Abstract
Do some patients benefit from an unrelated donor (URD) transplant because of a stronger graft-versus-leukemia (GVL) effect? We analyzed 4099 patients with acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), and chronic myeloid leukemia (CML) undergoing a myeloablative allogeneic hematopoietic cell transplantation (HCT) from an URD (8/8 human leukocyte antigen [HLA]–matched, n = 941) or HLA-identical sibling donor (n = 3158) between 1995 and 2004 reported to the CIBMTR. In the Cox regression model, acute and chronic GVHD were added as time-dependent variables. In multivariate analysis, URD transplant recipients had a higher risk for transplantation-related mortality (TRM; relative risk [RR], 2.76; P < .001) and relapse (RR, 1.50; P < .002) in patients with AML, but not ALL or CML. Chronic GVHD was associated with a lower relapse risk in all diagnoses. Leukemia-free survival (LFS) was decreased in patients with AML without acute GVHD receiving a URD transplant (RR, 2.02; P < .001) but was comparable to those receiving HLA-identical sibling transplants in patients with ALL and CML. In patients without GVHD, multivariate analysis showed similar risk of relapse but decreased LFS for URD transplants for all 3 diagnoses. In conclusion, risk of relapse was the same (ALL, CML) or worse (AML) in URD transplant recipients compared with HLA-identical sibling transplant recipients, suggesting a similar GVL effect.
Introduction
Both experimental and clinical studies demonstrate that the immune system may control cancer.1–4 This effect is most evident in the graft-versus-leukemia (GVL) effect, which is observed after allogeneic hematopoietic cell transplantation (HCT). For instance, patients with graft-versus-host disease (GVHD), especially chronic GVHD, have a lower risk of relapse compared with patients without GVHD.1,2,4 Furthermore, identical twins undergoing HCT run a higher risk of relapse than recipients of grafts from human leukocyte antigen [HLA]–identical sibling donors.5–7 T-cell depletion of bone marrow grafts, which may effectively prevent severe GVHD, increases the risk of relapse, especially in patients with chronic myeloid leukemia (CML).8–10 More effective immunosuppression, for instance by combining cyclosporine and methotrexate, which is more effective than monotherapy to prevent GVHD, also increases the risk of leukemic relapse in some studies, although conflicting data exist.11–15 A study by Bacigalupo and coworkers16 in acute myeloid leukemia (AML) showed that a high dose of cyclosporine compared with a low dose was associated with an increased risk of leukemic relapse. This observation has been used therapeutically, and it was reported that giving a low dose of cyclosporine of short duration increased the risk of mild acute and chronic GVHD and decreased the probability of relapse after HLA-identical sibling transplantations.17
Most studies addressed grafts from HLA-identical sibling donors, where risk factors for relapse and GVL effect have been extensively analyzed. However, the GVL effect has been less frequently evaluated using unrelated donor transplants (URD).18 Today, approximately one-third of the patients in need of HCT have an available HLA-identical sibling to serve as a donor. The growth of donor registries worldwide has improved the overall chance that a patient who lacks a family donor will be able to identify a suitable URD for transplantation.19 With better matching due to genomic tissue typing and improved immunosuppression, outcomes using URD have approached those using HLA-identical sibling donors.20–22 HLA-matched unrelated individuals are not identical by descent and have more genetic disparity compared with HLA-genotypical identical siblings for HLA-DPB1 that has been associated with a decreased risk of relapse, and for minor histocompatibility antigens (mHags), which may function as leukemia-associated specific antigens.23–26
It has been suggested that the GVL effect is more potent using URD compared with HLA-identical siblings, presumably related to a higher likelihood of mismatching at DPB1 and mHags.25,26 However, formal analysis of the potential beneficial effects of greater disparity and GVL effects is lacking. The aim of the present study was to determine whether the GVL effect is stronger in transplantations using URD, compared with HLA-identical sibling donors. If the GVL reaction is more potent in URD transplantation, and outcomes are otherwise similar, should, in patients with high-risk leukemia, a URD be selected instead of an HLA-identical sibling donor?
Methods
Data source
The CIBMTR is a research organization formed of more than 500 transplant centers worldwide that contribute detailed data on consecutive allogeneic HCT. Patients are followed longitudinally, with yearly follow-up. Computerized checks for errors, physician reviews of submitted data, and on-site audits of participating centers ensure data quality.
All patients registered with the National Marrow Donor Program (NMDP) were retrospectively contacted, and informed consent was obtained from patients in accordance with the Declaration of Helsinki for participation in the NMDP research program. Informed consent was waived by the NMDP Institutional Review Board for all deceased patients. Surviving patients who did not provide signed informed consent to allow analysis of their clinical data were excluded to adjust for the potential bias introduced by exclusion of nonconsenting surviving patients; a corrective action plan (CAP) modeling process randomly appropriately excluded the same percentage of deceased patients using a biased coin randomization with exclusion probabilities based on characteristics associated with not providing consent for use of the data in survivors.
Patient selection
The patient population consisted of adult patients (> 18 years) with AML, acute lymphoblastic leukemia (ALL), or CML undergoing an allogeneic HCT between 1995 and 2004 and reported to the CIBMTR. A total of 4099 cases fulfilling the inclusion criteria within the CIBMTR were identified. Of these, 3158 were HLA-identical siblings and 941 were 8/8 matched URD transplant recipients (high resolution matched at HLA-A, -B, -C, and -DPB1). All patients received myeloablative-conditioning regimens, methotrexate plus cyclosporine, or tacrolimus with or without other drugs for GVHD prophylaxis and had no previous transplantation. Patients receiving a T cell–depleted graft were excluded.
Study end points and definitions
The primary outcome was relapse and time to onset of leukemia recurrence. Relapse was defined by hematologic criteria for acute leukemia. Relapse for CML was defined by cytogenetic or hematologic evidence of disease. Transplantation-related mortality (TRM) was defined as death without evidence of leukemia recurrence. Patients were censored at relapse or at last follow-up. Leukemia-free survival (LFS) was defined as time to treatment failure (death or relapse); all living patients were censored at the last follow-up evaluation. Grade II-IV acute GVHD was graded according to Glücksberg criteria based on the pattern of severity of abnormalities in skin, gastrointestinal tract, and liver.27 Chronic GVHD was diagnosed according to standard criteria.28 Patients with evidence of donor engraftment who survived more than 90 days from transplantation were evaluated for chronic GVHD. Disease status at transplantation was classified as early, intermediate, or late. Early disease included patients undergoing HCT in first remission (acute leukemia) or first chronic phase (CML); intermediate disease was defined as second or later complete remission (acute leukemia), second or later chronic phase/accelerated phase (CML); and advanced disease included patients in relapse or primary induction failure (acute leukemia) or blast crisis (CML). For patients with AML, cytogenetic abnormalities were classified according to the classification proposed by Gale et al.29 Four categories were defined: good risk, intermediate risk, poor risk, and no abnormalities [good risk included t(8;21), t(8;21) + other, t(15;17) only, t(15;17) + other, inv or del (16) only, inv or del (16) + other];intermediate risk included + 8 only, + 8 + other, + 21 only, + 21 + other, t(6;9) + other, other translocations, other numerical abnormalities, other structural abnormalities; poor risk included t(9;22) + other, −5 or del (5) + other, −7 or del (7) only, −7 or del (7) + other, del(11) only, del(11) + other; no abnormalities included normal cytogenetics].
