Abstract
Purpose
To evaluate the in vivo efficacy of curcumin as an inhibitor of the multidrug-resistance-linked ATP Binding Cassette (ABC) drug transporter, ABCG2.
Methods
Photoaffinity labeling with [125I]-iodoarylazidoprazosin was used to characterize the interaction of sulfasalazine, a substrate of the mouse ABCG2, with human ABCG2. In addition, the inhibitory effect of curcumin on ABCG2 was evaluated in brain capillaries from rats. Furthermore, the effect of curcumin on absorption of orally administered sulfasalazine in wild-type and abcg2-/- mice was also determined.
Results
Sulfasalazine interacted at the drug-substrate site(s) of human ABCG2. Curcumin inhibited ABCG2 activity at nanomolar concentrations at the rat blood-brain barrier in the ex vivo assay. Based on studies in wild type and abcg2-/- mice, we observed that oral curcumin increased Cmax and relative bioavailability of sulfasalazine by selectively inhibiting ABCG2 function.
Conclusions
This study validates our previous in vitro results with human ABCG2 (Mol. Cancer Ther. 2006; 5:1995-2006) and provides the first in vivo evidence for the inhibition by curcumin of ABCG2-mediated efflux of sulfasalazine in mice. Based on these studies, we propose that non-toxic concentrations of curcumin may be used to enhance drug exposure when the rate-limiting step of drug absorption and/or tissue distribution is impacted by ABCG2.
Keywords: ABC transporter, multidrug resistance, ABCG2, curcumin, sulfasalazine
Introduction
Curcumin (Diferuoyl methane) is a naturally occurring polyphenol found in the rhizomes of Curcuma longa (turmeric). Curcumin is known for its antitumor, antioxidant, antiarthritic, anti-amyloid and anti-inflammatory properties (1-3). It has been found to suppress, retard, and even reverse cancer development at each stage of the disease (4). The anticancer properties of curcumin have been primarily attributed to its activity to block nuclear factor-kappa B (NF-kappa B) which regulates inflammation, cell proliferation and apoptosis in normal cells (5-7).
ATP-binding cassette (ABC) transporters belong to a superfamily which transports a wide variety of substrates across extra- and intracellular membranes, including ions, sugars, metabolic products, lipids, sterols, toxins and drugs (8). Some of the ABC transporters play a crucial role in the development of multidrug resistance (MDR), as patients that are undergoing chemotherapy can eventually develop resistance not only to the anticancer drug they are taking but also to several other types of drugs (9). P-glycoprotein (P-gp), breast cancer resistance protein (BCRP or ABCG2), and multidrug resistance protein (MRP1) are the major ABC drug transporters that have been linked with MDR (9).
In addition to conferring MDR in tumor cells, in normal physiology ABC transporters limit the absorption of many drugs from the intestine, and pump drugs from the liver cells into the bile as a means of removing foreign substances from the body (10). In this regard, a large number of drugs are substrates that are themselves transported by ABC transporters or that affect the transport of other therapeutic drugs thereby altering the bioavailability of these drugs.
We have recently shown that curcumin inhibits the function of three major ABC drug transporters (P-gp, ABCG2 and MRP1) and curcumin I was most effective in interacting with ABCG2 (11-14). Based on our in vitro data, we proposed that curcumin can prevent chemotherapeutic drug resistance mediated by these transporters and in addition, can also improve the systemic availability of the cancer drugs that have limited intestinal absorption due to active efflux by these transporters. Thus, the aim of the present study was to demonstrate the ability of curcumin to inhibit one of the above ABC drug transporters, ABCG2 in an in vivo system. In our experimental approach, we used brain capillaries from rats to assess the inhibitory effect of curcumin on the efflux of bodipy® FL prazosin ex vivo and also performed pharmacokinetic studies in mice using the ABCG2 specific substrate sulfasalazine (SASP) (15) to study the modulatory effect of curcumin on ABCG2 efflux activity in vivo. The data presented here strongly suggest that curcumin at clinically achievable non-toxic concentrations could be used to increase the bioavailability of ABCG2 substrates.
Materials and Methods
Chemicals
Curcumin was from Sigma Aldrich (St. Louis, MO). BODIPY® FL prazosin and FTC were from Molecular Probes (Eugene, OR, USA). Sulfasalazine (SASP; PNU-133) was from Pfizer Global research and Development (Kalamazoo, MI). [125I]-iodoarylazidoprazosin (IAAP) (2200 Ci/mM) was purchased from Perkin Elmer Life Sciences (Wellesley, MA). All other chemicals were of highest grade and were purchased from Sigma (St. Louis, MO, USA).
Cells
Control MCF7 and MCF7FLV1000 (482R) cells overexpressing ABCG2 were cultured in RPMI with 10% FBS in the absence or presence of 1 μg/ml flavopiridol, respectively (16, 17). These cell lines were provided by Dr. Susan Bates (NCI/NIH).
Isolation of crude membranes from ABCG2-expressing HEK 293 or MCF7 cells
Crude membranes from MCF7 and MCF7FLV1000 cells were prepared as described elsewhere (11, 18).
Animals
Male Sprague-Dawley rats (for brain capillary assays), male outbred mdr1aPGP (mdr1a-/-) and Crl:CF-1 WT mice (for SASP pharmacokinetic studies) were purchased from Charles River Laboratories (Wilmington, MA). Bcrp1-/- (FVB.129S6-Abcg2tm1Ahs) and WT (FVB) mice (for SASP pharmacokinetic studies) were obtained from Taconic Laboratories (Germantown, NY). Animals were housed and handled according to Pfizer Global Research & Development and University of Minnesota guidelines complying with the U.S. Public Health Service Policy for the Care and Use of Laboratory Animals and were kept under controlled environmental conditions (23°C, 35% relative humidity, 12 hour dark-light cycle) with free access to tap water and standard rodent chow. Before using animals for experiments they were allowed to adapt to the animal facility for at least five days after transportation to the facility. Animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Minnesota and were in accordance with AAALAC regulations and NIH animal guidelines.
