal adult Leydig cell size (94).
Despite increased Leydig cell cytoplasmic volume and increased Leydig cell steroidogenic activity in the testis of S-AR−/y mice, our study showed that the serum testosterone level is lower than AR+/y control (Fig. 2F). The possible explanation for these discrepancies could be that, although the Leydig cells might have gain-of-function in the environment of low serum testosterone levels in S-AR−/y mice, they cannot restore the normal serum testosterone values due to the 78.5% decline in testicular size (Fig. 2F and Table 1) and the 50% decrease in Leydig cell numbers.
Table 1.
Comparison of reproductive phenotypes in T-AR−/y, G-AR−/y, S-AR−/y, L-AR−/y, and PM-AR−/y mice
| T-AR−/y | G-AR−/y | S-AR−/y | L-AR−/y | PM-AR−/y | |
|---|---|---|---|---|---|
| H-P-T axis | |||||
| Serum FSH | Elevated | Normal | Normal | Elevated | Normal |
| Serum LH | Elevated | Normal | Elevated | Elevated | Normal |
| Serum testosterone | Decreased | Normal | Decreased | Decreased | Normal |
| Testicular function | |||||
| Testis size | Decreased | Normal | Decreased | Decreased | Decreased |
| Testis size/WT testis size (%) | 7 | Normal | 23.40 | 31.10 | 79.00 |
| Epididymal sperm count | No epididymis | Within normal range | No sperm | No sperm | Decreased by ∼57% of WT sperm count |
| General histology observation | No lumen formation; germ cell hypoplasia and development to pachytene spermatocyte | Normal full range of spermatogenesis | Decrease of lumen formation; germ cell development to diplotene spermatocyte | Decrease of lumen formation; germ cell development to round spermatid | Normal full range of spermatogenesis |
| Overall fertility | Infertile | Normal fertility | Infertile | Infertile | Normal fertility |
H-P-T, Hypothalamic-pituitary-testis; WT, wild-type. All of these results originated from our previous publications. The other studies also showed the similar trend of serum testosterone level changes in T-AR−/y mice and S-AR−/y mice as compared to AR+/y controls, although this was not statistically significant due to a wide variation of serum testosterone levels between animals within the same genotype groups.
F. L-AR−/y mice
The serum testosterone levels in L-AR−/y mice are decreased (20.14 ± 1.5 vs. 192 ± 13.1 ng/dl; P < 0.05) (only reached 10.5% of AR+/y values) (Fig. 2F), and the serum LH levels are increased compared with AR+/y controls (54,56). The results of quantitative RT-PCR for Leydig cell-specific steroidogenic enzyme and functional gene expressions revealed that adult L-AR−/y mice have significant decreases in P450c17, 3β-HSD VI, 17β-HSD III, and RLF, as well as significant increases in LHR and TSP-2 gene expressions compared with AR+/y controls (54). Earlier reports have shown that RLF, 3β-HSD VI, and 17β-HSD III are mainly expressed in the adult Leydig cells and barely detectable in the fetal Leydig cells; therefore they act as markers for adult Leydig cell differentiation. In contrast, the expression pattern of TSP-2 is predominantly in the fetal Leydig cell population, and the expression levels are gradually decreased after puberty due to the rapid increase of adult Leydig cell population (78). Looking at the results of L-AR−/y mice, the markers of adult Leydig cell differentiation are all significantly decreased, but they are still detectable, and the TSP-2 gene expression levels are significantly increased compared with AR+/y controls (54). In contrast, we found that the Leydig cell number and size in L-AR−/y mice are comparable with AR+/y controls. These findings fit the hypothesis that the early initiation of adult Leydig cell differentiation and prepubertal rise in adult Leydig cell numbers are normal, but the later differentiation of adult Leydig cells to establish the full capacity of steroidogenic function is affected by loss of functional AR in the Leydig cells.
G. PM-AR−/y mice and G-AR−/y mice
The serum testosterone levels in PM-AR−/y and G-AR−/y mice are comparable to AR+/y controls (Fig. 2F), which implies that the functional AR in PM cells and germ cells are not essential for Leydig cell development, differentiation, and steroidogenesis.
IV. Phenotypes of External Genitalia and Internal Male Accessory Genital Organ Size in Various Testicular Cell-Specific AR Knockout Mice
It is generally agreed that the testicular differentiation from the bipotential gonad in early embryonic development is not an androgen/AR-dependent process. However, the early differentiation of male accessory sex organs during embryonic development is dependent on testosterone when the enzymes necessary to convert testosterone to DHT do not yet exist. The major effect of testosterone at early embryonic stages involves directing the differentiation of male-specific internal genital structures derived from the Wolffian ducts, including the epididymis, vas deferens, and seminal vesicles (98,99,100). During later embryonic developmental stages, when the enzymes responsible for mediating testosterone to DHT conversion became available, DHT-dependent development of the prostate and prostatic urethra occurs internally, and the differentiation of genital structures externalizes (4). Failure of the AR to activate its target genes in the presence of androgen during these critical stages will result in severe defects in induction of male sex differentiation and development of the male phenotype.
The results from recent publications showed that T-AR−/y male mice, with AR knockout in all cell types, exhibited female-like external genitalia, such as shorter genito-anal distance (the distance from the sex papilla to the anus; 0.59 cm in T-AR−/y compared with 1.12 cm in AR+/y 8-wk-old mice), a vagina with a blind end, and a microphallus. The male internal reproductive organs originating from the Wolffian ducts (such as epididymis, vas deferens, and seminal vesicles) or urogenital sinus (prostate) were absent in T-AR−/y male mice (Fig. 2E) (47,48,51), which was similar to Tfm male mice and humans with CAIS. In contrast, results from various testicular cell-specific AR knockout mice showed that the external genitalia and internal reproductive organs are normally developed (Fig. 2, B–D). However, the epididymis size in S-AR−/y mice is decreased by 63% of AR+/y size (53), and in L-AR−/y mice is decreased by 44% of AR+/y size (54). This decrease in epididymis size in S-AR−/y and L-AR−/y mice could be due to the lower serum testosterone levels and no mature sperm production in these mice (details will be discussed in Section VII). These in vivo results display the clear evidence that androgen/AR signals in Wolffian ducts, genital tubercle, and urogenital sinus during embryonic developmental stage play a critical role in the differentiation and development of internal and external male genitalia.
V. Testis Position in Various Testicular Cell-Specific AR Knockout Mice
The testis position of T-AR−/y mice is similar to Tfm male mice and men with CAIS and is located anywhere in the abdomen along the pathway from the normal position of the ovaries in female mice to the inguinal region (47,48). The developing gonads are initially attached to the posterior abdominal wall around the pararenal position by two ligamentous structures derived from the genital mesenteries. One is the cranial suspensory ligament, a ligamentous structure connecting the upper tip of the testis to the posterior abdominal wall. The other is the gubernaculums, a ligamentous structure of condensed mesenchymal cells connecting the caudal pole of the testis to the precursor structure of the scrotum. Regulation of transabdominal descent centers on the control of gubernacular enlargement and regression of the cranial suspensory ligament (98,101,102). Testicular descent is suggested to be mediated by the gubernaculums in that androgen/AR activity promotes outgrowth of the gubernaculums to allow caudal testis migration and is believed to be required for the inguinoscrotal phase of testicular descent (103). Androgen/AR action also drives regression of the cranial suspensory ligament, another process necessary for testis migration. Notably, both T-AR−/y male mice and Tfm male mice, as well as the humans with CAIS, present an intraabdominal migration of testis but a failure of inguinoscrotal migration of testis (104). The lack of an androgenic effect might affect the normal involution of the cranial suspensory ligament and enlargement of the gubernaculum, causing the testis to remain in the peritoneal cavity (101,102). In contrast, the testis of S-AR−/y, L-AR−/y, PM-AR−/y, and G-AR−/y mice were normally descended compared with AR+/y control mice. This in vivo evidence further emphasizes that the extratesticular mesenchymal (gubernaculums and cranial suspensory ligament) androgen/AR signals are important in normal testis descent, especially in the phase of inguinoscrotal migration.