Statistical analysis
TRM and relapse were estimated as cumulative incidences, taking into account competing risks. Probability of LFS was calculated using the Kaplan-Meier estimator with variance estimated by the Greenwood formula. Comparison of survival curves was done using the log-rank test. In multivariate analysis, a stepwise selection procedure was performed using the proportional hazards model. The influence of acute GVHD and chronic GVHD was evaluated by treating occurrence of GVHD as a time-dependent variable. The model was built by forcing the main variables (HLA-identical sibling vs URD, acute GVHD, and chronic GVHD) into the model with adjustment for significant covariates (with the threshold 0.05) and nonsignificant variables being dropped out of the model.
The proportional hazards assumption was assessed for each variable using a time-dependent approach. Two-way interactions were checked between each selected variable and the main effects. SAS software, version 9.1 (SAS Institute, Cary, NC) was used in all analyses. Separate models were built for each disease of AML, ALL, and CML. Similarly, when studying only patients without any acute or chronic GVHD to look at the impact of URD versus HLA-identical sibling transplants, separate models were built for each disease of AML, ALL, and CML. Outcomes of TRM, relapse, and LFS were analyzed in the multivariate analysis. For each disease, a URD versus an HLA-identical sibling transplant was the main variable being forced into the model.
Results
Patients
Patient and donor characteristics are summarized in Table 1. The median age at transplantation was 39 years in URD recipients and 38 years in HLA-identical sibling recipients. The median donor age was 36 and 37 years in the 2 groups, respectively; 36% of URD and 40% of HLA-identical sibling recipients had AML; 20% and 15% had ALL and 44% in each category had CML, respectively; 51% of URD recipients versus 70% of HLA-identical sibling recipients had early-stage disease at the time of transplantation. Peripheral blood stem cell grafts were given to 21% of URD recipients versus 43% of HLA-identical sibling recipients. Median follow-up was 72 and 60 months in the 2 groups, respectively.
Table 1.
Characteristics of patients (> 18 y) who underwent a myeloablative 8/8 matched URD or HLA-identical sibling transplantation between 1995 and 2004 for AML, ALL, and CML
| Characteristics | HLA-identical siblings | URD | P |
|---|---|---|---|
| Total patients, n (%) | 3158 (77) | 941 (23) | |
| Median recipient age at transplantation, y (range) | |||
| Recipient | 38.1 (18-59.9) | 38.9 (18-59.9) | .09 |
| Donor | 37 (18-60) | 35.5 (19-59.3) | < .001 |
| Male sex | 1768 (56) | 526 (56) | |
| Donor-recipient sex match | < .001 | ||
| Male→male | 1039 (33) | 369 (39) | |
| Male→female | 723 (23) | 250 (27) | |
| Female→male | 728 (23) | 157 (17) | |
| Female→female | 659 (21) | 165 (18) | |
| Unknown | 9 (< 1) | 0 | |
| Disease | .001 | ||
| AML | 1271 (40) | 340 (36) | |
| ALL | 483 (15) | 189 (20) | |
| CML | 1404 (44) | 412 (44) | |
| Disease status at transplantation* | < .001 | ||
| Early | 2203 (70) | 476 (51) | |
| Intermediate | 468 (15) | 243 (26) | |
| Advanced | 449 (14) | 222 (24) | |
| Unknown | 38 (1) | 0 | |
| Karnofsky performance score | < .001 | ||
| < 90 | 678 (21) | 238 (25) | |
| 90-100 | 2441 (77) | 651 (69) | |
| Unknown | 39 (1) | 52 (6) | |
| Donor-recipient CMV match | < .001 | ||
| D(−)/R(−) | 766 (24) | 306 (33) | |
| D(−)/R(+) | 409 (13) | 286 (30) | |
| D(+)/R(−) | 276 (9) | 137 (15) | |
| D(+)/R(+) | 1527 (48) | 188 (20) | |
| Unknown | 180 (6) | 24 (3) | |
| Graft type | < .001 | ||
| Bone marrow | 1788 (57) | 748 (79) | |
| Peripheral blood | 1370 (43) | 193 (21) | |
| Conditioning regimen | < .001 | ||
| cy + tbi ± other | 1237 (39) | 738 (78) | |
| cy + bu ± other | 1921 (61) | 203 (22) | |
| ATG during conditioning | 31 (1) | 48 (5) | |
| GVHD prophylaxis | < .001 | ||
| CSA + MTX ± other | 3007 (95) | 648 (69) | |
| FK506 + MTX ± other | 151 (5) | 293 (31) | |
| Median follow-up of survivors, mo (range) | 60.3 (1.4-136.7) | 72.2 (10.8-135.3) | < .001 |
| Year of transplantation | < .001 | ||
| 1995-1997 | 1456 (46) | 250 (27) | |
| 1998-2000 | 972 (31) | 327 (35) | |
| 2001-2004 | 730 (23) | 364 (39) | |
| Acute leukemia | |||
| AML FAB subtype | < .001 | ||
| AML M1 | 189 (15) | 50 (15) | |
| AML M2 | 342 (28) | 71 (21) | |
| AML M3 | 117 (10) | 16 (5) | |
| AML M4 | 256 (21) | 63 (19) | |
| AML M5 | 154 (13) | 26 (8) | |
| AML M6 | 29 (2) | 18 (5) | |
| AML M7 | 15 (1) | 7 (2) | |
| Other AML | 48 (4) | 23 (7) | |
| Unclassified AML | 76 (6) | 57 (17) | |
| WBC at diagnosis | < .001 | ||
| < 25 × 109/L | 994 (57) | 246 (47) | |
| 25-50 × 109/L | 191 (11) | 60 (11) | |
| 50-100 × 109/L | 206 (12) | 49 (9) | |
| > 100 × 109/L | 144 (8) | 59 (11) | |
| Missing | 219 (12) | 115 (22) | |
| Median WBC at diagnosis, ×109/L (range) | 21.1 (0.1-900) | 14.7 (0.3-870) | .001 |
| Duration of CR1 (for patients beyond CR1) | < .001 | ||
| < 6 mo | 186 (35) | 77 (27) | |
| 6-12 mo | 92 (17) | 75 (27) | |
| > 12 mo | 157 (29) | 98 (35) | |
| Missing | 104 (19) | 32 (11) | |
| Median (range), mo | 8.0 (0.1-151.1) | 9.6 (0.6-145.2) | .008 |
| Time from remission to transplantation (for patients in CR1) | < .001 | ||
| < 3 mo | 463 (45) | 71 (39) | |
| 3-6 mo | 376 (36) | 77 (42) | |
| > 6 mo | 134 (13) | 34 (19) | |
| Missing | 60 (6) | 0 | |
| Median (range), mo | 3.1 (0.06-46.8) | 3.6 (0.2-20.5) | .249 |
| Cytogenetics: AML | < .001 | ||
| Good prognosis | 140 (11) | 35 (10) | |
| Intermediate prognosis | 188 (15) | 53 (16) | |
| Poor prognosis | 158 (12) | 60 (18) | |
| No abnormalities | 469 (37) | 72 (21) | |
| Unknown | 316 (25) | 120 (35) | |
| Cytogenetics: ALL | < .001 | ||
| No abnormalities | 154 (32) | 36 (19) | |
| Hyperdiploid | 36 (7) | 18 (10) | |
| Hypodiploid, t(9;22), t(4;11), t(8;14) | 73 (15) | 56 (30) | |
| Other abnormalities | 84 (17) | 24 (13) | |
| Unknown | 136 (28) | 55 (29) | |
| Chronic leukemia | |||
| CML: Time from diagnosis to transplantation | < .001 | ||
| < 12 mo | 893 (64) | 210 (51) | |
| 12-24 mo | 325 (23) | 110 (27) | |
| > 24 mo | 186 (13) | 91 (22) | |
| Missing | 0 | 1 (< 1) | |
| Median (range), mo | 8.7 (0.7-149.9) | 11.8 (0.9-138.7) | < .001 |
| Grade II-IV acute GVHD | 1083 (34) | 485 (52) | |
| Grade III-IV acute GVHD | 495 (16) | 194 (21) | |
| Chronic GVHD | 1308 (42) | 465 (49) |
Characteristics of URD or HLA-identical sibling transplantations and differences between the 2 groups regarding some prognostic factors are shown. Unless otherwise indicated, all values are no. (%).