Photoaffinity labeling of ABCG2 with [125I]-Iodoarylazidoprazosin (IAAP)
Crude membranes (1 mg protein/ml) from ABCG2-expressing MCF-7 FLV1000 cells were incubated with 0-100 μM SASP for 10 min at 21-23°C in 50 mM Tris-HCl, pH 7.5. 3-6 nM [125I]-IAAP (2200 Ci/mmole) (Perkin Elmer Life Sciences, Wellesley, MA) was added and incubated for an additional 5 min under subdued light. The samples were illuminated with a UV lamp (365 nm) for 10 min at room temperature. The labeled ABCG2 was immunoprecipitated as described previously (19). Samples were separated on a 7% Tris-acetate gel at constant voltage and gels were dried and exposed to X-ray film for 12-24 h at -80°C. The incorporation of [125I]-IAAP into the ABCG2 or P-gp band was quantified using the STORM 860 phosphor imager system (Molecular Dynamics, Sunnyvale, CA, USA) and the software ImageQuaNT, as described (20).
Isolation of brain capillaries
Brain capillaries from rats were isolated as described previously (21-23). For each preparation, 10 rats were euthanized by CO2 inhalation and decapitated. Brains were dissected and homogenized in PBS buffer (2.7 mM KCl, 1.46 mM KH2PO4, 136.9 mM NaCl, and 8.1 mM Na2HPO4 supplemented with 5 mM d-glucose and 1 mM sodium pyruvate, pH 7.4). After the addition of Ficoll (final concentration 15%), the homogenate was centrifuged at 5800×g for 20 min at 4°C. The pellet was resuspended in PBS containing 1% BSA and passed over a glass bead column. Capillaries adhering to the glass beads were collected by gentle agitation in PBS (1% BSA), washed with PBS, and then used for transport experiments.
Bodipy® FL prazosin transport assay in rat brain capillaries
Transport assays using freshly isolated, functionally intact rat brain capillaries were done as reported previously (24, 25) with minor modifications. Briefly, to measure ABCG2 transport function, isolated brain capillaries were incubated for 1 h at room temperature with 2 μM BODIPY® FL prazosin. For each treatment, images of 10 capillaries were acquired by confocal microscopy (Nikon C1 laser scanning confocal microscope unit, Nikon TE2000 inverted microscope, 40x oil immersion objective, numerical aperture: 1.3, 488 nm line of a Spectra Physics argon laser (model 163C), 515/30nm band pass filter; Nikon Instruments Inc., Melville, NY, USA). Images were analyzed by measuring fluorescence intensity of BODIPY® FL prazosin in the capillary lumen using Image J software (NIH, Bethesda, MD, USA) as described previously (25, 26).
Statistical analysis for bodipy® FL prazosin transport assays
Data are presented as mean ± SEM. Two-tailed unpaired Student’s t test was used to evaluate differences between controls and treated groups; differences were considered to be statistically significant when P < 0.05. Sigmoidal dose-response curves and IC50 values were calculated using GraphPad Prism Software Version 4.01 (GraphPad Software, Inc., La Jolla, CA, USA).
Pharmacokinetic experiments with wild-type, abcg2-/- and abcb1a (mdr1a-/-) mice
Curcumin or an equivalent volume of vehicle (in 0.5% methylcellulose in sterile phosphate buffer saline) was administered by oral gavage at a dose of 40 mg/kg or 400 mg/kg followed by administration of SASP after 1 h to groups of mice at a dose of 20 mg/kg of body weight as previously described (15). Using the administration of SASP as time zero, mice were anesthetized with isoflurane at pre-determined time points, blood samples obtained by cardiac puncture and transferred to EDTA tubes. The samples were centrifuged immediately at 3000 ×g for 15 min, and plasma was collected and stored at -80°C until the time of LC-MS/MS analysis.
Detection of sulfasalazine using LC-MS/MS and Pharmacokinetic calculations
LC-MS/MS analysis was carried out using a high-performance liquid chromatography system consisting of a Shimadzu binary pump with CTC PAL autosampler interfaced to an API 4000 SCIEX triple quadrupole tandem mass spectrometer (Applied Biosystems, Foster City, CA) as described earlier (15). Area under the concentration-time curve (AUC) from time zero to the last sampling time was calculated by the linear trapezoidal rule using non compartmental analysis of average plasma concentration in WinNonlin v5.1(Pharsight Corporation, Mountain View, CA 94041). Relative SASP exposures were estimated as the ratio of AUC in wild-type mice in the presence of curcumin versus wild-type mice in the absence of curcumin for each dose level examined in this study. Two Way ANOVA was used to evaluate the influence of curcumin on SASP Cmax in wild type, abcg2-/- and abcb1a (mdr1a-/-) mice (GraphPad Software, La Jolla, CA).