VI. Testis Size in Various Testicular Cell-Specific AR Knockout Mice
A. S-AR−/y mice
Recent studies point out that the Sertoli cell number is the main contributory factor for the capacity of sperm production per testis and the final testis size in adulthood (105,106,107). The exact regulatory mechanism of Sertoli cell proliferation is not clear yet, but several hormones are involved in these processes. These include FSH, which stimulates Sertoli cell proliferation (108,109), and thyroid hormones, which regulate the cessation of Sertoli cell proliferation (105,110). Several earlier studies showed that androgens/AR play an important role in the Sertoli cell maturation process (105,111), rather than Sertoli cell proliferation, because the Sertoli cells do not express AR during fetal and early neonatal time periods of Sertoli cell proliferation (33,35,112). The expression of AR is first detectable in fetal rat testis at 14–15 d after coitum (113,114), coincident with the time that the fetal Leydig cells begin to produce testosterone (115,116). The positive AR immunostaining in the fetal testis is mostly localized in the interstitial cells and PM cells. In contrast, the Sertoli cells do not obviously express AR until postnatal d 5 (114). However, recent studies using the transgenic Tfm mouse model (109), gene expression profiling of androgen-regulated transcripts in neonatal mice testis (117), and combinations of hormonal manipulations in neonatal rat (118) suggested that androgens/AR might play a role in Sertoli cell proliferation, especially during early postnatal testis development. Another study using gene expression profiling of AR antagonists in the fetal rat testis suggested that the fetal testis is not a major target for AR activity during this stage of testis development, and results indicated that there are no overt histology changes and no common set of gene targets among various treated groups and the control group (119).
The size of testis in adult S-AR−/y mice is decreased by 21.5% of AR+/y testis size (Fig. 2F and Table 1) (50,51,52,56); however, there are studies (51,120) to show that adult S-AR−/y mice develop nearly normal numbers of Sertoli cells as compared with AR+/y control. In agreement with the earlier studies in which androgens/AR play an important role in the Sertoli cell maturation rather than Sertoli cell proliferation, other studies also suggest that the decrease in testis size in S-AR−/y mice can be mainly due to the functional defect of Sertoli cells to support late meiotic and postmeiotic germ cells (50,53).
B. Tfm mice and T-AR−/y mice
Earlier studies indicated that Tfm mice have significant decreases in testis size as early as postnatal d 5 compared with AR+/y control (108,109), and the testis of adult T-AR−/y mice were markedly reduced in size (only reached 7% of AR+/y testis size at 14 wk old; Fig. 2F and Table 1) and cryptorchid (47,56). The testis sizes of AR+/y and T-AR−/y are comparable up to postnatal d 2 despite significant decreases in Sertoli cell numbers in T-AR−/y testis (120), but by postnatal d 5, the testis size of Tfm mice was significantly smaller as compared with AR+/y testis (109). The reasons for testis hypoplasia in these mice could be the result of multiple factor effects, including loss of AR function in whole testicular cell types and the effect of elevated testicular temperature due to the intraabdominal position of testis.
C. L-AR−/y mice, PM-AR−/y mice, and G-AR−/y mice
The size of testis in adult L-AR−/y mice is decreased to 32.0% of AR+/y testis size (Fig. 2F and Table 1). The result showed that the decrease in testis size in L-AR−/y mice is mainly due to the defect of Leydig cell steroidogenic function subsequently affecting Sertoli cell functions to support postmeiotic germ cells (54,56). The size of testis in adult PM-AR−/y mice is decreased to 79.3% of AR+/y testis size (Fig. 2F and Table 1). The result suggested that loss of functional AR in PM cells might impair the paracrine effect from PM cells to Sertoli cells, resulting in the influence of normal Sertoli cell nourishing function and reduction in testis germ cell number (55). Interestingly, the testis of G-AR−/y mice showed similar testis size compared with AR+/y testis (Fig. 2F and Table 1) (56). This result clearly demonstrated that androgen/AR signals in germ cells do not have significant effects on sperm maturation and testis development.
VII. Testis Morphology, Epididymal Sperm Count, and Fertility Test in Various Testicular Cell-Specific AR Knockout Mice
Qualitatively complete spermatogenesis is defined as the presence of all germ cell types, but the cell number might be subnormal, whereas quantitatively complete spermatogenesis is the presence of a full complement of germ cells both in type and number (121). Androgen replacement alone has been shown to initiate qualitatively complete spermatogenesis in the gonadotropin-deficient mice (122), but FSH replacement alone fails to rescue spermatogenesis beyond the meiotic stages in these mice (123,124). Moreover, earlier studies using hypophysectomy to remove androgens from adult rats demonstrated an initial display of loss of midstage round spermatids and elongated spermatids (125). After long-term hypophysectomy and elimination of residual testosterone activity by flutamide or ethane dimethanesulphonate treatment, spermatogenesis rarely proceeds beyond meiosis and only a few round spermatids can be observed as well as the absence of elongated spermatids (126,127). These results provide additional supportive evidence that androgens/AR are necessary for the completion of meiosis and the differentiation of round spermatids into the spermatozoa.
A. Humans with AIS, Tfm mice, and T-AR−/y mice
The histology of testis in humans with CAIS showed that a majority of the seminiferous tubules were dysgenic. Some seminiferous tubules contained only spermatogonia and occasional meiotic spermatocytes. There were no mature germ cells in any seminiferous tubules (128).
The testicular histology of 14-wk-old T-AR−/y mice showed that a majority of seminiferous tubules lack germ cells, whereas others contained few germ cells, had no lumen formation in seminiferous tubules as well as decreases in diameter, and germ cell development stopped at the pachytene primary spermatocyte stage of the first meiosis division (47,56), which was similar to Tfm mice (15,16). The epididymis in T-AR−/y and Tfm mice were absent. As mentioned previously, there are reduced Leydig cell numbers, size, and steroidogenic enzyme gene expressions in T-AR−/y mice compared with AR+/y controls. But these findings in T-AR−/y mice are not explained by the coincidental cryptorchidism (92).
B. S-AR−/y mice
The histology of S-AR−/y testis showed a decrease of lumen formation as well as a decrease in diameter of seminiferous tubules and reduced germ cell complement as compared with AR+/y littermates. The seminiferous tubules of S-AR−/y mice displayed poor germ cell differentiation, with the majority of germ cell maturation ceasing at the diplotene primary spermatocyte stage (Fig. 3G) (50). Occasionally, secondary spermatocytes occur, but in greatly reduced numbers, and very few round spermatids exist (51,52). Consequently, the results from epididymal sperm count indicated that there was no sperm existing in the epididymis of S-AR−/y mice, and the mating tests showed that S-AR−/y mice failed to impregnate the wild-type (AR+/+) C57BL/6 female mice (Table 2) (50,51,52). Using flow cytometric scanning of propidium iodide-labeled cells, S-AR−/y testes showed a 3-fold increase in diploid cells, 2-fold increase in tetraploid cells, and 11-fold reduction in haploid cells compared with AR+/y testes (Fig. 3B vs. Fig. 3A) (50). Moreover, recent studies suggest that androgen, acting through Sertoli cells AR, regulates the microenvironment of seminiferous epithelium by influencing a broad spectrum of gene changes in Sertoli cells (53,129 and 130). The results showed that loss of AR specifically in Sertoli cells could affect the following: 1) structural support elements of Sertoli cells leading to impaired normal supportive function for movement of developing germ cells; 2) junction complex formation and basement membrane development of Sertoli cells leading to impaired functional integrity of the blood-testis barrier; and 3) Sertoli cell-specific proteases, transport proteins, and paracrine factor production and/or secretion, leading to impaired Sertoli cell nursery functions for developing germ cells (53,129). The results of S-AR−/y mice studies (50,51,52,53,120,129,130) clearly demonstrated that AR function in Sertoli cells is essential for the maintenance of fully competent Sertoli cell functions as well as the appropriate hormone levels that support the completion of meiosis I during spermatogenesis.