URD indicates unrelated donor; AML, acute myeloid leukemia; ALL, acute lymphoid leukemia; CML, chronic myeloid leukemia; CMV, cytomegalovirus; cy, cyclophosphamide; tbi, total body irradiation; bu, busulpan; ATG, anti–T-cell immunoglobulin; CSA, cyclosporine; MTX, methotrexate; FK506, tacrolimus; FAB, French-American-British; WBC, white blood cell; CR1, first complete remission; and GVHD, graft-versus-host disease.
Disease status is categorized as follows: early includes AML and ALL in CR1 or CML in first chronic phase; intermediate includes AML and ALL in ≥ CR2 or CML in accelerated phase or ≥ second chronic phase; and advanced includes AML and ALL with relapse or primary induction failure or CML in blast crisis.
GVHD and transplantation-related mortality
Grade II-IV acute GVHD was present in 52% of URD and 34% of HLA-identical sibling recipients, whereas grade III-IV acute GVHD was seen in 21% and 16% in the 2 groups, respectively (Table 1). Chronic GVHD was present in 49% in URD recipients and 42% of HLA-identical sibling recipients, respectively.
Results of univariate analysis for TRM, relapse, and LFS are shown in Table 2. In the multivariate analysis, the relative risk for TRM was significantly increased in the URD group compared with the HLA-identical sibling group in patients with AML without acute GVHD (RR, 2.76; P < .001; Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). In AML patients with acute GVHD, TRM was the same in the 2 groups (RR, 1.19; P = .32). The risk of TRM was similar in patients with ALL (RR, 1.19; P = .25) or CML (RR, 1.02; P = .85) between recipients of grafts from HLA-identical siblings or URD.
Table 2.
Univariate analyses among patients who underwent a 8/8 matched unrelated donor (URD) or HLA identical sibling donor (Sib) myeloablative stem cell transplantation for AML, ALL, and CML between 1995 and 2004
| Disease status* |
TRM† |
Relapse† |
LFS† |
||||||
|---|---|---|---|---|---|---|---|---|---|
| (N: Sib/URD) | Sib | URD | P | Sib | URD | P | Sib | URD | P |
| AML | |||||||||
| Early (760/118) | 24 (20-27) | 40 (31-50) | .001 | 15 (13-18) | 22 (15-30) | .1 | 61 (57-65) | 38 (28-48) | < .001 |
| Intermediate (180/88) | 30 (23-37) | 39 (28-50) | .18 | 22 (16-29) | 21 (13-30) | .8 | 48 (40-56) | 41 (30-52) | .3 |
| Advanced (294/129) | 31 (25-36) | 44 (35-53) | .01 | 46 (40-52) | 43 (34-51) | .6 | 23 (18-29) | 13 (8-20) | .01 |
| ALL | |||||||||
| Early (271/62) | 31 (25-37) | 43 (30-57) | .09 | 23 (18-29) | 15 (7-25) | .13 | 46 (39-53) | 42 (29-55) | .6 |
| Intermediate (111/61) | 41 (32-51) | 40 (27-53) | .9 | 32 (24-42) | 36 (24-48) | .7 | 27 (18-36) | 24 (14-37) | .8 |
| Advanced (88/64) | 38 (28-48) | 48 (35-60) | .2 | 52 (42-63) | 48 (35-60) | .6 | 10 (4-18) | 5 (1-12) | .2 |
| CML | |||||||||
| Early (1122/281) | 30 (27-33) | 37 (31-43) | .02 | 10 (8-12) | 10 (6-13) | .81 | 60 (57-63) | 54 (48-60) | .06 |
| Intermediate (169/87) | 40 (32-48) | 47 (36-58) | .3 | 27 (20-34) | 21 (13-30) | .3 | 34 (26-42) | 32 (23-43) | .8 |
| Advanced (47/27) | 32 (19-47) | 67 (48-83) | .003 | 43 (29-58) | 33 (17-52) | .4 | 25 (13-39) | 0 | < .001 |
Differences between TRM and LFS but no difference in relapse between the 2 groups in univariate analysis are shown. Analysis is stratified by disease and disease stage; 5-year probability for treatment related mortality (TRM), relapse, and leukemia-free survival (LFS) is given (95% confidence interval).
N: Sib/URD indicates number of patients in HLA-identical sibling/unrelated donor groups; URD, unrelated donor; Sib, HLA-identical sibling; AML, acute myeloid leukemia; ALL, acute lymphoid leukemia; and CML, chronic myeloid leukemia.
Disease status is categorized as follows: early includes AML and ALL in CR1 or CML in first chronic phase; intermediate includes AML and ALL in ≥ CR2 or CML in accelerated phase or ≥ second chronic phase; and advanced includes AML and ALL with relapse or primary induction failure or CML in blast crisis.
Percentage of 5-year probability.