Results
Curcumin inhibits ABCG2 activity at the blood-brain barrier
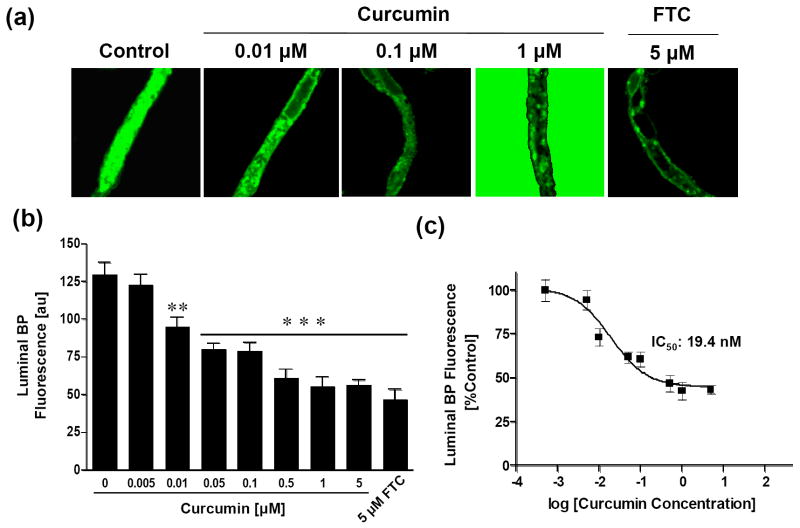
In our previous study using cell lines overexpressing ABCG2, we showed that curcumin inhibited the activity of ABCG2 in in vitro assays (13). The objective of the present study was to demonstrate the inhibitory activity of curcumin on ABCG2 function under normal physiological conditions in an in vivo system. To demonstrate this in a model close to the in vivo situation, we used freshly isolated, functionally active rat brain capillaries. Isolated brain capillaries are an established ex vivo model for the blood-brain barrier, a tissue well-known to express ABCG2 (25, 27, 28). At the blood-brain barrier, ABCG2 is localized to the luminal membrane, where it protects the brain by mediating substrate transport from brain-to-blood. Accordingly, in control capillaries that were incubated with fluorescent BODIPY® FL prazosin, an ABCG2 substrate, there was high fluorescence in the capillary lumen (Figure. 1a, left image). With our imaging conditions, no BODIPY® FL prazosin fluorescence was visible in the bath containing 2 μM BODIPY® FL prazosin. Fluorescence was low in the capillary endothelium and high in the capillary lumen indicating an active transport process. Quantitation of steady-state luminal BODIPY® FL prazosin accumulation showed that 5 μM fumitremorgin C (FTC), a known specific inhibitor of ABCG2 activity, reduced luminal fluorescence to approximately 50% of control capillaries (Figure 1a, right panel and Figure 1b). In capillaries treated with different concentrations of curcumin, luminal BODIPY® FL prazosin fluorescence was reduced in a dose-dependent manner (Figures 1a and b). Inhibition of ABCG2 activity by curcumin was concentration-dependent with an IC50 value of 19.4 nM (Figure 1c). Maximal inhibition of ABCG2 activity by 1-5 μM was comparable to the inhibition observed with 5 μM FTC (Figures 1a and b). Thus, the above findings suggest that the in vitro inhibitory activity of curcumin on ABCG2 could also be observed with in vivo models where the transporter is present at normal physiological levels.
Figure 1. Curcumin inhibits the activity of ABCG2 in rat brain capillaries.
(a) Isolated, functionally intact rat brain capillaries were incubated with 2 μM BODIPY® FL prazosin in the absence (control) or presence of varying concentrations of curcumin (middle three panels) and 5 μM FTC at room temperature for 1h and the images were acquired as described in ‘Materials and methods’. Representative images from a single experiment from three individual experiments are shown here. (b) Luminal BODIPY® FL prazosin fluorescence in capillaries in the presence and absence of varying concentrations of curcumin was quantitated as described in ‘Materials and Methods’ using Image J software and plotted as a function of curcumin concentrations. Statistical comparison: **, significantly lower than control capillaries, P < 0.01; ***, significantly lower than control capillaries, P < 0.001. (c) Non-linear regression curve; data are plotted on a logarithmic scale. Each data point represents the luminal BODIPY® FL prazosin fluorescence mean value from 10 capillaries (pooled capillary tissue from 10 rats of a single preparation); variability is given by SEM bars. Units are arbitrary fluorescence units (scale 0-255).
SASP, a specific substrate of murine ABCG2, also interacts with human ABCG2
Due to high presystemic clearance, it is known that curcumin has a low bioavailability (29) and therefore it is possible that when given orally, curcumin may not be an effective systemic inhibitor of ABC transporters throughout the body. In this regards, we and other groups have shown that the absorption and elimination of the sulfa drug, SASP in the mouse is determined by ABCG2 as it is a substrate of this transporter (15, 30). To further investigate the mechanism by which SASP may interact with human ABCG2, we monitored its effect on the photolabeling of ABCG2 with the known photoaffinity substrate, [125I]-Iodoarylazidoprazosin (IAAP) (19). As shown in Figure 2, SASP inhibited the binding of IAAP to ABCG2 in a concentration-dependent manner with an IC50 value of 1.02 μM. This confirmed that besides interacting with murine ABCG2, SASP also interacts with human ABCG2 at the substrate binding sites. In previous reports, we have shown that curcumin also interacts at the substrate binding pocket of ABCG2. Therefore it can probably be used as a competitive inhibitor to prevent SASP interactions with ABCG2, which was the basis for conducting the following in vivo experiments.
Figure 2. Sulfasalazine inhibits the photolabeling of ABCG2 with [125I]-IAAP binding.