Figure 3.
Analysis of germ cell DNA content of 14-wk-old male AR+/y (A), S-AR−/y (B), L-AR−/y (C), PM-AR−/y (D), and G-AR−/y (E) mice by using flow cytometry. 1N represents haploid cells, 2N represents diploid cells, and 4N represents tetraploid cells. Compared with AR+/y testis (A), S-AR−/y testis showed 3-fold increase in diploid cells, 2-fold increase in tetraploid cells, and 11-fold reduced haploid cells (B); L-AR−/y testis showed 4-fold increase in tetraploid cells and 2.8-fold reduced haploid cells (C). There were similar distributions of DNA content histogram picks between PM-AR−/y (D), G-AR−/y (E), and AR+/y testis. Histology of testis by hematoxylin and eosin staining in testicular sections from 14-wk-old AR+/y (F), S-AR−/y (G), L-AR−/y (H), PM-AR−/y (I), and G-AR−/y (J) mice. Four to six 14-wk-old mice from individual groups were killed, and testes were excised for histology section. Compared with AR+/y testis (F), S-AR−/y testis showed that decrease of lumen formation in seminiferous tubules as well as germ cell development stopped at diplotene primary spermatocyte (G); L-AR−/y testis showed that decrease of lumen formation in seminiferous tubules as well as germ cell development stopped at round spermatid and no further differentiated elongated spermatid or released spermatozoa can be found (H); PM-AR−/y (I) and G-AR−/y (J) testis showed relatively comparable seminiferous tubule diameters and full range of germ cell development. [Sections of figures were reproduced from Refs. 55 and 56. Copyright 2006, Proceedings of the National Academy of Sciences.] All of these results originated from our previous publications.
Table 2.
Fertility test (pup number per litter; mean ± sem) and epididymal sperm content analysis in AR+/y, G-AR−/y, PM-AR−/y, L-AR−/y, S-AR−/y, and T-AR−/y mice
| Genotype | Mate no.
|
Vaginal plug | Sperm count/epididymis (n = 5) | Motility | ||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||
| AR+/y | 8.5 ± 0.5 | 7.5 ± 0.5 | 7.2 ± 1.0 | + | 26 × 106/ml | Normal |
| G-AR−/y | 7.5 ± 0.5 | 7.8 ± 0.5 | 8.2 ± 0.5 | + | 25 × 106/ml | Normal |
| PM-AR−/y | 9.0 ± 2.6 | 8.6 ± 1.5 | 8.7 ± 2.1 | + | 12 × 106/mla | Normal |
| L-AR−/y | 0 | 0 | 0 | + | 0 | 0 |
| S-AR−/y | 0 | 0 | 0 | + | 0 | 0 |
| T-AR−/y | 0 | 0 | 0 | + | No epididymis | |
All of these results originated from our previous publications.
Significant difference (P < 0.05; t test) as compared to AR+/y.
C. L-AR−/y mice
Testicular testosterone is produced by the Leydig cells and is essential for qualitatively and quantitatively complete spermatogenesis and development of the male phenotype. Earlier studies have demonstrated that long-term intratesticular testosterone withdrawal causes the failure of progression of round spermatid to elongated spermatids, particularly during stages VII and VIII of the spermatogenic cycle (131,132). The histology of L-AR−/y testis revealed a decrease of lumen formation as well as a decrease in diameter of seminiferous tubules, and germ cell development stopped at the round spermatid stage as compared with AR+/y littermates. There are no further differentiated mature elongated spermatids or spermatozoa throughout the L-AR−/y testis (Fig. 3H). Consequently, the results from epididymal sperm count indicated that no sperm exists in the epididymis of L-AR−/y mice, and the mating tests showed that L-AR−/y mice failed to impregnate the AR+/+ C57BL/6 female mice (Table 2). The flow cytometric scanning showed that L-AR−/y testes have 4-fold increased tetraploid cells and 2.8-fold reduced haploid cells compared with AR+/y testes (Fig. 3C vs. Fig. 3A) (54). The results from L-AR−/y mice studies illustrated that AR function in Leydig cells is essential for the maintenance of appropriate Leydig cell steroidogenic function and subsequent affect on Sertoli cell functions to support the final differentiation of round spermatids to mature elongated spermatids, the so-called process of spermiogenesis, in testis (54). The results from L-AR−/y mice also confirmed previous in vitro findings (133) demonstrating that androgen/AR signaling in Leydig cells displays in an autocrine regulation manner.
D. PM-AR−/y mice
The testicular histology of 14-wk-old PM-AR−/y mice showed relatively comparable seminiferous tubule diameters and the full range of germ cell development (Fig. 3I), but the epididymal sperm count in PM-AR−/y mice revealed 57% decreases compared with AR+/y mice (Table 2). Despite decreased epididymal sperm count in PM-AR−/y mice, the mating test showed that there were no significant differences in the litter number and the pup number per litter compared with AR+/y male mice (Table 2). The flow cytometric scanning showed that there were similar distributions of DNA content histogram peaks between PM-AR−/y and AR+/y controls (Fig. 3D vs. Fig. 3A) (55). In addition, the result of selective gene expression changes of PM-AR−/y testis, such as the genes related to smooth muscle contractions (endothelin, endothelin receptor A and B, adrenomedullin, adrenomedullin receptor, and vasopressin receptor 1a) or genes related to Sertoli cells’ functional genes and cell junction genes (transferrin, epidermal fatty acid binding protein, tissue-type plasminogen activator, urokinase-type plasminogen activator, androgen-binding protein, occludin, testin, nectin, zyxin, vinculin, laminin γ3, gelsolin, connexin43, and N-cadherin), indicated that there might be impaired smooth muscle contractility, Sertoli cell nourishing function, and the integrity of Sertoli cell junctions. All of these consequently affect testis sperm production and slow down the germ cell movement (55). The results in PM-AR−/y mice clearly showed that lack of functional AR in PM cells might directly cause functional impairment of PM cell contractility and indirectly cause some functional defects of Sertoli cells to support germ cell development and spermatozoa output, which subsequently lead to decreased epididymal sperm count.
E. G-AR−/y mice
In comparison with AR+/y mice, testes from G-AR−/y mice have normal spermatogenesis at every spermatogenic stage (Fig. 3J). There are no significant differences in the litter number and the pup number per litter between G-AR−/y and AR+/y controls (Table 2). The flow cytometric scanning also showed that there were similar distributions of DNA content histogram peaks between G-AR−/y and AR+/y controls (Fig. 3E vs. Fig. 3A) (56). Although it was suggested that the presence of functional AR in germ cells was not essential for development into sperm in a chimera study (134) and in a spermatogonial stem cell transplantation study (135), studies from G-AR−/y mice clearly demonstrated that the quality and the quantity of spermatogenesis, as well as fertility, were almost normal if AR gene is deficient in nearly all of germ cells beyond the pachytene stage (56). The major concern about the chimera study (134) is that many germ cells from the pachytene stage to spermatids (the obvious androgen-dependent period during spermatogenesis) still contain normal AR that might support spermatogenesis through autocrine or paracrine effects. Another potential argument is that the floxed AR allele might not be deleted in spermatogonia and early spermatocytes in Sycp1-Cre (+) G-AR−/y mice. However, little AR expression was detected in spermatogonia and spermatocytes in any of the previous studies (34). The testicular morphology from T-AR−/y and Tfm mice also suggests that AR has little effect on this stage of spermatogenesis because their testis contained spermatogonia and the spermatocytes that reached the pachytene stage (47,91). However, the animal model might not be exactly the same as the human condition. The testicular morphology of humans with CAIS showed only Sertoli cells and very scant spermatogonia in seminiferous tubules (136,137). In the mouse counterpart, the testicular histology of T-AR−/y revealed maturation arrest, with the development of germ cells stopping at the pachytene stage of spermatocytes (47). Thus, the role of AR in the human spermatogenesis might be more critical than in mice. Earlier studies using immunofluorescence labeling and confocal microscopy localized AR in the midpiece and mitochondria of human sperm (38) and demonstrated that AR in human sperm have the ability to modulate the phosphatidylinositol-3-kinase/AKT pathway (40). Therefore, we cannot completely rule out the possibility of the presence of the AR nongenomic signaling within the germ cells. Manipulating the AR in the laboratory in developed human embryonic gonadal cells (138) or cultured human spermatogonia might help us to further clarify the AR role in human germ cells during spermatogenesis.