Relapse
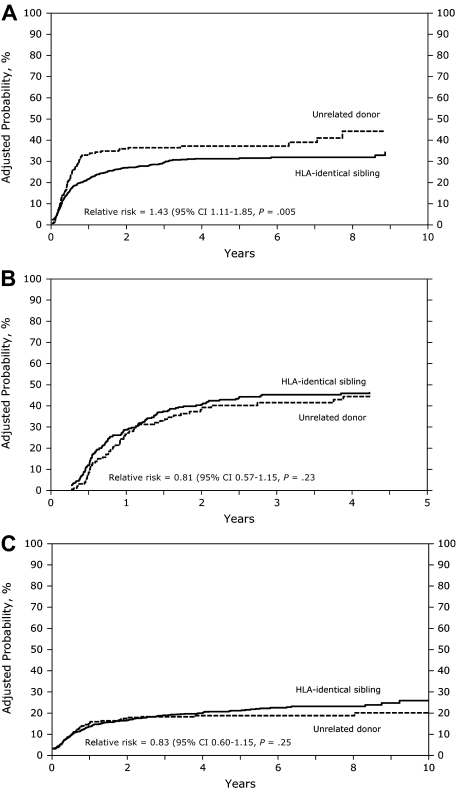
In the univariate analysis (Table 2), the cumulative incidence of relapse was similar between patients receiving grafts from HLA-identical sibling donors or URD in AML, ALL, or CML in early, intermediate, or advanced disease (Table 2). In the Cox model, the relative risk of relapse was significantly increased in URD transplant recipients with AML (RR, 1.5; P = .002; Figure 1A) but not in recipients with ALL or CML (Table 3, Figure 1B,C). Multivariate analysis, stratified by disease status, showed an increased relapse risk in the URD group for AML CR1 (RR, 1.73; P = .02) and in AML patients with more advanced disease (RR, 1.37; P = .04; data not shown). Risk of relapse was lower in patients with AML, ALL, and CML in the presence of chronic GVHD. Acute GVHD had no impact on relapse risk. In patients with AML, other factors associated with an increased risk of relapse were intermediate and advanced disease stage, high white blood cell count at diagnosis (> 50 × 109/L), shorter duration (< 6 months) of first remission (CR1) in patients with relapse, and poor cytogenetics.
Figure 1.
(A) Adjusted probability of relapse in patients with AML receiving grafts from an unrelated donor or an HLA-identical sibling (P = .005). (B) Adjusted probability of relapse in patients with ALL receiving grafts from an unrelated donor or an HLA-identical sibling (P = .23). (C) Adjusted probability of relapse in patients with CML receiving grafts from an unrelated donor or an HLA-identical sibling (P = .25).
Table 3.
Multivariate analysis for relapse in patients who underwent myeloablative URD or HLA-identical sibling transplantation for AML, ALL, and CML
| Factors | Relative risk | 95% CI | P |
|---|---|---|---|
| AML* | |||
| URD vs sib | 1.50 | 1.16-1.94 | .002 |
| Acute GVHD | 0.91 | 0.72-1.16 | .45 |
| Chronic GVHD | 0.75 | 0.56-0.99 | .046 |
| ALL† | |||
| URD vs sib | 0.95 | 0.68-1.33 | .78 |
| Acute GVHD | 0.78 | 0.57-1.07 | .13 |
| Chronic GVHD | 0.69 | 0.47-1.01 | .058 |
| CML‡ | |||
| URD vs sib | 0.91 | 0.65-1.27 | .56 |
| Acute GVHD | 0.84 | 0.62-1.13 | .25 |
| Chronic GVHD | 0.67 | 0.49-0.93 | .017 |
Relapse is increased in URD transplantations for AML but is similar between URD and sib for ALL and CML. The sib group was the reference group.
URD indicates unrelated donor; sib, HLA-identical sibling; AML, acute myeloid leukemia; ALL, acute lymphoid leukemia; CML, chronic myeloid leukemia, GVHD, graft-versus-host disease; CI, confidence interval; WBC, white blood cell; and CR1, first complete remission.
Model also adjusted for disease stage, WBC count, duration of CR1, and cytogenetics and stratified by graft type and Karnofsky score.
Model also adjusted for disease stage and stratified by graft type and Karnofsky score.
Model stratified by graft type, disease stage, and Karnofsky score.
Leukemia-free survival
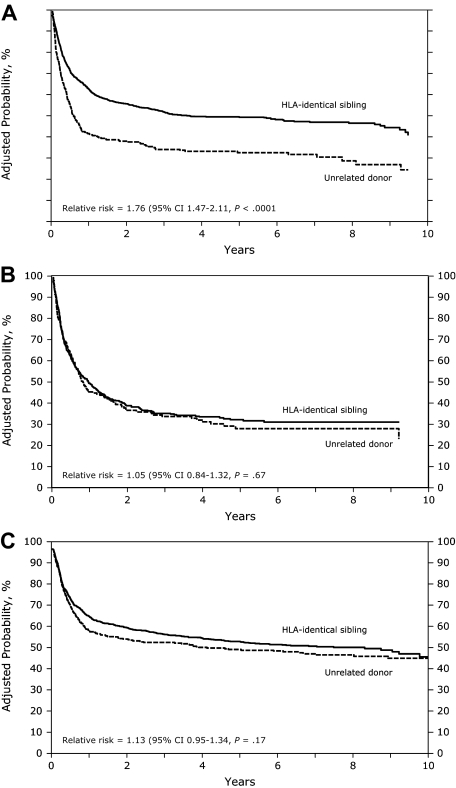
In the univariate analysis, URD recipients had a significantly worse leukemia-free survival (LFS) compared with HLA-identical sibling recipients with AML (early and advanced disease) and CML (advanced disease; Table 2). In multivariate analysis in patients with AML, the probability of LFS was lower in the URD group compared with the HLA-identical sibling group in patients with no acute GVHD (RR, 2.02; P < .001; Figure 2A). However, in patients with acute GVHD, LFS tended to be higher, although not statistically significant (RR, 1.27; P = .072; Table 4). Other factors associated with poor LFS were high WBC count at diagnosis (> 50 × 109/L), shorter duration (< 6 months) of CR1 in AML with relapse, poor-risk cytogenetics, and GVHD prophylaxis with cyclosporine plus methotrexate plus or minus other (vs tacrolimus + other ± other).
Figure 2.
(A) Adjusted probability of leukemia-free survival in patients with AML receiving grafts from HLA-identical siblings or an unrelated donor (P < .001). (B) Adjusted probability of leukemia-free survival in patients with ALL receiving grafts from HLA-identical siblings or an unrelated donor (P = .67). (C) Adjusted probability of leukemia-free survival in patients with CML receiving grafts from HLA-identical siblings or an unrelated donor (P = .17).
Table 4.