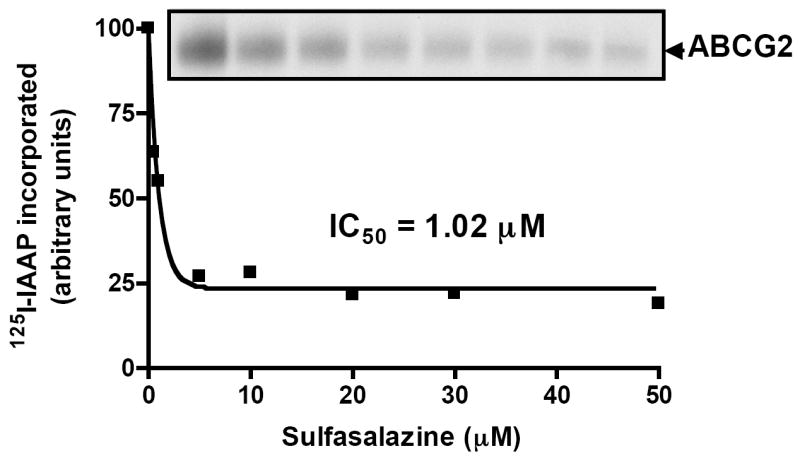
Crude membranes (500 μg/ml) from MCF-7 FLV1000 cells were incubated with 0–50 μM of SASP for 5 min at 21–23°C in 50 mM Tris-HCl, pH 7.5. 3–6 nM [125I]-IAAP (2200 Ci/mmole) was added and incubated for an additional 5 min under subdued light. The samples were then illuminated with a UV lamp (365 nm) for 10 min and were processed as described in ‘Materials and Methods’. A representative autoradiogram from one experiment is shown and similar results were obtained in two additional experiments. The arrow shows the position of the ABCG2 band. The incorporation of [125I]-IAAP (from autoradiogram, Y-axis) into the ABCG2 (■) band was quantified by estimating the radioactivity of this band plotted as a concentration of SASP using the software GRAPHPAD PRISM 2.0, as described previously (19).
Curcumin pretreatment enhances sulfasalazine exposure in mice
To investigate the interaction of curcumin with a known probe substrate whose systemic bioavailability is known to be greatly influenced by ABCG2 (15, 30, 31), we hypothesized that SASP absorption in the intestine could be enhanced through inhibition of the binding of SASP to mouse ABCG2. Briefly described, male FVB-WT mice were pretreated with a single oral gavage of curcumin (40 mg/kg or 400 mg/kg) 1 h prior to a single oral dose of SASP (20 mg/kg) whereby the plasma concentration of SASP was determined as a function of time in mice treated with SASP alone or a combination of curcumin and SASP. While no effect was observed when mice were pretreated with curcumin 40 mg/kg (data not shown), we report that the plasma concentration of SASP increased in the presence of curcumin (400 mg/kg) at all time points, suggesting that curcumin increased the relative bioavailability of SASP in the plasma probably by inhibiting intestinal ABCG2 (Figure 3). It should be noted that at 24 h, SASP concentrations in control mice were below the limits of quantification (1 ng/mL), however, these samples were within the lower limit of detection (0.5 ng/mL). It is important to note that SASP plasma levels were still high in curcumin-treated mice after 24 h. Additional pharmacokinetic parameters were also moderate to significantly altered in the curcumin-treated mice; these results are summarized in Table I. Co-administration of curcumin decreased the apparent oral clearance by ~10-fold (data not shown). Further, Tmax (the time to achieve maximal plasma concentration) of SASP was increased from 0.5 to 2 h, while the apparent SASP plasma half-life was increased from 1.08 to 5.02 h (data not shown). The area under concentration-time curve (AUC) of SASP in the curcumin-treated mice increased to 5-fold after 4 h and 13-fold after 24 h when compared to that of vehicle treated mice. In addition, 400 mg/kg curcumin treatment resulted in an increase in the maximal SASP plasma levels (Cmax) of SASP from 1230 ng/ml (vehicle) to 3350 ng/ml (curcumin) in these mice. Taken together, the above results suggest that oral administration of curcumin enhanced the intestinal absorption of SASP in mice thereby increasing the apparent bioavailability of this ABCG2-specific substrate in these animals.
Figure 3. Curcumin enhances plasma concentration of sulfasalazine in mice.
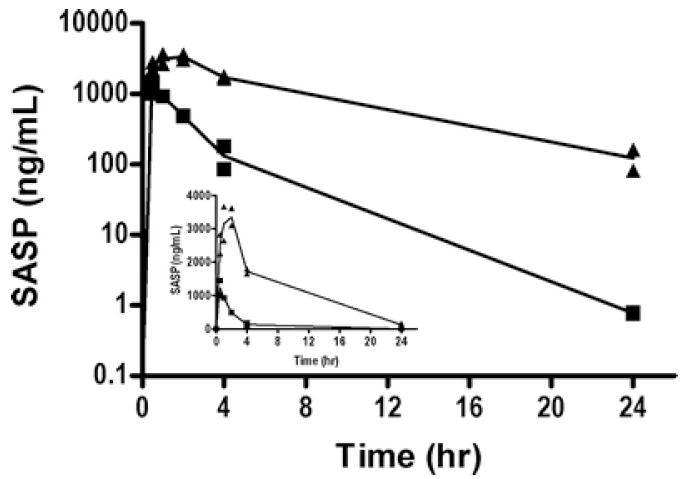
Wild-type animals were pretreated with a single oral dose of curcumin (400 mg/kg) or vehicle for 1 hr and all animals received a single oral dose of SASP (20 mg/kg). Line represents the average (N=2/time point). Blood collected by cardiac puncture and the concentration of SASP was detected by LC-MS/MS as described in ‘Materials and Methods’. The main graph represents the log plasma concentration of SASP (Y-axis) with respect to time (X-axis) in vehicle treated (■) or curcumin and SASP (▲) treated mice. Inset shows the SASP concentrations on Y-axis in linear scale.
Table I.