VIII. Concluding Remarks and Future Directions
The powerful new techniques of genetic manipulations have provided us new animal models with precisely defined gene ablations in specific cell types in the body. These animal models along with emerging phenotypes have led to the accumulation of a great deal of useful information on less well-known mechanisms involved in pathological effects of androgen/AR function in male reproductive health. This review clearly indicates that AR has differential roles in the different testicular cells responsible for spermatogenesis and male fertility (Fig. 4). The androgen/AR signaling in Sertoli cells plays the most important role in the process of meiosis I during spermatogenesis, and the testis from S-AR−/y mice shows the most detrimental phenotype in that the spermatogenesis arrests predominantly at the diplotene primary spermatocyte stage before the accomplishment of first meiotic division (50,51,53). The lack of functional AR in Leydig cells has a major influence on Leydig cells steroidogenic functions leading to spermatogenesis arrest predominately at the round spermatid stage (54). Male mice lacking functional AR only in the smooth muscle cells and testicular PM cells (PM-AR−/y) have similar fertility despite decreased sperm output compared with AR+/y controls (55). The functional AR in germ cells is not essential for spermatogenesis and male fertility in mice (56). However, there are still many questions that need to be answered regarding the molecular mechanisms for androgen/AR regulation of Sertoli cell, Leydig cell, and PM cell proliferation and/or differentiation. The exact mechanism(s) of stage-specific regulation of Sertoli cells via androgen/AR signals in testis are still largely unknown. It would also be of interest to determine the role of AR in the modulation of the complex paracrine signaling between different testicular cell-cell communications during testis development and spermatogenesis, or whether androgens/AR can regulate some aspects of spermatogonia stem cell proliferation or differentiation through the PM cell AR. The future studies towards understanding detailed molecular mechanisms in these in vivo animals with cell-specific AR knockout could possibly lead to useful insights for improvements in the treatment of male infertility, hypogonadism, testicular dysgenesis syndrome, and in the attempts to create a safe as well as effective male contraceptive method.
Figure 4.
Diagram of germ cell progression in T-AR−/y, S-AR−/y, L-AR−/y, PM-AR−/y, G-AR−/y, and AR+/y testis. AR+/y, G-AR−/y, and PM-AR−/y testis can achieve full germ cell progression. However, spermatogenesis in the T-AR−/y, S-AR−/y, and L-AR−/y testis ceases predominately at the pachytene, diplotene, and round spermatid stages, respectively.
Footnotes
This work was supported by National Institutes of Health Grants DK073414 and CA127300 and the George Whipple Professorship Endowment.
Disclosure Summary: The authors have nothing to disclose.
First Published Online January 27, 2009
Abbreviations: ACTB, β-Actin; AIS, androgen insensitivity syndrome; AMH, anti-Müllerian hormone; AR, androgen receptor; AR−/y, AR knockout male; AR+/y, wild-type AR male; AR+/+, wild-type AR female; CAIS, complete androgen insensitivity syndrome; Cre, cre recombinase; DHT, 5α-dihydroxytestosterone; floxed, flanked by lox site; G-AR−/y, germ cell-specific AR knockout; 3β-HSD, 3β-hydroxysteroid dehydrogenase; 17β-HSD, 17β-hydroxysteroid dehydrogenase; L-AR−/y, Leydig cell-specific AR knockout; LHR, LH receptor; PM, peritubular myoid; PM-AR−/y, PM cell-specific AR knockout; P450c17, 17α-hydroxylase or 17,20 lyase; P450scc, P450 side-chain cleavage; RLF, relaxin-like factor; S-AR−/y, Sertoli cell-specific AR knockout; StAR, steroidogenic acute regulatory protein; T-AR−/y, total AR knockout; Tfm, testicular feminization; TSP-2, thrombospondin type 2.
References
- Chang JA, Nguyen HT, Lue TF 2002 Androgens in penile development, penile erection, and erectile dysfunction. In: Chang C, ed. Androgens and androgen receptor: mechanisms, functions and clinical applications. Norwell, MA: Kluwer Academic Publishers; 289–298 [Google Scholar]
- Collins LL, Chang C 2002 Androgens and the androgen receptor in male sex development and fertility. In: Chang C, ed. Androgens and androgen receptor: mechanisms, functions and clinical applications. Norwell, MA: Kluwer Academic Publishers; 299–323 [Google Scholar]
- Shumazaki S 2002 The role of 5-α reductase in prostate disease and male pattern baldness. In: Chang C, ed. Androgens and androgen receptor: mechanisms, functions and clinical applications. Norwell, MA: Kluwer Academic Publishers; 155–196 [Google Scholar]
- Quigley CA 1998 The androgen receptor: physiology and pathophysiology. In: Nieschlag E, Behre HM, eds. Testosterone: action, deficiency, substitution. Heidelberg: Springer-Verlag; 33–106 [Google Scholar]
- Chang CS, Kokontis J, Liao ST 1988 Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science 240:324–326 [DOI] [PubMed] [Google Scholar]
- Chang CS, Kokontis J, Liao ST 1988 Structural analysis of complementary DNA and amino acid sequences of human and rat androgen receptors. Proc Natl Acad Sci USA 85:7211–7215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubahn DB, Joseph DR, Sullivan PM, Willard HF, French FS, Wilson EM 1988 Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science 240:327–330 [DOI] [PubMed] [Google Scholar]
- Heinlein CA, Chang C 2002 Androgen receptor (AR) coregulators: an overview. Endocr Rev 23:175–200 [DOI] [PubMed] [Google Scholar]
- Griffin JE 1992 Androgen resistance—the clinical and molecular spectrum. N Engl J Med326:611–618 [DOI] [PubMed] [Google Scholar]
- La Spada AR, Wilson EM, Lubahn DB, Harding AE, Fischbeck KH 1991 Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature 352:77–79 [DOI] [PubMed] [Google Scholar]
- McPhaul MJ 1999 Molecular defects of the androgen receptor. J Steroid Biochem Mol Biol 69:315–322 [DOI] [PubMed] [Google Scholar]
- Quigley CA, De Bellis A, Marschke KB, el-Awady MK, Wilson EM, French FS 1995 Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr Rev 16:271–321 [DOI] [PubMed] [Google Scholar]
- Schultz MG 1962 Male pseudohermaphroditism diagnosed with aid of sex chromatin technique. J Am Vet Med Assoc 140:241–244 [PubMed] [Google Scholar]
- Bardin CW, Bullock L, Schneider G, Allison JE, Stanley AJ 1970 Pseudohermaphrodite rat: end organ insensitivity to testosterone. Science 167:1136–1137 [DOI] [PubMed] [Google Scholar]