Multivariate analysis for leukemia-free survival (LFS) in patients who underwent a URD or HLA-identical sibling transplantation for AML, ALL, and CML
| Factors | Relative risk | 95% CI | P |
|---|---|---|---|
| AML* | |||
| URD vs sib (no acute GVHD) | 2.02 | 1.61-2.53 | < .001 |
| URD vs sib (acute GVHD present) | 1.27 | 0.98-1.65 | .072 |
| Chronic GVHD | 1.08 | 0.89-1.31 | .45 |
| ALL† | |||
| URD vs sib | 1.02 | 0.82-1.29 | .84 |
| Acute GVHD | 1.14 | 0.92-1.42 | .23 |
| Chronic GVHD | 1.12 | 0.86-1.46 | .41 |
| CML‡ | |||
| URD vs sib | 0.95 | 0.80-1.14 | .59 |
| Acute GVHD | 1.91 | 1.64-2.22 | < .001 |
| Chronic GVHD | 1.21 | 1.01-1.47 | .043 |
LFS is decreased in URD transplantations for AML without acute GVHD but is similar between URD and sib transplants for ALL and CML. RR for reference group (sib) is 1.0.
URD indicates unrelated donor; sib, HLA-identical sibling; AML, acute myeloid leukemia; ALL, acute lymphoid leukemia; CML, chronic myeloid leukemia; GVHD, graft-versus-host disease; CI, confidence interval; WBC, white blood cell; and CR1, first complete remission.
Interaction present between donor source and aGVHD. Model also adjusted for GVHD prophylaxis, WBC count, duration of CR1, and cytogenetics and stratified by disease stage, graft type, and Karnofsky score.
Model also adjusted for patient's age and stratified by disease stage, graft type, and Karnofsky score.
Model also adjusted for patient age and time from diagnosis to transplantation, and stratified by disease stage, graft type, and Karnofsky score.
In patients with ALL and CML, there was no difference in LFS between recipients of URD transplants and HLA-identical sibling transplants (Table 4, Figure 2B,C). In patients with CML, presence of acute GVHD (RR, 1.91; P < .001) and chronic GVHD (RR, 1.21; P = .043) were associated with a lower probability of LFS in the multivariate analysis (Table 4).
Relapse and leukemia-free survival in patients without acute and/or chronic GVHD
In patients without any acute and/or chronic GVHD, in multivariate analysis there was no difference in relapse risk in patients with AML, ALL, or CML, regardless of donor type (Table 5). Increased relapse was seen in patients with shorter duration of CR1 (< 6 months) and poor-risk cytogenetics (in AML), high WBC count (> 50 × 109/L) at diagnosis (in ALL), and with intermediate and advanced disease stage (in CML). In the multivariate analysis, the URD group had a lower probability of LFS in patients with all 3 diseases (Table 6).
Table 5.
Multivariate analysis for relapse in patients without any acute or chronic GVHD who underwent a URD or HLA-identical sibling transplantation for AML, ALL, and CML
| Factors | Relative risk | 95% CI | P |
|---|---|---|---|
| AML*: URD vs sib | 1.33 | 0.90-1.97 | .16 |
| ALL†: URD vs sib | 0.61 | 0.30-1.28 | .19 |
| CML‡: URD vs sib | 0.82 | 0.45-1.52 | .53 |
In patients without acute and chronic GVHD, relapse is similar for AML, ALL, and CML between URD and HLA-identical sibling transplations. RR for reference group (sib) is 1.0.
URD indicates unrelated donor; sib, HLA-identical sibling; AML, acute myeloid leukemia; ALL, acute lymphoid leukemia; CML, chronic myeloid leukemia, GVHD, graft-versus-host disease; CI, confidence interval; WBC, white blood cell; and CR1, first complete remission.
Model adjusted for duration of CR1 and cytogenetics, and stratified by disease stage, Karnofsky score, and graft type.
Model also adjusted for WBC count at diagnosis and stratified by disease stage, Karnofsky score, and graft type.
Model also adjusted for disease stage and stratified by Karnofsky score and graft type.
Table 6.
Multivariate analysis for leukemia-free survival (LFS) in patients without any acute or chronic GVHD who underwent a URD or HLA-identical sibling transplantation for AML, ALL, and CML
| Factors | Relative risk | 95% CI | P |
|---|---|---|---|
| AML*: URD vs sib | 2.17 | 1.67-2.80 | < .001 |
| ALL†: URD vs sib | 1.69 | 1.10-2.62 | .017 |
| CML‡: URD vs sib | 1.48 | 1.04-2.12 | .03 |
In patients without acute or chronic GVHD, LFS is decreased in URD compared with HLA-identical sibling transplantations for AML, ALL, and CML
URD indicates unrelated donor; Sib, HLA-identical sibling; AML, acute myeloid leukemia; ALL, acute lymphoid leukemia; CML, chronic myeloid leukemia, GVHD, graft-versus-host disease; CI, confidence interval; and CR1, first complete remission. RR for reference group (sib) is 1.0.
Model also adjusted for duration of CR1 and cytogenetics, and stratified by disease stage, Karnofsky score, and graft type.
Model also adjusted for patient age at transplant and stratified by disease stage, Karnofsky score, and graft type.
Model also adjusted for patient age and stratified by disease stage, Karnofsky score, and graft type.
Discussion
Although it is true that disease relapse remains the number one cause of treatment failure, it is followed very closely behind by GVHD. Indeed, in this study, TRM accounts for more deaths than does disease relapse (Table 2). Therefore, clinical GVHD and associated infections and other toxicities are still a major barrier to transplantation success. In the present study, recipients of URD transplants had a higher TRM than HLA-identical sibling transplant recipients for AML, but not for ALL or CML.
Higher TRM using URD compared with HLA-identical sibling donors may partly be due to URD transplantation being associated with more GVHD. However, even in patients without acute and chronic GVHD, recipients of URD transplants still had a similar relapse rate and lower LFS, suggesting an increased TRM in URD recipients compared with recipients of HLA-identical sibling grafts for all 3 diagnoses (Table 5). This discrepancy may be explained by the possibility that mHags are important for immune defense. This is further corroborated by reports of higher frequency of infectious complications and infectious deaths in URD transplant recipients, compared with recipients of HLA-identical sibling grafts.30 We want to stress another possible explanation, namely that the patients receiving URD transplants are treated with additional immunosuppression, such as anti–T-cell antibodies and high doses of immunosuppressive agents and, therefore, run a higher risk of infectious complications and TRM.31 In AML patients with acute GVHD, URD and HLA-identical sibling transplants had similar TRM. This may be attributed to the fact that patients with severe acute GVHD have a poor outcome, regardless of the type of donor. In AML patients without GVHD, TRM was increased in the URD group, which may be due to that infections are more common using URD for the above mentioned reasons.30
The most important finding from this study is that a similar risk of relapse was observed using URD or HLA-identical sibling donors in ALL and CML regardless of disease stage. This was observed in patients with and without acute and chronic GVHD. Hence, using high-resolution HLA-typing of URD results in similar GVL effect to transplantation using HLA-identical sibling donors. The data suggest that the GVL effect is mainly associated with chronic GVHD. A profound GVL effect in the presence of chronic GVHD is in keeping with previous studies in HLA-identical sibling transplantations.1,2,4,32 A previous IBMTR study, however, observed correlation of acute GVHD with GVL activity in patients with ALL and chronic GVHD in patients with AML and CML.1 The discrepant findings regarding the relative role of acute and chronic GVHD and the associated GVL effects may reflect a close correlation between acute and chronic GVHD.33–35 Therefore, in some patient populations it may be difficult to differentiate the GVL effect of acute GVHD from that of chronic GVHD. It is likely that both acute and chronic GVHD contribute to the GVL effect. The immunosuppressive therapy is also of importance for GVL.2,8,9,11,16,31 This study included patients of all stages. Most patients in this study had early disease. A GVL effect is most obvious in early disease.2 This is in agreement with the present study: chronic GVHD was associated with decreased relapse in AML CR1 (RR, 0.65; P = .05) but not in more advanced disease (RR, 0.82; P = .3).