Effect of curcumin on sulfasalazine, maximal plasma concentration (Cmax), Tmax and area under concentration-time curve (AUC) in mice.
| Curcumin PO Dose (mg/kg) | Cmax (ng/ml) |
Tmax (hr) |
Interval (hr) | AUC (ng.hr/ml) |
Relative exposure AUCC+SASP/AUCSASP | |||
|---|---|---|---|---|---|---|---|---|
| SASP | C+SASP | SASP | C+SASP | SASP | C+SASP | |||
| 400 | 1230 | 3350 | 0.5 | 2 | 0-4 | 2170 | 10380 | 5.0 |
| 0-24 | 2170* | 28802 | 13* | |||||
AUC 0-24 C + SASP /AUC0-24 SASP
Curcumin does not affect the plasma concentration of sulfasalazine in abcg2 knock-out mice
To further rule out the possibility of the other major ABC drug transporter, Pgp’s role in increasing the absorption of SASP, we did similar studies in wild-type, abcb1a-/-(32) and abcg2-/- mice (33). All mice received a single oral dose of vehicle or curcumin (400 mg/kg) followed by with a single oral dose of SASP (20 mg/kg) after 1 h. 90 minutes post SASP dosing, the animals were sacrificed and plasma collected for the analysis of SASP. As shown in Figure 4, the plasma concentrations of unchanged SASP in wild-type mice were increased from approximately 540 ng/ml (wild-type control) to approximately 2070 ng/ml (wild-type mice treated with curcumin) (figure 4). While abcb1a-/- mice showed similar levels of unchanged SASP with and without curcumin to wild-type mice, the abcg2-/- mice had 12350 ng/ml of SASP which was ~10-20 fold higher than the abcb1a-/- and wild-type groups (Figure 4). The presence of curcumin (400 ng/ml) did increase the plasma concentration of SASP 3-4 fold in both wild-type and abcb1a-/- mice, however, there were no significant effect on the levels of SASP in abcg2-/-mice. Two-Way ANOVA using the Bonferroni post-test was used to determine the influence of curcumin inhibition (present/absent) with respect to the influence of genotype (WT, abcb1a, and abcg2) on SASP Cmax (thus 3 × two factors). Collectively, these data suggest that the observed effects of curcumin on the plasma levels of SASP were primarily mediated by inhibition of ABCG2 function, which is consistent with our current and published observation that SASP is a specific substrate of ABCG2 (Figure 4 and (15, 30, 31)).
Figure 4. Effect of curcumin on the plasma concentration of sulfasalazine in abcb1a, and abcg2 knock-out mice.
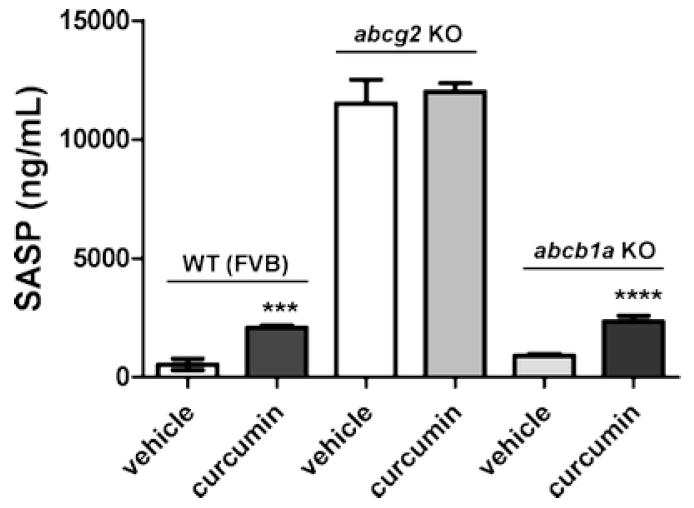
All animals were treated with a single oral dose of curcumin (400 mg/kg) for 1 hour followed by a single oral dose of sulfasalazine (20 mg/kg). At a pre-determined time point (90 minutes), mice were anesthetized with isoflurane, and blood samples were obtained by cardiac puncture and transferred to heparinized tubes. The samples were centrifuged immediately at 3000 ×g for 15 min, and plasma was collected and stored at -80°C until the time of LC-MS/MS analysis. N=5/treatment. Data reported as mean ± SD. Two way ANOVA was used to compare the effect of curcumin versus vehicle on SASP absorption in wild type (FVB), abcg2-/- (abcg2 KO), and abcb1a-/- (mdr1a KO) mice. Using Bonferroni’s post-hoc test, the effect of curcumin pretreatment significantly increased SASP concentration in the wild-type (P<0.001) and mdr1a (abcb1a-/-) (P<0.001) mice. However, there was no significant increase in SASP exposure in abcg2-/-mice after curcumin pretreatment (P>0.05). As previously reported, abcg2 genotype significantly influences SASP Cmax (P<0.0001) (15).
Discussion
Oral administration of anticancer drugs is a convenient mode of drug delivery because of the simplicity in taking the drug, its potential use on an outpatient basis, and for development of chronic treatment schedules. However, the therapeutic use of orally administered drugs is frequently limited by poor and/or highly variable bioavailability. ABC drug transporters may restrict the oral bioavailability of their substrates by inhibiting the intestinal absorption of orally administered drugs (34). It has been shown by several groups that the oral bioavailability of certain anticancer drugs can be increased by inhibiting these ABC drug transporters in the intestine, which leads to increased drug response and thus, improved therapeutic outcome (35-37).
We showed previously that curcumin, a natural polyphenolic compound can be used to inhibit major ABC drug transporters such as P-gp, ABCG2 and MRP1 which play a major role in the development of MDR (11-13). Since curcumin is a relatively non-toxic nutraceutical with anticancer properties, we proposed that it could be used to increase the therapeutic efficacy of chemotherapy in MDR cancer cells by inhibiting the ABC drug transporters. In addition, it could also be used to increase the oral bioavailability of drugs that are substrates of ABC drug transporters.