- Lyon MF, Hawkes SG 1970 X-linked gene for testicular feminization in the mouse. Nature 227:1217–1219 [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Wilson JD 1972 Studies on the pathogenesis of the pseudohermaphroditism in the mouse with testicular feminization. J Clin Invest 51:1647–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers-Wallen VN, Wilson JD, Griffin JE, Fisher S, Moorhead PH, Goldschmidt MH, Haskins ME, Patterson DF 1989 Testicular feminization in a cat. J Am Vet Med Assoc 195:631–634 [PubMed] [Google Scholar]
- de Kretser DM, Kerr JB 1988 The cytology of the testis. In: Knobil E, Neill JB, Ewing LL, Greenwald GS, Markert CL, Pfaff DW, ed. The physiology of reproduction. New York: Raven Press; 837–932 [Google Scholar]
- Saez JM 1994 Leydig cells: endocrine, paracrine, and autocrine regulation. Endocr Rev 15:574–626 [DOI] [PubMed] [Google Scholar]
- Vogl AW, Pfeiffer DC, Mulholland D, Kimel G, Guttman J 2000 Unique and multifunctional adhesion junctions in the testis: ectoplasmic specializations. Arch Histol Cytol 63:1–15 [DOI] [PubMed] [Google Scholar]
- Russell L 1993 Morphological and functional evidence for Sertoli-germ cell relationships. In: Russell LD, Griswold MD, eds. The Sertoli cell. Clearwater, FL: Cache River Press; 365–390 [Google Scholar]
- Mruk DD, Cheng CY 2004 Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev 25:747–806 [DOI] [PubMed] [Google Scholar]
- Griswold MD 1988 Protein secretions of Sertoli cells. Int Rev Cytol 110:133–156 [DOI] [PubMed] [Google Scholar]
- Skinner M 1993 Secretion of growth factors and other regulatory factor. In: Russell LD, Griswold MD, eds. The Sertoli cell. Clearwater, FL: Cache River Press; 237–247 [Google Scholar]
- Waites GM, Gladwell RT 1982 Physiological significance of fluid secretion in the testis and blood-testis barrier. Physiol Rev 62:624–671 [DOI] [PubMed] [Google Scholar]
- Skinner MK, Fritz IB 1985 Testicular peritubular cells secrete a protein under androgen control that modulates Sertoli cell functions. Proc Natl Acad Sci USA 82:114–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton JN, Skinner MK 1989 Regulation of Sertoli cell function and differentiation through the actions of a testicular paracrine factor P-Mod-S. Endocrinology 124:2711–2719 [DOI] [PubMed] [Google Scholar]
- Romano F, Tripiciano A, Muciaccia B, De Cesaris P, Ziparo E, Palombi F, Filippini A 2005 The contractile phenotype of peritubular smooth muscle cells is locally controlled: possible implications in male fertility. Contraception 72:294–297 [DOI] [PubMed] [Google Scholar]
- Skinner MK, Schlitz SM, Anthony CT 1989 Regulation of Sertoli cell differentiated function: testicular transferrin and androgen-binding protein expression. Endocrinology 124:3015–3024 [DOI] [PubMed] [Google Scholar]
- Anthony CT, Kovacs WJ, Skinner MK 1989 Analysis of the androgen receptor in isolated testicular cell types with a microassay that uses an affinity ligand. Endocrinology 125:2628–2635 [DOI] [PubMed] [Google Scholar]
- Sar M, Lubahn DB, French FS, Wilson EM 1990 Immunohistochemical localization of the androgen receptor in rat and human tissues. Endocrinology 127:3180–3186 [DOI] [PubMed] [Google Scholar]
- Kimura N, Mizokami A, Oonuma T, Sasano H, Nagura H 1993 Immunocytochemical localization of androgen receptor with polyclonal antibody in paraffin-embedded human tissues. J Histochem Cytochem 41:671–678 [DOI] [PubMed] [Google Scholar]
- Bremner WJ, Millar MR, Sharpe RM, Saunders PT 1994 Immunohistochemical localization of androgen receptors in the rat testis: evidence for stage-dependent expression and regulation by androgens. Endocrinology 135:1227–1234 [DOI] [PubMed] [Google Scholar]
- Vornberger W, Prins G, Musto NA, Suarez-Quian CA 1994 Androgen receptor distribution in rat testis: new implications for androgen regulation of spermatogenesis. Endocrinology 134:2307–2316 [DOI] [PubMed] [Google Scholar]
- Zhou X, Kudo A, Kawakami H, Hirano H 1996 Immunohistochemical localization of androgen receptor in mouse testicular germ cells during fetal and postnatal development. Anat Rec 245:509–518 [DOI] [PubMed] [Google Scholar]
- Janssen PJ, Brinkmann AO, Boersma WJ, Van der Kwast TH 1994 Immunohistochemical detection of the androgen receptor with monoclonal antibody F39.4 in routinely processed, paraffin- embedded human tissues after microwave pre-treatment. J Histochem Cytochem 42:1169–1175 [DOI] [PubMed] [Google Scholar]
- Arenas MI, Royuela M, Lobo MV, Alfaro JM, Fraile B, Paniagua R 2001 Androgen receptor (AR), estrogen receptor-α (ER-α) and estrogen receptor-β (ER-β) expression in the testis of the newt, Triturus marmoratus marmoratus, during the annual cycle. J Anat 199:465–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solakidi S, Psarra AM, Nikolaropoulos S, Sekeris CE 2005 Estrogen receptors α and β (ERα and ERβ) and androgen receptor (AR) in human sperm: localization of ERβ and AR in mitochondria of the midpiece. Hum Reprod 20:3481–3487 [DOI] [PubMed] [Google Scholar]
- Merlet J, Racine C, Moreau E, Moreno SG, Habert R 2007 Male fetal germ cells are targets for androgens that physiologically inhibit their proliferation. Proc Natl Acad Sci USA 104:3615–3620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquila S, Middea E, Catalano S, Marsico S, Lanzino M, Casaburi I, Barone I, Bruno R, Zupo S, Ando S 2007 Human sperm express a functional androgen receptor: effects on PI3K/AKT pathway. Hum Reprod 22:2594–2605 [DOI] [PubMed] [Google Scholar]
- Warikoo PK, Majumdar SS, Allag IS, Das RP, Roy S 1986 Interactions among motility, fertilizing ability, and testosterone binding on spermatozoa of bonnet monkey (Macaca radiata). Arch Androl 16:135–141 [DOI] [PubMed] [Google Scholar]
- Galena HJ, Pillai AK, Terner C 1974 Progesterone and androgen receptors in non-flagellate germ cells of the rat testis. J Endocrinol 63:223–237 [DOI] [PubMed] [Google Scholar]
- Grootegoed JA, Peters MJ, Mulder E, Rommerts FF, Van der Molen HJ 1977 Absence of a nuclear androgen receptor in isolated germinal cells of rat testis. Mol Cell Endocrinol 9:159–167 [DOI] [PubMed] [Google Scholar]
- Suarez-Quian CA, Martinez-Garcia F, Nistal M, Regadera J 1999 Androgen receptor distribution in adult human testis. J Clin Endocrinol Metab 84:350–358 [DOI] [PubMed] [Google Scholar]
- Van Roijen JH, Van Assen S, Van Der Kwast TH, De Rooij DG, Boersma WJ, Vreeburg JT, Weber RF 1995 Androgen receptor immunoexpression in the testes of subfertile men. J Androl 16:510–516 [PubMed] [Google Scholar]
- Pelletier G, Labrie C, Labrie F 2000 Localization of oestrogen receptor α, oestrogen receptor β and androgen receptors in the rat reproductive organs. J Endocrinol 165:359–370 [DOI] [PubMed] [Google Scholar]