We have to interpret these data with caution, because of limitations using retrospective registry analyses. Although multivariate models may have adjusted for the various disease and biological factors including WBC count, cytogenetics, disease stage, and duration of CR1, there may be factors of importance that we have not been able to take into account. For example, we do not know the number of courses of chemotherapy required to achieve CR in patients with acute leukemia and the number of patients with extramedullary leukemia. Furthermore, approximately 30% of the patients also had unknown cytogenetics (Table 1).
Some of these may have contributed to higher relapse observed with URD transplants in patients with AML. To support this, there was a trend for more patients with intermediate and advanced disease stage and fewer patients with early disease stage in the URD group. Furthermore, it is not the standard practice to perform a transplantion in a patient with AML with normal karyotype in first CR using an unrelated donor, unless the patient has other poor prognostic factors, which could include more than one course of chemotherapy to achieve CR, hyperleukocytosis, or extramedullary disease. An increased relapse risk in the URD patients was seen in AML CR1 and also in more advanced disease. This may suggest that there may be several reasons for the increased risk for relapse in the URD AML group, such as selection bias and more heavy immunosuppressive therapy. With increased immunosuppression, the GVL effect may be decreased.36 Relapse in the URD AML group was statistically significantly increased in all patients (RR, 1.5; P = .002; Table 4) but not in those without acute or chronic GVHD (RR, 1.33; P = .16; Table 5). The reason for this discrepancy is most probably the lower number of patients in the latter analysis.
Some investigators propose that it is possible to separate the beneficial effects of GVL and the deleterious effects of clinical severe GVHD.23–25,37 Broadly expressed mHags and malignancy-associated minor antigens may be involved in a complex interaction that define the given patients' risk of clinical GVHD and relapse.24 Minor antigens expressed by the recipients' malignant cells and tumor-specific minor antigens may be a target for graft-versus-host immunoresponses. Our data suggest that the GVL effect is closely linked with GVHD. Therefore, to best utilize the GVL effect with minimal toxicity would require limiting GVHD to mild acute and mild chronic.1,17,32 Although chronic GVHD decreased relapse for all patients with leukemia in this study, acute and chronic GVHD had a negative impact on LFS in patients with CML (Table 4). Thus, chronic GVHD is not associated with improved LFS for all patients with leukemia.1,2,4,32
Some studies have suggested a GVL effect in the absence of GVHD, because HLA-identical sibling transplants without GVHD had a reduced relapse risk compared with syngeneic transplants.1,38 Therefore, we compared relapse and LFS in URD transplants and HLA-identical sibling transplants in patients without any acute or chronic GVHD (Tables 5,6). In this analysis, relapse was the same in patients receiving URD and HLA-identical sibling transplants, suggesting similar GVL effects also in this cohort of patients.
We conclude that GVL effect is not superior using URD compared with an HLA-identical sibling donor. This is done with the reservations that recipients of URD had more poor risk factors. Some were obvious and controlled for, and some probable risk factors, especially in the AML group, were not reported and collected. These data may also raise questions regarding the relative importance of mHags as targets for GVL effects and generate a new impetus for more research in this area. Finally, URD is a good alternative in the absence of an available matched sibling for patients with acute leukemia with an indication for HCT.
Supplementary Material
Acknowledgments
This project has been supported by funding from the National Marrow Donor Program and the Department of the Navy, Office of Naval Research Grant N00014-05-1-0859 to the NMDP. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the Office of Naval Research or the National Marrow Donor Program.
The study was also supported by the Swedish Cancer Society (Stockholm, Sweden; 0070-B06-20XBC), the Children's Cancer Foundation (Sweden; 06/094), the Swedish Research Council (Stockholm, Sweden; K2007-64X-05 971-27-1), the Cancer Society in Stockholm (Stockholm, Sweden), the Cancer and Allergy Foundation, and Karolinska Institutet (Stockholm, Sweden).
The CIBMTR is supported by Public Health Service grant U24-CA76518 from the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung, and Blood Institute (Bethesda, MD); Office of Naval Research (Arlington, VA); Health Resources and Services Administration (DHHS); and grants from AABB, Advancing Transfusion and Cellular Therapies Worldwide (Bethesda, MD); Aetna (Hartford, CT); American Society for Blood and Marrow Transplantation (Arlington Heights, IL); and Amgen (Thousand Oaks, CA). Anonymous donation from the Medical College of Wisconsin (Milwaukee, WI); Association of Medical Microbiology and Infectious Disease Canada (Ottawa, ON); Astellas Pharma US (Deerfield, IL); Baxter International (Deerfield, IL); Bayer HealthCare Pharmaceuticals (Wayne, NJ); BloodCenter of Wisconsin (Milwaukee, WI); Blue Cross and Blue Shield Association (Chicago, IL); Bone Marrow Foundation (New York, NY); Canadian Blood and Marrow Transplant Group (Vancouver, BC); Celgene Corporation (Summit, NJ); CellGenix (Freiburg, Germany); Centers for Disease Control and Prevention (Atlanta, GA); ClinImmune Labs (Denver, CO); CTI Clinical Trial and Consulting Services (Cincinnati, OH); Cubist Pharmaceuticals (Lexington, MA); Cylex (Columbia, MD); CytoTherm (Trenton, NJ); DOR BioPharma (Ewing, NJ); Dynal Biotech, an Invitrogen company (Carlsbad, CA); Enzon Pharmaceuticals (Bridgewater, NJ); European Group for Blood and Marrow Transplantation (Maastricht, The Netherlands); Gambro BCT (Lakewood, CO); Gamida Cell (Jerusalem, Israel); Genzyme Corporation (Cambridge, MA); Histogenetics (Ossining, NY); HKS Medical Information Systems (Omaha, NE); Hospira (Lake Forest, IL); Infectious Diseases Society of America (Arlington, VA); Kiadis Pharma (Amsterdam, The Netherlands); Kirin Brewery Company (Tokyo, Japan); Merck & Co (Whitehouse Station, NJ); The Medical College of Wisconsin (Milwaukee, WI); MGI Pharma (Bloomington, MN); Michigan Community Blood Centers (Grand Rapids, MI); Millennium Pharmaceuticals (Cambridge, MA); Miller Pharmacal Group (Carol Stream, IL); Milliman USA (Seattle, WA); Miltenyi Biotec (Bergisch Gladbach, Germany); National Marrow Donor Program (Minneapolis, MN); Nature Publishing Group (New York, NY); New York Blood Center (New York, NY); Novartis Oncology (East Hanover, NJ); Oncology Nursing Society (Pittsburgh, PA); Osiris Therapeutics (Columbia, MD); Otsuka Pharmaceutical Development and Commercialization (Princeton, NJ); Pall Life Sciences (East Hills, NY); PDL BioPharma (Incline Village, NV); Pfizer (New York, NY); Pharmion Corporation (Summit, NJ); Saladax Biomedical (Bethlehem, PA); Schering Plough Corporation (Kenilworth, NJ); Society for Healthcare Epidemiology of America (Arlington, VA); StemCyte (Covina, CA); StemSoft Software (Vancouver, BC); Sysmex (Mundelein, IL); Teva Pharmaceutical Industries (Petach-Tikva, Israel); The Marrow Foundation (Minneapolis, MN); THERAKOS (Exton, PA); Vidacare Corporation (San Antonio, TX); Vion Pharmaceuticals (New Haven, CT); ViraCor Laboratories (Lee's Summit, MO); ViroPharma (Exton, PA); and WellPoint (Indianapolis, IN).