Both P-gp and ABCG2 are known to be expressed on the apical membranes of brain capillary endothelial cells (38). We therefore used this system to demonstrate the in vivo efficacy of curcumin in inhibiting ABCG2 at the blood brain barrier of rats. Curcumin was effective in inhibiting the ABCG2-mediated efflux of BODIPY® FL prazosin with an IC50 of 0.019 μM. The IC50 value of curcumin in these ex vivo assays for inhibiting ABCG2 activity is different from our previous results where we reported that curcumin inhibited ABCG2 activity in in vitro cultured cell lines with an IC50 of 1-2 μM (13). These differences in the IC50 values could be due to differences in the ABCG2 expression levels and altered substrate specificities between species. In addition, the brain capillaries used in this study have normal physiological levels of rat ABCG2, while in our previous study, we used either drug-selected or transfected cell lines that overexpress human ABCG2 at high levels. Although FTC inhibited 50% of the efflux of Bodipyprazosin in the brain capillaries, we did observe a residual fluorescence in these cells. This may be due to passive diffusion, non specific binding, presence of secondary transporters responsible for the uptake of the fluorescent compound or other active ABC drug transporters at the blood brain barrier which were not inhibited by FTC (24, 26).
In this study, we also assessed the effect of curcumin on the oral absorption of the ABCG2-specific substrate SASP in mice. Our data suggest that pretreatment of mice with a single oral dose of curcumin resulted in a 2-fold higher plasma level of unchanged SASP in both wild-type and abcb1-/- mice. Our observation is also in agreement with previously published results indicating a minor role for P-gp in SASP availability after oral intake (15). Moreover, pretreatment of abcg2-/- mice with curcumin resulted in similar plasma concentrations of SASP in both treatment groups, also supporting a lesser role of P-gp in SASP oral absorption. Taken together, our data strongly support an important role of abcg2 in sulfasalazine oral absorption. We have previously shown that abcg2 not only limits the oral absorption of SASP in mice (15) but also influences the distribution and elimination processes of this drug in the same animals (14). Therefore, it is not surprising that our current data shows a decrease in oral clearance and volume of distribution and an increase in the half-life of SASP in the wild-type mice treated with curcumin.
This indicates that our in vitro results can be translated to in vivo systems in which, curcumin enhances the oral bioavailability of substrates that otherwise are hampered by active efflux by ABC drug transporters in the intestine. Although clinical trials in humans indicated that the systemic bioavailability of orally administered curcumin is relatively low (29), but it has been shown that curcumin formulation with phosphatidylcholine is 5-fold more bioavailable than normal curcumin (39). In addition, it has recently been shown that oral administration of 8 g curcumin daily up to 18 months is well tolerated and despite its limited bioavailability, also inhibited progression of pancreatic cancer (40). Several other studies have shown that curcumin bioavailability can be enhanced considerably by using different approaches such as using adjuvants, making nanoparticles, liposomal formulation or making derivatives (reviewed in (41)).
Although curcumin is known to posses anticancer properties by virtue of its ability to inhibit key targets in cancer cells, this study provides additional in vivo evidence supporting its effectiveness in inhibiting ABC drug transporters. Both experiments using brain capillaries ex vivo assays and studies with mice in vivo showed convincingly that curcumin can enhance the drug (SASP) accumulation by inhibiting ABCG2 thereby increasing its intracellular concentrations.
Taken together, these non-clinical observations may be potentially clinically translatable with respect to curcumin administered orally in combination with other anticancer agents in cancer chemotherapy and hence could enhance the effectiveness of the drug treatment. The concept of using combination therapy to treat drug-resistant cancer or to improve the oral availability of certain drugs is not new and several inhibitors have been generated in the last ten years (reviewed in (42)). However, the usefulness of such inhibitors to overcome MDR is still controversial and perhaps limited by the inhibition of ABCB1 in a non-tumor selective manor. It is noteworthy to recognize that a large number of these inhibitors are natural products or their purified constituents derived from varying sources ranging from fungi to plants and vegetables (42). Therefore, these natural inhibitors are expected to be much less toxic to the host than synthetic compounds. The advantage of using curcuminoids as inhibitors lies in their relatively large therapeutic-index compared to most of the other third generation MDR inhibitors. For example, Ko143 and GF120918, which are well characterized and potent inhibitors of ABCG2 and ABCB1, have an IC50 value of 19 and 18 μM for MCF-7 cells (43), respectively, while curcuminoids have IC50 values in the range of 25 – 50 μM for the same cells (13). Therefore, curcumin or its formulation with improved bioavailability such as curcumin encapsulated into liposomes may represent an ideal lead compound that could be developed as a broad spectrum inhibitor of ABC drug transporters.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research. We thank Dr. Krishnamachary Nandigama for providing ABCG2-expressing Hi-five insect cell crude membranes and George Leiman for editorial assistance. The authors wish to acknowledge the contribution of Joe Palandra of Pfizer Global Research and Development for his assistance in determination of SASP bioanalysis and for the input of Lisa Bernstein, Non-clinical Biostatistics, Genentech, Inc.