- Yeh S, Tsai MY, Xu Q, Mu XM, Lardy H, Huang KE, Lin H, Yeh SD, Altuwaijri S, Zhou X, Xing L, Boyce BF, Hung MC, Zhang S, Gan L, Chang C 2002 Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci USA 99:13498–13503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Takeyama K, Sato T, Kato S 2003 Androgen receptor functions from reverse genetic models. J Steroid Biochem Mol Biol 85:95–99 [DOI] [PubMed] [Google Scholar]
- Holt CL, May GS 1993 A novel phage λ replacement Cre-lox vector that has automatic subcloning capabilities. Gene 133:95–97 [DOI] [PubMed] [Google Scholar]
- Chang C, Chen YT, Yeh SD, Xu Q, Wang RS, Guillou F, Lardy H, Yeh S 2004 Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc Natl Acad Sci USA 101:6876–6881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lecureuil C, Heyns W, Carmeliet P, Guillou F, Sharpe RM, Verhoeven G 2004 A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci USA 101:1327–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdcraft RW, Braun RE 2004 Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development 131:459–467 [DOI] [PubMed] [Google Scholar]
- Wang RS, Yeh S, Chen LM, Lin HY, Zhang C, Ni J, Wu CC, di Sant'Agnese PA, DeMesy-Bentley KL, Tzeng CR, Chang C 2006 Androgen receptor in Sertoli cell is essential for germ cell nursery and junctional complex formation in mouse testes. Endocrinology 147:5624–5633 [DOI] [PubMed] [Google Scholar]
- Xu Q, Lin HY, Yeh SD, Yu IC, Wang RS, Chen YT, Zhang C, Altuwaijri S, Chen LM, Chuang KH, Chiang HS, Yeh S, Chang C 2007 Infertility with defective spermatogenesis and steroidogenesis in male mice lacking androgen receptor in Leydig cells. Endocrine 32:96–106 [DOI] [PubMed] [Google Scholar]
- Zhang C, Yeh S, Chen YT, Wu CC, Chuang KH, Lin HY, Wang RS, Chang YJ, Mendis-Handagama C, Hu L, Lardy H, Chang C 2006 Oligozoospermia with normal fertility in male mice lacking the androgen receptor in testis peritubular myoid cells. Proc Natl Acad Sci USA 103:17718–17723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MY, Yeh SD, Wang RS, Yeh S, Zhang C, Lin HY, Tzeng CR, Chang C 2006 Differential effects of spermatogenesis and fertility in mice lacking androgen receptor in individual testis cells. Proc Natl Acad Sci USA 103:18975–18980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco DM, Clark BJ 1996 Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev 17:221–244 [DOI] [PubMed] [Google Scholar]
- Liu J, Rone MB, Papadopoulos V 2006 Protein-protein interactions mediate mitochondrial cholesterol transport and steroid biosynthesis. J Biol Chem 281:38879–38893 [DOI] [PubMed] [Google Scholar]
- Chung BC, Matteson KJ, Voutilainen R, Mohandas TK, Miller WL 1986 Human cholesterol side-chain cleavage enzyme, P450scc: cDNA cloning, assignment of the gene to chromosome 15, and expression in the placenta. Proc Natl Acad Sci USA 83:8962–8966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weusten JJ, Smals AG, Hofman JA, Kloppenborg PW, Benraad TJ 1987 Early time sequence in pregnenolone metabolism to testosterone in homogenates of human and rat testis. Endocrinology 120:1909–1913 [DOI] [PubMed] [Google Scholar]
- Fluck CE, Miller WL, Auchus RJ 2003 The 17, 20-lyase activity of cytochrome p450c17 from human fetal testis favors the Δ5 steroidogenic pathway. J Clin Endocrinol Metab 88:3762–3766 [DOI] [PubMed] [Google Scholar]
- Fevold HR, Lorence MC, McCarthy JL, Trant JM, Kagimoto M, Waterman MR, Mason JI 1989 Rat P450(17 α) from testis: characterization of a full-length cDNA encoding a unique steroid hydroxylase capable of catalyzing both Δ4- and Δ5-steroid-17,20-lyase reactions. Mol Endocrinol 3:968–975 [DOI] [PubMed] [Google Scholar]
- Zhang LH, Rodriguez H, Ohno S, Miller WL 1995 Serine phosphorylation of human P450c17 increases 17,20-lyase activity: implications for adrenarche and the polycystic ovary syndrome. Proc Natl Acad Sci USA 92:10619–10623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey AV, Mellon SH, Miller WL 2003 Protein phosphatase 2A and phosphoprotein SET regulate androgen production by P450c17. J Biol Chem 278:2837–2844 [DOI] [PubMed] [Google Scholar]
- Luu-The V, Labrie C, Simard J, Lachance Y, Zhao HF, Couet J, Leblanc G, Labrie F 1990 Structure of two in tandem human 17 β-hydroxysteroid dehydrogenase genes. Mol Endocrinol 4:268–275 [DOI] [PubMed] [Google Scholar]
- Lorence MC, Corbin CJ, Kamimura N, Mahendroo MS, Mason JI 1990 Structural analysis of the gene encoding human 3 β-hydroxysteroid dehydrogenase/Δ5-4-isomerase. Mol Endocrinol 4:1850–1855 [DOI] [PubMed] [Google Scholar]
- Baker PJ, Sha JA, McBride MW, Peng L, Payne AH, O'Shaughnessy PJ 1999 Expression of 3β-hydroxysteroid dehydrogenase type I and type VI isoforms in the mouse testis during development. Eur J Biochem 260:911–917 [DOI] [PubMed] [Google Scholar]
- Sriraman V, Sairam MR, Rao AJ 2003 Evaluation of relative roles of LH and FSH in regulation of differentiation of Leydig cells using an ethane 1,2-dimethylsulfonate-treated adult rat model. J Endocrinol 176:151–161 [DOI] [PubMed] [Google Scholar]
- Tong MH, Christenson LK, Song WC 2004 Aberrant cholesterol transport and impaired steroidogenesis in Leydig cells lacking estrogen sulfotransferase. Endocrinology 145:2487–2497 [DOI] [PubMed] [Google Scholar]
- Huhtaniemi I, Pelliniemi LJ 1992 Fetal Leydig cells: cellular origin, morphology, life span, and special functional features. Proc Soc Exp Biol Med 201:125–140 [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy PJ, Baker PJ, Johnston H 2006 The foetal Leydig cell—differentiation, function and regulation. Int J Androl 29:90–95; discussion, 105–108 [DOI] [PubMed] [Google Scholar]
- Gondos B 1980 Development and differentiation of the testis and male reproductive tract. In: Steinberger A, Steinberger E, eds. Testicular development, structure and function. New York: Raven Press; 3–20 [Google Scholar]
- Payne AH, O'Shaughnessy PJ, Chase DJ, Dixon GE, Christensen AK 1982 LH receptors and steroidogenesis in distinct populations of Leydig cells. Ann NY Acad Sci 383:174–203 [DOI] [PubMed] [Google Scholar]
- Tilmann C, Capel B 2002 Cellular and molecular pathways regulating mammalian sex determination. Recent Prog Horm Res 57:1–18 [DOI] [PubMed] [Google Scholar]
- Vergouwen RP, Jacobs SG, Huiskamp R, Davids JA, de Rooij DG 1991 Proliferative activity of gonocytes, Sertoli cells and interstitial cells during testicular development in mice. J Reprod Fertil 93:233–243 [DOI] [PubMed] [Google Scholar]
- Dong L, Jelinsky SA, Finger JN, Johnston DS, Kopf GS, Sottas CM, Hardy MP, Ge RS 2007 Gene expression during development of fetal and adult Leydig cells. Ann NY Acad Sci 1120:16–35 [DOI] [PubMed] [Google Scholar]