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, or any other agency of the US Government.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: O.R. designed the study and prepared the manuscript; T.W. and M.A. prepared the data and performed the statistical analysis; and O.R., S.Z.P., C.A., A.J.B., T.W., D.W., J.H.A., P.D.B., B.J.B., C.B., M.S.C., R.P.G., V.G., T.H., G.A.H., J.H., M.J., M.R.L., F.L., D.I.M., P.L.M., M.J.C., E.W.P., J.A.R., G.J.S., H.S., S.S., L.F.V., J.R.W., M.M.H., and M.A. participated in interpretation of data and approval of final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Olle Ringdén, Karolinska Institutet, Division of Clinical Immunology, F79, Karolinska University Hospital Huddinge, SE-141 86 Stockholm, Sweden; e-mail: Olle.Ringden@ki.se.
References
- 1.Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. [PubMed] [Google Scholar]
- 2.Ringden O, Labopin M, Gluckman E, et al. Graft-versus-leukemia effect in allogeneic marrow transplant recipients with acute leukemia is maintained using cyclosporin A combined with methotrexate as prophylaxis. Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 1996;18:921–929. [PubMed] [Google Scholar]
- 3.Truitt RL, Johnson BD, McCabe CM, Weiler MB. Graft-versus-leukemia. In: Ferrara JLM, Deeg HJ, Burakoff S, editors. Graft vs. Host Disease. 2nd Ed. New York: Marcel Dekker; 1996. pp. 385–423. [Google Scholar]
- 4.Weiden PL, Sullivan KM, Flournoy N, Storb R, Thomas ED. Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med. 1981;304:1529–1533. doi: 10.1056/NEJM198106183042507. [DOI] [PubMed] [Google Scholar]
- 5.Fefer A, Sullivan KM, Weiden PL, et al. Graft versus leukemia effect in man: the relapse rate of acute leukemia is lower after allogeneic than syngeneic marrow transplantation. In: Truitt RL, Gale RP, Bortin MM, editors. Cellular Immunotherapy of Cancer. New York: AR Liss; 1987. pp. 401–408. [PubMed] [Google Scholar]
- 6.Gale RP, Horowitz MM, Ash RC, et al. Identical-twin bone marrow transplants for leukemia. Ann Intern Med. 1994;120:646–652. doi: 10.7326/0003-4819-120-8-199404150-00004. [DOI] [PubMed] [Google Scholar]
- 7.Ringden O, Zwaan F, Hermans J, Gratwohl A. European experience of bone marrow transplantation for leukemia. Transplant Proc. 1987;19:2600–2604. [PubMed] [Google Scholar]
- 8.Aschan J, Ringden O, Sundberg B, Klaesson S, Ljungman P, Lonnqvist B. Increased risk of relapse in patients with chronic myelogenous leukemia given T-cell depleted marrow compared to methotrexate combined with cyclosporin or monotherapy for the prevention of graft-versus-host disease. Eur J Haematol. 1993;50:269–274. doi: 10.1111/j.1600-0609.1993.tb00161.x. [DOI] [PubMed] [Google Scholar]
- 9.Goldman JM, Gale RP, Horowitz MM, et al. Bone marrow transplantation for chronic myelogenous leukemia in chronic phase: increased risk for relapse associated with T-cell depletion. Ann Intern Med. 1988;108:806–814. doi: 10.7326/0003-4819-108-6-806. [DOI] [PubMed] [Google Scholar]
- 10.Marmont AM, Horowitz MM, Gale RP, et al. T-cell depletion of HLA-identical transplants in leukemia. Blood. 1991;78:2120–2130. [PubMed] [Google Scholar]
- 11.Aschan J, Ringden O, Sundberg B, Gahrton G, Ljungman P, Winiarski J. Methotrexate combined with cyclosporin A decreases graft-versus-host disease, but increases leukemic relapse compared to monotherapy. Bone Marrow Transplant. 1991;7:113–119. [PubMed] [Google Scholar]
- 12.Fefer A, Einstein AB, Cheever MA. Adoptive chemoimmunotherapy of cancer in animals: a review of results, principles, and problems. Ann N Y Acad Sci. 1976;277:492–504. doi: 10.1111/j.1749-6632.1976.tb41723.x. [DOI] [PubMed] [Google Scholar]
- 13.Ringden O, Horowitz MM, Sondel P, et al. Methotrexate, cyclosporine, or both to prevent graft-versus-host disease after HLA-identical sibling bone marrow transplants for early leukemia? Blood. 1993;81:1094–1101. [PubMed] [Google Scholar]
- 14.Storb R, Deeg HJ, Fisher L, et al. Cyclosporine v methotrexate for graft-v-host disease prevention in patients given marrow grafts for leukemia: long-term follow-up of three controlled trials. Blood. 1988;71:293–298. [PubMed] [Google Scholar]
- 15.Weaver CH, Clift RA, Deeg HJ, et al. Effect of graft-versus-host disease prophylaxis on relapse in patients transplanted for acute myeloid leukemia. Bone Marrow Transplant. 1994;14:885–893. [PubMed] [Google Scholar]
- 16.Bacigalupo A, Van Lint MT, Occhini D, et al. Increased risk of leukemia relapse with high-dose cyclosporine A after allogeneic marrow transplantation for acute leukemia. Blood. 1991;77:1423–1428. [PubMed] [Google Scholar]
- 17.Carlens S, Aschan J, Remberger M, Dilber M, Ringden O. Low-dose cyclosporine of short duration increases the risk of mild and moderate GVHD and reduces the risk of relapse in HLA-identical sibling marrow transplant recipients with leukaemia. Bone Marrow Transplant. 1999;24:629–635. doi: 10.1038/sj.bmt.1701954. [DOI] [PubMed] [Google Scholar]
- 18.Remberger M, Mattsson J, Hentschke P, et al. The graft-versus-leukaemia effect in haematopoietic stem cell transplantation using unrelated donors. Bone Marrow Transplant. 2002;30:761–768. doi: 10.1038/sj.bmt.1703735. [DOI] [PubMed] [Google Scholar]