Abbreviations
- ABC
ATP Binding Cassette
- AUC
area under concentration-time curve
- BBB
blood-brain barrier
- IAAP
Iodoarylazidoprazosin
- MDR
Multidrug resistance
- P-gp
P-glycoprotein
- SASP
sulfasalazine
References
- 1.Shishodia S, Chaturvedi MM, Aggarwal BB. Role of Curcumin in Cancer Therapy. Current Problems in Cancer. 2007;31:243–305. doi: 10.1016/j.currproblcancer.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwaland BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Choi H, Chun Y-S, Kim S-W, Kim M-S, Park J-W. Curcumin Inhibits Hypoxia-Inducible Factor-1 by Degrading Aryl Hydrocarbon Receptor Nuclear Translocator: A Mechanism of Tumor Growth Inhibition. Mol Pharmacol. 2006;70:1664–1671. doi: 10.1124/mol.106.025817. [DOI] [PubMed] [Google Scholar]
- 4.Duvoix A, Blasius R, Delhalle S, Schnekenburger M, Morceau F, Henry E, Dicato M, Diederich M. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005;223:181–90. doi: 10.1016/j.canlet.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–98. [PubMed] [Google Scholar]
- 6.Aggarwal BB, Shishodia S, Takada Y, Banerjee S, Newman RA, Bueso-Ramos CE, Price JE. Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin Cancer Res. 2005;11:7490–8. doi: 10.1158/1078-0432.CCR-05-1192. [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol Pharmacol. 2006;69:195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- 8.Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE, Gottesman MM. P-glycoprotein: from genomics to mechanism. Oncogene. 2003;22:7468–85. doi: 10.1038/sj.onc.1206948. [DOI] [PubMed] [Google Scholar]
- 9.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 10.Velamakanni S, Wei S, Janvilisri T, van Veen H. ABCG transporters: structure, substrate specificities and physiological roles. JBioenerg Biomembr. 2007;39:465–471. doi: 10.1007/s10863-007-9122-x. [DOI] [PubMed] [Google Scholar]
- 11.Chearwae W, Anuchapreeda S, Nandigama K, Ambudkar SV, Limtrakul P. Biochemical mechanism of modulation of human P-glycoprotein (ABCB1) by curcumin I, II, and III purified from Turmeric powder. Biochem Pharmacol. 2004;68:2043–52. doi: 10.1016/j.bcp.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Chearwae W, Wu CP, Chu HY, Lee TR, Ambudkar SV, Limtrakul P. Curcuminoids purified from turmeric powder modulate the function of human multidrug resistance protein 1 (ABCC1) Cancer Chemother Pharmacol. 2005;57:376–388. doi: 10.1007/s00280-005-0052-1. [DOI] [PubMed] [Google Scholar]
- 13.Chearwae W, Shukla S, Limtrakul P, Ambudkar SV. Modulation of the function of the multidrug resistance-linked ATP-binding cassette transporter ABCG2 by the cancer chemopreventive agent curcumin. Mol Cancer Ther. 2006;5:1995–2006. doi: 10.1158/1535-7163.MCT-06-0087. [DOI] [PubMed] [Google Scholar]
- 14.Limtrakul P, Chearwae W, Shukla S, Phisalphong C, Ambudkar S. Modulation of function of three ABC drug transporters, P-glycoprotein (ABCB1), mitoxantrone resistance protein (ABCG2) and multidrug resistance protein 1 (ABCC1) by tetrahydrocurcumin, a major metabolite of curcumin. Mol Cell Biochem. 2007;296:85–95. doi: 10.1007/s11010-006-9302-8. [DOI] [PubMed] [Google Scholar]
- 15.Zaher H, Khan AA, Palandra J, Brayman TG, Yu L, Ware JA. Breast cancer resistance protein (Bcrp/abcg2) is a major determinant of sulfasalazine absorption and elimination in the mouse. Mol Pharm. 2006;3:55–61. doi: 10.1021/mp050113v. [DOI] [PubMed] [Google Scholar]
- 16.Robey RW, Medina-Perez WY, Nishiyama K, Lahusen T, Miyake K, Litman T, Senderowicz AM, Ross DD, Bates SE. Overexpression of the ATP-binding cassette half-transporter, ABCG2 (Mxr/BCrp/ABCP1), in flavopiridol-resistant human breast cancer cells. Clin Cancer Res. 2001;7:145–52. [PubMed] [Google Scholar]
- 17.Robey RW, Honjo Y, van de Laar A, Miyake K, Regis JT, Litman T, Bates SE. A functional assay for detection of the mitoxantrone resistance protein, MXR (ABCG2) Biochim Biophys Acta. 2001;1512:171–82. doi: 10.1016/s0005-2736(01)00308-x. [DOI] [PubMed] [Google Scholar]
- 18.Ambudkar SV. Drug-stimulatable ATPase activity in crude membranes of human MDR1- transfected mammalian cells. Methods Enzymol. 1998;292:504–14. doi: 10.1016/s0076-6879(98)92039-0. [DOI] [PubMed] [Google Scholar]
- 19.Shukla S, Robey RW, Bates SE, Ambudkar SV. The calcium channel blockers, 1,4-dihydropyridines, are substrates of the multidrug resistance-linked ABC drug transporter, ABCG2. Biochemistry. 2006;45:8940–51. doi: 10.1021/bi060552f. [DOI] [PubMed] [Google Scholar]
- 20.Sauna ZE, Peng XH, Nandigama K, Tekle S, Ambudkar SV. The molecular basis of the action of disulfiram as a modulator of the multidrug resistance-linked ATP binding cassette transporters MDR1 (ABCB1) and MRP1 (ABCC1) Mol Pharmacol. 2004;65:675–84. doi: 10.1124/mol.65.3.675. [DOI] [PubMed] [Google Scholar]
- 21.Bauer B, Hartz AM, Miller DS. Tumor necrosis factor alpha and endothelin-1 increase P-glycoprotein expression and transport activity at the blood-brain barrier. Mol Pharmacol. 2007;71:667–75. doi: 10.1124/mol.106.029512. [DOI] [PubMed] [Google Scholar]
- 22.Bauer B, Yang X, Hartz AM, Olson ER, Zhao R, Kalvass JC, Pollack GM, Miller DS. In vivo activation of human pregnane X receptor tightens the blood-brain barrier to methadone through P-glycoprotein up-regulation. Mol Pharmacol. 2006;70:1212–9. doi: 10.1124/mol.106.023796. [DOI] [PubMed] [Google Scholar]