- Vergouwen RP, Huiskamp R, Bas RJ, Roepers-Gajadien HL, Davids JA, de Rooij DG 1993 Postnatal development of testicular cell populations in mice. J Reprod Fertil 99:479–485 [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy PJ, Willerton L, Baker PJ 2002 Changes in Leydig cell gene expression during development in the mouse. Biol Reprod 66:966–975 [DOI] [PubMed] [Google Scholar]
- Mendis-Handagama SM, Ariyaratne HB 2001 Differentiation of the adult Leydig cell population in the postnatal testis. Biol Reprod 65:660–671 [DOI] [PubMed] [Google Scholar]
- Teerds KJ 1996 Regeneration of Leydig cells after depletion by EDS: a model for postnatal Leydig cell renewal. In: Payne AH, Hardy MP, Russell LD, eds. The Leydig cell. 1st ed. Clearwater, FL: Cache River Press; 203–219 [Google Scholar]
- O'Shaughnessy PJ, Baker PJ, Johnston H 2005 Neuroendocrine regulation of Leydig cell development. Ann NY Acad Sci 1061:109–119 [DOI] [PubMed] [Google Scholar]
- Sharpe RM 1993 Experimental evidence for Sertoli cell-germ cell and Sertoli cell-Leydig cell interactions. In: Russell LD, Griswold MD, eds. The Sertoli cell. 1st ed. Clearwater, FL: Cache River Press; 391–419 [Google Scholar]
- Saez JM, Lejeune H 1996 Regulation of Leydig cell functions by hormones and growth factors other than LH and IGF-1. In: Payne AH, Hardy MP, Russell LD, eds. The Leydig cell. 1st ed. Clearwater, FL: Cache River Press; 383–406 [Google Scholar]
- Teerds KJ, de Rooij DG, de Jong FH, van Haaster LH 1998 Development of the adult-type Leydig cell population in the rat is affected by neonatal thyroid hormone levels. Biol Reprod 59:344–350 [DOI] [PubMed] [Google Scholar]
- Ariyaratne HB, Mills N, Mason JI, Mendis-Handagama SM 2000 Effects of thyroid hormone on Leydig cell regeneration in the adult rat following ethane dimethane sulphonate treatment. Biol Reprod 63:1115–1123 [DOI] [PubMed] [Google Scholar]
- Abney TO 1999 The potential roles of estrogens in regulating Leydig cell development and function: a review. Steroids 64:610–617 [DOI] [PubMed] [Google Scholar]
- Ge RS, Shan LX, Hardy MP 1996 Pubertal development of Leydig cells. In: Payne AH, Hardy MP, Russell LD, eds. The Leydig cell. 1st ed. Clearwater, FL: Cache River Press; 159–172 [Google Scholar]
- Hardy MP, Kelce WR, Klinefelter GR, Ewing LL 1990 Differentiation of Leydig cell precursors in vitro: a role for androgen. Endocrinology 127:488–490 [DOI] [PubMed] [Google Scholar]
- Murphy L, Jeffcoate IA, O'Shaughnessy PJ 1994 Abnormal Leydig cell development at puberty in the androgen-resistant Tfm mouse. Endocrinology 135:1372–1377 [DOI] [PubMed] [Google Scholar]
- Jones RD, Pugh PJ, Hall J, Channer KS, Jones TH 2003 Altered circulating hormone levels, endothelial function and vascular reactivity in the testicular feminised mouse. Eur J Endocrinol 148:111–120 [DOI] [PubMed] [Google Scholar]
- Murphy L, O'Shaughnessy PJ 1991 Testicular steroidogenesis in the testicular feminized (Tfm) mouse: loss of 17 α-hydroxylase activity. J Endocrinol 131:443–449 [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy PJ, Johnston H, Willerton L, Baker PJ 2002 Failure of normal adult Leydig cell development in androgen-receptor-deficient mice. J Cell Sci 115:3491–3496 [DOI] [PubMed] [Google Scholar]
- Blackburn WR, Chung KW, Bullock L, Bardin CW 1973 Testicular feminization in the mouse: studies of Leydig cell structure and function. Biol Reprod 9:9–23 [DOI] [PubMed] [Google Scholar]
- De Gendt K, Atanassova N, Tan KA, de Franca LR, Parreira GG, McKinnell C, Sharpe RM, Saunders PT, Mason JI, Hartung S, Ivell R, Denolet E, Verhoeven G 2005 Development and function of the adult generation of Leydig cells in mice with Sertoli cell-selective or total ablation of the androgen receptor. Endocrinology 146:4117–4126 [DOI] [PubMed] [Google Scholar]
- Morris JM, Mahesh VB 1963 Further observations on the syndrome, “testicular feminization.” Am J Obstet Gynecol 87:731–748 [PubMed] [Google Scholar]
- Savage MO, Chaussain JL, Evain D, Roger M, Canlorbe P, Job JC 1978 Endocrine studies in male pseudohermaphroditism in childhood and adolescence. Clin Endocrinol (Oxf) 8:219–231 [DOI] [PubMed] [Google Scholar]
- Migeon CJ, Berkovitz GD, Brown TR 1994 Sexual differentiation and ambiguity. In: Kappy MS, Blizzard RM, Migeon CJ, eds. The diagnosis and treatment of endocrine disorders in childhood and adolescence. Springfield, IL: Thomas; 573–716 [Google Scholar]
- Hutson JM, Hasthorpe S, Heyns CF 1997 Anatomical and functional aspects of testicular descent and cryptorchidism. Endocr Rev 18:259–280 [DOI] [PubMed] [Google Scholar]
- Tong SY, Hutson JM, Watts LM 1996 Does testosterone diffuse down the Wolffian duct during sexual differentiation? J Urol 155:2057–2059 [PubMed] [Google Scholar]
- Wilson JD, George FW, Griffin JE 1981 The hormonal control of sexual development. Science 211:1278–1284 [DOI] [PubMed] [Google Scholar]
- Ivell R, Hartung S 2003 The molecular basis of cryptorchidism. Mol Hum Reprod 9:175–181 [DOI] [PubMed] [Google Scholar]
- Foresta C, Zuccarello D, Garolla A, Ferlin A 2008 Role of hormones, genes, and environment in human cryptorchidism. Endocr Rev 29:560–580 [DOI] [PubMed] [Google Scholar]
- Klonisch T, Fowler PA, Hombach-Klonisch S 2004 Molecular and genetic regulation of testis descent and external genitalia development. Dev Biol 270:1–18 [DOI] [PubMed] [Google Scholar]
- Hutson JM 1986 Testicular feminization: a model for testicular descent in mice and men. J Pediatr Surg 21:195–198 [DOI] [PubMed] [Google Scholar]
- Sharpe RM, McKinnell C, Kivlin C, Fisher JS 2003 Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 125:769–784 [DOI] [PubMed] [Google Scholar]
- Johnson L, Zane RS, Petty CS, Neaves WB 1984 Quantification of the human Sertoli cell population: its distribution, relation to germ cell numbers, and age-related decline. Biol Reprod 31:785–795 [DOI] [PubMed] [Google Scholar]
- Orth JM, Gunsalus GL, Lamperti AA 1988 Evidence from Sertoli cell-depleted rats indicates that spermatid number in adults depends on numbers of Sertoli cells produced during perinatal development. Endocrinology 122:787–794 [DOI] [PubMed] [Google Scholar]
- Baker PJ, O'Shaughnessy PJ 2001 Role of gonadotrophins in regulating numbers of Leydig and Sertoli cells during fetal and postnatal development in mice. Reproduction 122:227–234 [DOI] [PubMed] [Google Scholar]
- Johnston H, Baker PJ, Abel M, Charlton HM, Jackson G, Fleming L, Kumar TR, O'Shaughnessy PJ 2004 Regulation of Sertoli cell number and activity by follicle-stimulating hormone and androgen during postnatal development in the mouse. Endocrinology 145:318–329 [DOI] [PubMed] [Google Scholar]
- Holsberger DR, Cooke PS 2005 Understanding the role of thyroid hormone in Sertoli cell development: a mechanistic hypothesis. Cell Tissue Res 322:133–140 [DOI] [PubMed] [Google Scholar]