- 19.van Rood JJ, Oudshoorn M. Eleven million donors in Bone Marrow Donors Worldwide! Time for reassessment? Bone Marrow Transplant. 2008;41:1–9. doi: 10.1038/sj.bmt.1705866. [DOI] [PubMed] [Google Scholar]
- 20.Kiehl MG, Kraut L, Schwerdtfeger R, et al. Outcome of allogeneic hematopoietic stem-cell transplantation in adult patients with acute lymphoblastic leukemia: no difference in related compared with unrelated transplant in first complete remission. J Clin Oncol. 2004;22:2816–2825. doi: 10.1200/JCO.2004.07.130. [DOI] [PubMed] [Google Scholar]
- 21.Saarinen-Pihkala UM, Gustafsson G, Ringden O, et al. No disadvantage in outcome of using matched unrelated donors as compared with matched sibling donors for bone marrow transplantation in children with acute lymphoblastic leukemia in second remission. J Clin Oncol. 2001;19:3406–3414. doi: 10.1200/JCO.2001.19.14.3406. [DOI] [PubMed] [Google Scholar]
- 22.Yakoub-Agha I, Mesnil F, Kuentz M, et al. Allogeneic marrow stem-cell transplantation from human leukocyte antigen-identical siblings versus human leukocyte antigen-allelic-matched unrelated donors (10/10) in patients with standard-risk hematologic malignancy: a prospective study from the French Society of Bone Marrow Transplantation and Cell Therapy. J Clin Oncol. 2006;24:5695–5702. doi: 10.1200/JCO.2006.08.0952. [DOI] [PubMed] [Google Scholar]
- 23.Farag SS, Fehniger TA, Ruggeri L, Velardi A, Caligiuri MA. Natural killer cell receptors: new biology and insights into the graft-versus-leukemia effect. Blood. 2002;100:1935–1947. doi: 10.1182/blood-2002-02-0350. [DOI] [PubMed] [Google Scholar]
- 24.Goulmy E, Schipper R, Pool J, et al. Mismatches of minor histocompatibility antigens between HLA-identical donors and recipients and the development of graft-versus-host disease after bone marrow transplantation. N Engl J Med. 1996;334:281–285. doi: 10.1056/NEJM199602013340501. [DOI] [PubMed] [Google Scholar]
- 25.Hambach L, Spierings E, Goulmy E. Risk assessment in haematopoietic stem cell transplantation: minor histocompatibility antigens. Best Pract Res Clin Haematol. 2007;20:171–187. doi: 10.1016/j.beha.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Shaw BE, Gooley TA, Malkki M, et al. The importance of HLA-DPB1 in unrelated donor hematopoietic cell transplantation. Blood. 2007;110:4560–4566. doi: 10.1182/blood-2007-06-095265. [DOI] [PubMed] [Google Scholar]
- 27.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man: a long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 29.Gale RP, Horowitz MM, Weiner RS, et al. Impact of cytogenetic abnormalities on outcome of bone marrow transplants in acute myelogenous leukemia in first remission. Bone Marrow Transplant. 1995;16:203–208. [PubMed] [Google Scholar]
- 30.Marks DI, Cullis JO, Ward KN, et al. Allogeneic bone marrow transplantation for chronic myeloid leukemia using sibling and volunteer unrelated donors: a comparison of complications in the first 2 years. Ann Intern Med. 1993;119:207–214. doi: 10.7326/0003-4819-119-3-199308010-00005. [DOI] [PubMed] [Google Scholar]
- 31.Remberger M, Svahn BM, Mattsson J, Ringden O. Dose study of thymoglobulin during conditioning for unrelated donor allogeneic stem-cell transplantation. Transplantation. 2004;78:122–127. [PubMed] [Google Scholar]
- 32.Ringden O, Hermans J, Labopin M, Apperley J, Gorin NC, Gratwohl A. The highest leukaemia-free survival after allogeneic bone marrow transplantation is seen in patients with grade I acute graft-versus-host disease. Acute and Chronic Leukaemia Working Parties of the European Group for Blood and Marrow Transplantation (EBMT). Leuk Lymphoma. 1996;24:71–79. doi: 10.3109/10428199609045715. [DOI] [PubMed] [Google Scholar]
- 33.Atkinson K, Horowitz MM, Gale RP, et al. Risk factors for chronic graft-versus-host disease after HLA-identical sibling bone marrow transplantation. Blood. 1990;75:2459–2464. [PubMed] [Google Scholar]
- 34.Ringden O, Paulin T, Lonnqvist B, Nilsson B. An analysis of factors predisposing to chronic graft-versus-host disease. Exp Hematol. 1985;13:1062–1067. [PubMed] [Google Scholar]
- 35.Storb R, Prentice RL, Sullivan KM, et al. Predictive factors in chronic graft-versus-host disease in patients with aplastic anemia treated by marrow transplantation from HLA-identical siblings. Ann Intern Med. 1983;98:461–466. doi: 10.7326/0003-4819-98-4-461. [DOI] [PubMed] [Google Scholar]
- 36.Remberger M, Storer B, Ringden O, Anasetti C. Association between pretransplant Thymoglobulin and reduced non-relapse mortality rate after marrow transplantation from unrelated donors. Bone Marrow Transplant. 2002;29:391–397. doi: 10.1038/sj.bmt.1703374. [DOI] [PubMed] [Google Scholar]
- 37.Riddell SR, Berger C, Murata M, Randolph S, Warren EH. The graft versus leukemia response after allogeneic hematopoietic stem cell transplantation. Blood Rev. 2003;17:153–162. doi: 10.1016/s0268-960x(03)00007-9. [DOI] [PubMed] [Google Scholar]
- 38.Ringden O, Labopin M, Gorin NC, et al. Is there a graft-versus-leukaemia effect in the absence of graft-versus-host disease in patients undergoing bone marrow transplantation for acute leukaemia? Br J Haematol. 2000;111:1130–1137. doi: 10.1046/j.1365-2141.2000.02493.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.