- 23.Bauer B, Hartz AM, Fricker G, Miller DS. Pregnane X receptor up-regulation of P-glycoprotein expression and transport function at the blood-brain barrier. Mol Pharmacol. 2004;66:413–9. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 24.Hartz AM, Bauer B, Block ML, Hong JS, Miller DS. Diesel exhaust particles induce oxidative stress, proinflammatory signaling, and P-glycoprotein up-regulation at the blood-brain barrier. Faseb J. 2008 doi: 10.1096/fj.08-106997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartz AM, Bauer B, Fricker G, Miller DS. Rapid modulation of P-glycoprotein-mediated transport at the blood-brain barrier by tumor necrosis factor-alpha and lipopolysaccharide. Mol Pharmacol. 2006;69:462–70. doi: 10.1124/mol.105.017954. [DOI] [PubMed] [Google Scholar]
- 26.Bauer B, Hartz AM, Pekcec A, Toellner K, Miller DS, Potschka H. Seizure-induced up-regulation of P-glycoprotein at the blood-brain barrier through glutamate and cyclooxygenase-2 signaling. Mol Pharmacol. 2008;73:1444–53. doi: 10.1124/mol.107.041210. [DOI] [PubMed] [Google Scholar]
- 27.Eisenblatterand T, Galla HJ. A new multidrug resistance protein at the blood-brain barrier. Biochem Biophys Res Commun. 2002;293:1273–8. doi: 10.1016/S0006-291X(02)00376-5. [DOI] [PubMed] [Google Scholar]
- 28.Zhang W, Mojsilovic-Petrovic J, Andrade MF, Zhang H, Ball M, Stanimirovic DB. The expression and functional characterization of ABCG2 in brain endothelial cells and vessels. Faseb J. 2003;17:2085–7. doi: 10.1096/fj.02-1131fje. [DOI] [PubMed] [Google Scholar]
- 29.Sharma RA, Gescher AJ, Steward WP. Curcumin:The story so far. European Journal of Cancer. 2005;41:1955–1968. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Yamasaki Y, Ieiri I, Kusuhara H, Sasaki T, Kimura M, Tabuchi H, Ando Y, Irie S, Ware JA, Nakai Y, Higuchi S, Sugiyama Y. Pharmacogenetic Characterization of Sulfasalazine Disposition Based on NAT2 and ABCG2 (BCRP) Gene Polymorphisms in Humans. Clin Pharmacol Ther. 2008;84:95–103. doi: 10.1038/sj.clpt.6100459. [DOI] [PubMed] [Google Scholar]
- 31.Urquhart BL, Ware JA, Tirona RG, Ho RH, Leake BF, Schwarz UI, Zaher H, Palandra J, Gregor JC, Dresser GK, Kim RB. Breast cancer resistance protein (ABCG2) and drug disposition: intestinal expression, polymorphisms and sulfasalazine as an in vivo probe. Pharmacogenet Genomics. 2008;18:439–48. doi: 10.1097/FPC.0b013e3282f974dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Umbenhauer DR, Lankas GR, Pippert TR, Wise LD, Cartwright ME, Hall SJ, Beare CM. Identification of a P-Glycoprotein-Deficient Subpopulation in the CF-1 Mouse Strain Using a Restriction Fragment Length Polymorphism. Toxicol Appl Pharmacol. 1997;146:88–94. doi: 10.1006/taap.1997.8225. [DOI] [PubMed] [Google Scholar]
- 33.Jonker JW, Buitelaar M, Wagenaar E, Van Der Valk MA, Scheffer GL, Scheper RJ, Plosch T, Kuipers F, Elferink RP, Rosing H, Beijnen JH, Schinkel AH. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc Natl Acad Sci U S A. 2002;99:15649–54. doi: 10.1073/pnas.202607599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan LMS, Lowes S, Hirst BH. The ABCs of drug transport in intestine and liver: efflux proteins limiting drug absorption and bioavailability. Eur J Pharmaceut Sci. 2004;21:25–51. doi: 10.1016/j.ejps.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Jonker JW, Smit JW, Brinkhuis RF, Maliepaard M, Beijnen JH, Schellens JH, Schinkel AH. Role of breast cancer resistance protein in the bioavailability and fetal penetration of topotecan. J Natl Cancer Inst. 2000;92:1651–6. doi: 10.1093/jnci/92.20.1651. [DOI] [PubMed] [Google Scholar]
- 36.Kruijtzer CM, Beijnen JH, Schellens JH. Improvement of oral drug treatment by temporary inhibition of drug transporters and/or cytochrome P450 in the gastrointestinal tract and liver: an overview. Oncologist. 2002;7:516–30. doi: 10.1634/theoncologist.7-6-516. [DOI] [PubMed] [Google Scholar]
- 37.Kruijtzer CM, Beijnen JH, Rosing H, ten Bokkel Huinink WW, Schot M, Jewell RC, Paul EM, Schellens JH. Increased oral bioavailability of topotecan in combination with the breast cancer resistance protein and P-glycoprotein inhibitor GF120918. J Clin Oncol. 2002;20:2943–50. doi: 10.1200/JCO.2002.12.116. [DOI] [PubMed] [Google Scholar]
- 38.Miller DS, Bauer B, Hartz AMS. Modulation of P-Glycoprotein at the Blood-Brain Barrier: Opportunities to Improve Central Nervous System Pharmacotherapy. Pharmacol Rev. 2008 doi: 10.1124/pr.107.07109. pr.107.07109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marczylo TH, Verschoyle RD, Cooke DN, Morazzoni P, Steward WP, Gescher AJ. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother Pharmacol. 2007;60:171–7. doi: 10.1007/s00280-006-0355-x. [DOI] [PubMed] [Google Scholar]
- 40.Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V, Kurzrock R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491–9. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 41.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–18. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 42.Shukla S, Wu CP, Ambudkar SV. Development of inhibitors of ATP-binding cassette drug transporters: present status and challenges. Expert Opin Drug Metab Toxicol. 2008;4:205–23. doi: 10.1517/17425255.4.2.205. [DOI] [PubMed] [Google Scholar]
- 43.Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, Reid G, Schellens JH, Koomen GJ, Schinkel AH. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther. 2002;1:417–25. [PubMed] [Google Scholar]