- Hill CM, Anway MD, Zirkin BR, Brown TR 2004 Intratesticular androgen levels, androgen receptor localization, and androgen receptor expression in adult rat Sertoli cells. Biol Reprod 71:1348–1358 [DOI] [PubMed] [Google Scholar]
- McKinnell C, Saunders PT, Fraser HM, Kelnar CJ, Kivlin C, Morris KD, Sharpe RM 2001 Comparison of androgen receptor and oestrogen receptor β immunoexpression in the testes of the common marmoset (Callithrix jacchus) from birth to adulthood: low androgen receptor immunoexpression in Sertoli cells during the neonatal increase in testosterone concentrations. Reproduction 122:419–429 [DOI] [PubMed] [Google Scholar]
- Majdic G, Millar MR, Saunders PT 1995 Immunolocalisation of androgen receptor to interstitial cells in fetal rat testes and to mesenchymal and epithelial cells of associated ducts. J Endocrinol 147:285–293 [DOI] [PubMed] [Google Scholar]
- You L, Sar M 1998 Androgen receptor expression in the testes and epididymides of prenatal and postnatal Sprague-Dawley rats. Endocrine 9:253–261 [DOI] [PubMed] [Google Scholar]
- Warren DW, Haltmeyer GC, Eik-Nes KB 1973 Testosterone in the fetal rat testis. Biol Reprod 8:560–565 [DOI] [PubMed] [Google Scholar]
- Feldman SC, Bloch E 1978 Developmental pattern of testosterone synthesis by fetal rat testes in response to luteinizing hormone. Endocrinology 102:999–1007 [DOI] [PubMed] [Google Scholar]
- Zhou Q, Shima JE, Nie R, Friel PJ, Griswold MD 2005 Androgen-regulated transcripts in the neonatal mouse testis as determined through microarray analysis. Biol Reprod 72:1010–1019 [DOI] [PubMed] [Google Scholar]
- Atanassova NN, Walker M, McKinnell C, Fisher JS, Sharpe RM 2005 Evidence that androgens and oestrogens, as well as follicle-stimulating hormone, can alter Sertoli cell number in the neonatal rat. J Endocrinol 184:107–117 [DOI] [PubMed] [Google Scholar]
- Mu X, Liu K, Kleymenova E, Sar M, Young SS, Gaido KW 2006 Gene expression profiling of androgen receptor antagonists in the rat fetal testis reveals few common gene targets. J Biochem Mol Toxicol 20:7–17 [DOI] [PubMed] [Google Scholar]
- Tan KA, De Gendt K, Atanassova N, Walker M, Sharpe RM, Saunders PT, Denolet E, Verhoeven G 2005 The role of androgens in Sertoli cell proliferation and functional maturation: studies in mice with total or Sertoli cell-selective ablation of the androgen receptor. Endocrinology 146:2674–2683 [DOI] [PubMed] [Google Scholar]
- Weinbauer GF, Nieschlag E 1993 Hormonal control of spermatogenesis. In: de Kretser D, ed. Molecular biology of the male reproductive system. San Diego: Academic Press Inc.; 101–142 [Google Scholar]
- Singh J, O'Neill C, Handelsman DJ 1995 Induction of spermatogenesis by androgens in gonadotropin-deficient (hpg) mice. Endocrinology 136:5311–5321 [DOI] [PubMed] [Google Scholar]
- Singh J, Handelsman DJ 1996 The effects of recombinant FSH on testosterone-induced spermatogenesis in gonadotrophin-deficient (hpg) mice. J Androl 17:382–393 [PubMed] [Google Scholar]
- Haywood M, Spaliviero J, Jimemez M, King NJ, Handelsman DJ, Allan CM 2003 Sertoli and germ cell development in hypogonadal (hpg) mice expressing transgenic follicle-stimulating hormone alone or in combination with testosterone. Endocrinology 144:509–517 [DOI] [PubMed] [Google Scholar]
- Ghosh S, Sinha-Hikim AP, Russell LD 1991 Further observations of stage-specific effects seen after short-term hypophysectomy in the rat. Tissue Cell 23:613–630 [DOI] [PubMed] [Google Scholar]
- Kerr JB, Maddocks S, Sharpe RM 1992 Testosterone and FSH have independent, synergistic and stage-dependent effects upon spermatogenesis in the rat testis. Cell Tissue Res 268:179–189 [DOI] [PubMed] [Google Scholar]
- Franca LR, Parreira GG, Gates RJ, Russell LD 1998 Hormonal regulation of spermatogenesis in the hypophysectomized rat: quantitation of germ-cell population and effect of elimination of residual testosterone after long-term hypophysectomy. J Androl 19:335–340; discussion, 341–332 [PubMed] [Google Scholar]
- Hannema SE, Scott IS, Rajpert-De Meyts E, Skakkebaek NE, Coleman N, Hughes IA 2006 Testicular development in the complete androgen insensitivity syndrome. J Pathol 208:518–527 [DOI] [PubMed] [Google Scholar]
- Denolet E, De Gendt K, Allemeersch J, Engelen K, Marchal K, Van Hummelen P, Tan KA, Sharpe RM, Saunders PT, Swinnen JV, Verhoeven G 2006 The effect of a Sertoli cell-selective knockout of the androgen receptor on testicular gene expression in prepubertal mice. Mol Endocrinol 20:321–334 [DOI] [PubMed] [Google Scholar]
- Eacker SM, Shima JE, Connolly CM, Sharma M, Holdcraft RW, Griswold MD, Braun RE 2007 Transcriptional profiling of androgen receptor (AR) mutants suggests instructive and permissive roles of AR signaling in germ cell development. Mol Endocrinol 21:895–907 [DOI] [PubMed] [Google Scholar]
- McLachlan RI, Wreford NG, Meachem SJ, De Kretser DM, Robertson DM 1994 Effects of testosterone on spermatogenic cell populations in the adult rat. Biol Reprod 51:945–955 [DOI] [PubMed] [Google Scholar]
- O'Donnell L, McLachlan RI, Wreford NG, Robertson DM 1994 Testosterone promotes the conversion of round spermatids between stages VII and VIII of the rat spermatogenic cycle. Endocrinology 135:2608–2614 [DOI] [PubMed] [Google Scholar]
- Hales DB, Sha LL, Payne AH 1987 Testosterone inhibits cAMP-induced de novo synthesis of Leydig cell cytochrome P-450(17 α) by an androgen receptor-mediated mechanism. J Biol Chem 262:11200–11206 [PubMed] [Google Scholar]
- Lyon MF, Glenister PH, Lamoreux ML 1975 Normal spermatozoa from androgen-resistant germ cells of chimaeric mice and the role of androgen in spermatogenesis. Nature 258:620–622 [DOI] [PubMed] [Google Scholar]
- Johnston DS, Russell LD, Friel PJ, Griswold MD 2001 Murine germ cells do not require functional androgen receptors to complete spermatogenesis following spermatogonial stem cell transplantation. Endocrinology 142:2405–2408 [DOI] [PubMed] [Google Scholar]
- Justrabo E, Cabanne F, Michiels R, Bastien H, Dusserre P, Pansiot F, Cayot F 1978 A complete form of testicular feminisation syndrome; a light and electron microscopy study. J Pathol 126:165–171 [DOI] [PubMed] [Google Scholar]
- Rutgers JL, Scully RE 1991 The androgen insensitivity syndrome (testicular feminization): a clinicopathologic study of 43 cases. Int J Gynecol Pathol 10:126–144 [DOI] [PubMed] [Google Scholar]
- Shamblott MJ, Axelman J, Wang S, Bugg EM, Littlefield JW, Donovan PJ, Blumenthal PD, Huggins GR, Gearhart JD 1998 Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci USA 95:13726–13731 [DOI] [PMC free article] [PubMed] [Google Scholar]